Physiological Mechanisms of Citrus Fruit Cracking: Study on Cell Wall Components, Osmoregulatory Substances, and Antioxidant Enzyme Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Determination of Cell Wall Material
2.3. Determination of Cell Wall Polysaccharides
2.4. Determination of Lignin
2.5. Determination of Osmoregulatory Substances
2.6. Determination of Antioxidant Enzyme Activity
2.7. Statistical Analysis
3. Results
3.1. Changes in the Cell Wall Components and Contents in the Pericarp during Fruit Development in ‘Mingrijian’ and ‘Daya’
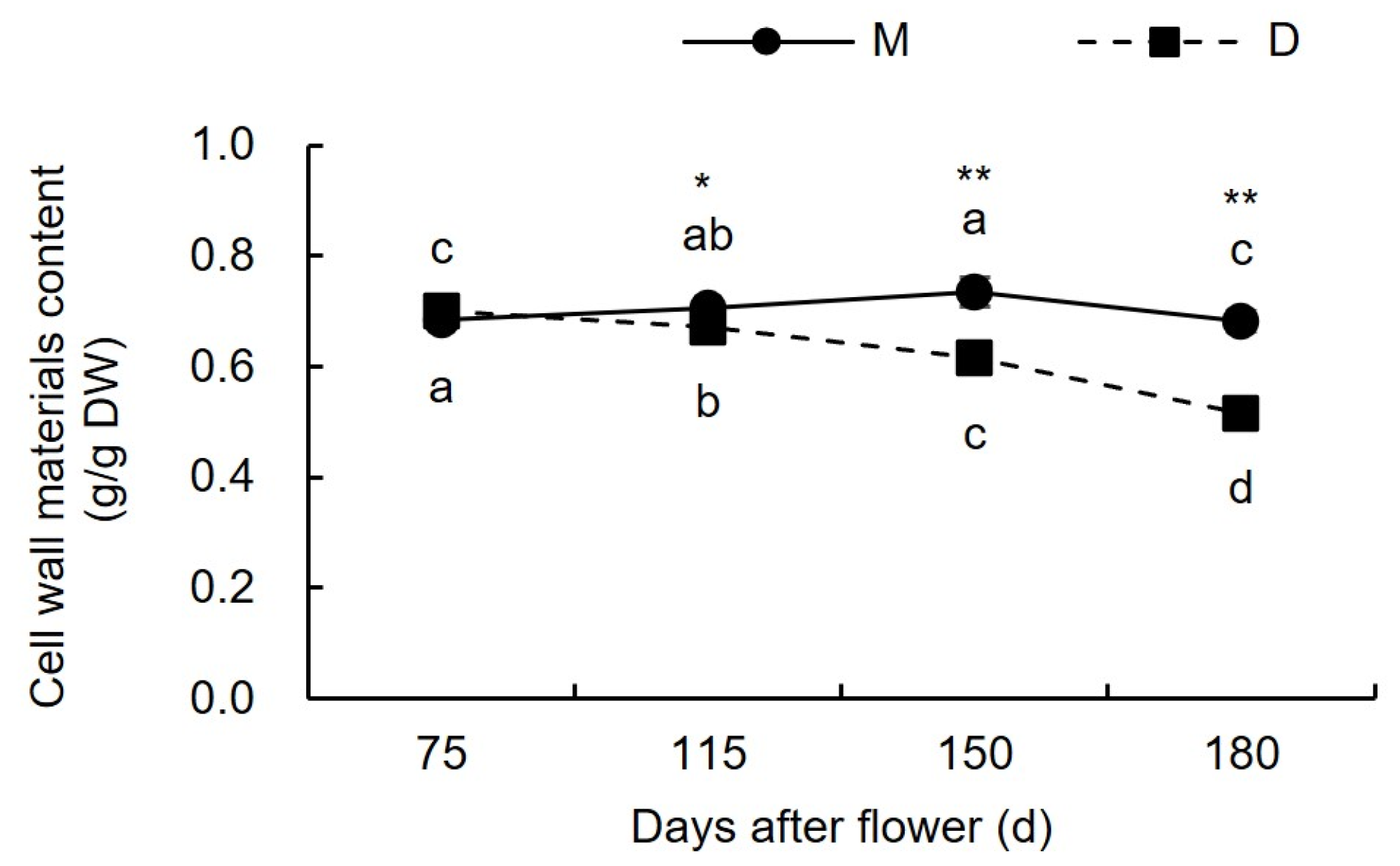
3.1.1. Changes in Cell Wall Material Content
3.1.2. Changes in Pectin Content
3.1.3. Changes in Cellulose and Hemicellulose Contents
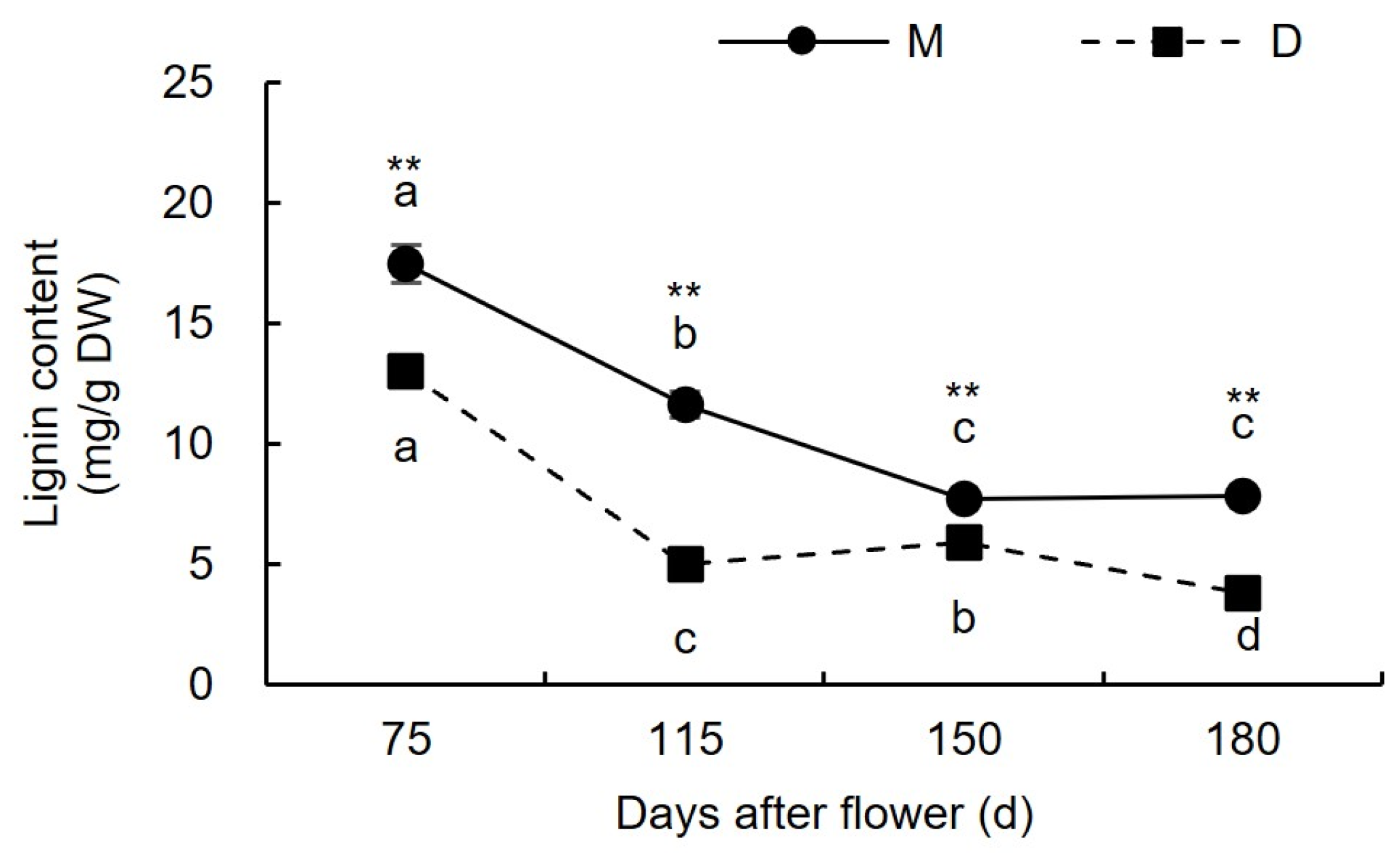
3.1.4. Changes in Lignin Content
3.1.5. Correlation Analysis between Cell Wall Components and Fruit Cracking Rate
3.2. Variations in Osmoregulatory Substances during Fruit Development of ‘Mingrijian’ and ‘Daya’
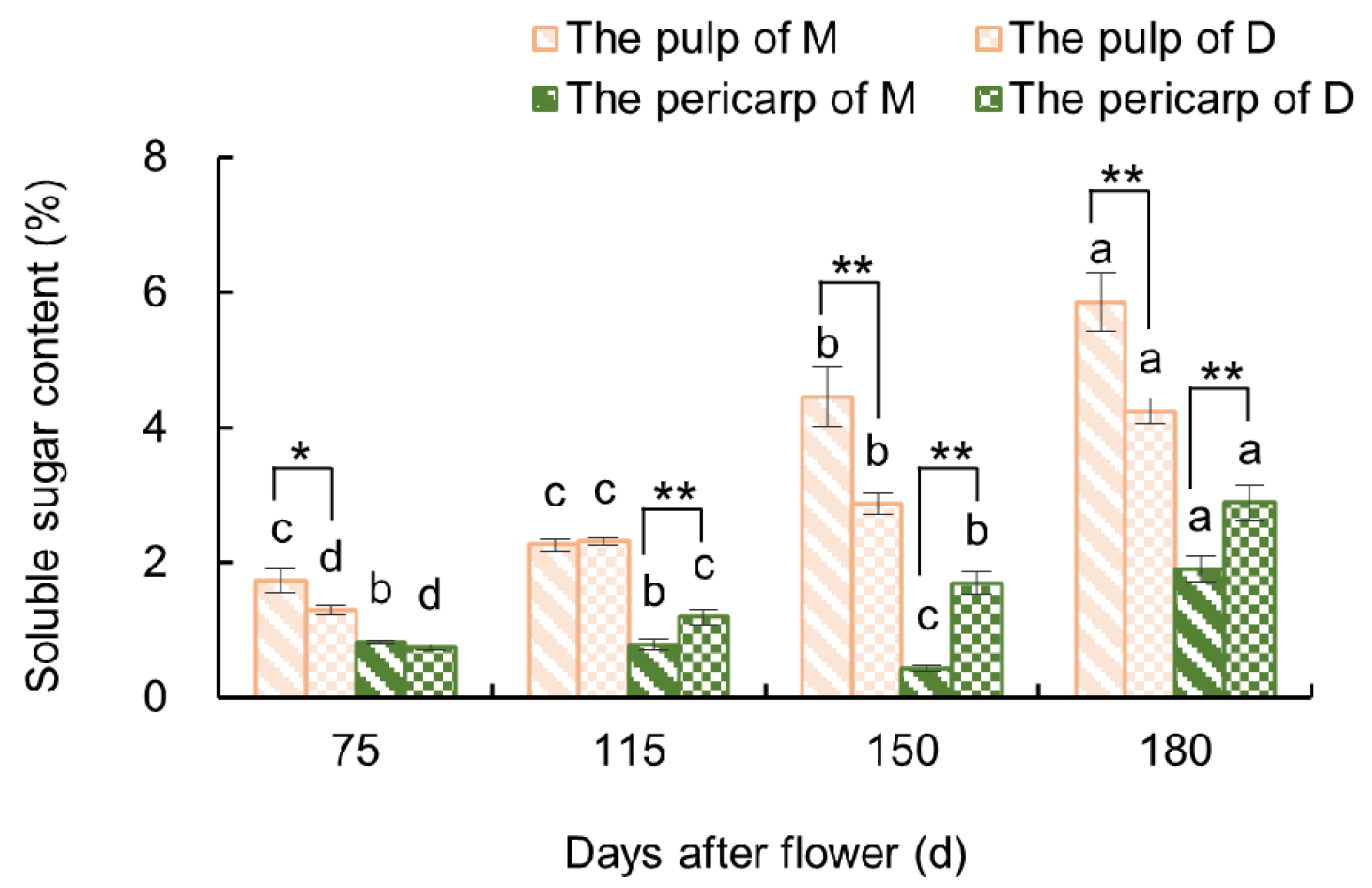
3.2.1. Variations in Soluble Sugar Content in the Fruit
3.2.2. Variations in Soluble Protein Content in the Fruit
3.3. Changes in Antioxidant Enzyme Activities in the Pericarp during Fruit Development in ‘Mingrijian’ and ‘Daya’
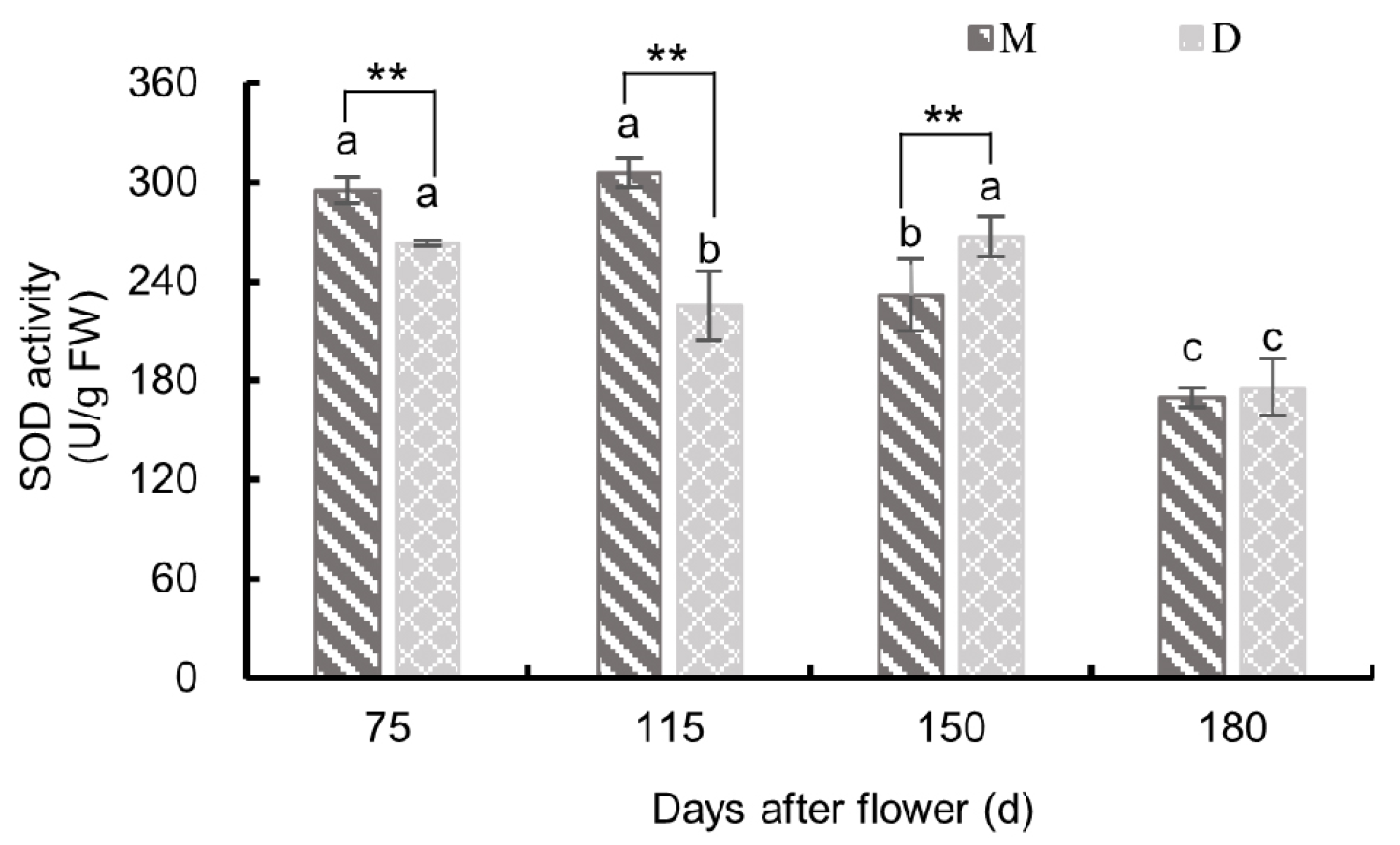
3.3.1. Changes in Superoxide Dismutase Activity
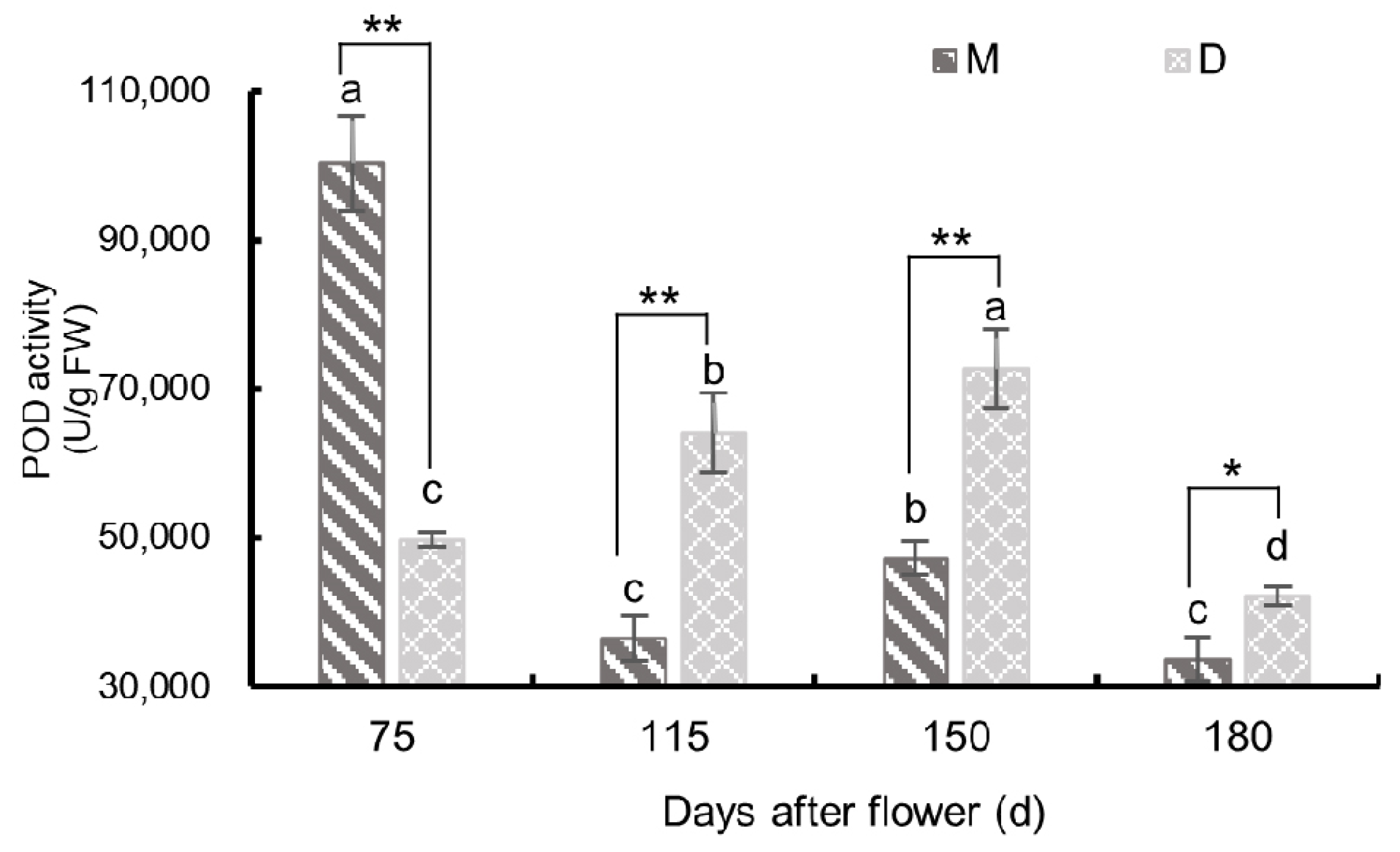
