Clinopodium L. Taxa from the Balkans—Are There Unique Leaf Micromorphological and Phytochemical Patterns?
Abstract
1. Introduction
2. Results
2.1. Epicuticular Wax Composition
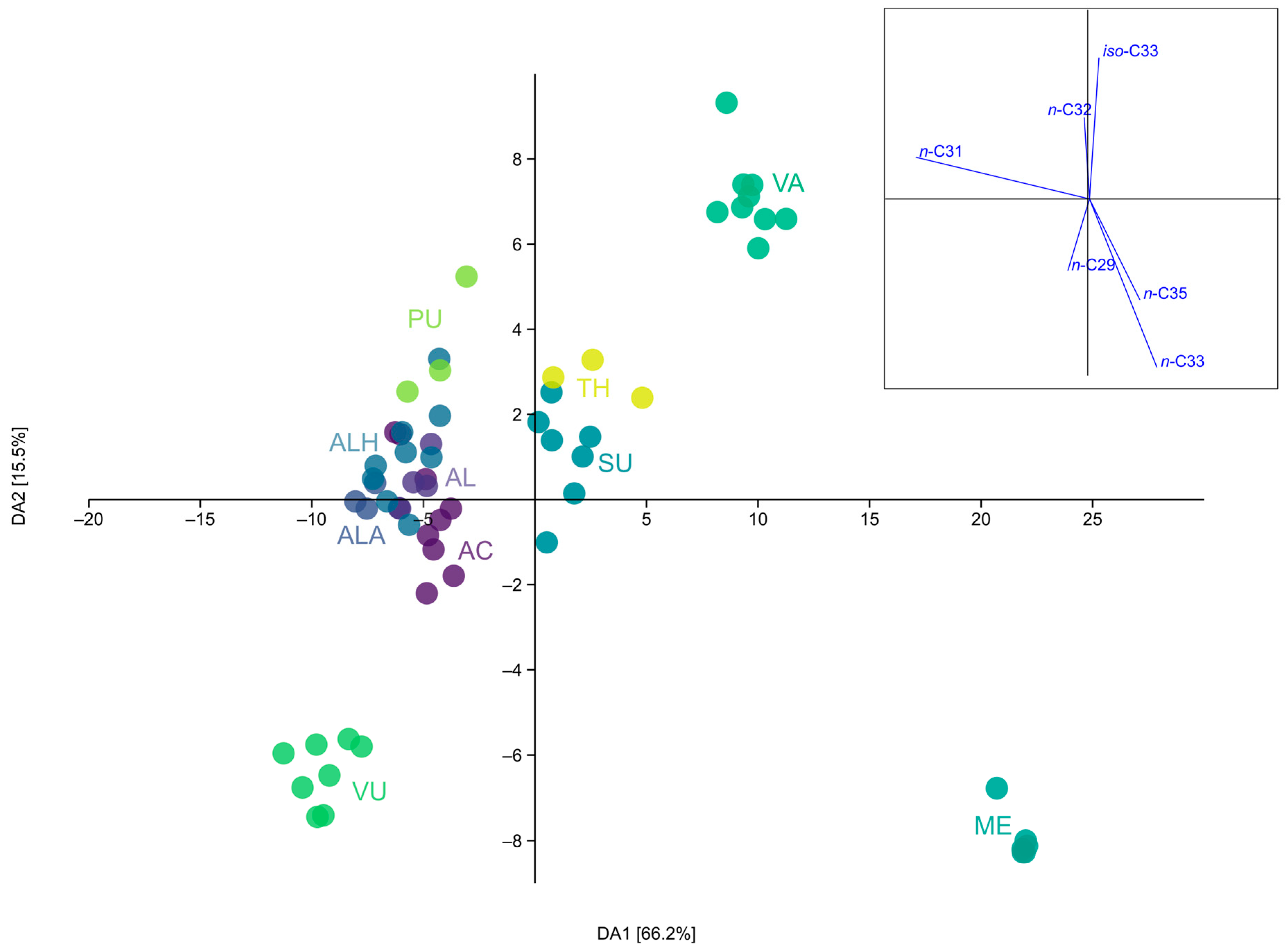
Statistical Analysis of Alkane Profile
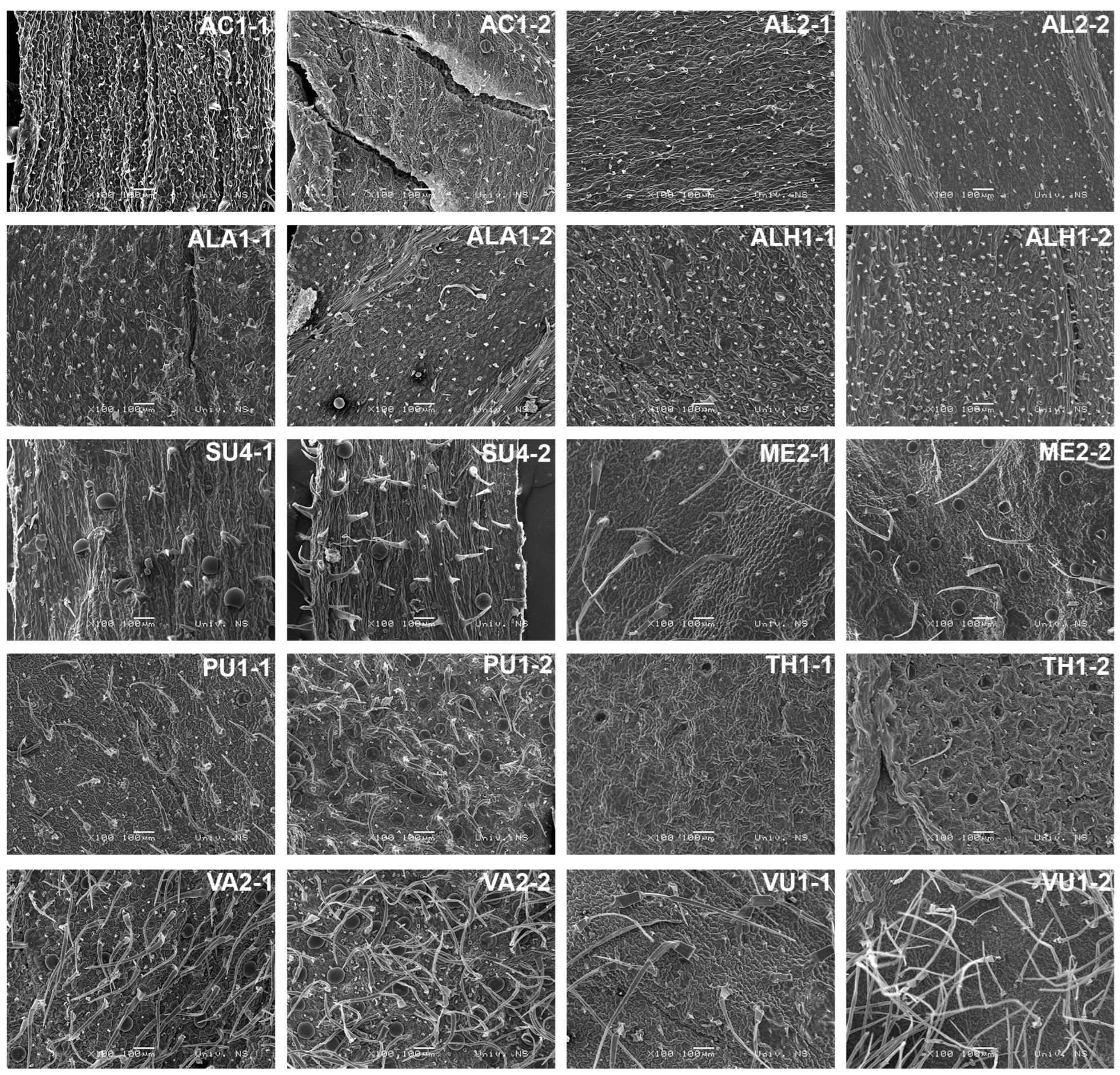
2.2. Micromorphological Features
Statistical Analysis of Leaves’ Indumentums
2.3. Environmental Parameters
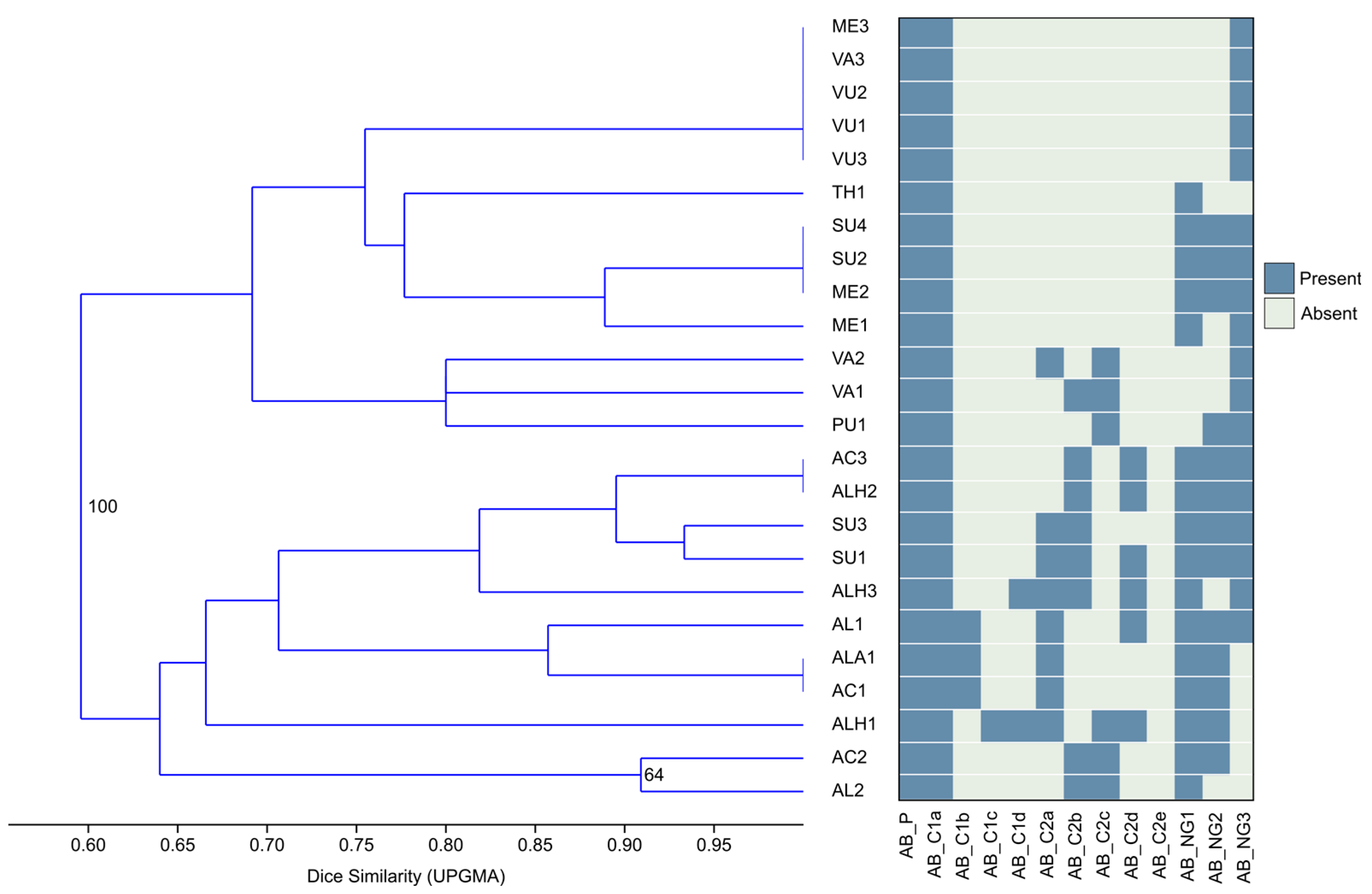
2.4. Correlations of the Data
Correlation between Micromorphological Parameters
3. Discussion
3.1. Normal Chain and Branched Alkanes
3.2. Micromorphological Features
3.3. Correlations
