In Vitro Safety and Efficacy Evaluation of a Juniperus communis Callus Culture Extract and Matricaria recutita Processing Waste Extract Combination as a Cosmetic Ingredient
Abstract
1. Introduction
2. Results
2.1. Chemical Characterization of Individual Extracts and Extract Combination
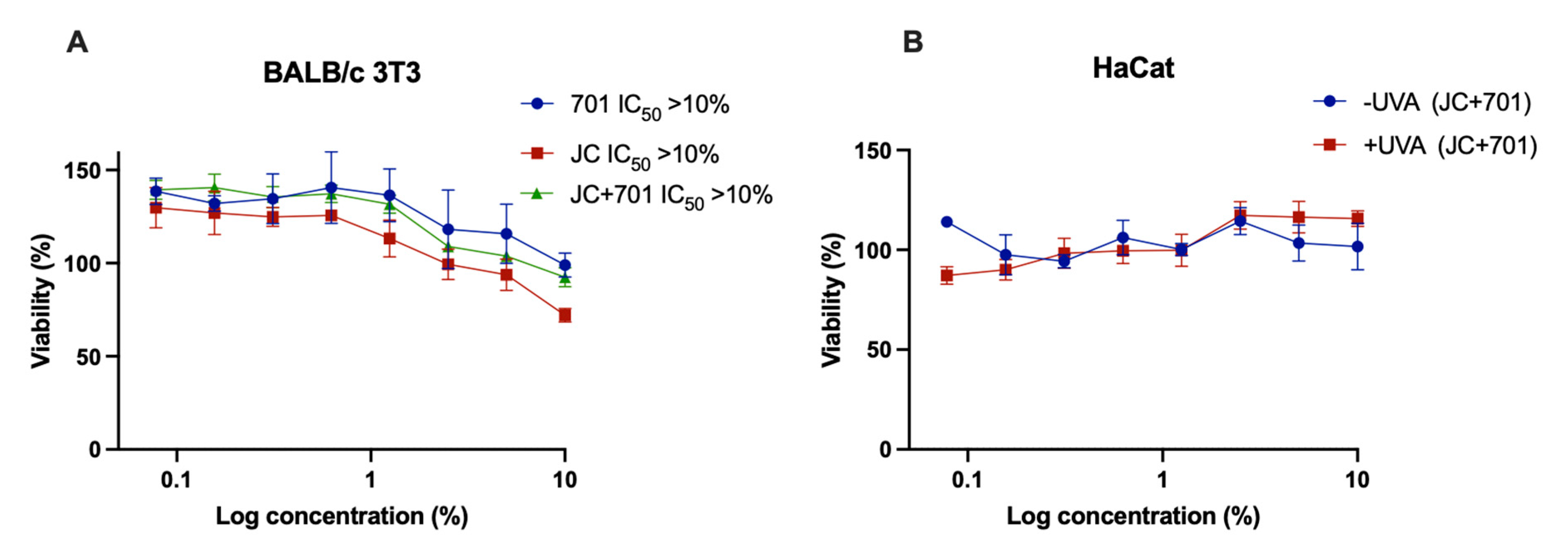
2.2. Cytotoxicity and Phototoxicity
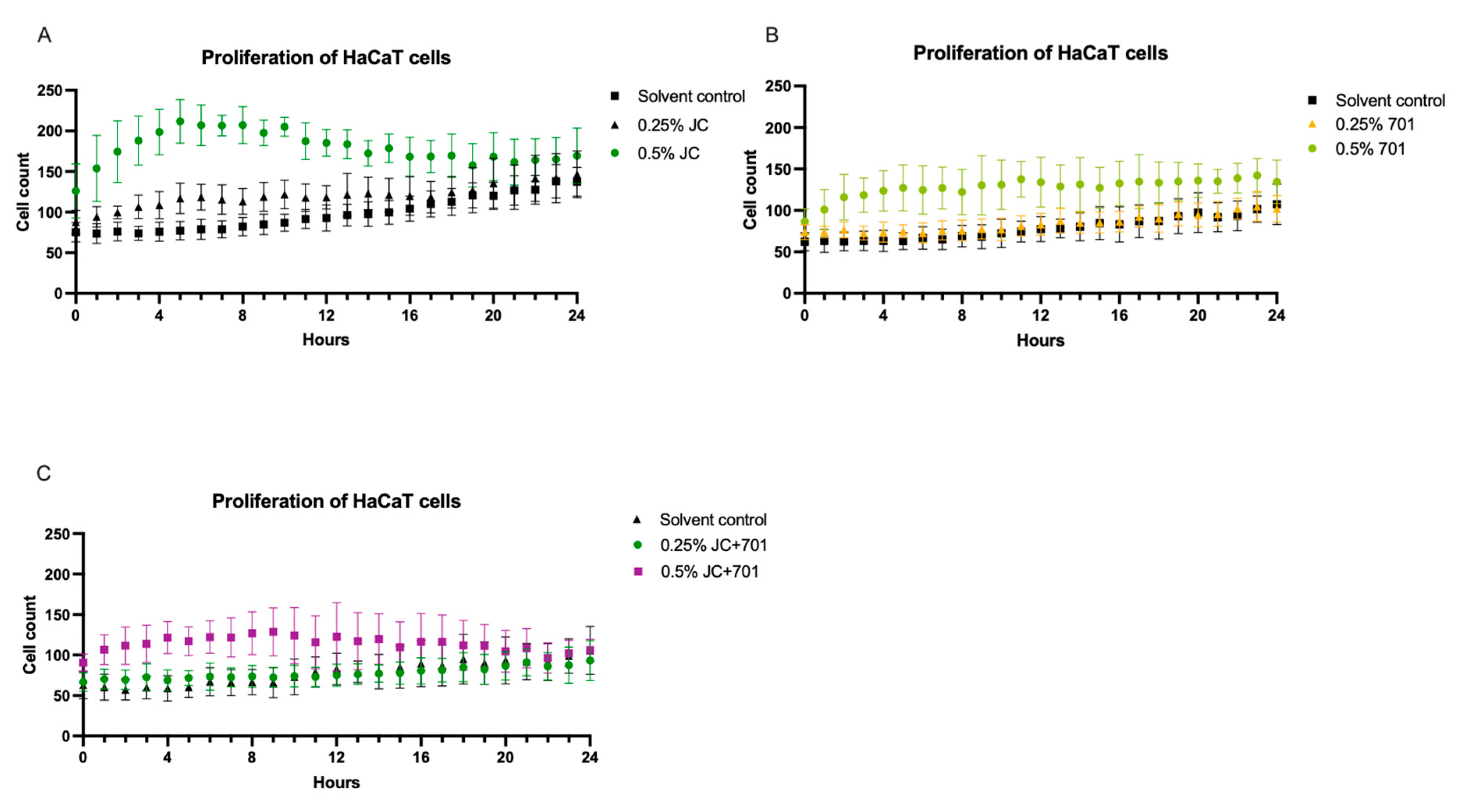
2.3. Proliferation of Keratinocytes
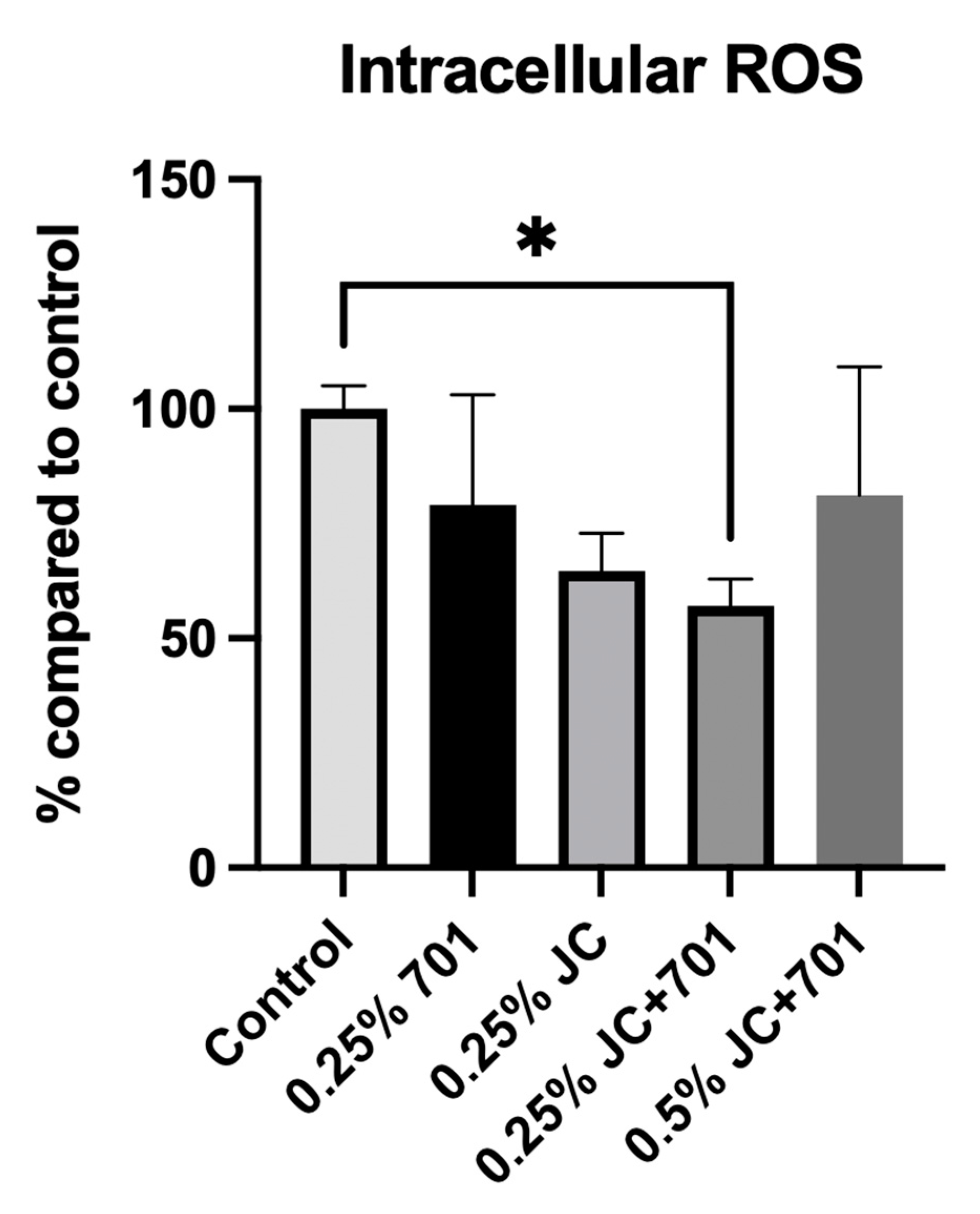
2.4. In Vitro Antioxidative Activity
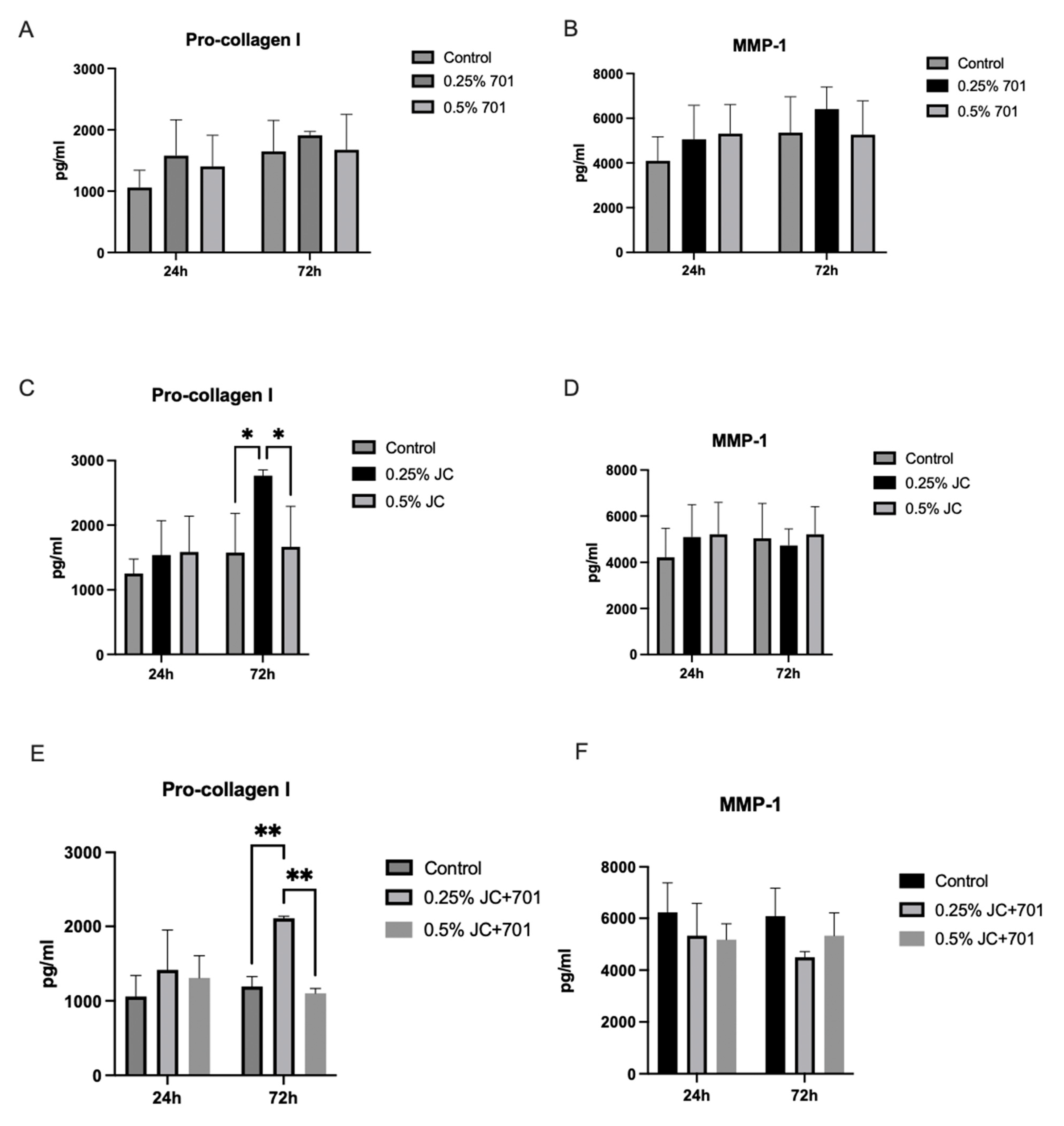
2.5. Secretion of Pro-Collagen I Alpha 1 and Matrix Metalloproteinase 1 (MMP-1)
3. Discussion
4. Materials and Methods
4.1. Extracts and Development of the Combination
4.2. Spectrophotometric Screening of Total Phenolics, Flavonoids, Tannins, Sugars, and Antioxidant Activity
4.3. Phytochemical Characterization of Extracts Using LC-qTOF-MS
4.4. Cytotoxicity
4.5. Phototoxicity
4.6. Proliferation Analysis
4.7. Flow Cytometric Quantification of Reactive Oxygen Species (ROS) in HaCaT Keratinocytes
4.8. Secretion of Pro-Collagen I and MMP-1
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martins, M.A.; Marto, J.M. A sustainable life cycle for cosmetics: From design and development to post-use phase. Sustain. Chem. Pharm. 2023, 35, 101178. [Google Scholar] [CrossRef]
- Zollo, L.; Carranza, R.; Faraoni, M.; Díaz, E.; Martín-Consuegra, D. What influences consumers’ intention to purchase organic personal care products? The role of social reassurance. J. Retail. Consum. Serv. 2021, 60, 102432. [Google Scholar] [CrossRef]
- Liu, J.K. Natural products in cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- McMullen, R.L.; Dell’Acqua, G. History of Natural Ingredients in Cosmetics. Cosmetics 2023, 10, 71. [Google Scholar] [CrossRef]
- Andersen, F.A. Final Report on the Safety Assessment of Juniperus communis Extract, Juniperus oxycedrus Extract, Juniperus oxycedrus Tar, Juniperus phoenicea Extract, and Juniperus virginiana Extract. Int. J. Toxicol. 2001, 20, 41–56. [Google Scholar] [CrossRef]
- Raina, R.; Verma, P.K.; Peshin, R.; Kour, H. Potential of Juniperus communis L. as a nutraceutical in human and veterinary medicine. Heliyon 2019, 5, e02376. [Google Scholar] [CrossRef] [PubMed]
- Garbossa, W.A.C.; Campos, P.M.M.B.G. Euterpe oleracea, Matricaria chamomilla, and Camellia sinensis as promising ingredients for development of skin care formulations. Ind. Crops Prod. 2016, 83, 1–10. [Google Scholar] [CrossRef]
