Relationship between Cumulative Temperature and Light Intensity and G93 Parameters of Isoprene Emission for the Tropical Tree Ficus septica
Abstract
:1. Introduction
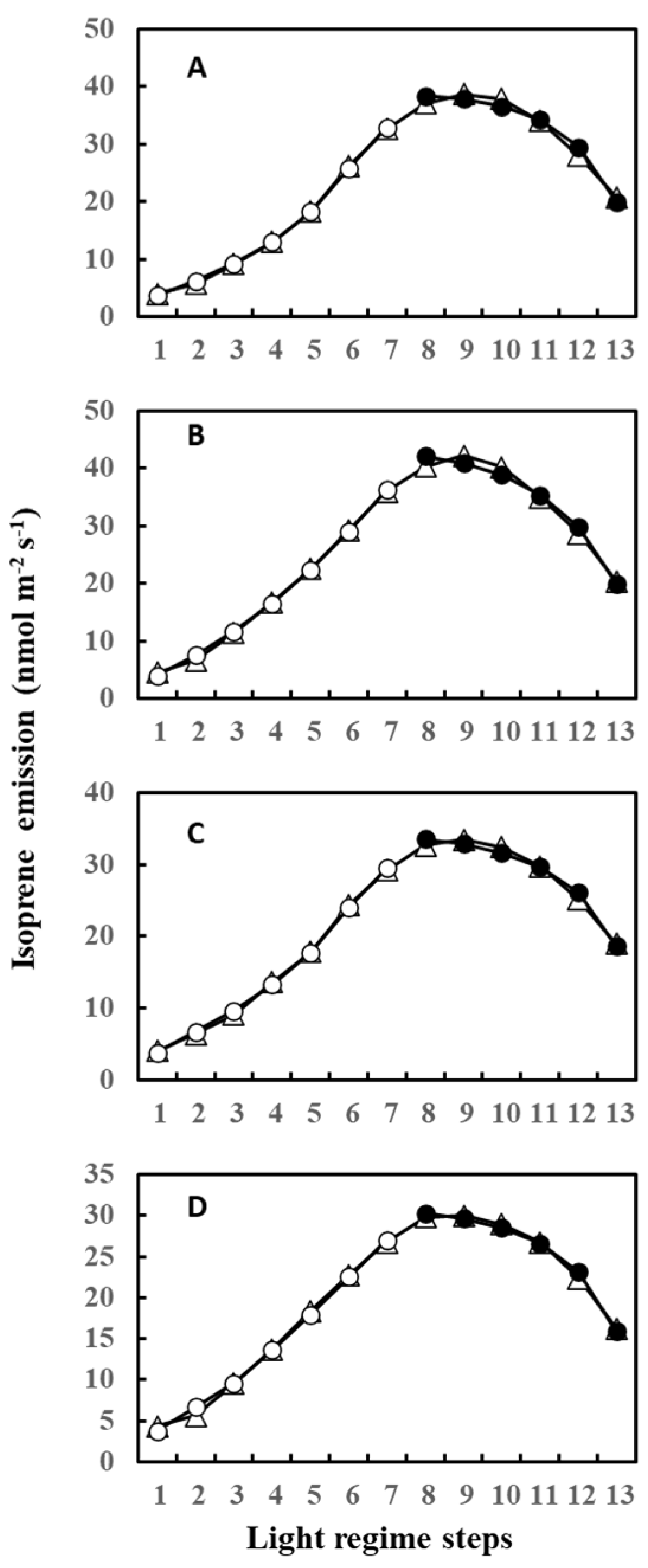
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Experimental Design
4.3. Measurement of Isoprene Emission and Photosynthesis
4.4. Parameterization of G93
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 2006, 6, 3181–3210. [Google Scholar] [CrossRef]
- Loreto, F.; Pollastri, S.; Fineschi, S.; Velikova, V. Volatile isoprenoids and their importance for protection against environmental constraints in the Mediterranean area. Environ. Exp. Bot. 2014, 103, 99–106. [Google Scholar] [CrossRef]
- Zuo, Z.; Weraduwage, S.M.; Lantz, A.T.; Sanchez, L.M.; Weise, S.E.; Wang, J.; Childs, K.L.; Sharkey, T.D. Isoprene Acts as a Signaling Molecule in Gene Networks Important for Stress Responses and Plant Growth. Plant Physiol. 2019, 180, 124–152. [Google Scholar] [CrossRef]
- Behnke, K.; Kaiser, A.; Zimmer, I.; Brüggemann, N.; Janz, D.; Polle, A.; Hampp, R.; Hänsch, R.; Popko, J.; Schmitt-Kopplin, P.; et al. RNAi-mediated suppression of isoprene emission in poplar transiently impacts phenolic metabolism under high temperature and high light intensities: A transcriptomic and metabolomic analysis. Plant Mol. Biol. 2010, 74, 61–75. [Google Scholar] [CrossRef]
- Harvey, C.M.; Sharkey, T.D. Exogenous isoprene modulates gene expression in unstressed Arabidopsis thaliana plants. Plant Cell Environ. 2016, 39, 1251–1263. [Google Scholar] [CrossRef]
- Sasaki, K.; Saito, T.; Lämsä, M.; Oksman-Caldentey, K.M.; Suzuki, M.; Ohyama, K.; Muranaka, T.; Ohara, K.; Yazaki, K. Plants utilize isoprene emission as a thermotolerance mechanism. Plant Cell Physiol. 2007, 48, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, E.; Merl-Pham, J.; Velikova, V.; Ghirardo, A.; Lindermayr, C.; Hauck, S.M.; Bernhardt, J.; Riedel, K.; Durner, J.; Schnitzler, J.P. Modulation of protein s-nitrosylation by isoprene emission in poplar. Plant Physiol. 2016, 170, 1945–1961. [Google Scholar] [CrossRef]
- Velikova, V.; Ghirardo, A.; Vanzo, E.; Merl, J.; Hauck, S.M.; Schnitzler, J.P. Genetic manipulation of isoprene emissions in poplar plants remodels the chloroplast proteome. J. Proteome Res. 2014, 13, 2005–2018. [Google Scholar] [CrossRef]
- Monson, R.K.; Weraduwage, S.M.; Rosenkranz, M.; Schnitzler, J.P.; Sharkey, T.D. Leaf isoprene emission as a trait that mediates the growth-defense tradeoff in the face of climate stress. Oecologia 2021, 197, 885–902. [Google Scholar] [CrossRef]
- Pollastri, S.; Velikova, V.; Castaldini, M.; Fineschi, S.; Ghirardo, A.; Renaut, J.; Schnitzler, J.P.; Sergeant, K.; Winkler, J.B.; Zorzan, S.; et al. Isoprene-Emitting Tobacco Plants Are Less Affected by Moderate Water Deficit under Future Climate Change Scenario and Show Adjustments of Stress-Related Proteins in Actual Climate. Plants 2023, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Achakulwisut, P.; Mickley, L.J.; Murray, L.T.; Alexander, B. Uncertainties in isoprene photochemistry and emissions: Implications for the oxidative capacity of past and present atmospheres and for trends in climate forcing agents. Atmos. Chem. Phys. Discuss. 2015, 15, 2197. [Google Scholar] [CrossRef]
