Growth, Physiological, and Biochemical Variations in Tomatoes after Infection with Different Density Levels of Meloidogyne enterolobii
Abstract
1. Introduction
2. Results
2.1. Growth Parameters
2.2. Nematode Infection
2.3. Photosynthetic Pigments
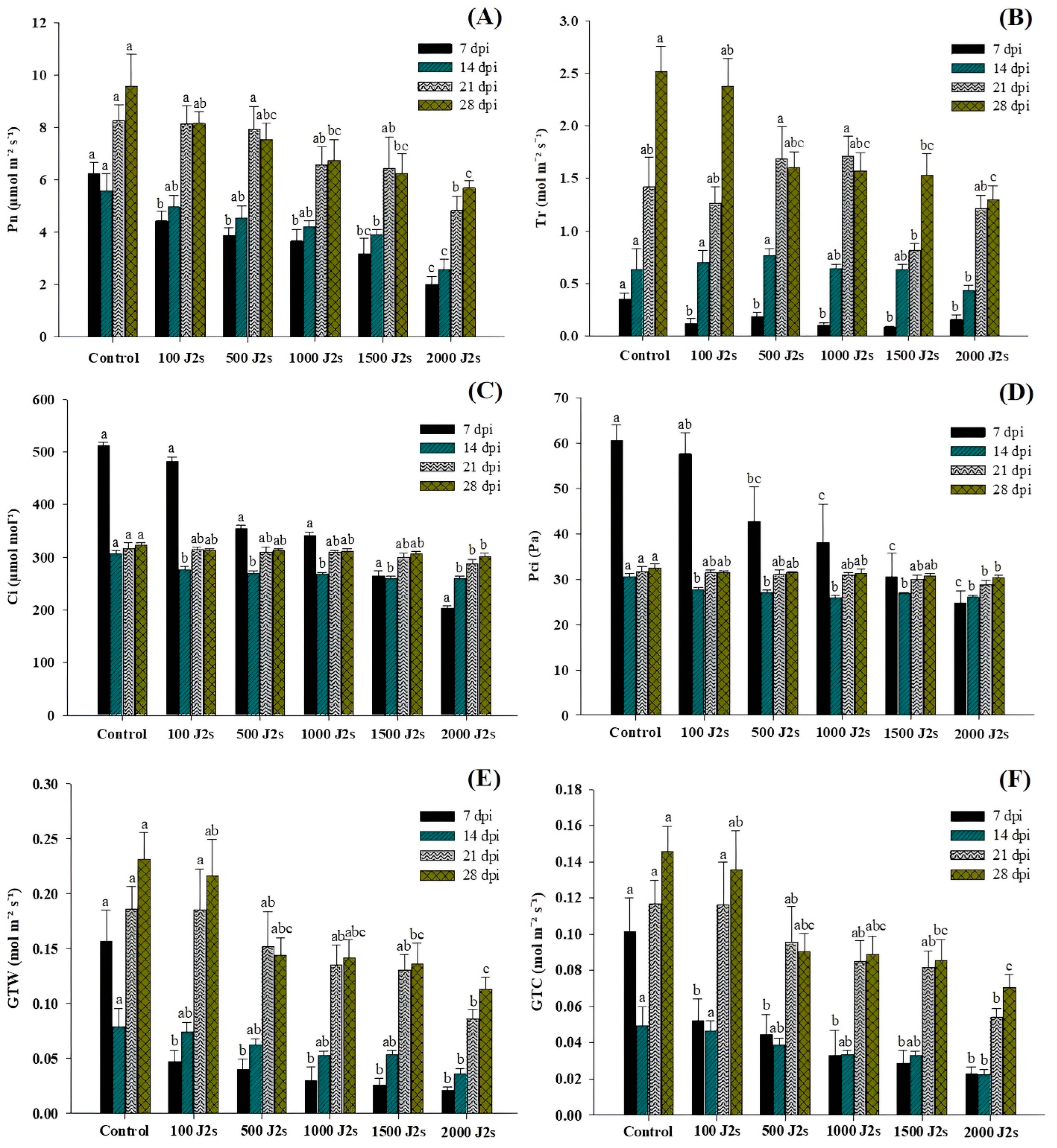
2.4. Gas Exchange Parameters
2.5. Chlorophyll Fluorescence Traits
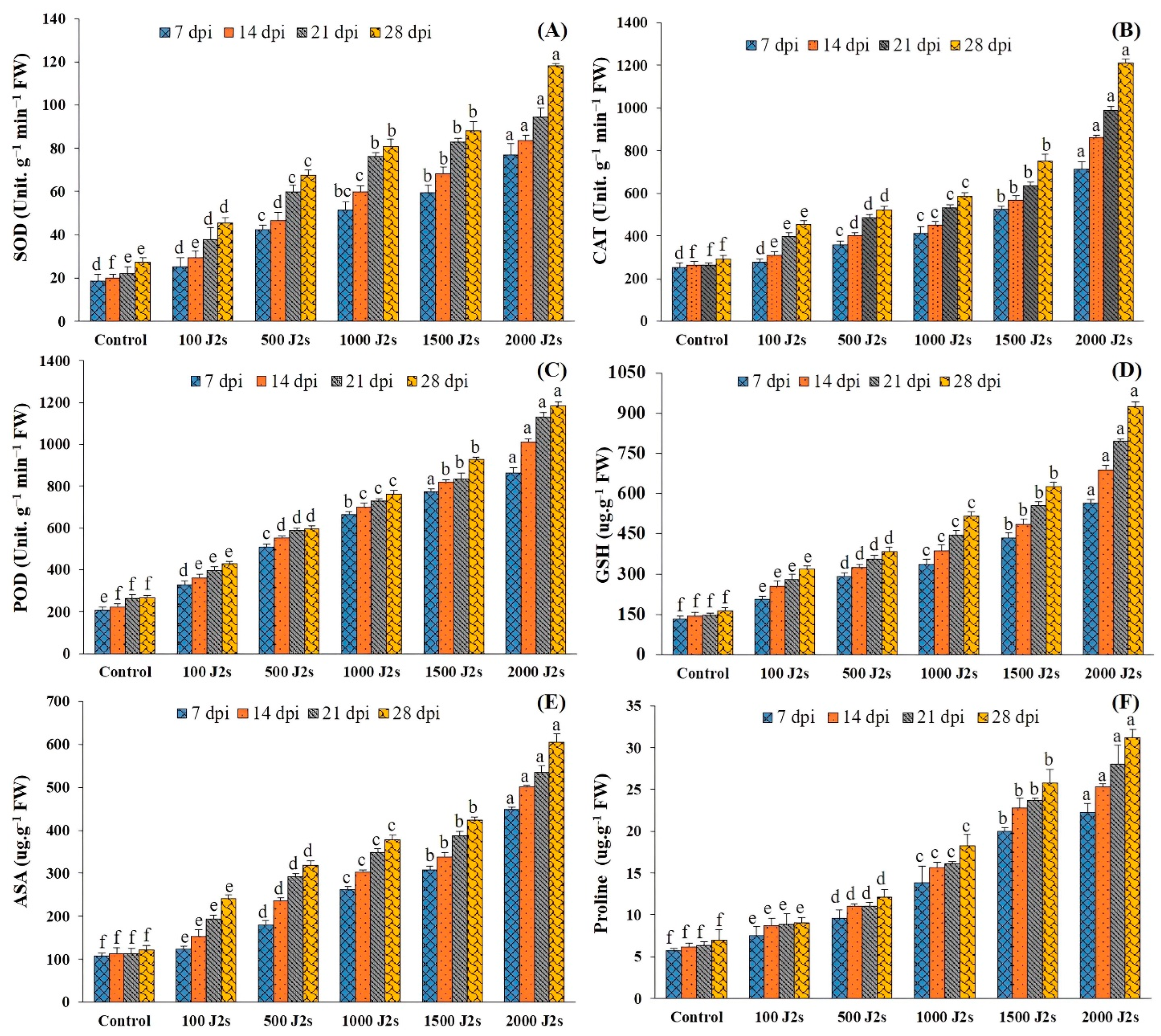
2.6. Antioxidant Enzymes Activities
2.7. Non-Enzymatic Antioxidants
2.8. Proline Content (Osmolyte)
2.9. Stress Indices
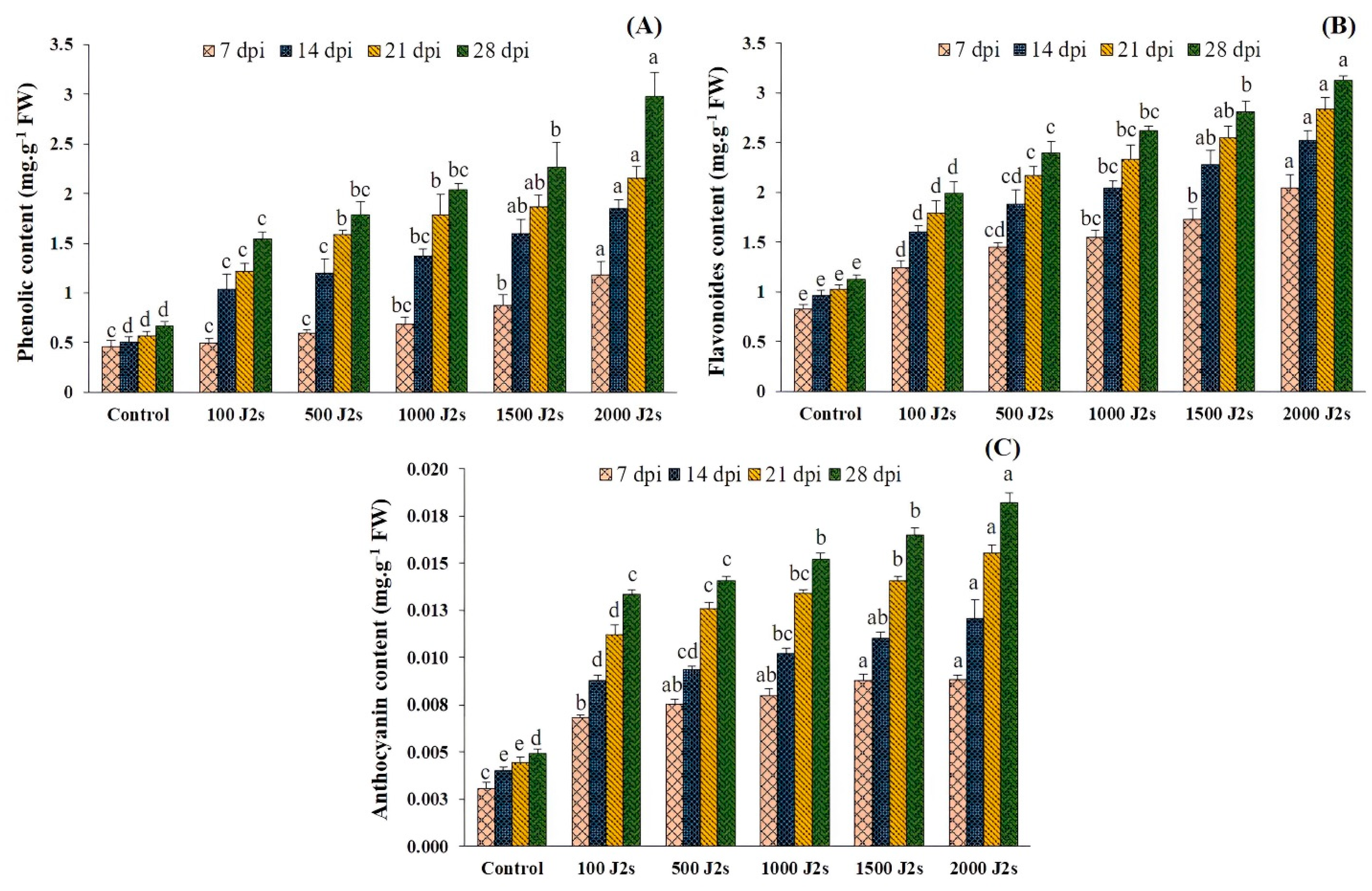
2.10. Secondary Metabolites
3. Discussion
4. Materials and Methods
4.1. Plant Material and Nematodes Inoculum
4.2. Treatment Plan
4.3. Assessment of Growth Parameter and Determination of Nematode Infection
4.4. Total Chlorophyll Contents
4.5. Leaf Gas Exchange and Chlorophyll Fluorescence Parameters Characteristics
4.6. Measurement of Antioxidant Enzymes
4.7. Measurement of Non-Enzymatic Antioxidants
4.8. Proline Contents
4.9. Stress Indices
4.10. Secondary Metabolites
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, A.; Khan, A.; Ali, A.; Fatima, S.; Siddiqui, M.A. Root-knot nematodes (Meloidogyne spp.): Biology, plant-nematode interactions and their environmentally benign management strategies. Gesunde Pflanz. 2023, 75, 2187–2205. [Google Scholar] [CrossRef]
- Sikandar, A.; Zhang, M.; Yang, R.; Liu, D.; Zhu, X.; Liu, X.; Fan, H.; Duan, Y.; Wang, Y. Analysis of gene expression in cucumber roots interacting with Penicillium chrysogenum strain Snef1216 through seed coating, which induced resistance to Meloidogyne incognita. Nematology 2021, 24, 121–135. [Google Scholar] [CrossRef]
- Song, P.; Peng, D.-l.; Li, Y.-m.; Chen, Z.-j.; Zhai, Y.-y.; Chen, L.; Bo, H. Potential global distribution of the guava root-knot nematode Meloidogyne enterolobii under different climate change scenarios using MaxEnt ecological niche modeling. J. Integr. Agric. 2023, 22, 2138–2150. [Google Scholar] [CrossRef]
- Collett, R.L.; Marais, M.; Daneel, M.; Rashidifard, M.; Fourie, H. Meloidogyne enterolobii, a threat to crop production with particular reference to sub-Saharan Africa: An extensive, critical and updated review. Nematology 2021, 23, 247–285. [Google Scholar] [CrossRef]
- Pinto, T.J.B.; Silva, G.O.d.; Vendrame, L.P.d.C.; Pinheiro, J.B.; Santos, L.A.; Cunha, D.F.; Melo, R.A.d.C.; Cares, J.E. Sources of root-knot nematode (Meloidogyne enterolobii) resistance in sweetpotato genotypes. Hortic. Bras. 2023, 41, e2588. [Google Scholar] [CrossRef]
- Jia, L.; Wang, Y.; Gao, F.; Chen, Q.; Yang, S.; Wu, H. First report of the root-knot nematode Meloidogyne enterolobii infecting Acalypha australis in China. Plant Dis. 2023, 107, 587. [Google Scholar] [CrossRef]
- Castagnone-Sereno, P. Meloidogyne enterolobii (=M. mayaguensis): Profile of an emerging, highly pathogenic, root-knot nematode species. Nematology 2012, 14, 133–138. [Google Scholar] [CrossRef]
- Costa, J.M.; Heuvelink, E. The Global Tomato Industry, 2nd ed.; CABI: Wallingford UK, 2018; pp. 1–26. [Google Scholar] [CrossRef]