3.3.2. Changes in Peroxidase Activity
3.3.3. Changes in Catalase Activity
3.4. Correlation Analysis of Osmoregulatory Substances, Antioxidant Enzymes, and Fruit Cracking Rates
4. Discussion
4.1. Relationship between Cell Wall Material and Fruit Cracking
4.2. Relationship between Osmoregulatory Substances and Fruit Cracking
4.3. Relationship between Antioxidant Enzymes and Fruit Cracking
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.Z.; Shi, M.M.; Duan, T.T.; Zhang, D.X. Isolation and characterization of ten microsatellite loci for wild Citrus japonica (Rutaceae). J. Genet. 2014, 93, 41–43. [Google Scholar] [CrossRef]
- Biswas, M.K.; Chai, L.J.; Amar, M.H.; Zhang, X.L.; Deng, X.X. Comparative analysis of genetic diversity in Citrus germplasm collection using AFLP, SSAP, SAMPL and SSR markers. Sci. Hortic. 2011, 129, 798–803. [Google Scholar] [CrossRef]
- Odemis, B.; Turhan, S.; Buyuktas, D. The effects of irrigation and fertilizer applications on yield, pomological characteristics and fruit cracking in Nova mandarin. Agric. Water Manag. 2014, 135, 54–60. [Google Scholar] [CrossRef]
- Khadivi-Khub, A. Physiological and genetic factors influencing fruit cracking. Acta Physiol. Plant. 2014, 37, 1718. [Google Scholar] [CrossRef]
- Cronje, P.J.; Stander, O.P.; Theron, K.I. Fruit splitting in citrus. Hortic. Rev. 2013, 41, 177–200. [Google Scholar] [CrossRef]