- Nakurte, I.; Berga, M.; Pastare, L.; Kienkas, L.; Senkovs, M.; Boroduskis, M.; Ramata-Stunda, A. Valorization of Bioactive Compounds from By-Products of Matricaria recutita White Ray Florets. Plants 2023, 12, 396. [Google Scholar] [CrossRef]
- Pastare, L.; Berga, M.; Kienkas, L.; Boroduskis, M.; Ramata-Stunda, A.; Reihmane, D.; Senkovs, M.; Skudrins, G.; Nakurte, I. Exploring the Potential of Supercritical Fluid Extraction of Matricaria chamomilla White Ray Florets as a Source of Bioactive (Cosmetic) Ingredients. Antioxidants 2023, 12, 1092. [Google Scholar] [CrossRef]
- Slavov, A.; Yantcheva, N.; Vasileva, I. Chamomile Wastes (Matricaria chamomilla): New Source of Polysaccharides. Waste Biomass Valor. 2019, 10, 2583–2594. [Google Scholar] [CrossRef]
- Molnar, M.; Mendešević, N.; Šubarić, D.; Banjari, I.; Jokić, S. Comparison of Various Techniques for the Extraction of Umbelliferone and Herniarin in Matricaria chamomilla Processing Fractions. Chem. Cent. J. 2017, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Rajčević, N.; Bukvički, D.; Dodoš, T.; Marin, P.D. Interactions between Natural Products—A Review. Metabolites 2022, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- Ferid, A.; Arifullah, M.; Mohd, F.H.R.; Zulhisyam, A.K.; Lee, S.W.; Khang, W.G. Plant cell culture technologies: A promising alternatives to produce high-value secondary metabolites. Arab. J. Chem. 2022, 15, 104161. [Google Scholar] [CrossRef]
- Apone, F.; Tito, A.; Arciello, S.; Carotenuto, G.; Colucci, M.G. Plant Tissue Cultures as Sources of Ingredients for Skin Care Applications. Annu. Plant Rev. Online 2020, 3, 135–150. [Google Scholar] [CrossRef]
- Barbulova, A.; Apone, F.; Colucci, G. Plant Cell Cultures as Source of Cosmetic Active Ingredients. Cosmetics 2014, 1, 94–104. [Google Scholar] [CrossRef]
- Diaz, I.; Namkoong, J.; Wu, J.Q.; Giancola, G. Amino acid complex (AAComplex) benefits in cosmetic products: In vitro and in vivo clinical studies. J. Cosmet. Dermatol. 2022, 21, 3046. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-S. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Kim, J.M.; Heo, H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022, 31, 957. [Google Scholar] [CrossRef]
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from Microalgae for Cosmetic Applications. Cosmetics 2021, 8, 52. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N. Biopolysaccharides for skin hydrating cosmetics. In Polysaccharides; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Cham, Switzerland, 2015; pp. 1867–1892. [Google Scholar]