- Kissoudis, C.; Chowdhury, R.; van Heusden, S.; van de Wiel, C.; Finkers, R.; Visser, R.G.; Bai, Y.; van der Linden, G. Combined biotic and abiotic stress resistance in tomato. Euphytica 2015, 202, 317–332. [Google Scholar] [CrossRef]
- Sikandar, A.; Jia, L.M.; Wu, H.Y.; Yang, S. Meloidogyne enterolobii risk to agriculture, its present status and future prospective for management. Front. Plant Sci. 2023, 13, 1093657. [Google Scholar] [CrossRef]
- Philbrick, A.N.; Adhikari, T.B.; Louws, F.J.; Gorny, A.M. Meloidogyne enterolobii, a major threat to tomato production: Current status and future prospects for its management. Front. Plant Sci. 2020, 11, 606395. [Google Scholar] [CrossRef]
- Hussain, M.A.; Parveen, G. Determining the damage threshold of root-knot nematode, Meloidogyne arenaria on Vigna unguiculata (L.) Walp. Rhizosphere 2023, 27, 100714. [Google Scholar] [CrossRef]
- Jin, Y.; Li, D.; Liu, M.; Cui, Z.; Sun, D.; Li, C.; Zhang, A.; Cao, H.; Ruan, Y. Genome-wide association study identified novel SNPs associated with chlorophyll content in maize. Genes 2023, 14, 1010. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant 2016, 38, 102. [Google Scholar] [CrossRef]
- Senesi, G.S.; De Pascale, O.; Marangoni, B.S.; Caires, A.R.L.; Nicolodelli, G.; Pantaleo, V.; Leonetti, P. Chlorophyll fluorescence imaging (CFI) and laser-induced breakdown spectroscopy (LIBS) applied to investigate tomato plants infected by the root knot nematode (RKN) Meloidogyne incognita and tobacco plants infected by Cymbidium ringspot virus. Photonics 2022, 9, 627. [Google Scholar] [CrossRef]
- Carrillo-Fasio, J.; Martínez-Gallardo, J.; Ayala-Tafoya, F.; López-Orona, C.; Allende-Molar, R.; Retes-Manjarrez, J. Screening for resistance to Meloidogyne enterolobii in Capsicum annuum landraces from Mexico. Plant Dis. 2020, 104, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, J.; Kiewnick, S. Virulence of Meloidogyne incognita populations and Meloidogyne enterolobii on resistant cucurbitaceous and solanaceous plant genotypes. J. Plant Dis. Prot. 2018, 125, 415–424. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Suman, S.; Bagal, D.; Jain, D.; Singh, R.; Singh, I.K.; Singh, A. Biotic stresses on plants: Reactive oxygen species generation and antioxidant mechanism. In Frontiers in Plant-Soil Interaction; Aftab, T., Hakeem, K.R., Eds.; Academic Press: London, UK, 2021; pp. 381–411. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Farahat, A.A.; Alsayed, A.A.; Mahfoud, N.M. Response of antioxidant substances and enzymes activities as a defense mechanism against root-knot nematode infection. Not. Bot. Horti Agrobot. 2012, 40, 132–142. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive oxygen species, antioxidant responses and implications from a microbial modulation perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Biswas, K.; Adhikari, S.; Tarafdar, A.; Kumar, R.; Saha, S.; Ghosh, P. Reactive oxygen species and antioxidant defence systems in plants: Role and crosstalk under biotic stress. In Sustainable Agriculture in the Era of Climate Change; Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S., Eds.; Springer: Cham, Switzerland, 2020; pp. 265–292. [Google Scholar] [CrossRef]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef]
