Exogenous Spermidine and Amino-Ethoxyvinylglycine Improve Nutritional Quality via Increasing Amino Acids in Rice Grains
Abstract
1. Introduction
2. Materials and Methods
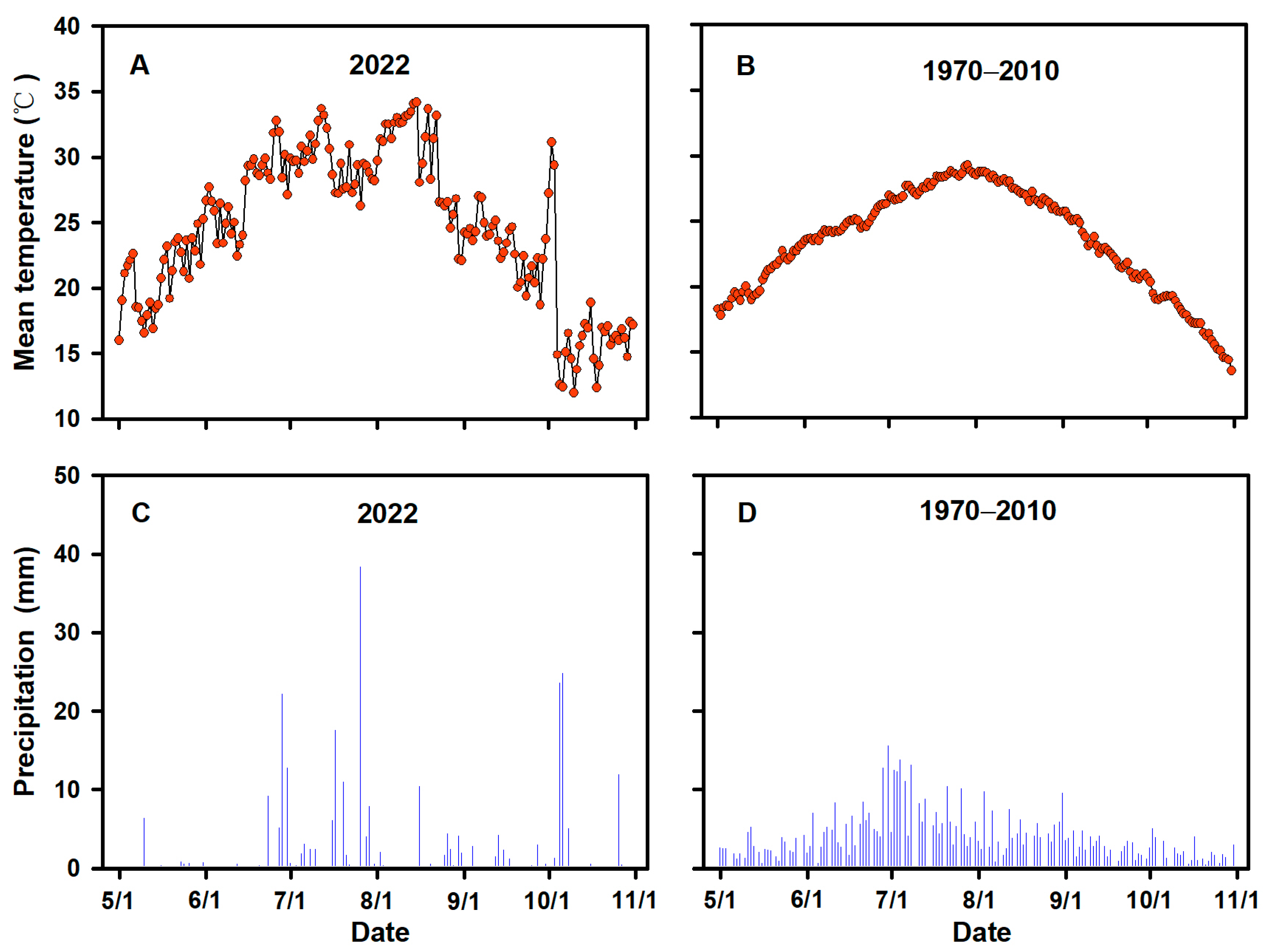
2.1. Plant Materials and Treatments
2.2. Spd and AVG Application
2.3. Sampling
2.4. Determination of PA Contents
2.5. Determination of Arginine Decarboxylase (ADC), S-Adenosylmethionine (SAMDC) and Polyamine Oxidase (PAO) Activities
2.6. Determination of Ethylene Release Rate and ACC Content
2.7. Determination of Hydrolyzed Amino Acid Content and Yield
2.8. Final Harvest
2.9. Statistical Analysis