- Pike, R.C.; Young, P.J. How plants can influence tropospheric chemistry: The role of isoprene emissions from the biosphere. Weather 2009, 64, 332–336. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Apel, E.C.; Schwantes, R.H.; Hornbrook, R.S.; Hills, A.J.; DeMarsh, K.E.; Moo, Z.; Ortega, J.; Brune, W.H.; et al. Probing isoprene photochemistry at atmospherically relevant nitric oxide levels. Chem 2022, 8, 3225–3240. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Wu, C.; Cao, C.; Ren, Y.; Wang, J.; Li, J.; Cao, J.; Zeng, L.; Zhu, T. Characterization of isoprene-derived secondary organic aerosols at a rural site in North China Plain with implications for anthropogenic pollution effects. Sci. Rep. 2018, 8, 535. [Google Scholar] [CrossRef] [PubMed]
- Surratt, J.D.; Chan, A.W.; Eddingsaas, N.C.; Chan, M.; Loza, C.L.; Kwan, A.J.; Hersey, S.P.; Flagan, R.C.; Wennberg, P.O.; Seinfeld, J.H. Reactive intermediates revealed in secondary organic aerosol formation from isoprene. Proc. Natl. Acad. Sci. USA 2010, 107, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Claeys, M.; Graham, B.; Vas, G.; Wang, W.; Vermeylen, R.; Pashynska, V.; Cafmeyer, J.; Guyon, P.; Andreae, M.O.; Artaxo, P.; et al. Formation of Secondary Organic Aerosols Through Photooxidation of Isoprene. Science 2004, 303, 1173–1176. [Google Scholar] [CrossRef]
- Claeys, M.; Wang, W.; Ion, A.C.; Kourtchev, I.; Gelencsér, A.; Maenhaut, W. Formation of secondary organic aerosols from isoprene and its gas-phase oxidation products through reaction with hydrogen peroxide. Atmos. Environ. 2004, 38, 4093–4098. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Schwender, J.; Disch, A.; Rohmer, M. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett. 1997, 400, 271–274. [Google Scholar] [CrossRef]
- Rohmer, M. Diversity in isoprene unit biosynthesis: The methylerythritol phosphate pathway in bacteria and plastids. Pure Appl. Chem. 2007, 79, 739–751. [Google Scholar] [CrossRef]
- Zeidler, J.G.; Lichtenthaler, H.K.; May, H.U.; Lichtenthaler, F.W. Is isoprene emitted by plants synthesized via the novel isopentenyl pyrophosphate pathway? Z. Fur Naturforschung Sect. C—J. Biosci. 1997, 52, 15–23. [Google Scholar] [CrossRef]
- Delwiche, C.F.; Sharkey, T.D. Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ. 2006, 16, 587–591. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Preiser, A.L.; Weraduwage, S.M.; Gog, L. Source of 12C in Calvin-Benson cycle intermediates and isoprene emitted from plant leaves fed with 13CO2. Biochem. J. 2020, 477, 3237–3252. [Google Scholar] [CrossRef] [PubMed]