3.3.1. Influence of the Environment
3.3.2. Correlation of the Micromorphological Features and Alkane Profile
4. Materials and Methods
4.1. Plant Material
4.2. Extraction of Leaf Alkanes
4.3. The GC-FID and GC/MS Analysis
4.4. The Average Chain Length and Carbon Preference Index Calculations
4.5. Bioclimatic Data
4.6. Micromorphological Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harley, R.M.; Atkins, S.; Budantsev, A.L.; Cantino, P.D.; Conn, B.J.; Grayer, R.; Harley, M.M.; de Kok, R.; Krestovskaja, T.; Morales, R.; et al. Labiatae. In Flowering Plants·Dicotyledons; Kadereit, J.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 167–275. ISBN 978-3-642-62200-7. [Google Scholar]
- Clinopodium L. |Plants of the World Online|Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30008690-2 (accessed on 8 December 2023).
- Clinopodium, L. Wfo. Available online: https://www.worldfloraonline.org/taxon/wfo-4000008626 (accessed on 8 December 2023).
- Bentham, G. Labiatarum Genera et Species: Or, a Description of the Genera and Species of Plants of the Order Labiatae; with their General History, Characters, Affinities, and Geographical Distribution. Available online: https://bibdigital.rjb.csic.es/records/item/12582-redirection (accessed on 8 December 2023).