3. Results
3.1. Grain Yield
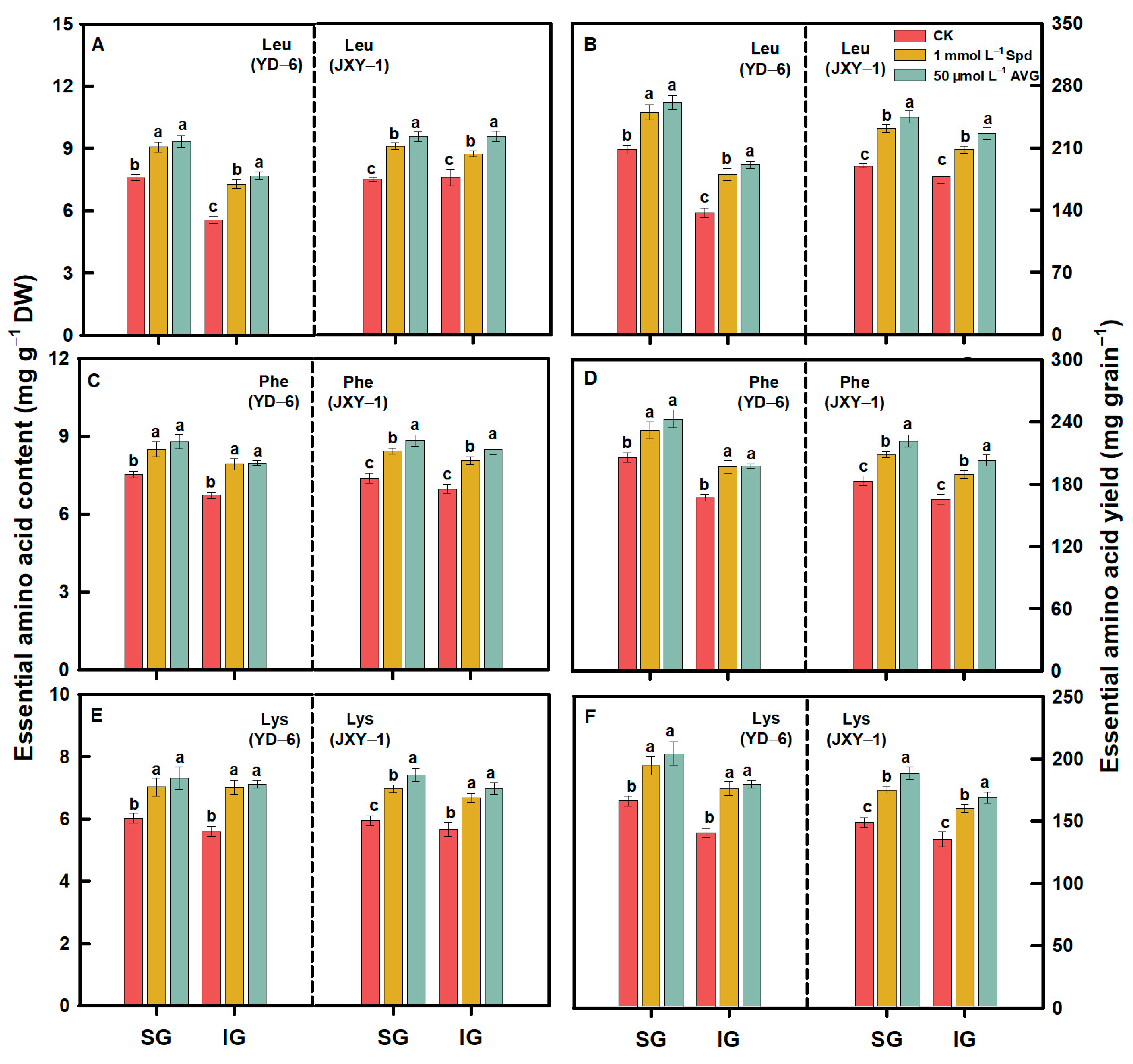
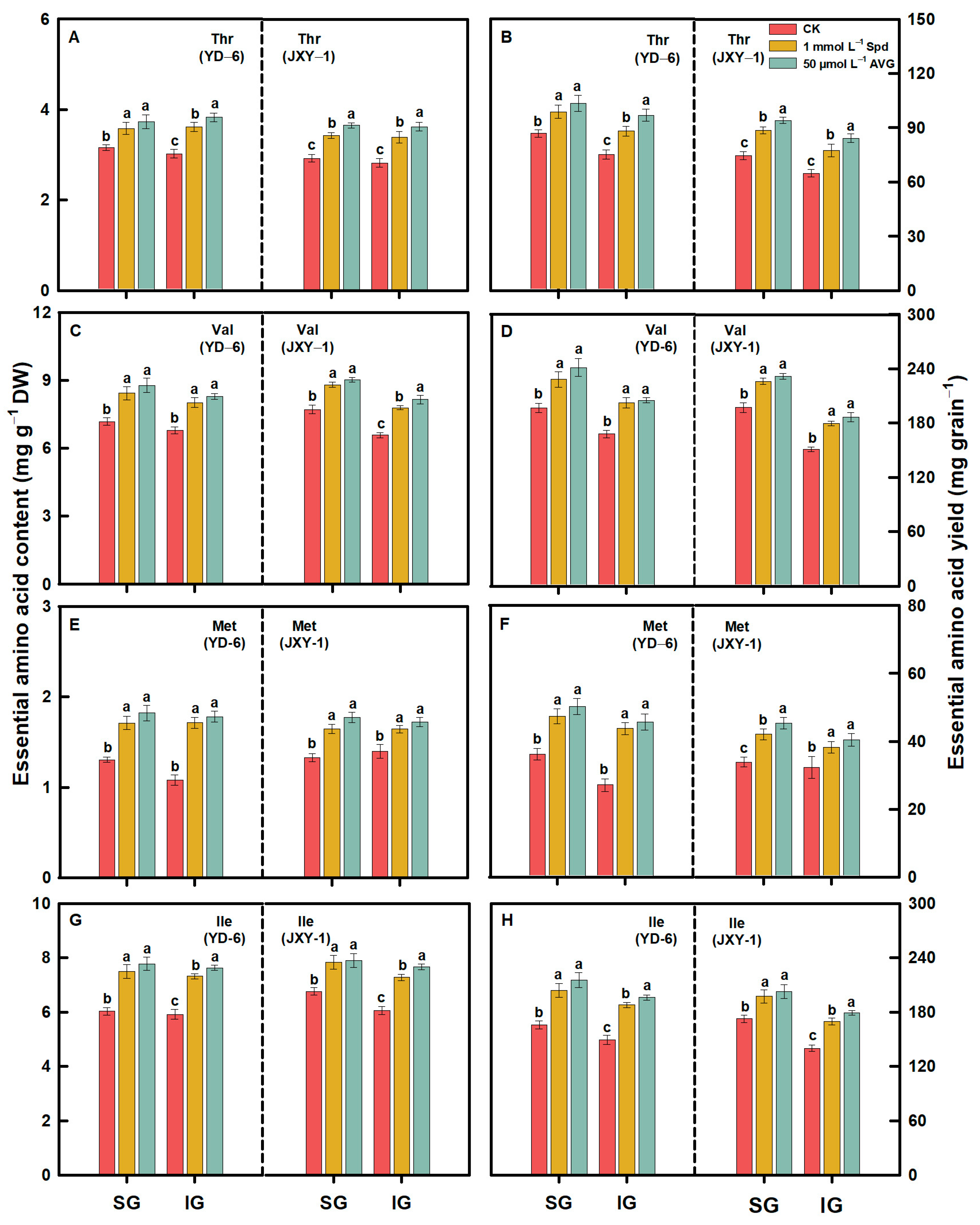
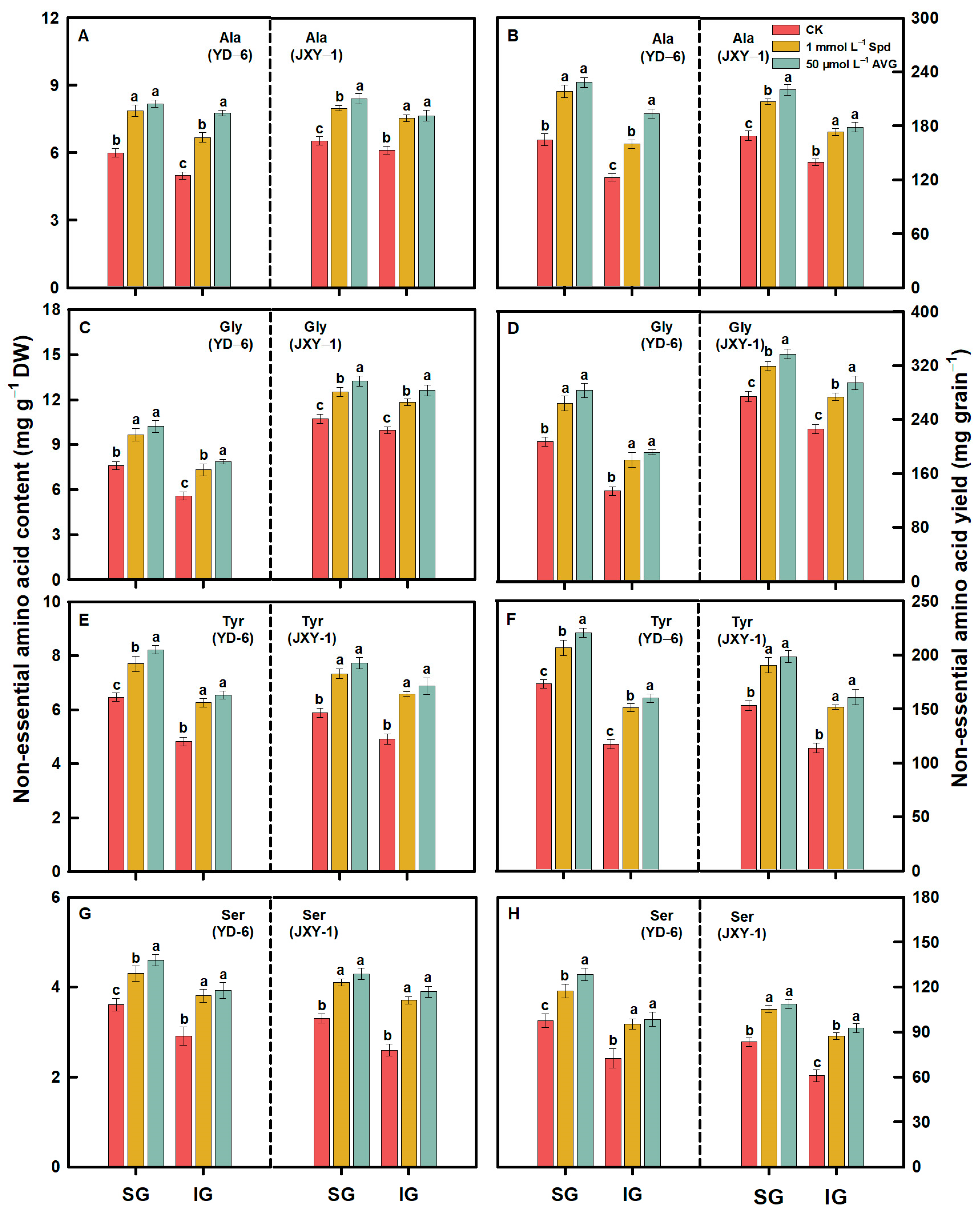
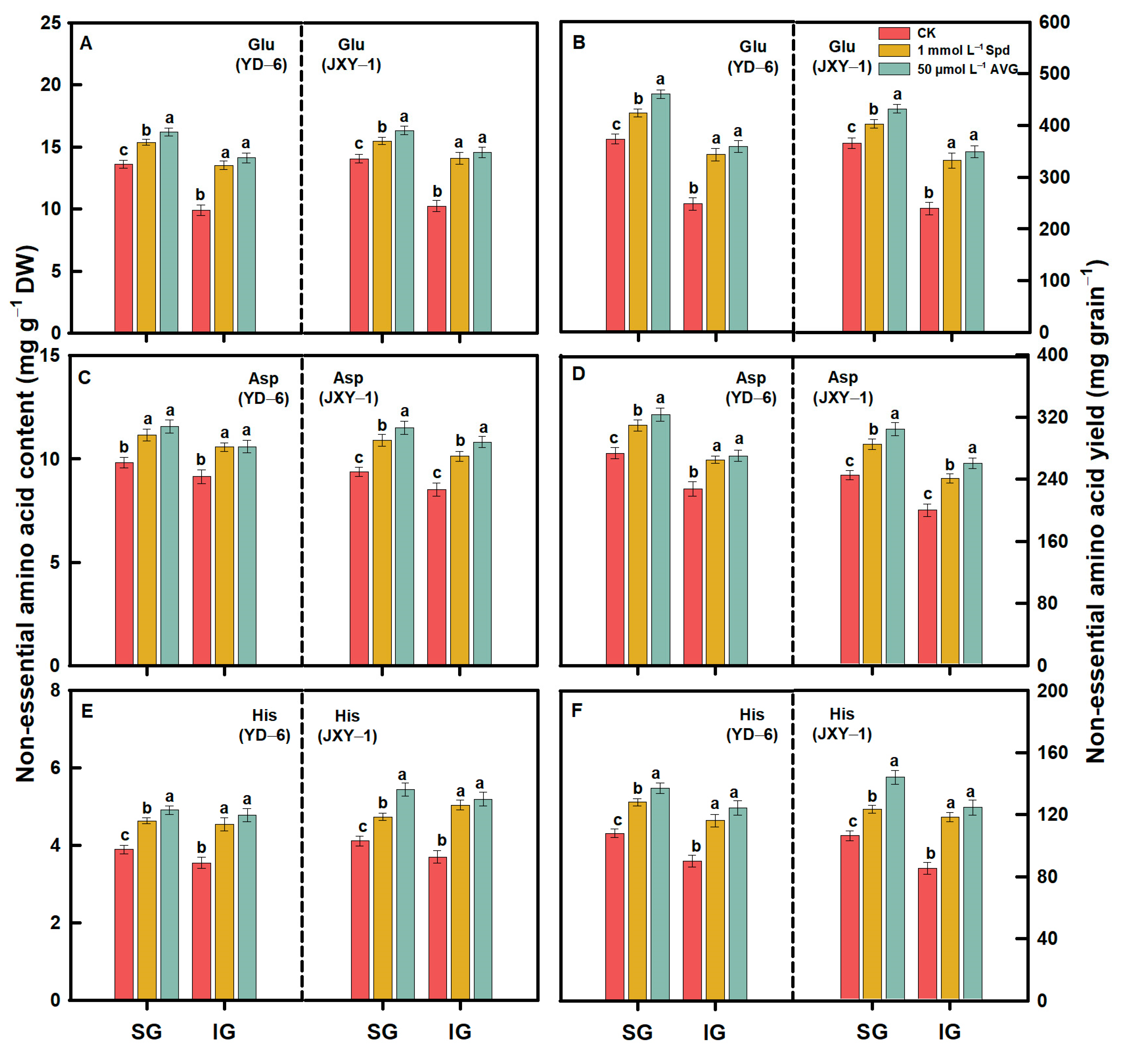
3.2. Amino Acid Content
3.3. PA Content
3.4. Ethylene Release Rate and ACC Content
3.5. Activities of ADC, SAMDC and PAO
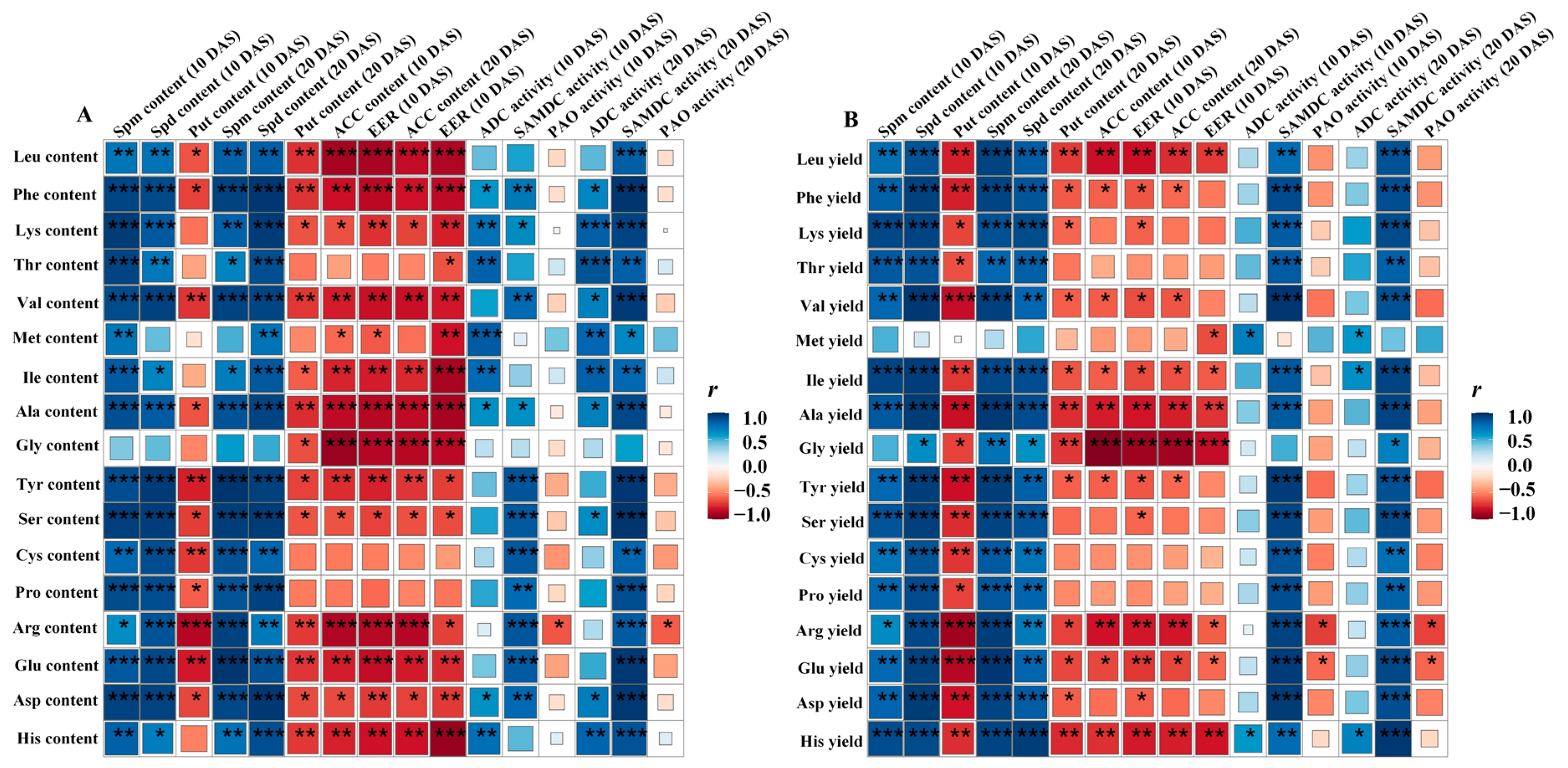
3.6. Relationship of PA and ACC Contents, Ethylene Release Rate and Activities of ADC, SAMDC, and PAO with the Amino Acid Content and Yield in Milled Rice
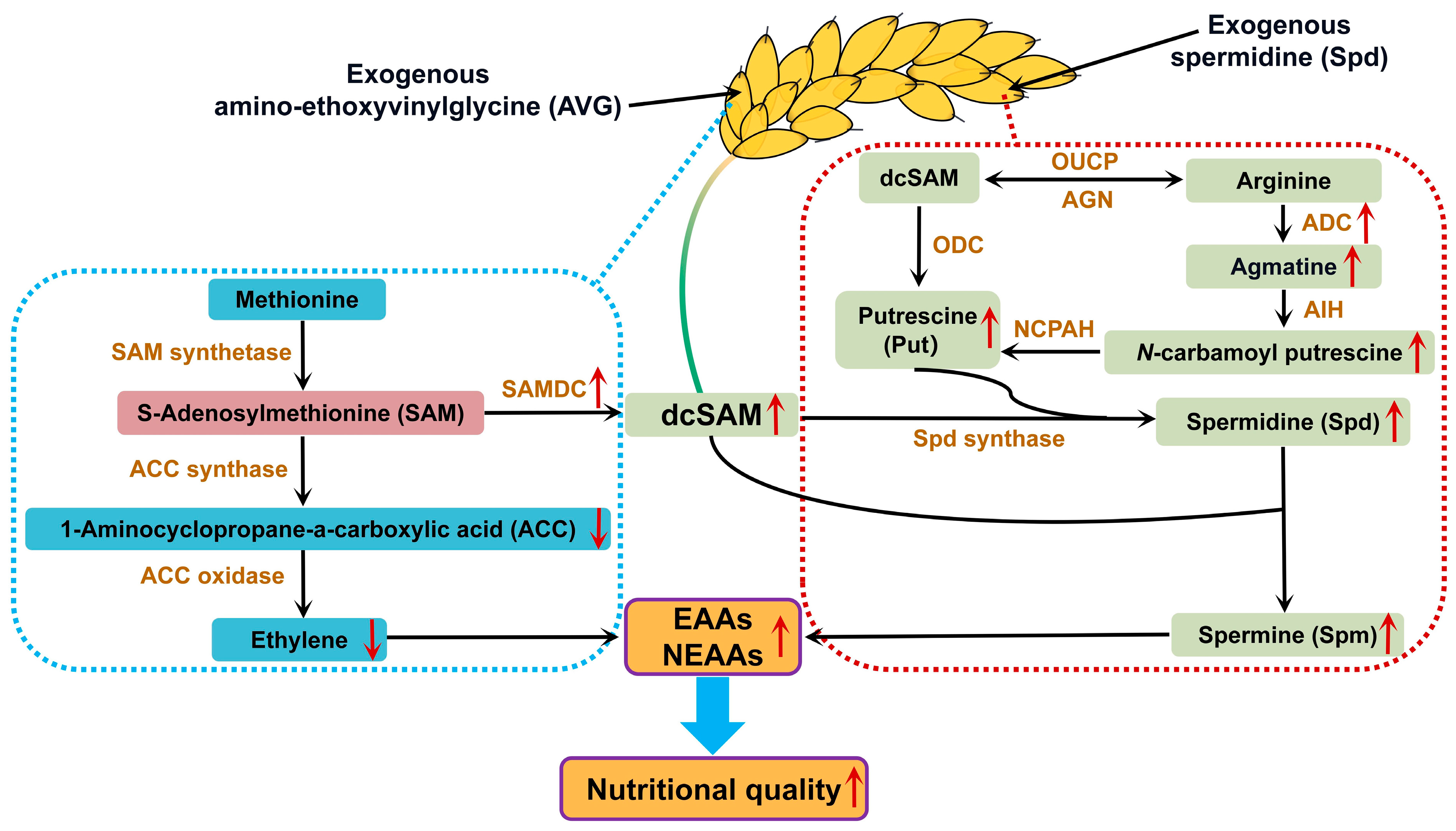