- Sharma, I.; Ahmad, P. Catalase: A versatile antioxidant in plants. In Oxidative Damage to Plants; Ahmad, P., Ed.; Elsevier: London, UK, 2014; pp. 131–148. [Google Scholar] [CrossRef]
- Mhamdi, A.; Noctor, G.; Baker, A. Plant catalases: Peroxisomal redox guardians. Arch. Biochem. Biophys. 2012, 525, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Gondim, F.A.; Gomes-Filho, E.; Costa, J.H.; Alencar, N.L.M.; Prisco, J.T. Catalase plays a key role in salt stress acclimation induced by hydrogen peroxide pretreatment in maize. Plant Physiol. Biochem. 2012, 56, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Saberi Riseh, R.; Skorik, Y.A.; Thakur, V.K.; Moradi Pour, M.; Tamanadar, E.; Noghabi, S.S. Encapsulation of plant biocontrol bacteria with alginate as a main polymer material. Int. J. Mol. Sci. 2021, 22, 11165. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Shumayla; Singh, S.P.; Upadhyay, S.K. Role of superoxide dismutases (SODs) in stress tolerance in plants. In Molecular Approaches in Plant Biology and Environmental Challenges; Singh, S., Upadhyay, S., Pandey, A., Kumar, S., Eds.; Springer: Singapore, 2019; pp. 51–77. [Google Scholar] [CrossRef]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Superoxide dismutases and catalase. In Reactions, Processes: Oxidants and Antioxidant Defense Systems; Grune, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 109–149. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, M.E.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Hatem, E.; Berthonaud, V.; Dardalhon, M.; Lagniel, G.; Baudouin-Cornu, P.; Huang, M.-E.; Labarre, J.; Chédin, S. Glutathione is essential to preserve nuclear function and cell survival under oxidative stress. Free Radic. Biol. Med. 2014, 67, 103–114. [Google Scholar] [CrossRef]
- Kilili, K.G.; Atanassova, N.; Vardanyan, A.; Clatot, N.; Al-Sabarna, K.; Kanellopoulos, P.N.; Makris, A.M.; Kampranis, S.C. Differential roles of tau class glutathione S-transferases in oxidative stress. J. Biol. Chem. 2004, 279, 24540–24551. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Reavy, B.; Smant, G.; Prior, A. Glutathione peroxidases of the potato cyst nematode Globodera rostochiensis. Gene 2004, 324, 47–54. [Google Scholar] [CrossRef]
- Hasan, M.S.; Chopra, D.; Damm, A.; Koprivova, A.; Kopriva, S.; Meyer, A.J.; Müller-Schüssele, S.; Grundler, F.M.; Siddique, S. Glutathione contributes to plant defence against parasitic cyst nematodes. Mol. Plant Pathol. 2022, 23, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and Its Management: Perspectives and Strategies; Venkateswarlu, B., Shanker, A., Shanker, C., Maheswari, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The multiple roles of ascorbate in the abiotic stress response of plants: Antioxidant, cofactor, and regulator. Front. Plant Sci. 2021, 12, 598173. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Espín, A.; Sánchez-Guerrero, A.; Sevilla, F.; Jiménez, A. The role of ascorbate in plant growth and development. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M., Munné-Bosch, S., Burritt, D., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 25–45. [Google Scholar] [CrossRef]