- Monson, R.K.; Fall, R. Isoprene emission from aspen leaves: Influence of environment and relation to photosynthesis and photorespiration. Plant Physiol. 1989, 90, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Monson, R.K.; Jaeger, C.H.; Adams, W.W.; Driggers, E.M.; Silver, G.M.; Fall, R. Relationships among isoprene emission rate, photosynthesis, and isoprene synthase activity as influenced by temperature. Plant Physiol. 1992, 98, 1175–1180. [Google Scholar] [CrossRef]
- Tingey, D.T.; Manning, M.; Grothaus, L.C.; Burns, W.F. The Influence of Light and Temperature on Isoprene Emission Rates from Live Oak. Physiol. Plant. 1979, 47, 112–118. [Google Scholar] [CrossRef]
- Sahu, A.; Mostofa, M.G.; Weraduwage, S.M.; Sharkey, T.D. Hydroxymethylbutenyl diphosphate accumulation reveals MEP pathway regulation for high CO2-induced suppression of isoprene emission. Proc. Natl. Acad. Sci. USA 2023, 120, e2309536120. [Google Scholar] [CrossRef] [PubMed]
- Lantz, A.T.; Allman, J.; Weraduwage, S.M.; Sharkey, T.D. Isoprene: New insights into the control of emission and mediation of stress tolerance by gene expression. Plant Cell Environ. 2019, 42, 2808–2826. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Monson, R.K. Isoprene research—60 years later, the biology is still enigmatic. Plant Cell Environ. 2017, 40, 1671–1678. [Google Scholar] [CrossRef]
- Monson, R.K.; Grote, R.; Niinemets, Ü.; Schnitzler, J.P. Modeling the isoprene emission rate from leaves. New Phytol. 2012, 195, 541–559. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, Y.S.; Tan, Y.R.; Tan, Y.; Bai, J.H.; Gu, D.S.; Ma, Z.Z.; Du, J.H.; Han, Z.Y. Effects of light on the emissions of biogenic isoprene and monoterpenes: A review. Atmos. Pollut. Res. 2022, 13, 101397. [Google Scholar] [CrossRef]
- Grote, R.; Monson, R.K.; Niinemets, Ü. Leaf-Level Models of Constitutive and Stress-Driven Volatile Organic Compound Emissions; Springer: Dordrecht, The Netherlands, 2013; p. 41. [Google Scholar]
- Guenther, A.B.; Zimmerman, P.R.; Harley, P.C.; Monson, R.K.; Fall, R. Isoprene and monoterpene emission rate variability: Model evaluations and sensitivity analyses. J. Geophys. Res. 1993, 98, 12609–12617. [Google Scholar] [CrossRef]
- Guenther, A.; Nicholas, C.; Fall, R.; Klinger, L.; McKay, W.A.; Scholes, B. A global model of natural volatile organic compound emissions s Raja the balance Triangle changes in the atmospheric accumulation rates of greenhouse Triangle Several inventories of natural and Exposure Assessment global scales have been two classes Fores. J. Geophys. Res. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Guenther, A. Isoprene emission estimates and uncertainties for the Central African EXPRESSO study domain. J. Geophys. Res. Atmos. 1999, 104, 30625–30639. [Google Scholar] [CrossRef]
- Seco, R.; Holst, T.; Davie-Martin, C.L.; Simin, T.; Guenther, A.; Pirk, N.; Rinne, J.; Rinnan, R. Strong isoprene emission response to temperature in tundra vegetation. Proc. Natl. Acad. Sci. USA 2022, 119, e2118014119. [Google Scholar] [CrossRef]