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ladha, J.K.; Radanielson, A.M.; Rutkoski, J.E.; Roland, J.; Buresh, B.J.; Dobermann, A.; Angeles, O.; Pabuayon, I.L.B.; Santos-Medellín, C.; Fritsche-Neto, R.; et al. Steady agronomic and genetic interventions are essential for sustaining productivity in intensive rice cropping. Proc. Natl. Acad. Sci. USA 2021, 118, e2110807118. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhao, D.S.; Zhang, C.Q.; Sun, S.S.M.; Liu, Q.Q. Lysine biofortification of crops to promote sustained human health in the 21st century. J. Exp. Bot. 2022, 73, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Jang, Y.H.; Kim, E.G.; Park, J.R.; Eom, G.H.; Zhao, D.-D.; Kim, K.M. Evaluation of amino acid profiles of rice genotypes under different salt stress conditions. Plants 2023, 12, 1315. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, Y.; Sun, Y.; Xie, Z.; Luo, Y.; Long, Q.; Feng, J.; Liu, X.; Wang, B.; He, D.; et al. Natural variations of OsAUX5, a target gene of OsWRKY78, control the neutral essential amino acid content in rice grains. Mol. Plant 2023, 16, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Schop, M.; De Boer, I.J.M.; Huppertz, T. Protein quality in perspective: A review of protein quality metrics and their applications. Nutrients 2022, 14, 947. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Zhang, X.F.; Cheng, Y.; Du, X.X.; Teotia, S.; Miao, C.B.; Sun, H.W.; Fan, G.Q.; Tang, G.L.; Xue, H.W.; et al. The miR167-OsARF12 module regulates rice grain filling and grain size downstream of miR159. Plant Commun. 2023, 4, 100604. [Google Scholar] [CrossRef] [PubMed]
- Min, X.M.; Xu, H.L.; Huang, F.L.; Wei, Y.D.; Lin, W.X.; Zhang, Z.X. GC-MS-based metabolite profiling of key differential metabolites between superior and inferior spikelets of rice during the grain filling stage. BMC Plant Biol. 2021, 21, 439. [Google Scholar] [CrossRef]
- Xu, H.F.; Wang, Z.X.; Xiao, F.; Yang, F.; Li, G.H.; Ding, Y.F.; Paul, M.J.; Li, W.W.; Liu, Z. Dynamics of dry matter accumulation in internodes indicates source and sink relations during grain-filling stage of japonica rice. Field Crops Res. 2021, 263, 108009. [Google Scholar] [CrossRef]
- Liu, J.L.; Yang, R.C.; Jian, N.; Wei, L.; Ye, L.L.; Wang, R.H.; Gao, H.L.; Zheng, Q.S. Putrescine metabolism modulates the biphasic effects of brassinosteroids on canola and arabidopsis salt tolerance. Plant Cell Environ. 2020, 43, 1348–1359. [Google Scholar] [CrossRef]