- De Tullio, M.C.; Guether, M.; Balestrini, R. Ascorbate oxidase is the potential conductor of a symphony of signaling pathways. Plant Signal. Behav. 2013, 8, e23213. [Google Scholar] [CrossRef] [PubMed]
- Labudda, M. Ascorbate-glutathione pathway as an important player in redox regulation in nematode-infested plants: What we have learned so far. Physiol. Mol. Plant Pathol. 2018, 103, 47–53. [Google Scholar] [CrossRef]
- Teoh, E.S. Secondary metabolites of plants. In Medicinal Orchids of Asia; Teoh, E.S., Ed.; Springer: Singapore, 2016; pp. 59–73. [Google Scholar] [CrossRef]
- Khare, S.; Singh, N.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Schulze, E.-D.; Beck, E.; Buchmann, N.; Clemens, S.; Müller-Hohenstein, K.; Scherer-Lorenzen, M.; Schulze, E.-D.; Beck, E.; Buchmann, N.; Clemens, S. Biotic stress. In Plant Ecology; Schulze, E.-D., Beck, E., Buchmann, N., Clemens, S., Müller-Hohenstein, K., Schulze, E.-D., Beck, E., Buchmann, N., Clemens, S., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 257–299. [Google Scholar] [CrossRef]
- Aguirre-Becerra, H.; Vazquez-Hernandez, M.C.; Saenz de la O, D.; Alvarado-Mariana, A.; Guevara-Gonzalez, R.G.; Garcia-Trejo, J.F.; Feregrino-Perez, A.A. Role of stress and defense in plant secondary metabolites production. In Bioactive Natural Products for Pharmaceutical Applications; Pal, D., Nayak, A.K., Eds.; Springer: Cham, Switzerland, 2021; pp. 151–195. [Google Scholar] [CrossRef]
- Jha, Y.; Mohamed, H.I. Plant secondary metabolites as a tool to investigate biotic stress tolerance in plants: A review. Gesunde Pflanz. 2022, 74, 771–790. [Google Scholar] [CrossRef]
- Arraes, F.B.M.; Vasquez, D.D.N.; Tahir, M.; Pinheiro, D.H.; Faheem, M.; Freitas-Alves, N.S.; Moreira-Pinto, C.E.; Moreira, V.J.V.; Paes-de-Melo, B.; Lisei-de-Sa, M.E.; et al. Integrated Omic Approaches Reveal Molecular Mechanisms of Tolerance during Soybean and Meloidogyne incognita Interactions. Plants 2022, 11, 2744. [Google Scholar] [CrossRef]
- Singh, G.; Kanwar, R.S.; Sharma, L.; Neeraj, L.K.C.; Kaushik, P. Biochemical changes induced by Meloidogyne graminicola in resistant and susceptible pearl millet (Pennisetum glaucum L.) hybrids. Plant Pathol. J. 2020, 19, 132–139. [Google Scholar] [CrossRef]
- Tapia-Vázquez, I.; Montoya-Martínez, A.C.; De los Santos-Villalobos, S.; Ek-Ramos, M.J.; Montesinos-Matías, R.; Martínez-Anaya, C. Root-knot nematodes (Meloidogyne spp.) a threat to agriculture in Mexico: Biology, current control strategies, and perspectives. World J. Microbiol. Biotechnol. 2022, 38, 26. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Poornima, K.; Somvanshi, V.S.; Walia, R.K. Meloidogyne enterolobii: A threat to guava (Psidium guajava) cultivation. Arch. Phytopathol. Plant Prot. 2022, 55, 1961–1997. [Google Scholar] [CrossRef]