- De Candolle, A. Prodromus Systematis Naturalis Regni Vegetabilis; Sumptibus Sociorum, Treuttel et Würtz: Paris, France, 1848. [Google Scholar]
- Boissier, E. Flora Orientalis; Biodiversity Heritage Library: Washington, DC, USA, 1879. [Google Scholar]
- Visiani, R.D. Flora Dalmatica-Sive Enumeratio Stirpium Vascularium Quas Hactenus in Dalmatia Lectas et Sibi Digessit; Hofmeister: Leipzig, Germany, 1847. [Google Scholar]
- Šilić, Č. Monografija Rodova Satureja L., Calamintha Miller, Micromeria Bentham, Acinos Miller I Clinopodium L. u Flori Jugoslavije; Zemaljski muzej BiH: Sarajevo, Bosnia and Herzegovina, 1979. [Google Scholar]
- Engler, A.; Prantl, K. Die Natürlichen Pflanzenfamilien; W. Engelmann: Leipzig, Germany, 1897; pp. 369–372. [Google Scholar]
- Kuntze, O. Revisio Generum Plantarum: Vascularium Omnium Atque Cellularium Multarum Secundum Leges Nomenclaturae Internationales Cum Enumeratione Plantarum Exoticarum in Itinere Mundi Collectarum, miterläuterungen; A. Felix [etc.]: Leipzig, Germany, 1891. [Google Scholar]
- Bräuchler, C.; Meimberg, H.; Heubl, G. New Names in Old World Clinopodium—The Transfer of the Species of Micromeria Sect. Pseudomelissa to Clinopodium. Taxon 2006, 55, 977–981. [Google Scholar] [CrossRef]
- Bräuchler, C.; Meimberg, H.; Heubl, G. Molecular Phylogeny of Menthinae (Lamiaceae, Nepetoideae, Mentheae) –Taxonomy, Biogeography and Conflicts. Mol. Phylogenet. Evol. 2010, 55, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Fırat, M.; Akçiçek, E.; Kaya, A. Clinopodium serpyllifolium subsp. sirnakense (Lamiaceae), a New Taxon from South-Eastern Anatolia, Turkey. Phytotaxa 2015, 201, 131. [Google Scholar] [CrossRef]
- Gülz, P.G. Epicuticular Leaf Waxes in the Evolution of the Plant Kingdom. J. Plant Physiol. 1994, 143, 453–464. [Google Scholar] [CrossRef]
- Bhanot, V.; Fadanavis, S.V.; Panwar, J. Revisiting the Architecture, Biosynthesis and Functional Aspects of the Plant Cuticle: There Is More Scope. Environ. Exp. Bot. 2021, 183, 104364. [Google Scholar] [CrossRef]
- Nishiyama, T.; Sakayama, H.; De Vries, J.; Buschmann, H.; Saint-Marcoux, D.; Ullrich, K.K.; Haas, F.B.; Vanderstraeten, L.; Becker, D.; Lang, D.; et al. The Chara Genome: Secondary Complexity and Implications for Plant Terrestrialization. Cell 2018, 174, 448–464. e24. [Google Scholar] [CrossRef]
- Lewandowska, M.; Keyl, A.; Feussner, I. Wax Biosynthesis in Response to Danger: Its Regulation upon Abiotic and BioticStress. New Phytol. 2020, 227, 698–713. [Google Scholar] [CrossRef]
- Sharma, P.; Kothari, S.L.; Rathore, M.; Gour, V. Properties, Variations, Roles, and Potential Applications of Epicuticular Wax: A Review. Turk. J. Bot. 2018, 42, 135–149. [Google Scholar] [CrossRef]
- Bush, R.T.; McInerney, F.A. Leaf Wax N-Alkane Distributions in and across Modern Plants: Implications for Paleoecology and Chemotaxonomy. Geochim. Cosmochim. Acta 2013, 117, 161–179. [Google Scholar] [CrossRef]
- Eglinton, G.; Hamilton, R.J. Leaf Epicuticular Waxes: The Waxy Outer Surfaces of Most Plants Display a Wide Diversity ofFine Structure and Chemical Constituents. Science 1967, 156, 1322–1335. [Google Scholar] [CrossRef]
- Reddy, C.M.; Eglinton, T.I.; PalicÂ, R.; Benitez-Nelson, B.C.; StojanovicÂ, G.; PalicÂ, I. Even Carbon Number Predominance of Plant Wax n-Alkanes: A Correction. Org. Geochem. 2000, 31, 331–336. [Google Scholar] [CrossRef]