- Kanlayavattanakul, M.; Lourith, N. Cordyceps militaris polysaccharides: Preparation and topical product application. Fungal Biol. Biotechnol. 2023, 10, 3. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Fungpaisalpong, K.; Pumcharoen, M.; Lourith, N. Preparation and efficacy assessment of malva nut polysaccharide for skin hydrating products. Ann. Pharm. Françaises 2017, 75, 436–445. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, B. Skin Health Promoting Effects of Natural Polysaccharides and Their Potential Application in the Cosmetic Industry. Polysaccharides 2022, 3, 818–830. [Google Scholar] [CrossRef]
- Albuquerque, P.B.S.; de Oliveira, W.F.; Dos Santos Silva, P.M.; Dos Santos Correia, M.T.; Kennedy, J.F.; Coelho, L.C.B.B. Skincare application of medicinal plant polysaccharides—A review. Carbohydr. Polym. 2022, 277, 118824. [Google Scholar] [CrossRef]
- Švehlíková, V.; Bennett, R.N.; Mellon, F.A.; Needs, P.W.; Piacente, S.; Kroon, P.A.; Bao, Y. Isolation, Identification and Stability of Acylated Derivatives of Apigenin 7-O-Glucoside from Chamomile (Chamomilla recutita [L.] Rauschert). Phytochemistry 2004, 65, 2323–2332. [Google Scholar] [CrossRef]
- Tsivelika, N.; Irakli, M.; Mavromatis, A.; Chatzopoulou, P.; Karioti, A. Phenolic Profile by HPLC-PDA-MS of Greek Chamomile Populations and Commercial Varieties and Their Antioxidant Activity. Foods 2021, 10, 2345. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, N.S.; Megremi, S.F.; Tarantilis, P. Evaluation of Antioxidant Activity, Toxicity, and Phenolic Profile of Aqueous Extracts of Chamomile (Matricaria chamomilla L.) and Sage (Salvia officinalis L.) Prepared at Different Temperatures. Appl. Sci. 2020, 10, 2270. [Google Scholar] [CrossRef]
- Catani, M.V.; Rinaldi, F.; Tullio, V.; Gasperi, V.; Savini, I. Comparative Analysis of Phenolic Composition of Six Commercially Available Chamomile (Matricaria chamomilla L.) Extracts: Potential Biological Implications. Int. J. Mol. Sci. 2021, 22, 10601. [Google Scholar] [CrossRef]
- Nóbrega, A.T.; Wagemaker, T.A.L.; Maia Campos, P.M.B.G. Antioxidant activity of Matricaria chamomilla L. extract and clinical efficacy of cosmetic formulations containing this extract and its isolated compounds. Biomed. Biopharm. Res. 2013, 10, 249–261. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 5. [Google Scholar] [CrossRef]
- Che, D.N.; Cho, B.O.; Shin, J.Y.; Kang, H.J.; Kim, J.-S.; Oh, H.; Kim, Y.-S.; Jang, S.I. Apigenin Inhibits IL-31 Cytokine in Human Mast Cell and Mouse Skin Tissues. Molecules 2019, 24, 1290. [Google Scholar] [CrossRef]