- Krajewski, A.; Ebert, T.; Schumann, A.; Waldo, L. Pre-Harvest Fruit Splitting of Citrus. Agronomy 2022, 12, 1505. [Google Scholar] [CrossRef]
- Brüggenwirth, M.; Knoche, M. Cell wall swelling, fracture mode, and the mechanical properties of cherry fruit skins are closely related. Planta 2017, 245, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Vicens, A.; Fournand, D.; Williams, P.; Sidhoum, L.; Moutounet, M.; Doco, T. Changes in polysaccharide and protein composition of cell walls in grape berry skin (Cv. Shiraz) during ripening and over-ripening. J. Agric. Food Chem. 2009, 57, 2955–2960. [Google Scholar] [CrossRef]
- Jiang, F.; Lopez, A.; Jeon, S.; de Freitas, S.T.; Yu, Q.; Wu, Z.; Labavitch, J.M.; Tian, S.; Powell, A.L.T.; Mitcham, E. Disassembly of the fruit cell wall by the ripening-associated polygalacturonase and expansin influences tomato cracking. Hortic. Res. 2019, 6, 17. [Google Scholar] [CrossRef]
- Wang, B.; Ding, G.; Wang, X.; Fu, C.; Qin, G.; Yang, J.; Cang, G.; Wen, P. Changes of histological structure and water potential of huping jujube fruit cracking. Sci. Agric. Sin. 2013, 46, 4558–4568. [Google Scholar]
- Huang, X.M.; Wang, H.C.; Gao, F.F.; Huang, H.B. A comparative study of the pericarp of litchi cultivars susceptible and resistant to fruit cracking. J. Hortic. Sci. Biotechnol. 1999, 74, 351–354. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Z.; Zhang, C.; Hu, E.; Zhou, R.; Jiang, F. The composition of pericarp, cell aging, and changes in water absorption in two tomato genotypes: Mechanism, factors, and potential role in fruit cracking. Acta Physiol. Plant. 2016, 38, 215. [Google Scholar] [CrossRef]
- Huo, J.; Shi, Y.; Zhang, H.; Hu, R.; Huang, L.; Zhao, Y.; Zhang, Z. More sensitive to drought of young tissues with weak water potential adjustment capacity in two desert shrubs. Sci. Total Environ. 2021, 790, 148103. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, M.T.; Wang, M.J.; Tang, W.Y.; Wu, S.; Zhang, K.; Yang, G.S. Effect of nordihydroguaiaretic acid on grape berry cracking. Sci. Hortic. 2020, 261, 108979. [Google Scholar] [CrossRef]
- Richardson, D.G. Rain-cracking of ‘Royal Ann’ sweet cherries: Fruit physiological relationships, water temperature, orchard treatments, and cracking index. Acta Hortic. 1998, 468, 677–682. [Google Scholar] [CrossRef]
- Liu, J.; Liang, L.; Jiang, Y.M.; Chen, J.J. Changes in metabolisms of antioxidant and cell wall in three pummelo cultivars during postharvest storage. Biomolecules 2019, 9, 319. [Google Scholar] [CrossRef]
- Xu, H.; Gao, X.; Yu, C. Physiological and transcriptomic analysis of Pinus massoniana seedling response to osmotic stress. Biol. Plantarum. 2021, 65, 145–156. [Google Scholar] [CrossRef]