- Benjamin, M.T.; Sudol, M.; Bloch, L.; Winer, A.M. Low-emitting urban forests: A taxonomic methodology for assigning isoprene and monoterpene emission rates. Atmos. Environ. 1996, 30, 1437–1452. [Google Scholar] [CrossRef]
- Xiaoshan, Z.; Yujing, M.; Wenzhi, S.; Yahui, Z. Seasonal variations of isoprene emissions from deciduous trees. Atmos. Environ. 2000, 34, 3027–3032. [Google Scholar] [CrossRef]
- Chang, K.H.; Chen, T.F.; Huang, H.C. Estimation of biogenic volatile organic compounds emissions in subtropical island—Taiwan. Sci. Total Environ. 2005, 346, 184–199. [Google Scholar] [CrossRef]
- Padhy, P.K.; Varshney, C.K. Isoprene emission from tropical tree species. Environ. Pollut. 2005, 135, 101–109. [Google Scholar] [CrossRef]
- Lerdau, M.; Keller, M. Controls on isoprene emission from trees in a subtropical dry forest. Plant Cell Environ. 1997, 20, 569–578. [Google Scholar] [CrossRef]
- Lerdau, M.T.; Throop, H.L. Isoprene emission and photosynthesis in a tropical forest canopy: Implications for model development. Ecol. Appl. 1999, 9, 1109–1117. [Google Scholar] [CrossRef]
- Alves, E.G.; Harley, P.; Gonçalves, J.F.d.C.; Moura, C.E.d.S.; Jardine, K. Effects of light and temperature on isoprene emission at different leaf developmental stages of eschweilera coriacea in central Amazon. Acta Amaz. 2014, 44, 9–18. [Google Scholar] [CrossRef]
- Mutanda, I.; Inafuku, M.; Iwasaki, H.; Saitoh, S.; Fukuta, M.; Watanabe, K.; Oku, H. Parameterization of G-93 isoprene emission formula for tropical trees Casuarina equisetifolia and Ficus septica. Atmos. Environ. 2016, 141, 287–296. [Google Scholar] [CrossRef]
- Higa, T.; Parveen, S.; Mutanda, I.; Iqbal, M.A.; Inafuku, M.; Hashimoto, F.; Oku, H. Evaluation of isoprene emission rates of tropical trees by an iterative optimization procedure for G-93 parameters. Atmos. Environ. 2018, 192, 209–217. [Google Scholar] [CrossRef]
- Oku, H.; Iwai, S.; Uehara, M.; Iqbal, A.; Mutanda, I.; Inafuku, M. Growth condition controls on G-93 parameters of isoprene emission from tropical trees. J. Plant Res. 2021, 134, 1225–1242. [Google Scholar] [CrossRef] [PubMed]
- Hanson, D.T.; Sharkey, T.D. Effect of growth conditions on isoprene emission and other thermotolerance-enhancing compounds. Plant Cell Environ. 2001, 24, 929–936. [Google Scholar] [CrossRef]
- Hanson, D.T.; Sharkey, T.D. Rate of acclimation of the capacity for isoprene emission in response to light and temperature. Plant Cell Environ. 2001, 24, 937–946. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Singsaas, E.L.; Lerdau, M.T.; Geron, C.D. Weather effects on isoprene emission capacity and applications in emissions algorithms. Ecol. Appl. 1999, 9, 1132–1137. [Google Scholar] [CrossRef]
- Wiberley, A.E.; Donohue, A.R.; Meier, M.E.; Westphal, M.M.; Sharkey, T.D. Regulation of isoprene emission in Populus trichocarpa leaves subjected to changing growth temperature. Plant Cell Environ. 2008, 31, 258–267. [Google Scholar] [CrossRef]