- Majma Sanaye, P.; Mojaveri, M.R.; Ahmadian, R.; Sabet Jahromi, M.; Bahramsoltani, R. Apigenin and Its Dermatological Applications: A Comprehensive Review. Phytochemistry 2022, 203, 113390. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Kim, M.Y.; Cho, J.Y. Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2023, 24, 1498. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-H.; Min, S.-Y.; Yu, H.-W.; Kim, K.; Kim, S.; Lee, H.-J.; Kim, J.-H.; Park, Y.-J. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, S.; Haramizu, S.; Sasaoka, S.; Yasuda, Y.; Tsujimura, H.; Murase, T. Coffee polyphenols extracted from green coffee beans improve skin properties and microcirculatory function. Biosci. Biotechnol. Biochem. 2017, 81, 1814. [Google Scholar] [CrossRef]
- Lee, K.-H. Impact of chlorogenic acid on modulation of significant genes in dermal fibroblasts and epidermal keratinocytes. Biochem. Biophys. Res. Commun. 2021, 583, 22. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H.J. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Peres, D.D.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B Biol. 2018, 185, 46. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332. [Google Scholar] [CrossRef]
- Oresajo, C.; Stephens, T.; Hino, P.D.; Law, R.M.; Yatskayer, M.; Foltis, P.; Pillai, S.; Pinnell, S.R. Protective effects of a topical antioxidant mixture containing vitamin C, ferulic acid, and phloretin against ultraviolet-induced photodamage in human skin. J. Cosmet. Dermatol. 2008, 7, 290. [Google Scholar] [CrossRef]
- Alshibl, H.M.; Al-Abdullah, E.S.; Haiba, M.E.; Alkahtani, H.M.; Awad, G.E.A.; Mahmoud, A.H.; Ibrahim, B.M.M.; Bari, A.; Villinger, A. Synthesis and Evaluation of New Coumarin Derivatives as Antioxidant, Antimicrobial, and Anti-Inflammatory Agents. Molecules 2020, 25, 3251. [Google Scholar] [CrossRef]
- Roh, E.-J. Inhibitory Effects of Coumarin Derivatives on Tyrosinase. Molecules 2021, 26, 2346. [Google Scholar] [CrossRef]
- Ford, R.A.; Hawkins, D.R.; Mayo, B.C.; Api, A.M. The in vivo dermal absorption and metabolism of [4-14C] coumarin by rats and by human volunteers under simulated conditions of use in fragrances. Food Chem. Toxicol. 2001, 39, 153–162. [Google Scholar] [CrossRef]
- Arct, J.; Bielenda, B.; Oborska, A.; Pytkowska, K. The tea and its cosmetic application. J. Appl. Cosmetol. 2003, 21, 117–127. [Google Scholar]
