Tryptophan Seed Treatment Improves Morphological, Biochemical, and Photosynthetic Attributes of the Sunflower under Cadmium Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Layout
2.2. Morphological Attributes
2.3. Photosynthetic Pigment Determination
2.4. Analysis of Biochemical Parameters
2.4.1. Malondialdehyde (MDA) Content Estimation
2.4.2. Estimation of Hydrogen Peroxide Content
2.5. Laboratory Analysis of Osmoprotectant
2.5.1. Phenolic Contents Determination (mg/g FW)
2.5.2. Total Flavonoids (mg/g FW)
2.5.3. Anthocyanin Determination (mg/g FW)
2.5.4. Ascorbic Acid Determination (mg/g FW)
2.5.5. Total Soluble Proteins Determination (mg/g FW)
Preparation of Bradford Reagent
2.5.6. Determination of Total Soluble Sugar (TSS) (mg/g FW)
2.6. Statistical Analysis
3. Results
3.1. Root and Shoot Characteristics
3.2. Photosynthetic Pigments
3.3. Germination and Leaf Parameters
3.4. Biochemical Attributes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeleke, B.S.; Babalola, O.O. Oilseed crop sunflower (Helianthus annuus) as a source of food: Nutritional and health benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. World Agricultural Production; United States Department of Agriculture: Washington, DC, USA, 2023; p. 33. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 1 December 2023).
- Rizwan, M.; Ali, S.; Rizvi, H.; Rinklebe, J.; Tsang, D.C.; Meers, E.; Ok, Y.S.; Ishaque, W. Phytomanagement of heavy metals in contaminated soils using sunflower: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1498–1528. [Google Scholar] [CrossRef]
- Rezapour, S.; Siavash Moghaddam, S.; Nouri, A.; Khosravi Aqdam, K. Urbanization influences the distribution, enrichment, and ecological health risk of heavy metals in croplands. Sci. Rep. 2022, 12, 3868. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.T.; Nauman, M.; Nazir, N.; Ali, S.; Bangash, N. Environmental hazards of cadmium: Past, present, and future. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–183. [Google Scholar]
- Hocaoğlu-Özyiğit, A.; Genç, B.N. Cadmium in plants, humans and the environment. Front. Life Sci. Relat. Technol. 2020, 1, 12–21. [Google Scholar]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Benoit, D.L.; Watson, A.K. Effect of heavy metals on seed germination and seedling growth of common ragweed and roadside ground cover legumes. Environ. Pollut. 2016, 213, 112–118. [Google Scholar] [CrossRef]