- Yang, Y.; Han, X.; Liang, Y.; Ghosh, A.; Chen, J.; Tang, M. The combined effects of arbuscular mycorrhizal fungi (AMF) and lead (Pb) stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. PLoS ONE 2015, 10, e0145726. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.Z. Citrus fruit-cracking: Causes and occurrence. Hortic. Plant J. 2017, 3, 255–260. [Google Scholar] [CrossRef]
- Huber, D.J. Polyuronide degradation and hemicellulose modifications in ripening tomato fruit. J. Am. Soc. Hortic. Sci. 1983, 108, 405–409. [Google Scholar] [CrossRef]
- Siddiqui, S.; Brackmann, A.; Streif, J.; Bangerth, F. Controlled atmosphere storage of apples: Cell wall composition and fruit softening. J. Hortic. Sci. 1996, 71, 613–620. [Google Scholar] [CrossRef]
- Iii, P.K.K.; Buren, J.P.V. Carbohydrate interference and its correction in pectin analysis using the m-hydroxydiphenyl method. J. Food Ence 2010, 47, 756–759. [Google Scholar]
- Wen-qian, L.; Ming-ming, H.; Dang-wei, P.; Jin, C.; Yuan-yuan, W.; He-he, D.; Yong-lan, C.; Min, J.; Yong-li, L.; Yong, L.; et al. Characteristics of lodging resistance of high-yield winter wheat as affected by nitrogen rate and irrigation managements. J. Integr. Agric. 2022, 21, 1290–1309. [Google Scholar]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant-tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Stewart, R.R.C.; Bewley, J.D. Lipid-peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef]