- Dodoš, T.; Rajčević, N.; Tešević, V.; Matevski, V.; Janaćković, P.; Marin, P.D. Composition of Leaf n-Alkanes in Three Satureja montana L. Subspecies from the Balkan Peninsula: Ecological and Taxonomic Aspects. Chem. Biodivers. 2015, 12, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Rajčević, N.; Dodoš, T.; Novaković, J.; Janaćković, P.; Marin, P.D. Epicuticular Wax Variability of Juniperus Deltoides R. P. Adams from the Central Balkan—Ecology and Chemophenetics. Biochem. Syst. Ecol. 2020, 89, 104008. [Google Scholar] [CrossRef]
- Teunissen van Manen, M.L.; Jansen, B.; Cuesta, F.; León-Yánez, S.; Gosling, W.D. Leaf Wax n-Alkane Patterns of Six Tropical Montane Tree Species Show Species-specific Environmental Response. Ecol. Evol. 2019, 9, 9120–9128. [Google Scholar] [CrossRef] [PubMed]
- Brieskorn, C.H.; Beck, K.R. Die kohlenwasserstoffe des blattwachses von Rosmarinus officinalis. Phytochemistry 1970, 9, 1633–1640. [Google Scholar] [CrossRef]
- Maffei, M. Discriminant Analysis of Leaf Wax Alkanes in the Lamiaceae and Four Other Plant Families. Biochem. Syst. Ecol. 1994, 22, 711–728. [Google Scholar] [CrossRef]
- Huang, X.; Meyers, P.A.; Wu, W.; Jia, C.; Xie, S. Significance of Long Chain Iso and Anteiso Monomethyl Alkanes in the Lamiaceae (Mint Family). Org. Geochem. 2011, 42, 156–165. [Google Scholar] [CrossRef]
- Goodwin, W. Explanation in Organic Chemistry. Ann. N. Y. Acad. Sci. 2003, 988, 141–153. [Google Scholar] [CrossRef]
- Kolattukudy, P.E.; Walton, T.J. The Biochemistry of Plant Cuticular Lipids. Prog. Chem. Fats Other Lipids 1973, 13, 119–175. [Google Scholar] [CrossRef]
- Nelson, D.R. Long-Chain Methyl-Branched Hydrocarbons: Occurrence, Biosynthesis, and Function. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 13, pp. 1–33. ISBN 978-0-12-024213-9. [Google Scholar]
- Payne, W.W. A Glossary of Plant Hair Terminology. Brittonia 1978, 30, 239. [Google Scholar] [CrossRef]
- Kang, J.H.; Shi, F.; Jones, A.D.; Marks, M.D.; Howe, G.A. Distortion of Trichome Morphology by the Hairless Mutation of Tomato Affects Leaf Surface Chemistry. J. Exp. Bot. 2010, 61, 1053–1064. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.; Meng, P.; Tan, G.; Lv, L. Analysis and Review of Trichomes in Plants. BMC Plant Biol. 2021, 21, 70. [Google Scholar] [CrossRef]
- Alakoon, U.I.; Taheri, A.; Nayidu, N.K.; Epp, D.; Yu, M.; Parkin, I.; Hegedus, D.; Bonham-Smith, P.; Gruber, M.Y. Hairy Canola (Brasssica napus) Re-Visited: Down-Regulating TTG1 in an AtGL3-Enhanced Hairy Leaf Back ground Improves Growth, Leaf Trichome Coverage, and Metabolite Gene Expression Diversity. BMC Plant Biol. 2016, 16, 12. [Google Scholar] [CrossRef]
- Gao, C.; Li, D.; Jin, C.; Duan, S.; Qi, S.; Liu, K.; Wang, H.; Ma, H.; Hai, J.; Chen, M. Genome-Wide Identification of GLABRA3 Downstream Genes for Anthocyanin Biosynthesis and Trichome Formation in Arabidopsis. Biochem. Biophys. Res. Commun. 2017, 485, 360–365. [Google Scholar] [CrossRef]
- Gul, S.; Ahmad, M.; Zafar, M.; Bahadur, S.; Celep, F.; Sultana, S.; Begum, N.; Hanif, U.; Zaman, W.; Shuaib, M.; et al. Taxonomic Significance of Foliar Epidermal Morphology in Lamiaceae from Pakistan. Microsc. Res. Tech. 2019, 82, 1507–1528. [Google Scholar] [CrossRef] [PubMed]
- Marin, P. Orasice I Trihome u Familiji Lamiaceae; Biološki fakultet: Beograd, Serbia, 1996; pp. 159–168. [Google Scholar]
- Moon, H.-K.; Hong, S.-P.; Smets, E.; Huysmans, S. Micromorphology and Character Evolution of Nutlets in Tribe Mentheae (Nepetoideae, Lamiaceae). Syst. Bot. 2009, 34, 760–776. [Google Scholar] [CrossRef]