- Moradi, R.; Pourghasemian, N.; Naghizadeh, M. Effect of beeswax waste biochar on growth, physiology and cadmium uptake in saffron. J. Clean. Prod. 2019, 229, 1251–1261. [Google Scholar] [CrossRef]
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef]
- Sabreena; Hassan, S.; Bhat, S.A.; Kumar, V.; Ganai, B.A.; Ameen, F. Phytoremediation of heavy metals: An indispensable contrivance in green remediation technology. Plants 2022, 11, 1255. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Bashir, T.; Hashem, A.; Abd_Allah, E.F.; Khan, A.L.; Al-Harrasi, A.S. Early events in plant abiotic stress signaling: Interplay between calcium, reactive oxygen species and phytohormones. J. Plant Growth Regul. 2018, 37, 1033–1049. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.; Sarwar, T.; Nawaz, A.; Ijaz, M.; Sattar, A.; Ahmad, S. Methods of seed priming. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants 2019; Springer: Singapore, 2019; pp. 1–10. [Google Scholar] [CrossRef]
- Blum, R.; Beck, A.; Korte, A.; Stengel, A.; Letzel, T.; Lendzian, K.; Grill, E. Function of phytochelatin synthase in catabolism of glutathione-conjugates. Plant J. 2007, 49, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Kamran, M.; Rizwan, M.; Ali, S.; Parveen, A.; Malik, Z.; Wang, X. Cadmium uptake and translocation: Selenium and silicon roles in Cd detoxification for the production of low Cd crops: A critical review. Chemosphere 2021, 273, 129690. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kumar, V.; Bose, B.; Singhal, R.K. Cadmium toxicity in plants and alleviation through seed priming approach. Plant Physiol. Rep. 2021, 26, 647–660. [Google Scholar] [CrossRef]
- Arnao, M.; Hernández-Ruiz, J. Melatonin: Synthesis from tryptophan and its role in higher plant. In Amino Acids in Higher Plants; CAB International: Wallingford, UK, 2015; pp. 390–435. [Google Scholar]
- Rai, V. Role of amino acids in plant responses to stresses. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Sadak, M.S.; Ramadan, A.A.E.-M. Impact of melatonin and tryptophan on water stress tolerance in white lupine (Lupinus termis L.). Physiol. Mol. Biol. Plants 2021, 27, 469–481. [Google Scholar] [CrossRef]
- Zahir, A.; Malik, M.; Arshad, M. Effect of auxins on the growth and yield of rice. Pak. J. Agric. Sci. 1999, 36, 3–4. [Google Scholar]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef]
- Muneer, M.; Saleem, M.; Abbas, S.H.; Hussain, I.; Asim, M. Using L-Tryptophan to influence the crop growth of maize at different harvesting stages. Int. J. Biol. Biotechnol. 2009, 6, 251–255. [Google Scholar]