- Zhang, W.; Yang, Y.; Lv, T.; Fan, Z.; Xu, Y.; Yin, J. Sucrose esters improve the colloidal stability of nanoethosomal suspensions of (−)-epigallocatechin gallate for enhancing the effectiveness against UVB-induced skin damage. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 105, 2416–2425. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, S.; Mitoma, T.; Tsuruta, K.; Todo, H.; Sugibayashi, K. Effect of emulsification on the skin permeation and UV protection of catechin. Pharm. Dev. Technol. 2013, 19, 395–400. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.; Shin, D.W. The Molecular Mechanism of Polyphenols with Anti-Aging Activity in Aged Human Dermal Fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Cegliński, T.; Zajdel, R. Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.R.; Kang, Y.J.; Kim, J.H.; Lee, H.T.; Cho, S.G. Modulation of apoptosis in HaCaT keratinocytes via differential regulation of ERK signaling pathway by flavonoids. J. Biol. Chem. 2005, 9, 280–31498. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, T. Antimicrobial/Antibiofilm Activity and Cytotoxic Studies of β-Thujaplicin Derivatives. Arch. Pharm. 2016, 349, 698. [Google Scholar] [CrossRef]
- Domon, H.; Hiyoshi, T.; Maekawa, T.; Yonezawa, D.; Tamura, H.; Kawabata, S.; Yanagihara, K.; Kimura, O.; Kunitomo, E.; Terao, Y. Antibacterial activity of hinokitiol against both antibiotic-resistant and -susceptible pathogenic bacteria that predominate in the oral cavity and upper airways. Microbiol. Immunol. 2019, 63, 213. [Google Scholar] [CrossRef]
- Suzuki, H.; Ueda, T.; Juránek, I.; Yamamoto, S.; Katoh, T.; Node, M. Hinokitiol, a Selective Inhibitor of the Platelet-Type Isozyme of Arachidonate 12-Lipoxygenase. Biochem. Biophys. Res. Commun. 2000, 275, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y. Biological activity of α-thujaplicin, the minor component of Thujopsis dolabrata Sieb. et Zucc. var. hondai Makino. Biol. Pharm. Bull. 2001, 24, 607. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Qiu, L.; Zhou, J.J.; Guo, H.Y.; Hu, Y.H.; Li, Z.C.; Wang, Q.; Chen, Q.X.; Liu, B. Inhibitory effects of hinokitiol on tyrosinase activity and melanin biosynthesis and its antimicrobial activities. J. Enzym. Inhib. Med. Chem. 2010, 25, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.G.; Bae, E.J.; Kim, D.S.; Park, S.H.; Kwon, S.B.; Na, J.I.; Park, K.C. Differential regulation of melanosomal proteins after hinokitiol treatment. J. Dermatol. Sci. 2006, 43, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Dank, C.; Sanichar, R.; Choo, K.L.; Olsen, M.; Lautens, M. Recent advances towards syntheses of diterpenoid alkaloids. Synthesis 2019, 51, 3915–3946. [Google Scholar]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, S.; Miao, Q. Protective Role of Hinokitiol Against H2O2-Induced Injury in Human Corneal Epithelium. Curr. Eye Res. 2017, 42, 47–53. [Google Scholar] [CrossRef]