- Dodoš, T.; Rajčević, N.; Tešević, V.; Marin, P.D. Chemodiversity of Epicuticular n-Alkanes and Morphological Traits of Natural Populations of Satureja subspicata BARTL. Ex VIS. Along Dinaric Alps- Ecological and Taxonomic Aspects. Chem. Biodivers. 2017, 14, e1600201. [Google Scholar] [CrossRef] [PubMed]
- Dodoš, T.; Rajčević, N.; Janaćković, P.; Novaković, J.; Marin, P.D. Intra- and Interpopulation Variability of Balkan Endemic—Satureja kitaibelii Based on–Alkane Profile. Biochem. Syst. Ecol. 2019, 85, 68–71. [Google Scholar] [CrossRef]
- Carruthers, W.; Johnstone, R.A.W. Composition of a Paraffin Wax Fraction From Tobacco Leaf and Tobacco Smoke. Nature 1959, 184, 1131–1132. [Google Scholar] [CrossRef]
- Mold, J.D.; Stevens, R.K.; Means, R.E.; Ruth, J.M. The Paraffin Hydrocarbons of Tobacco; Normal, Iso-, and Anteiso-Homologs. Biochemistry 1963, 2, 605–610. [Google Scholar] [CrossRef]
- Busta, L.; Jetter, R. Structure and Biosynthesis of Branched Wax Compounds on Wild Type and Wax Biosynthesis Mutants of Arabidopsis thaliana. Plant Cell Physiol. 2017, 58, 1059–1074. [Google Scholar] [CrossRef]
- Acensão, L. Glandular Trichomes on the Leaves and Flowers of Plectranthus ornatus: Morphology, Distribution and Histochemistry. Ann. Bot. 1999, 84, 437–447. [Google Scholar] [CrossRef]
- Telepova, M.N.; Budantzev, A.L.; Shavarda, A.L. Etude comparative de la secretion des terpènes par les elements glandulaires foliaires chez différentes espèces du genre Dracocephalum L. (Labiatae). Bull. Soc. Bot. Fr. Lett. Bot. 1992, 139, 247–264. [Google Scholar] [CrossRef]
- Bourett, T.M.; Howard, R.J.; O’Keefe, D.P.; Hallahan, D.L. Gland Development on Leaf Surfaces of Nepeta Racemosa. Int. J. Plant Sci. 1994, 155, 623–632. [Google Scholar] [CrossRef]
- Husain, S.Z.; Marin, P.D.; Šilić, Č.; Qaiser, M.; Petcović, B. A Micromorphological Study of Some Representative Genera in the Tribe Saturejeae (Lamiaceae). Bot. J. Linn. Soc. 1990, 103, 59–80. [Google Scholar] [CrossRef]
- Kaya, A. Morphological Characteristics of Clinopodium acinos and Clinopodium suaveolens (Lamiaceae) Growing in Turkey. J. Res. Pharm. 2018, 23, 62–68. [Google Scholar] [CrossRef]
- Cantino, P.D. The Phylogenetic Significance of Stomata and Trichomes in the Labiatae and Verbenaceae. J. Arnold Arbor. 1990, 71, 323–370. [Google Scholar] [CrossRef]
- Kremer, D.; Stabentheiner, E.; Bogunić, F.; Ballian, D.; Eleftheriadou, E.; Stešević, D.; Matevski, V.; Ranđelović, V.; Ivanova, D.; Ruščić, M.; et al. Micromorphological Traits of Balcanic Micromeria and Closely Related Clinopodium Species (Lamiaceae). Plants 2021, 10, 1666. [Google Scholar] [CrossRef]
- Giuliani, C.; Maleci Bini, L. Insight into the Structure and Chemistry of Glandular Trichomes of Labiatae, with Emphasis on Subfamily Lamioideae. Plant Syst. Evol. 2008, 276, 199–208. [Google Scholar] [CrossRef]
- Stojičić, D.; Tošić, S.; Stojanović, G.; Zlatković, B.; Jovanović, S.; Budimir, S.; Uzelac, B. Volatile Organic Compound Composition and Glandular Trichome Characteristics of In Vitro Propagated Clinopodium pulegium (Rochel) Bräuchler: Effect of Carbon Source. Plants 2022, 11, 198. [Google Scholar] [CrossRef]
- Pascal, S.; Bernard, A.; Deslous, P.; Gronnier, J.; Fournier-Goss, A.; Domergue, F.; Rowland, O.; Joubès, J. Arabidopsis CER1-LIKE1 Functions in a Cuticular Very-Long-Chain Alkane-Forming Complex. Plant Physiol. 2019, 179, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, L.; Chen, Y.; Liu, T.; Jiang, Q.; Wei, Z.; Li, C.; Wang, Z. Tomato SlCER1–1 Catalyzes the Synthesis of Wax Alkanes, Increasing Drought Tolerance and Fruit Storability. Hortic. Res. 2022, 9, uhac004. [Google Scholar] [CrossRef] [PubMed]