- El-Awadi, M.; El-Bassiony, A.; Fawzy, Z.; El-Nemr, M. Response of snap bean (Phaseolus vulgaris L.) plants to nitrogen fertilizer and foliar application with methionine and tryptophan. Nat. Sci. 2011, 9, 87–94. [Google Scholar]
- Abbas, S.H.; Sohail, M.; Saleem, M.; Mahmood, T.; Aziz, I.; Qamar, M.; Majeed, A.; Arif, M. Effect of L-tryptophan on plant weight and pod weight in chickpea under rainfed conditions. Sci. Tech. Dev. 2013, 32, 277–280. [Google Scholar]
- Mustafa, A.; Hussain, A.; Naveed, M.; Ditta, A.; Nazli, Z.-e.-H.; Sattar, A. Response of okra (Abelmoschus esculentus L.) to soil and foliar applied L-tryptophan. Soil Environ. 2016, 35, 76–84. [Google Scholar]
- Frankenberger, W.; Arshad, M. Yield response of watermelon and muskmelon to L-tryptophan applied to soil. HortScience 1991, 26, 35–37. [Google Scholar] [CrossRef]
- Solanki, M.; Shukla, L.I. Recent advances in auxin biosynthesis and homeostasis. 3 Biotech 2023, 13, 290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, S.; Shi, L.; Gong, D.; Zhang, S.; Zhao, Q.; Zhan, D.; Vasseur, L.; Wang, Y.; Yu, J. Haplotype-resolved genome assembly provides insights into evolutionary history of the tea plant Camellia sinensis. Nat. Genet. 2021, 53, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, Z.; Kong, X.; Chen, Y.; Li, J. Exogenous tryptophan application improves cadmium tolerance and inhibits cadmium upward transport in broccoli (Brassica oleracea var. italica). Front. Plant Sci. 2022, 13, 969675. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.M.; Mazhar, A.A.; Mahgoub, M.H.; Abd El-Aziz, N.G.; Darwish, M.A.; Shanan, N.T. Investigation the effect of L-tryptophan on growth and chemical composition of Eucalyptus gomphocephala plants under cadmium stress. Middle East J. Agric. Res 2019, 8, 106–116. [Google Scholar]
- Jaffar, M.T.; Mushtaq, Z.; Waheed, A.; Asghar, H.N.; Zhang, J.; Han, J. Pseudomonas fluorescens and L-tryptophan application triggered the phytoremediation potential of sunflower (Heliantus annuus L.) in lead-contaminated soil. Environ. Sci. Pollut. Res. 2023, 30, 120461–120471. [Google Scholar] [CrossRef]
- Khattab, M.; Shehata, A.; El-Saadate, A.; Al-Hasni, K. Effect of glycine, methionine and tryptophan on the vegetative growth, flowering and corms production of gladiolus plant. Alex. Sci. Exch. J. 2016, 37, 647–659. [Google Scholar]