- Arima, Y.; Hatanaka, A.; Tsukihara, S.; Fujimoto, K.; Fukuda, K.; Sakurai, H. Scavenging activities of α-, β-and γ-thujaplicins against active oxygen species. Chem. Pharm. Bull. 1997, 45, 1881–1886. [Google Scholar] [CrossRef]
- Martín, M.A.; Serrano, A.B.; Ramos, S.; Pulido, M.I.; Bravo, L.; Goya, L. Cocoa flavonoids up-regulate antioxidant enzyme activity via the ERK1/2 pathway to protect against oxidative stress-induced apoptosis in HepG2 cells. J. Nutr. Biochem. 2010, 21, 196. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 3, 1379–1391. [Google Scholar] [CrossRef]
- Jomová, K.; Hudecova, L.; Lauro, P. A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3′,4′-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper(II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study. Molecules 2019, 24, 4335. [Google Scholar] [CrossRef] [PubMed]
- Xue, N.; Liu, Y.; Jin, J.; Ji, M.; Chen, X. Chlorogenic Acid Prevents UVA-Induced Skin Photoaging through Regulating Collagen Metabolism and Apoptosis in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2022, 23, 6941. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yu, J.S.; Phung, H.M.; Lee, J.G.; Kim, K.H.; Kang, K.S. Potential Anti-Skin Aging Effect of (−)-Catechin Isolated from the Root Bark of Ulmus davidiana var. japonica in Tumor Necrosis Factor-α-Stimulated Normal Human Dermal Fibroblasts. Antioxidants 2020, 9, 981. [Google Scholar] [CrossRef]
- Stipcevic, T.; Piljac, J.; Berghe, D.V. Effect of Different Flavonoids on Collagen Synthesis in Human Fibroblasts. Plant Foods Hum. Nutr. 2006, 61, 27–32. [Google Scholar] [CrossRef]
- Galicka, A.; Nazaruk, J. Stimulation of collagen biosynthesis by flavonoid glycosides in skin fibroblasts of osteogenesis imperfecta type I and the potential mechanism of their action. Int. J. Mol. Med. 2007, 20, 889–895. [Google Scholar]
- Zhang, Y.; Wang, J.; Cheng, X.; Yi, B.; Zhang, X.; Li, Q. Apigenin induces dermal collagen synthesis via smad2/3 signaling pathway. Eur. J. Histochem. 2015, 59, 2467. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.Y.; Chen, L.Y.; Shih, M.F. Preventive effects of β-thujaplicin against UVB-induced MMP-1 and MMP-3 mRNA expressions in skin fibroblasts. Am. J. Chin. Med. 2012, 40, 387–398. [Google Scholar] [CrossRef]
- OECD Series on Testing and Assessment No. 129. Guidance Document on Using Cytotoxicity Tests to Estimate Starting Doses for Acute Oral Systemic Toxicity Tests. 2010. Available online: https://ntp.niehs.nih.gov/pubhealth/evalatm/test-method-evaluations/acute-systemic-tox/in-vitro-validation/index.html (accessed on 11 December 2023).