- Danish, M.; Robab, M.I.; Marraiki, N.; Shahid, M.; Zaghloul, N.S.; Nishat, Y.; Shaikh, H. Root-knot nematode Meloidogyne incognita induced changes in morpho-anatomy and antioxidant enzymes activities in Trachyspermum ammi (L.) plant: A microscopic observation. Physiol. Mol. Plant Pathol. 2021, 116, 101725. [Google Scholar] [CrossRef]
- Jagdale, S.; Rao, U.; Giri, A.P. Effectors of root-knot nematodes: An arsenal for successful parasitism. Front. Plant Sci. 2021, 12, 800030. [Google Scholar] [CrossRef] [PubMed]
- Duggal, P.; Ram, S.; Bhatia, A.; Patil, J. Life Cycle and Pathogenicity of Meloidogyne incognita on Capsicum frutescens under Poly-House as Compared to Screen-House Conditions. Int. J. Pure Appl. Biosci. 2017, 5, 1017–1024. [Google Scholar] [CrossRef]
- Tikoria, R.; Kaur, A.; Ohri, P. Modulation of various phytoconstituents in tomato seedling growth and Meloidogyne incognita–induced stress alleviation by vermicompost application. Front. Environ. Sci. 2022, 10, 891195. [Google Scholar] [CrossRef]
- Chen, B.; Sikandar, A.; Ahmad, S.; Luo, M.; Wu, H. Meloidogyne graminicola’s effect on growth performance of rice under low population density. Agronomy 2022, 12, 587. [Google Scholar] [CrossRef]
- Ralmi, N.H.A.A.; Khandaker, M.M.; Mat, N. Occurrence and control of root knot nematode in crops: A review. Aust. J. Crop Sci. 2016, 11, 1649. [Google Scholar] [CrossRef]
- dos Santos Pereira, T.; Monteiro de Paula, A.; Ferrari, L.H.; da Silva, J.; Borges Pinheiro, J.; Navas Cajamarca, S.M.; Jindo, K.; Pupo Santos, M.; Zandonadi, D.B.; Busato, J.G. Trichoderma-enriched vermicompost extracts reduces nematode biotic stress in tomato and bell pepper crops. Agronomy 2021, 11, 1655. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jamali, S.; Esfahani, M.; Pedramfar, H. Assessment of photosynthetic fluorescence in tomato cultivars infested with root-knot nematode. Pak. J. Nematol. 2021, 39, 147. [Google Scholar] [CrossRef]
- Khajuria, A.; Ohri, P. Exogenously applied putrescine improves the physiological responses of tomato plant during nematode pathogenesis. Sci. Hortic. 2018, 230, 35–42. [Google Scholar] [CrossRef]
- Afifi, A.; Al-Sayed, A.; Mahfoud, N.; Farahat, A. Enzymatic and non-enzymatic oxidants and antioxidants involved in defense mechanisms against root-knot, reniform and citrus nematodes in their hosts. Egypt J. Agronematol. 2014, 13, 172–188. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Jamwal, V.L.; Kohli, S.K.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Role of plant growth promoting Bacteria (PGPRs) as biocontrol agents of Meloidogyne incognita through improved plant defense of Lycopersicon esculentum. Plant Soil 2019, 436, 325–345. [Google Scholar] [CrossRef]
- Sharma, N.; Khanna, K.; Manhas, R.K.; Bhardwaj, R.; Ohri, P.; Alkahtani, J.; Alwahibi, M.S.; Ahmad, P. Insights into the role of Streptomyces hydrogenans as the plant growth promoter, photosynthetic pigment enhancer and biocontrol agent against Meloidogyne incognita in Solanum lycopersicum seedlings. Plants 2020, 9, 1109. [Google Scholar] [CrossRef]
- Tsaniklidis, G.; Chatzistathis, T.; Fanourakis, D.; Nikoloudakis, N.; Kotsiras, A.; Delis, C.; Tzortzakakis, E.A. Leaf antioxidant machinery stimulation by Meloidogyne javanica infestation: A case study on Cucumis melo seedlings. Plant Stress 2021, 1, 100002. [Google Scholar] [CrossRef]