- Rahmatzadeh, S.; Khara, J.; Kazemitabar, S. Effects of tryptophan on growth and some physiological parameters in mycorrhizal inoculated plants of Catharanthus roseus (L.) G. Don. Int. J. Agric. Res. Rev. 2012, 2, 564–572. [Google Scholar]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.-D.; Wang, W.-Z.; Hu, J.-D.; Zhang, X.-M.; Wang, J.-B.; Wang, B.-S. Nondestructive determination of total chlorophyll content in maize using three-wavelength diffuse reflectance. J. Appl. Spectrosc. 2016, 83, 541–547. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: II. Role of electron transfer. Arch. Biochem. Biophys. 1968, 125, 850–857. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Hodges, D.M.; Nozzolillo, C. Anthocyanin and anthocyanoplast content of cruciferous seedlings subjected to mineral nutrient deficiencies. J. Plant Physiol. 1996, 147, 749–754. [Google Scholar] [CrossRef]
- Mukherjee, S.; Choudhuri, M. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Riazi, A.; Matsuda, K.; Arslan, A. Water-stress induced changes in concentrations of proline and other solutes in growing regions of young barley leaves. J. Exp. Bot. 1985, 36, 1716–1725. [Google Scholar] [CrossRef]
- Hu, B.; Jia, X.; Hu, J.; Xu, D.; Xia, F.; Li, Y. Assessment of heavy metal pollution and health risks in the soil-plant-human system in the Yangtze River Delta, China. Int. J. Environ. Res. Public Health 2017, 14, 1042. [Google Scholar] [CrossRef] [PubMed]
- Hussaan, M.; Tanwir, K.; Abbas, S.; Javed, M.T.; Iqbal, N. Zinc–Lysine (Zn–Lys) Decipher Cadmium Tolerance by Improved Antioxidants, Nutrient Acquisition, and Diminished Cd Retention in Two Contrasting Wheat Cultivars. J. Plant Growth Regul. 2021, 41, 3479–3497. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.S.; Wang, P.; Yin, N.; Rahman, M.H.; Du, H.; Cai, X.; Fu, Y.; Cui, Y. Assessment of nutrients effect on the bioaccessibility of Cd and Cu in contaminated soil. Ecotoxicol. Environ. Saf. 2020, 202, 110913. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.J.; Zahir, Z.A.; Jamil, A. Effect of cadmium on seed germination and seedling growth of four wheat (Triticum aestivum L.) cultivars. Pak. J. Bot. 2012, 44, 1569–1574. [Google Scholar]
- Catiempo, R.; Photchanachai, S.; Bayogan, E.R.; Wongs-Aree, C. Impact of hydropriming on germination and seedling establishment of sunflower seeds at elevated temperature. Plant Soil Environ. 2021, 67, 491–498. [Google Scholar] [CrossRef]
- Ding, Y.; Feng, R.; Wang, R.; Guo, J.; Zheng, X. A dual effect of Se on Cd toxicity: Evidence from plant growth, root morphology and responses of the antioxidative systems of paddy rice. Plant Soil 2014, 375, 289–301. [Google Scholar] [CrossRef]