- OECD Guidelines for the Testing of Chemicals, Section 4. Test Guideline No. 432: In Vitro 3T3 NRU Phototoxicity Test. 2019. Available online: https://www.oecd-ilibrary.org/docserver/9789264071162-en.pdf?expires=1705533292&id=id&accname=guest&checksum=F0372C589DA69F3CB3ACF20D64224C6F (accessed on 20 December 2023).
- Wang, J.; Roberts, S.; Li, W.; Wright, K. Phenotypic characterization of regional human meniscus progenitor cells. Front. Bioeng. Biotechnol. 2022, 10, 1003966. [Google Scholar] [CrossRef]
| Extract | TPC a, GAE mg mL−1 | TFC b, APE mg mL−1 | TFC c, CAE mg mL−1 | TTC d, TAE mg mL−1 | Sugars e, GLE mg mL−1 |
|---|---|---|---|---|---|
| 701 | 1.83 * ± 0.01 | 1.79 * ± 0.03 | 1.54 * ± 0.01 | 2.21 * ± 0.02 | 78.88 * ± 0.04 |
| JC | 0.35 * ± 0.01 | 0.19 * ± 0.01 | 0.48 * ± 0.01 | 2.64 * ± 0.01 | 5.60 * ± 0.01 |
| JC + 701 | 1.57 * ± 0.01 | 0.79 * ± 0.04 | 1.33 * ± 0.01 | 1.45 * ± 0.02 | 24.22 * ± 0.03 |
| Extract | ARA a, TE µg mL−1 | DPPH b Quenched, % | IC 50, mg mL−1 |
|---|---|---|---|
| 701 | 22.1 * ± 0.01 | 57.6 * ± 0.13 | 0.97 * ± 0.05 |
| JC | 91.3 * ± 0.02 | 62.0 * ± 0.15 | 2.00 * ± 0.06 |
| JC + 701 (1%) c | 16.9 * ± 0.02 | 13.7 * ± 0.02 | 1.91 * ± 0.05 |
| JC + 701 (5%) c | 50.5 * ± 0.03 | 35.5 * ± 0.02 | |
| JC + 701 (10%) c | 90.8 * ± 0.04 | 61.6 * ± 0.06 | |
| JC + 701 (25%) c | 110.9 * ± 0.06 | 74.6 * ± 0.05 |
| Number | Proposed Compound | Formula | JC + 701, µg mL−1 | Source |
|---|---|---|---|---|
| Amino Acids and Derivatives a, µg mL−1 | ||||
| 1 | Homostachydrine | C8H15NO2 | 16.98 ± 0.19 | 701 |
| 2 | N-(1-deoxy-D-fructos-1-yl)-L-Serine | C9H17NO8 | 14.52 ± 0.33 | JC; 701 |
| 3 | Isoleucine | C6H13NO2 | 11.07 ± 0.09 | JC; 701 |
| 4 | Leucine | C6H13NO2 | 20.20 ± 0.11 | JC; 701 |
| 5 | Tyrosine | C9H11NO3 | 3.49 ± 0.08 | JC; 701 |
| 6 | Phenylalanine | C9H11NO2 | 6.74 ± 0.09 | JC; 701 |
| 7 | Tyrosyl-Tyrosine | C18H20N2O5 | 2.54 ± 0.04 | JC; 701 |
| Total | 75.53 ± 0.45 | |||
| Flavonoids/Epigallocatechins b, µg mL−1 | ||||
| 8 | (−)-Epigallocatechin | C15H14O7 | 2.72 ± 0.09 | JC; 701 |
| 9 | Epicatechin-(4β⟶8)-gallocatechin | C30H26O13 | 1.59 ± 0.06 | JC |
| 10 | (+)-Gallocatechin | C15H14O7 | 1.00 ± 0.06 | JC |
| 11 | (+)-Catechin | C15H14O6 | 0.95 ± 0.05 | JC |
| Total | 6.26 ± 0.12 | |||
| Flavonoids c, µg mL−1 | ||||
| 12 | Apigenin 7-O-glucoside | C21H20O10 | 14.39 ± 0.23 | 701 |
| Total | 14.39 ± 0.23 | |||
| Quinic acids and derivatives d, µg mL−1 | ||||
| 13 | Neochlorogenic acid | C16H18O9 | 15.41 ± 0.28 | 701 |
| 14 | Chlorogenic acid | C16H18O9 | 36.29 ± 0.34 | 701 |