- Yang, Z.F.; Zheng, Y.H.; Cao, S.F. Effect of high oxygen atmosphere storage on quality, antioxidant enzymes, and DPPH-radical scavenging activity of chinese bayberry fruit. J. Agric. Food Chem. 2009, 57, 176–181. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, M.; Bai, M.; Xu, Y.; Fan, S.; Yang, G. Effect of calcium on relieving berry cracking in grape (Vitis vinifera L.) ‘Xiangfei’. PeerJ 2020, 8, e9896. [Google Scholar] [CrossRef]
- Ng, J.K.T.; Schroder, R.; Sutherland, P.W.; Hallett, I.C.; Hall, M.I.; Prakash, R.; Smith, B.G.; Melton, L.D.; Johnston, J.W. Cell wall structures leading to cultivar differences in softening rates develop early during apple (Malus x domestica) fruit growth. BMC Plant Biol. 2013, 13, 183. [Google Scholar] [CrossRef]
- Lu, P.-L.; Lin, C.-H. Physiology of fruit cracking in wax apple (Syzygium samarangense). Bot. Orient. J. Plant Sci. 2012, 8, 70–76. [Google Scholar] [CrossRef]
- Considine, J.A. Physical aspects of fruit growth: Cuticular fracture and fracture patterns in relation to fruit structure in vitis vinifera. J. Hortic. Sci. 1982, 57, 79–91. [Google Scholar] [CrossRef]