- Sikandar, A.; Zhang, M.; Zhu, X.; Wang, Y.; Ahmed, M.; Iqbal, M.; Javeed, A.; Xuan, Y.; Fan, H.; Liu, X. Efficacy of Penicillium chrysogenum strain Snef1216 against root-knot nematodes (Meloidogyne incognita) in cucumber (Cucumis sativus L.) under greenhouse conditions. Appl. Ecol. Environ. Res. 2019, 17, 12451–12464. [Google Scholar] [CrossRef]
- Sikandar, A.; Zhang, M.; Wang, Y.; Zhu, X.; Liu, X.; Fan, H.; Xuan, Y.; Chen, L.; Duan, Y. In vitro evaluation of Penicillium chrysogenum Snef1216 against Meloidogyne incognita (root-knot nematode). Sci. Rep. 2020, 10, 8342. [Google Scholar] [CrossRef]
- Maqsood, A.; Wu, H.; Kamran, M.; Altaf, H.; Mustafa, A.; Ahmar, S.; Hong, N.T.T.; Tariq, K.; He, Q.; Chen, J.-T. Variations in growth, physiology, and antioxidative defense responses of two tomato (Solanum lycopersicum L.) cultivars after co-infection of Fusarium oxysporum and Meloidogyne incognita. Agronomy 2020, 10, 159. [Google Scholar] [CrossRef]
- Yong, Z.; Hao-Ru, T.; Ya, L. Variation in antioxidant enzyme activities of two strawberry cultivars with short-term low temperature stress. World J. Agric. Sci. 2008, 4, 458–462. [Google Scholar]
- Korayem, A.; El-Bassiouny, H.; Abd El-Monem, A.A.; Mohamed, M. Physiological and biochemical changes in different sugar beet genotypes infected with root-knot nematode. Acta Physiol. Plant 2012, 34, 1847–1861. [Google Scholar] [CrossRef]
- Abou-Sreea, A.I.; Azzam, C.R.; Al-Taweel, S.K.; Abdel-Aziz, R.M.; Belal, H.E.; Rady, M.M.; Abdel-Kader, A.A.; Majrashi, A.; Khaled, K.A. Natural biostimulant attenuates salinity stress effects in chili pepper by remodeling antioxidant, ion, and phytohormone balances, and augments gene expression. Plants 2021, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Xu, B.; Li, J.; Huang, B. Exogenous melatonin suppresses dark-induced leaf senescence by activating the superoxide dismutase-catalase antioxidant pathway and down-regulating chlorophyll degradation in excised leaves of perennial ryegrass (Lolium perenne L.). Front. Plant Sci. 2016, 7, 1500. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ma, L.Q.; Srivastava, M.; Rathinasabapathi, B. Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L and Pteris ensiformis L. Plant Sci. 2006, 170, 274–282. [Google Scholar] [CrossRef]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for Determination of Proline in Plants; Humana Press: Totowa, NJ, USA, 2010; Volume 639, pp. 317–331. [Google Scholar] [CrossRef]
- Hashem, A.; Alqarawi, A.A.; Radhakrishnan, R.; Al-Arjani, A.-B.F.; Aldehaish, H.A.; Egamberdieva, D.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 2018, 25, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, F.; Cheng, J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011, 127, 1237–1242. [Google Scholar] [CrossRef]
- Ahmed, M.; Ji, M.; Qin, P.; Gu, Z.; Liu, Y.; Sikandar, A.; Iqbal, M.; Javeed, A.; Shafi, J.; Du, Y. Determination of phytochemicals, antioxidant activity and biochemical composition of Chinese mugwort (Artemisia argyi L.) leaf extract from Northeast China. Appl. Ecol. Environ. Res. 2019, 17, 15349–15362. [Google Scholar] [CrossRef]