- Bayat, M.; Faramarzi, A.; Ajalli, J.; Abdi, M.; Nourafcan, H. Bioremediation of potentially toxic elements of sewage sludge using sunflower (Heliantus annus L.) in greenhouse and field conditions. Environ. Geochem. Health 2022, 44, 1217–1227. [Google Scholar] [CrossRef]
- Benavides, B.J.; Drohan, P.; Spargo, J.; Maximova, S.; Guiltinan, M.; Miller, D. Cadmium phytoextraction by Helianthus annuus (sunflower), Brassica napus cv Wichita (rapeseed), and Chyrsopogon zizanioides (vetiver). Chemosphere 2021, 265, 129086. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Mazhar, A.A.; Abd El-Aziz, N.G.; Mahgoub, M.H.; Darwish, M.A.; Shanan, N.T. Response of Khaya senegalensis plants to growth improvement by L-Tryptophan under cadmium stress condition. Middle East J. 2018, 7, 847–857. [Google Scholar]
- Kahveci, H.; Bilginer, N.; Diraz-Yildirim, E.; Kulak, M.; Yazar, E.; Kocacinar, F.; Karaman, S. Priming with salicylic acid, β-carotene and tryptophan modulates growth, phenolics and essential oil components of Ocimum basilicum L. grown under salinity. Sci. Hortic. 2021, 281, 109964. [Google Scholar] [CrossRef]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int. J. Plant Prod. 2009, 3, 65–76. [Google Scholar] [CrossRef]
- Jacob, R.H.; Afify, A.S.; Shanab, S.M.; Shalaby, E.A. Chelated amino acids: Biomass sources, preparation, properties, and biological activities. Biomass Convers. Biorefinery 2022, 1–15. [Google Scholar] [CrossRef]
- Al Mahmud, J.; Bhuyan, M.B.; Anee, T.I.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Reactive oxygen species metabolism and antioxidant defense in plants under metal/metalloid stress. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches 2019; Springer: Cham, Switzerland, 2019; pp. 221–257. [Google Scholar] [CrossRef]
- Ni, H.; Liu, F.; Liang, X.; Yin, Y.; Liu, G. The role of zinc chelate of hydroxy analogue of methionine in cadmium toxicity: Effects on cadmium absorption on intestinal health in piglets. Animal 2020, 14, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Pandey, K.; Singh, L.K. Microbial Production and Applications of L-lysine. Innov. Food Technol. Curr. Perspect. Future Goals 2020, 211–229. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, D.; Liu, Q. Connections between amino acid metabolisms in plants: Lysine as an example. Front. Plant Sci. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, F.; Ali, Q.; Ali, S.; Al-Misned, F.A.; Maqbool, S. Fertigation with Zn-Lysine Confers Better Photosynthetic Efficiency and Yield in Water Stressed Maize: Water Relations, Antioxidative Defense Mechanism and Nutrient Acquisition. Plants 2022, 11, 404. [Google Scholar] [CrossRef]