| 15 | Cryptochlorogenic acid | C16H18O9 | 18.78 ± 0.09 | 701 |
| Total | 70.48 ± 0.63 | |||
| Coumarins and derivatives e, µg mL−1 | ||||
| 16 | Skimmin | C15H16O8 | 8.28 ± 0.08 | 701 |
| 17 | Herniarin | C10H8O3 | 13.71 ± 0.11 | 701 |
| Total | 21.99 ± 0.10 | |||
| Phenolic glycosides f, µg mL−1 | ||||
| 18 | Ferulic acid O-glucoside I | C16H20O9 | 880.8 ± 0.67 | 701 |
| 19 | Ferulic acid O-glucoside II | C16H20O9 | 223.4 ± 0.25 | 701 |
| Total | 1104.21 ± 0.95 | |||
| Tropones g, µg mL−1 | ||||
| 20 | β-Thujaplicin glycoside I | C16H22O7 | 0.97 ± 0.02 | JC |
| 21 | β-Thujaplicin glycoside II | C16H22O7 | 1.04 ± 0.02 | JC |
| Total | 2.01 ± 0.02 | |||
| Diterpenoid alkaloids i, µg mL−1 | ||||
| 22 | Dihydroajaconine I | C22H35NO3 | 0.27 ± 0.01 | JC |
| 23 | Dihydroajaconine II | C22H35NO3 | 0.03 ± 0.00 | JC |
| Total | 0.30 ± 0.01 | |||
| Fatty Acyls j, µg mL−1 | ||||
| 24 | 3-Hydroxyphenyl-valeric acid | C11H14O3 | 14.30 ± 0.03 | 701 |
| 25 | Isobutyl-2-methylbutyrate | C9H18O2 | 28.20 ± 0.07 | 701 |
| Total | 42.50 ± 0.15 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramata-Stunda, A.; Boroduskis, M.; Pastare, L.; Berga, M.; Kienkas, L.; Patetko, L.; Skudrins, G.; Reihmane, D.; Nakurte, I. In Vitro Safety and Efficacy Evaluation of a Juniperus communis Callus Culture Extract and Matricaria recutita Processing Waste Extract Combination as a Cosmetic Ingredient. Plants 2024, 13, 287. https://doi.org/10.3390/plants13020287
Ramata-Stunda A, Boroduskis M, Pastare L, Berga M, Kienkas L, Patetko L, Skudrins G, Reihmane D, Nakurte I. In Vitro Safety and Efficacy Evaluation of a Juniperus communis Callus Culture Extract and Matricaria recutita Processing Waste Extract Combination as a Cosmetic Ingredient. Plants. 2024; 13(2):287. https://doi.org/10.3390/plants13020287
Chicago/Turabian StyleRamata-Stunda, Anna, Martins Boroduskis, Laura Pastare, Marta Berga, Liene Kienkas, Liene Patetko, Gundars Skudrins, Dace Reihmane, and Ilva Nakurte. 2024. "In Vitro Safety and Efficacy Evaluation of a Juniperus communis Callus Culture Extract and Matricaria recutita Processing Waste Extract Combination as a Cosmetic Ingredient" Plants 13, no. 2: 287. https://doi.org/10.3390/plants13020287
APA StyleRamata-Stunda, A., Boroduskis, M., Pastare, L., Berga, M., Kienkas, L., Patetko, L., Skudrins, G., Reihmane, D., & Nakurte, I. (2024). In Vitro Safety and Efficacy Evaluation of a Juniperus communis Callus Culture Extract and Matricaria recutita Processing Waste Extract Combination as a Cosmetic Ingredient. Plants, 13(2), 287. https://doi.org/10.3390/plants13020287