- Sikandar, A.; Zhang, M.; Wang, Y.; Zhu, X.; Liu, X.; Fan, H.; Xuan, Y.; Chen, L.; Duan, Y. Mycochemical Screening and Analysis, Antioxidant Activity, and Biochemical Composition of Fermentation Strain Snef1216 (Penicillium chrysogenum). J. Anal. Methods Chem. 2020, 2020, 3073906. [Google Scholar] [CrossRef]
- Taghavi, T.; Patel, H.; Akande, O.E.; Galam, D.C.A. Total anthocyanin content of strawberry and the profile changes by extraction methods and sample processing. Foods 2022, 11, 1072. [Google Scholar] [CrossRef]



| Days Post-Inoculation (dpi) | Level of Inoculum (Number of J2s) | Growth Parameters | ||||
|---|---|---|---|---|---|---|
| Shoot Length (cm) | Shoot Weight (g) | Leaf Area (cm2) | Root Length (cm) | Root Weight (g) | ||
| 7 | Control | 15.71 ± 1.96 a | 14.11 ± 1.33 a | 4.23 ± 0.69 a | 9.92 ± 0.97 a | 3.11 ± 0.17 a |
| 100 | 11.97 ± 1.26 b | 12.15 ± 1.10 b | 4.12 ± 0.76 a | 8.74 ± 0.59 ab | 2.98 ± 0.96 a | |
| 500 | 10.93 ± 1.16 bc | 10.57 ± 1.57 b | 3.72 ± 0.95 ab | 7.54 ± 1.08 bc | 2.35 ± 0.08 b | |
| 1000 | 9.49 ± 2.58 bcd | 8.54 ± 1.25 c | 3.63 ± 0.59 ab | 7.02 ± 1.86 bc | 2.03 ± 0.03 bc | |
| 1500 | 8.698 ± 2.37 cd | 6.06 ± 1.37 d | 3.59 ± 0.64 ab | 6.56 ± 2.43 cd | 1.59 ± 0.02 cd | |
| 2000 | 7.41 ± 1.26 d | 3.16 ± 1.41 e | 2.76 ± 0.85 b | 4.84 ± 1.05 d | 1.29 ± 0.01 d | |
| 14 | Control | 18.45 ± 1.64 a | 14.22 ± 0.95 a | 5.12 ± 0.69 a | 11.32 ± 1.08 a | 3.72 ± 0.77 a |
| 100 | 15.71 ± 2.03 b | 12.25 ± 1.35 b | 4.77 ± 0.99 a | 9.78 ± 1.43 ab | 3.58 ± 0.94 a | |
| 500 | 13.25 ± 2.82 bc | 11.26 ± 1.35 b | 4.16 ± 0.78 ab | 8.90 ± 0.94 b | 3.10 ± 0.12 a | |
| 1000 | 11.01 ± 1.59 cd | 8.45 ± 1.66 c | 4.15 ± 0.65 ab | 8.82 ± 1.37 b | 2.39 ± 0.05 b | |
| 1500 | 10.73 ± 1.71 cd | 6.70 ± 0.99 d | 4.15 ± 0.61 ab | 8.42 ± 1.04 b | 2.01 ± 0.03 b | |
| 2000 | 9.65 ± 1.59 d | 3.96 ± 1.40 e | 3.44 ± 0.65 b | 5.82 ± 1.54 c | 1.92 ± 0.01 b | |
| 21 | Control | 21.22 ± 2.03 a | 21.27 ± 1.83 a | 6.45 ± 0.50 a | 12.06 ± 0.95 a | 4.49 ± 0.42 a |
| 100 | 19.82 ± 1.89 a | 19.97 ± 0.62 ab | 5.87 ± 0.52 b | 10.44 ± 0.76 ab | 3.98 ± 0.21 b | |
| 500 | 18.64 ± 2.63 ab | 18.65 ± 1.67 bc | 5.83 ± 0.29 b | 9.16 ± 1.91 b | 3.40 ± 0.17 c | |
| 1000 | 15.68 ± 2.91 bc | 16.59 ± 1.43 c | 5.58 ± 0.40 bc | 8.54 ± 1.26 bc | 2.56 ± 0.06 d | |
| 1500 | 13.64 ± 2.22 cd | 11.88 ± 1.57 d | 5.17 ± 0.35 cd | 6.96 ± 1.68 c | 2.14 ± 0.02 e | |
| 2000 | 11.88 ± 1.66 d | 9.50 ± 1.47 d | 4.64 ± 0.52 d | 6.80 ± 2.10 c | 2.05 ± 0.01 e | |
| 28 | Control | 27.49 ± 1.26 a | 22.59 ± 1.55 a | 7.09 ± 0.75 a | 13.38 ± 1.15 a | 5.69 ± 0.22 a |
| 100 | 22.15 ± 1.44 b | 21.23 ± 1.71 ab | 6.95 ± 0.91 ab | 11.70 ± 0.83 ab | 4.27 ± 0.37 b | |
| 500 | 21.17 ± 1.98 bc | 19.61 ± 1.91 b | 6.40 ± 0.55 abc | 10.84 ± 1.70 bc | 3.58 ± 0.09 c | |
| 1000 | 19.55 ± 2.08 cd | 16.93 ± 1.46 c | 6.32 ± 0.42 abc | 10.74 ± 2.27 bc | 3.06 ± 0.05 d | |
| 1500 | 18.75 ± 2.07 de | 13.62 ± 1.60 d | 6.06 ± 0.48 bc | 9.16 ± 2.18 cd | 2.22 ± 0.02 e | |
| 2000 | 16.93 ± 1.06 e | 11.77 ± 1.14 d | 5.55 ± 0.52 c | 7.86 ± 1.63 d | 2.24 ± 0.01 e | |