- Grajek, H.; Rydzyński, D.; Piotrowicz-Cieślak, A.; Herman, A.; Maciejczyk, M.; Wieczorek, Z. Cadmium ion-chlorophyll interaction–Examination of spectral properties and structure of the cadmium-chlorophyll complex and their relevance to photosynthesis inhibition. Chemosphere 2020, 261, 127434. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, F.; Adrees, M.; Zia-ur-Rehman, M.; Farid, M.; Gill, R.A.; Ali, B. Role of organic and inorganic amendments in alleviating heavy metal stress in oilseed crops. In Oilseed Crops: Yield and Adaptations under Environmental Stress; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 224–235. [Google Scholar] [CrossRef]
- Sowmya, R.; Warke, V.G.; Mahajan, G.B.; Annapure, U.S. Effect of amino acids on growth, elemental content, functional groups, and essential oils composition on hydroponically cultivated coriander under different conditions. Ind. Crops Prod. 2023, 197, 116577. [Google Scholar] [CrossRef]
- Mahmood, S.; Wahid, A.; Azeem, M.; Zafar, S.; Bashir, R.; Bajwa, M.O.S.; Ali, S. Tyrosine or lysine priming modulated phenolic metabolism and improved cadmium stress tolerance in mung bean (Vigna radiata L.). S. Afr. J. Bot. 2022, 149, 397–406. [Google Scholar] [CrossRef]
- Huybrechts, M.; Hendrix, S.; Kyndt, T.; Demeestere, K.; Vandamme, D.; Cuypers, A. Short-term effects of cadmium on leaf growth and nutrient transport in rice plants. Plant Sci. 2021, 313, 111054. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Bareen, F.-E.; Abbas, Z.; Bukhari, S.A.H.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Zinc-lysine prevents chromium-induced morphological, photosynthetic, and oxidative alterations in spinach irrigated with tannery wastewater. Environ. Sci. Pollut. Res. 2019, 26, 28951–28961. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.E.-A.H.; Abo-Shady, A.M.; Gaafar, R.M.; Ismail, G.A.; El-Nagar, M.M. Assessment of cyanobacteria and tryptophan role in the alleviation of the toxic action of brominal herbicide on wheat plants. Gesunde Pflanz. 2023, 75, 785–799. [Google Scholar] [CrossRef]
- Byeon, Y.; Back, K. An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield. J. Pineal Res. 2014, 56, 408–414. [Google Scholar] [CrossRef]
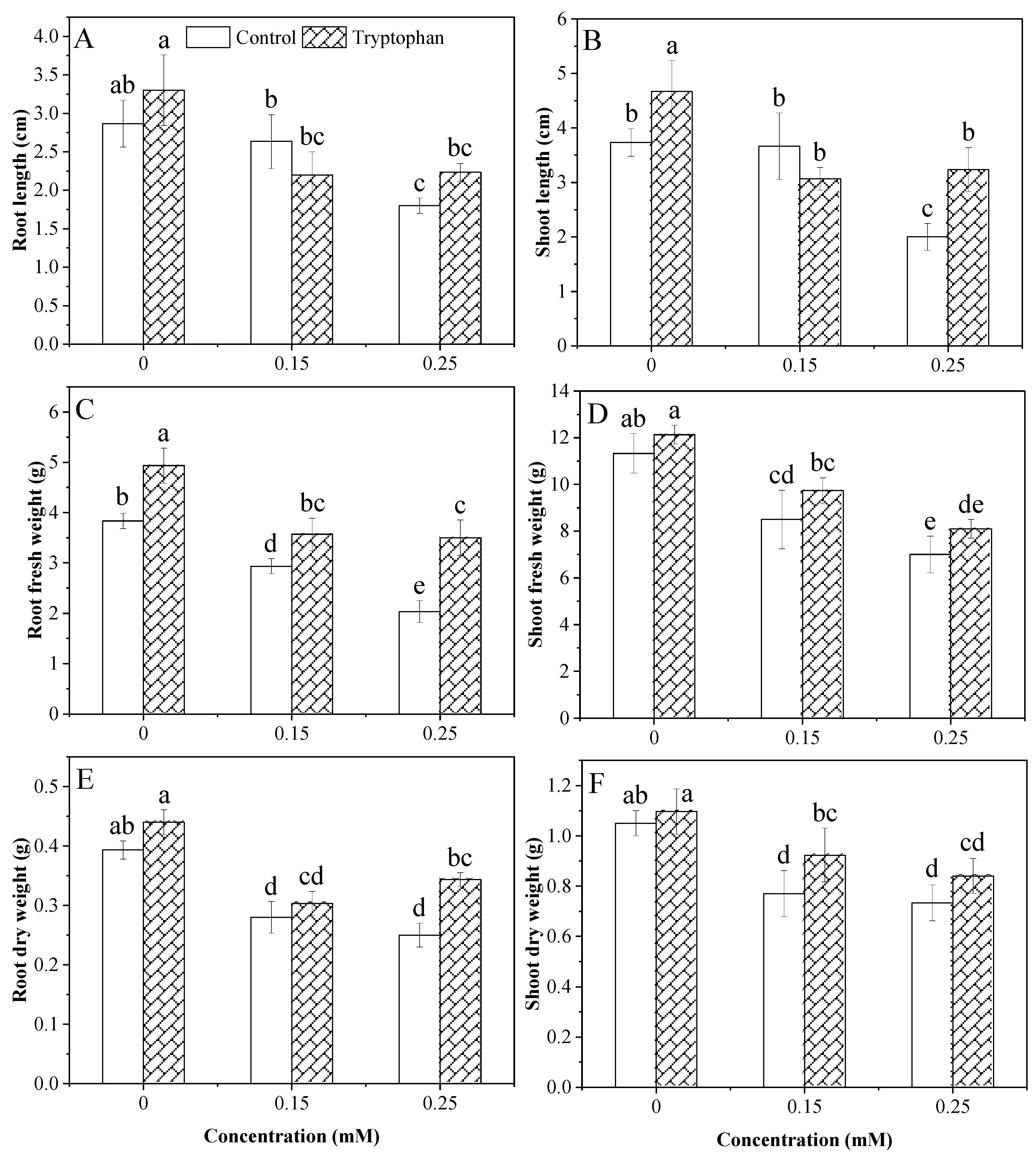



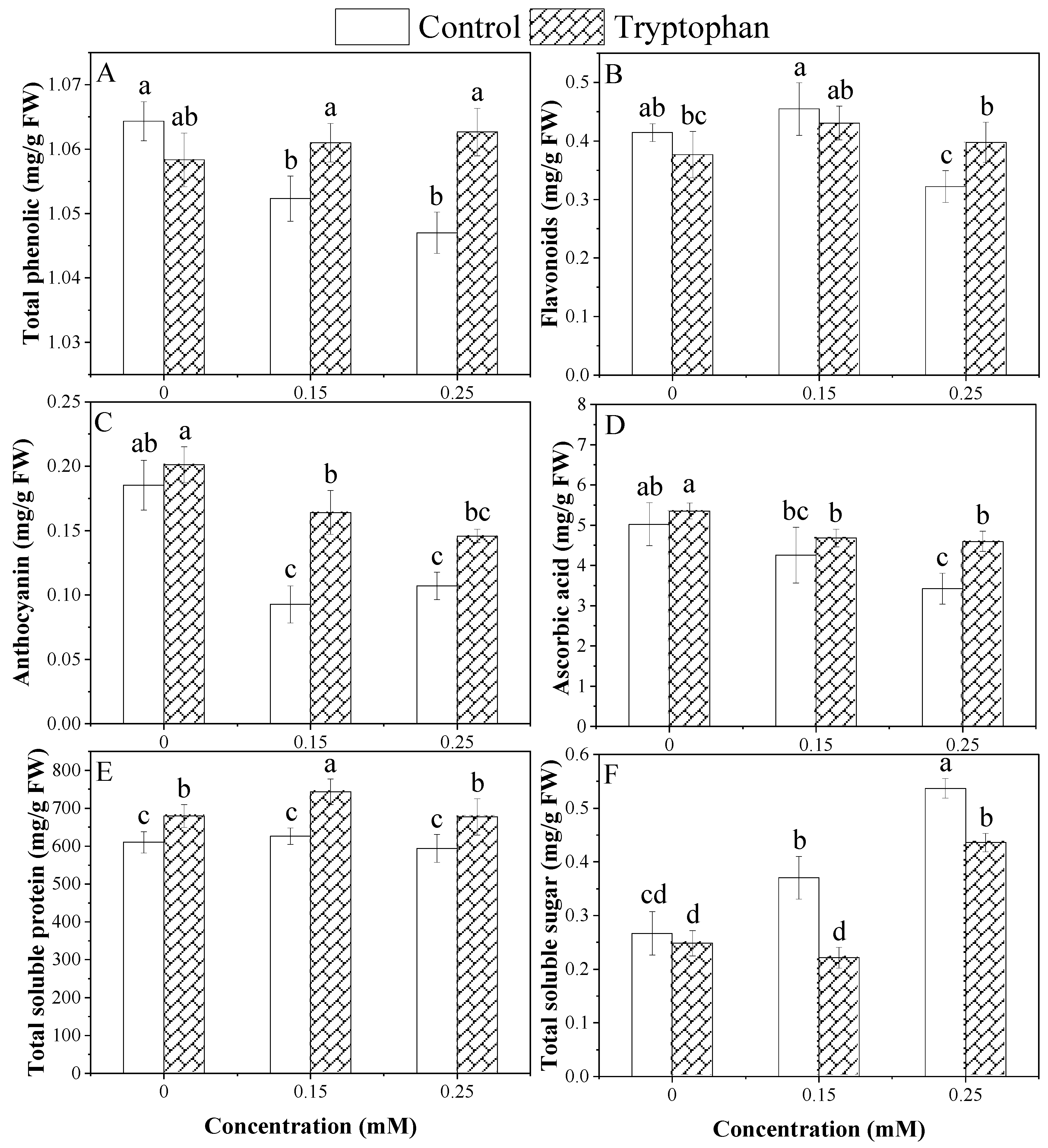