- Pétron, G.; Harley, P.; Greenberg, J.; Guenther, A. Seasonal temperature variations influence isoprene emission. Geophys. Res. Lett. 2001, 28, 1707–1710. [Google Scholar] [CrossRef]
- Rasulov, B.; Bichele, I.; Laisk, A.; Niinemets, Ü. Competition between isoprene emission and pigment synthesis during leaf development in aspen. Plant Cell Environ. 2014, 37, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Rasulov, B.; Hüve, K.; Välbe, M.; Laisk, A.; Niinemets, Ü. Evidence that light, carbon dioxide, and oxygen dependencies of leaf isoprene emission are driven by energy status in hybrid aspen. Plant Physiol. 2009, 151, 448–460. [Google Scholar] [CrossRef]
- Vickers, C.E.; Possell, M.; Hewitt, C.N.; Mullineaux, P.M. Genetic structure and regulation of isoprene synthase in poplar (Populus spp.). Plant Mol. Biol. 2010, 73, 547–558. [Google Scholar] [CrossRef]
- Jardine, K.; Chambers, J.; Alves, E.G.; Teixeira, A.; Garcia, S.; Holm, J.; Higuchi, N.; Manzi, A.; Abrell, L.; Fuentes, J.D.; et al. Dynamic balancing of isoprene carbon sources reflects photosynthetic and photorespiratory responses to temperature stress. Plant Physiol. 2014, 166, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Oku, H.; Inafuku, M.; Takamine, T.; Nagamine, M.; Saitoh, S.; Fukuta, M. Temperature threshold of isoprene emission from tropical trees, Ficus virgata and Ficus septica. Chemosphere 2014, 95, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Mutanda, I.; Inafuku, M.; Saitoh, S.; Iwasaki, H.; Fukuta, M.; Watanabe, K.; Oku, H. Temperature controls on the basal emission rate of isoprene in a tropical tree Ficus septica: Exploring molecular regulatory mechanisms. Plant Cell Environ. 2016, 39, 2260–2275. [Google Scholar] [CrossRef]
- Mutanda, I.; Saitoh, S.; Inafuku, M.; Aoyama, H.; Takamine, T.; Satou, K.; Akutsu, M.; Teruya, K.; Tamotsu, H.; Shimoji, M.; et al. Gene expression analysis of disabled and re-induced isoprene emission by the tropical tree Ficus septica before and after cold ambient temperature exposure. Tree Physiol. 2016, 36, 873–882. [Google Scholar] [CrossRef]
- Hills, A.J.; Zimmerman, P.R. Isoprene measurement by ozone-induced chemiluminescence. Anal. Chem. 1990, 62, 1055–1060. [Google Scholar] [CrossRef]











| Past Days | CT1 | CT2 | α |
|---|---|---|---|
| 1 | 0.01 | 0.32 | 0.65 |
| 2 | 0.02 | 0.44 | 0.40 |
| 3 | 0.04 | 0.61 | 0.40 |
| 4 | 0.05 | 0.64 | 0.52 |
| 5 | 0.07 | 0.65 | 0.73 |
| 6 | 0.10 | 0.52 | 0.87 |
| 7 | 0.13 | 0.44 | 0.90 |
| 8 | 0.16 | 0.46 | 0.78 |
| 9 | 0.20 | 0.41 | 0.42 |
| 10 | 0.18 | 0.33 | 0.05 |
| Past Days | CT1 | CT2 | α |
|---|---|---|---|
| 1 | −0.21 | 0.24 | 0.09 |
| 2 | 0.07 | 0.30 | 0.06 |
| 3 | 0.09 | 0.28 | −0.13 |
| 4 | −0.03 | 0.35 | −0.19 |
| 5 | −0.13 | 0.42 | −0.11 |
| 6 | −0.09 | 0.31 | 0.14 |
| 7 | 0.11 | 0.30 | 0.07 |
| 8 | 0.21 | −0.01 | −0.14 |