| Days Post-Inoculation (dpi) | Nematodes Population | ||||
|---|---|---|---|---|---|
| Level of Inoculum (Number of J2s) | Number of Juveniles per Root System | Number of Galls per Root System | Egg Masses per Root System | Nematodes Population per Gram Root Weight | |
| 7 | Control | 0 ± 0 f | 0 ± 0 f | - | 0 ± 0 f |
| 100 | 40.80 ± 5.45 e | 9.60 ± 2.08 e | - | 14.37 ± 2.68 e | |
| 500 | 130.40 ± 5.03 d | 15.20 ± 3.27 d | - | 55.63 ± 2.34 d | |
| 1000 | 490.20 ± 3.49 c | 27.60 ± 2.08 c | - | 242.09 ± 4.72 c | |
| 1500 | 595.20 ± 4.49 b | 47.20 ± 4.44 b | - | 372.93 ± 5.19 b | |
| 2000 | 870.60 ± 4.39 a | 71.60 ± 3.36 a | - | 674.91 ± 4.85 a | |
| 14 | Control | 0 ± 0 f | 0 ± 0 f | - | 0 ± 0 f |
| 100 | 55.20 ± 4.60 e | 20.40 ± 4.51 e | - | 15.53 ± 4.54 e | |
| 500 | 233.00 ± 3.39 d | 24.80 ± 4.15 d | - | 75.17 ± 3.30 d | |
| 1000 | 581.60 ± 5.03 c | 46.00 ± 3.16 c | - | 243.06 ± 4.37 c | |
| 1500 | 704.80 ± 4.32 b | 61.60 ± 3.51 b | - | 350.13 ± 3.77 b | |
| 2000 | 1307.40 ± 3.58 a | 92.20 ± 3.70 a | - | 680.96 ± 3.87 a | |
| 21 | Control | 0 ± 0 f | 0 ± 0 f | 0 ± 0 f | 0 ± 0 f |
| 100 | 63.40 ± 3.21 e | 29.80 ± 3.19 e | 11.40 ± 2.88 e | 15.97 ± 1.19 e | |
| 500 | 311.20 ± 4.32 d | 47.60 ± 3.64 d | 24.60 ± 3.21 d | 91.69 ± 5.35 d | |
| 1000 | 643.80 ± 4.92 c | 54.80 ± 2.59 c | 31.20 ± 3.35 c | 251.55 ± 5.34 c | |
| 1500 | 791.40 ± 3.85 b | 86.40 ± 4.62 b | 44.40 ± 3.71 b | 369.33 ± 4.61 b | |
| 2000 | 1376.60 ± 3.97 a | 104.80 ± 3.11 a | 50.80 ± 3.19 a | 672.97 ± 3.59 a | |
| 28 | Control | 0 ± 0 f | 0 ± 0 f | 0 ± 0 f | 0 ± 0 f |
| 100 | 80.20 ± 2.17 e | 30.60 ± 3.71 e | 27.20 ± 3.49 e | 18.88 ± 1.55 e | |
| 500 | 397.00 ± 2.92 d | 63.20 ± 3.35 d | 35.40 ± 2.88 d | 110.99 ± 2.86 d | |
| 1000 | 703.80 ± 4.32 c | 70.20 ± 4.55 c | 44.20 ± 3.35 c | 230.34 ± 3.31 c | |
| 1500 | 893.60 ± 4.22 b | 105.80 ± 4.98 b | 50.20 ± 4.55 b | 402.79 ± 3.22 b | |
| 2000 | 1576.60 ± 4.83 a | 117.40 ± 5.31 a | 63.6 ± 3.29 a | 704.86 ± 2.97 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikandar, A.; Wu, F.; He, H.; Ullah, R.M.K.; Wu, H. Growth, Physiological, and Biochemical Variations in Tomatoes after Infection with Different Density Levels of Meloidogyne enterolobii. Plants 2024, 13, 293. https://doi.org/10.3390/plants13020293
Sikandar A, Wu F, He H, Ullah RMK, Wu H. Growth, Physiological, and Biochemical Variations in Tomatoes after Infection with Different Density Levels of Meloidogyne enterolobii. Plants. 2024; 13(2):293. https://doi.org/10.3390/plants13020293
Chicago/Turabian StyleSikandar, Aatika, Fangcao Wu, Heliang He, Rana Muhammad Kaleem Ullah, and Haiyan Wu. 2024. "Growth, Physiological, and Biochemical Variations in Tomatoes after Infection with Different Density Levels of Meloidogyne enterolobii" Plants 13, no. 2: 293. https://doi.org/10.3390/plants13020293
APA StyleSikandar, A., Wu, F., He, H., Ullah, R. M. K., & Wu, H. (2024). Growth, Physiological, and Biochemical Variations in Tomatoes after Infection with Different Density Levels of Meloidogyne enterolobii. Plants, 13(2), 293. https://doi.org/10.3390/plants13020293