| Treatments | Root Length (cm) | Shoot Length (cm) | Root Fresh Weight (g) | Shoot Fresh Weight (g) | Root Dry Weight (g) | Shoot Dry Weight (g) | Chl a (mg/g FW) | Chl b (mg/g FW) | Chl a/b (mg/g FW) | Total Chl (mg/g FW) | Total Carotenoid (mg/g FW) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ANOVA | |||||||||||
| Cd Stress (A) | *** | *** | *** | *** | *** | *** | NS | *** | *** | *** | *** |
| Tryptophan (B) | NS | * | *** | * | *** | * | *** | ** | NS | * | *** |
| A × B | NS | ** | NS | NS | NS | NS | ** | *** | *** | *** | ** |
| Treatments | Seed Germination | No. of Leaves/Plant | Average Leaf Area (cm2) | Average Leaf Length (cm) | Average Leaf Fresh Weight (g) | Average Leaf Dry Weight (g) |
|---|---|---|---|---|---|---|
| ANOVA | ||||||
| Cd Stress (A) | ** | *** | ** | * | * | * |
| Tryptophan (B) | * | * | * | * | * | * |
| A × B | NS | ** | NS | NS | NS | NS |
| Treatments | MDA (µmol/mL FW) | H2O2 (µmol/mL FW) | Total Phenolic (mg/g FW) | Total Flavonoid (mg/g FW) | Anthocyanin (mg/g FW) | Ascorbic Acid (mg/g FW) | Total Soluble Protein (mg/g FW) | Total Soluble Sugar (mg/g FW) |
|---|---|---|---|---|---|---|---|---|
| ANOVA | ||||||||
| Cd Stress (A) | *** | NS | NS | ** | *** | ** | ** | *** |
| Tryptophan (B) | *** | NS | ** | NS | *** | ** | *** | *** |
| A × B | ** | * | * | * | * | * | NS | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.; Kaousar, R.; Ali, S.; Shan, C.; Wang, G.; Wang, S.; Lan, Y. Tryptophan Seed Treatment Improves Morphological, Biochemical, and Photosynthetic Attributes of the Sunflower under Cadmium Stress. Plants 2024, 13, 237. https://doi.org/10.3390/plants13020237
Hussain M, Kaousar R, Ali S, Shan C, Wang G, Wang S, Lan Y. Tryptophan Seed Treatment Improves Morphological, Biochemical, and Photosynthetic Attributes of the Sunflower under Cadmium Stress. Plants. 2024; 13(2):237. https://doi.org/10.3390/plants13020237
Chicago/Turabian StyleHussain, Mujahid, Rehana Kaousar, Sharafat Ali, Changfeng Shan, Guobin Wang, Shizhou Wang, and Yubin Lan. 2024. "Tryptophan Seed Treatment Improves Morphological, Biochemical, and Photosynthetic Attributes of the Sunflower under Cadmium Stress" Plants 13, no. 2: 237. https://doi.org/10.3390/plants13020237
APA StyleHussain, M., Kaousar, R., Ali, S., Shan, C., Wang, G., Wang, S., & Lan, Y. (2024). Tryptophan Seed Treatment Improves Morphological, Biochemical, and Photosynthetic Attributes of the Sunflower under Cadmium Stress. Plants, 13(2), 237. https://doi.org/10.3390/plants13020237