- Cochran, J.R.; Stow, D.A.V.; Auroux, C.; Amano, K.; Balson, P.S.; Boulègue, J.J.; Brass, G.W.; Corrigan, J.; Gartner, S.; Hall, S. (Eds.) Proceedings of the Ocean Drilling Program; 116 Scientific Results; Ocean Drilling Program: College Station TX, USA, 1990; Volume 116. [Google Scholar]
- Fick, S.; Hijmans, R. World Clim 2: New 1—Km Spatial Resolution Climate Surfaces for Global and Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and Future Köppen-Geiger Climate Classification Map sat1-Km Resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. (Eds.) Soil Sampling and Methods of Analysis, 2nd ed.; Canadian Society of Soil Science: Pinawa, MB, Canada; CRC Press: BocaRaton, FL, USA, 2008; ISBN 978-0-8493-3586-0. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]


| n-Alkanes | iso-Alkanes | anteiso-Alkanes | ||||||
|---|---|---|---|---|---|---|---|---|
| Range a | Total %b | Range a | Total %b | Range a | Total % b | ACL c | CPI d | |
| AC | 21–35 | 90.4 ± 1.5 | 29–37 | 7.1 ± 1.4 | 30–36 | 2.5 ± 0.3 | 31.7 ± 0.2 | 7.4 ± 1.3 |
| AL | 21–35 | 90.0 ± 1.7 | 29–37 | 6.5 ± 1.8 | 30–36 | 3.5 ± 0.6 | 31.8 ± 0.1 | 9.4 ± 2.3 |
| ALA | 21–35 | 93.0 ± 1.0 | 30–37 | 4.5 ± 0.6 | 30–36 | 2.5 ± 0.5 | 32.0 ± 0.1 | 7.1 ± 1.0 |
| ALH | 21–35 | 88.7 ± 2.5 | 27–37 | 7.6 ± 1.6 | 30–36 | 3.6 ± 1.3 | 31.8 ± 0.2 | 8.2 ± 1.8 |
| ME | 21–36 | 89.9 ± 4.9 | 27–37 | 5.6 ± 2.5 | 30–36 | 4.5 ± 2.5 | 32.4 ± 0.3 | 7.5 ± 2.6 |
| PU | 21–35 | 88.4 ± 1.2 | 29–37 | 7.7 ± 1.4 | 30–36 | 3.9 ± 0.3 | 32.1 ± 0.1 | 10.0 ± 0.2 |
| SU | 21–35 | 90.1 ± 2.8 | 29–37 | 5.6 ± 1.2 | 29–36 | 4.3 ± 1.8 | 32.1 ± 0.1 | 7.1 ± 1.1 |
| TH | 21–35 | 91.7 ± 1.0 | 29–37 | 4.5 ± 1.0 | 29–36 | 3.8 ± 0.2 | 32.3 ± 0.1 | 10.8 ± 2.9 |
| VA | 21–35 | 87.0 ± 2.9 | 29–37 | 9.3 ± 2.6 | 29–36 | 3.6 ± 1.2 | 32.2 ± 0.1 | 6.8 ± 0.5 |
| VU | 21–36 | 94.4 ± 0.8 | 29–37 | 2.3 ± 0.8 | 30–36 | 3.3 ± 0.6 | 32.1 ± 0.1 | 11.4 ± 1.6 |
| Taxa | Cuticle Thickness | Leaf Side | P | C | NG | Density of Peltate Trichomes | Presence of C-Type Trichomes | Presence of NG-Type Trichomes |
|---|---|---|---|---|---|---|---|---|
| AC | 1.415–3.819 | AD | - | + | + | 1.89 + 1.1 | C1a, C2a, C2b, C2c | NG1, NG2, NG3 |
| AB | + | + | + | 3.22 + 0.70 | C1a, C1b, C2a, C2b, C2c, C2d | NG1, NG2, NG3 | ||
| AL | 1.467–3.357 | AD | - | +/++ | +/++ | - | C1a | NG1, NG2, NG3 |
| AB | + | +/++ | +/++ | 2.49 ± 0.52 | C1a, C1b, C2a, C2b, C2c, C2d | NG1, NG2, NG3 | ||
| ALA | 1.558–2.570 | AD | - | + | ++ | - | C1a | NG1, NG2 |
| AB | + | + | + | 5.38 | C1a, C1b, C2a | NG1, NG3 | ||
| ALH | 0.885–3.109 | AD | -/+ | ++ | +/++ | - | C1a, C1c, C2a, C2b, C2c, C2d, C2e | NG1, NG2, NG3 |
| AB | + | ++ | + | 2.67 ± 0.69 | C1a, C1c, C1d, C2a, C2b, C2c, C2d | NG1, NG2, NG3 | ||
| SU | 0.504–4.077 | AD | -/+ | + | +/++ | 3.43 ± 3.97 | C1a | NG1, NG2, NG3 |
| AB | + | +/++ | +/++ | 5.91 ± 2.49 | C1a, C2a, C2b, C2d | NG1, NG2, NG3 | ||
| ME | 1.226–5.156 | AD | - | + | + | - | C1a | NG1, NG2, NG3 |
| AB | ++ | + | +/++ | 16.52 ± 5.53 | C1a | NG1, NG2, NG3 | ||
| VA | 1.007–4.466 | AD | + | ++ | +/++ | 3.91 ± 0.72 | C1a, C2a, C2b, C2c | NG3 |
| AB | ++ | ++ | +/++ | 18.85 ± 1.75 | C1a, C2a, C2b, C2c | NG3 | ||
| VU | 1.427–3.521 | AD | - | + | +/++ | - | C1a | NG1, NG3 |
| AB | + | + | ++ | 3.11 ± 0.94 | C1a | NG1, NG3 | ||
| PU | 2.293–5.019 | AD | + | ++ | ++ | 4.19 ± 1.84 | C1a, C2c | NG2, NG3 |