| 9 | 0.14 | −0.27 | −0.57 |
| 10 | 0.05 | −0.09 | −0.65 |
| Past Days | DXP | MEP | MEcDP | HMBDP | DMADP | IspS | IspS Protein |
|---|---|---|---|---|---|---|---|
| 1 | 0.39 | 0.49 | 0.47 | 0.36 | 0.36 | 0.57 | 0.27 |
| 2 | 0.36 | 0.40 | 0.44 | 0.32 | 0.37 | 0.52 | 0.28 |
| 3 | 0.18 | 0.32 | 0.28 | 0.16 | 0.37 | 0.45 | 0.32 |
| 4 | −0.05 | 0.23 | 0.13 | 0.05 | 0.33 | 0.34 | 0.35 |
| 5 | −0.27 | 0.18 | 0.01 | −0.02 | 0.33 | 0.24 | 0.37 |
| 6 | −0.33 | 0.12 | 0.01 | 0.03 | 0.27 | 0.16 | 0.33 |
| 7 | −0.29 | 0.07 | 0.05 | 0.07 | 0.26 | 0.17 | 0.32 |
| 8 | −0.24 | 0.00 | 0.07 | 0.05 | 0.22 | 0.18 | 0.30 |
| 9 | −0.15 | −0.14 | −0.02 | −0.09 | 0.10 | 0.14 | 0.24 |
| 10 | −0.14 | −0.26 | −0.19 | −0.26 | −0.05 | 0.01 | 0.13 |
| Past Days | DXP | MEP | MEcDP | HMBDP | DMADP | IspS | IspS Protein |
|---|---|---|---|---|---|---|---|
| 1 | 0.38 | 0.46 | 0.51 | 0.42 | 0.30 | 0.45 | 0.14 |
| 2 | 0.35 | 0.29 | 0.37 | 0.25 | 0.27 | 0.40 | 0.19 |
| 3 | 0.21 | 0.08 | 0.12 | −0.01 | 0.15 | 0.24 | 0.14 |
| 4 | 0.01 | 0.00 | −0.10 | −0.17 | 0.02 | 0.04 | 0.07 |
| 5 | −0.27 | −0.07 | −0.29 | −0.30 | −0.09 | −0.16 | −0.01 |
| 6 | −0.40 | −0.35 | −0.32 | −0.24 | −0.30 | −0.47 | −0.17 |
| 7 | −0.39 | −0.53 | −0.38 | −0.29 | −0.27 | −0.51 | −0.16 |
| 8 | −0.37 | −0.53 | −0.43 | −0.37 | −0.37 | −0.54 | −0.23 |
| 9 | −0.11 | −0.55 | −0.30 | −0.27 | −0.42 | −0.47 | −0.28 |
| 10 | −0.10 | −0.49 | −0.33 | −0.32 | −0.35 | −0.43 | −0.28 |
| Parameter | Whole (Steps 1–13) | Ascending (Steps 1–7) | Descending (Steps 8–13) |
|---|---|---|---|
| CT1 | 196,750 ± 4553 a | 189,250 ± 8199 a | 47,500 ± 5545 b |
| CT2 | 237,000 ± 2646 a | 249,750 ± 4250 a | 280,250 ± 5344 b |
| α | 0.0851 ± 0.0002 a | 0.0034 ± 0.0004 b | 0.0039 ± 0.0002 b |
| BER | 10.6 ± 0.4 a | 9.7 ± 0.7 a | 28.6 ± 2.1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oku, H.; Iqbal, A.; Oogai, S.; Inafuku, M.; Mutanda, I. Relationship between Cumulative Temperature and Light Intensity and G93 Parameters of Isoprene Emission for the Tropical Tree Ficus septica. Plants 2024, 13, 243. https://doi.org/10.3390/plants13020243
Oku H, Iqbal A, Oogai S, Inafuku M, Mutanda I. Relationship between Cumulative Temperature and Light Intensity and G93 Parameters of Isoprene Emission for the Tropical Tree Ficus septica. Plants. 2024; 13(2):243. https://doi.org/10.3390/plants13020243
Chicago/Turabian StyleOku, Hirosuke, Asif Iqbal, Shigeki Oogai, Masashi Inafuku, and Ishmael Mutanda. 2024. "Relationship between Cumulative Temperature and Light Intensity and G93 Parameters of Isoprene Emission for the Tropical Tree Ficus septica" Plants 13, no. 2: 243. https://doi.org/10.3390/plants13020243
APA StyleOku, H., Iqbal, A., Oogai, S., Inafuku, M., & Mutanda, I. (2024). Relationship between Cumulative Temperature and Light Intensity and G93 Parameters of Isoprene Emission for the Tropical Tree Ficus septica. Plants, 13(2), 243. https://doi.org/10.3390/plants13020243