- Cai, H.M.; Zhou, Y.; Xiao, J.H.; Li, X.H.; Zhang, Q.F.; Lian, X.M. Overexpressed glutamine synthetase gene modifies nitrogen metabolism and abiotic stress responses in rice. Plant Cell Rep. 2009, 28, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, H.; Wang, D.; Zhong, Y.; Deng, Y. Exploring the mechanism of Akebia trifoliata fruit cracking based on cell-wall metabolism. Food Res. Int. 2022, 157, 111219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, Y.J.; Jiang, F.L.; Wu, Z.; Cui, S.Y.; Lv, H.M.; Yu, L. Differences of reactive oxygen species metabolism in top, middle and bottom part of epicarp and mesocarp influence tomato fruit cracking. J. Hortic. Sci. Biotechnol. 2020, 95, 746–756. [Google Scholar] [CrossRef]
- Zhu, M.T.; Yu, J.; Zhao, M.; Wang, M.J.; Yang, G.S. Transcriptome analysis of metabolisms related to fruit cracking during ripening of a cracking-susceptible grape berry cv. Xiangfei (Vitis vinifera L.). Genes Genom. 2020, 42, 639–650. [Google Scholar] [CrossRef]
- Elstner, E.F. Oxygen activation and oxygen toxicity. Annu. Rev. Plant Physiol. 1982, 33, 73–96. [Google Scholar] [CrossRef]
- Karkonen, A.; Kuchitsu, K. Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 2015, 112, 22–32. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Meybodi, N.D.H.; Abadia, J.; Germ, M.; Gholami, R.; Abdelrahman, M. Evaluation of drought tolerance in three commercial pomegranate cultivars using photosynthetic pigments, yield parameters and biochemical traits as biomarkers. Agric. Water Manag. 2022, 261, 107357. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Yang, X.; Wang, T.; Li, H.; Deng, L.; Bi, X.; Hu, J.; Gong, Y.; Li, Y.; Qin, Z.; et al. Physiological Mechanisms of Citrus Fruit Cracking: Study on Cell Wall Components, Osmoregulatory Substances, and Antioxidant Enzyme Activities. Plants 2024, 13, 257. https://doi.org/10.3390/plants13020257
Huang S, Yang X, Wang T, Li H, Deng L, Bi X, Hu J, Gong Y, Li Y, Qin Z, et al. Physiological Mechanisms of Citrus Fruit Cracking: Study on Cell Wall Components, Osmoregulatory Substances, and Antioxidant Enzyme Activities. Plants. 2024; 13(2):257. https://doi.org/10.3390/plants13020257
Chicago/Turabian StyleHuang, Shengjia, Xinxia Yang, Tie Wang, Hang Li, Lijun Deng, Xiaoyi Bi, Juan Hu, Yan Gong, Yunjie Li, Zeyu Qin, and et al. 2024. "Physiological Mechanisms of Citrus Fruit Cracking: Study on Cell Wall Components, Osmoregulatory Substances, and Antioxidant Enzyme Activities" Plants 13, no. 2: 257. https://doi.org/10.3390/plants13020257
APA StyleHuang, S., Yang, X., Wang, T., Li, H., Deng, L., Bi, X., Hu, J., Gong, Y., Li, Y., Qin, Z., Yao, Y., Sun, G., Liao, L., Zhang, M., He, S., Jiang, L., & Wang, Z. (2024). Physiological Mechanisms of Citrus Fruit Cracking: Study on Cell Wall Components, Osmoregulatory Substances, and Antioxidant Enzyme Activities. Plants, 13(2), 257. https://doi.org/10.3390/plants13020257