| AB | ++ | ++ | ++ | 19.54 ± 4.47 | C1a, C2c | NG2, NG3 | ||
| TH | 0.846–3.387 | AD | + | + | - | 6.09 ± 1.77 | C1a | - |
| AB | ++ | ++ | + | 24.92 ± 7.15 | C1a | NG1 |
| Taxon | Code | Locality | Longitude [°N] | Latitude [°E] | Alt [m.a.s.l.] | BEOU |
|---|---|---|---|---|---|---|
| Clinopodium vulgare L. | VU1 | Serbia, Mt. Kosmaj | 44.476 | 20.577 | 580 | 18,008 |
| VU2 | Serbia, Tekija | 44.632 | 22.376 | 488 | 18,009 | |
| VU3 | Serbia, Mt. Rogozna | 43.026 | 20.567 | 1060 | 18,010 | |
| Acinos-group | ||||||
| C. acinos Kuntze | AC1 | Serbia, Mt. Tara | 43.865 | 19.406 | 872 | 17,987 |
| AC2 | Serbia, Subotica sands | 46.139 | 19.615 | 133 | 17,988 | |
| AC3 | Serbia, Svrljig gorge | 43.542 | 22.177 | 259 | 17,989 | |
| C. alpinum Kuntze subsp. alpinum | AL1 | Serbia, Topli Do | 43.334 | 22.664 | 690 | 17,990 |
| AL2 | Serbia, Mt. Stolovi | 43.607 | 20.611 | 1308 | 17,991 | |
| C. alpinum subsp. albanicum (Kümmerle & Jáv.) Govaerts | ALA1 | Serbia, Mt. Rogozna | 43.045 | 20.521 | 882 | 17,992 |
| C. alpinum subsp. hungaricum (Simonk.) Govaerts | ALH1 | Serbia, Mt. Fruska gora | 45.156 | 19.778 | 387 | 17,993 |
| ALH2 | Serbia, Milesevka gorge | 43.367 | 19.719 | 604 | 17,994 | |
| ALH3 | Serbia, Gradasnica | 43.189 | 22.597 | 488 | 17,995 | |
| C. suaveolens Kuntze | SU1 | Serbia, Soko grad fortress | 43.636 | 21.897 | 414 | 18,000 |
| SU2 | Serbia, Mt. Rtanj | 43.767 | 21.926 | 791 | 18,001 | |
| SU3 | North Macedonia, Raec gorge | 41.437 | 21.878 | 257 | 18,002 | |
| SU4 | North Macedonia, Dojran | 41.197 | 22.72 | 166 | 18,003 | |
| Calamintha group | ||||||
| C. menthifolium(Host) Stace | ME1 | Serbia, Zlot | 44.029 | 21.961 | 303 | 17,996 |
| ME2 | Serbia, Mt. Rogozna | 43.026 | 20.567 | 1060 | 17,997 | |
| ME3 | Serbia, Milesevka gorge | 43.367 | 19.719 | 604 | 17,998 | |
| C. vardarense (Šilić) Govaerts | VA1 | North Macedonia, Kaj-Baba | 41.665 | 20.605 | 796 | 18,005 |
| VA2 | North Macedonia, Dojran | 41.197 | 22.72 | 166 | 18,006 | |
| VA3 | Serbia, Dag Banjica | 43.203 | 22.606 | 499 | 18,007 | |
| Pseudomellisa group | ||||||
| C. pulegium (Rochel) Bräuchler | PU1 | Serbia, Svrljig gorge | 43.543 | 22.178 | 259 | 17,999 |
| C. thymifolium Kuntze | TH1 | Serbia, Mt. Tara | 43.866 | 19.407 | 872 | 18,004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janković, S.; Alimpić Aradski, A.; Dodoš, T.; Novaković, J.; Ivanović, S.; Vujisić, L.; Marin, P.D.; Rajčević, N. Clinopodium L. Taxa from the Balkans—Are There Unique Leaf Micromorphological and Phytochemical Patterns? Plants 2024, 13, 251. https://doi.org/10.3390/plants13020251
Janković S, Alimpić Aradski A, Dodoš T, Novaković J, Ivanović S, Vujisić L, Marin PD, Rajčević N. Clinopodium L. Taxa from the Balkans—Are There Unique Leaf Micromorphological and Phytochemical Patterns? Plants. 2024; 13(2):251. https://doi.org/10.3390/plants13020251
Chicago/Turabian StyleJanković, Smiljana, Ana Alimpić Aradski, Tanja Dodoš, Jelica Novaković, Stefan Ivanović, Ljubodrag Vujisić, Petar D. Marin, and Nemanja Rajčević. 2024. "Clinopodium L. Taxa from the Balkans—Are There Unique Leaf Micromorphological and Phytochemical Patterns?" Plants 13, no. 2: 251. https://doi.org/10.3390/plants13020251
APA StyleJanković, S., Alimpić Aradski, A., Dodoš, T., Novaković, J., Ivanović, S., Vujisić, L., Marin, P. D., & Rajčević, N. (2024). Clinopodium L. Taxa from the Balkans—Are There Unique Leaf Micromorphological and Phytochemical Patterns? Plants, 13(2), 251. https://doi.org/10.3390/plants13020251