- Huang, Y.T.; Mei, G.F.; Cao, D.D.; Qin, Y.B.; Yang, L.; Ruan, X.L. Spermidine enhances heat tolerance of rice seeds during mid-filling stage and promote subsequent seed germination. Front. Plant Sci. 2023, 14, 1230331. [Google Scholar] [CrossRef]
- Islam, M.A.; Pang, J.H.; Meng, F.W.; Li, Y.W.; Xu, N.; Yang, C.; Liu, J. Putrescine, spermidine, and spermine play distinct roles in rice salt tolerance. J. Integr. Agric. 2020, 19, 643–655. [Google Scholar] [CrossRef]
- Du, H.Y.; Liu, G.T.; Liu, D.X.; Liu, H.P.; Kurtenbach, R. Significance of putrescine conversion in filling grain embryos of wheat plants subjected to drought stress. Plant Soil 2023, 484, 589–610. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhou, Y.J.; Jiang, Y. Amino acids in rice grains and their regulation by polyamines and phytohormones. Plants 2022, 11, 1581. [Google Scholar] [CrossRef]
- Chu, G.; Chen, S.; Xu, C.M.; Zhang, X.F.; Wang, D.Y. Ethylene and polyamines interact in rice spikelet degeneration in response to water stress during meiosis. Plant Growth Regul. 2023, 101, 617–628. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, H.Y.; Lv, X.K.; Liu, D.D.; Wen, X.X.; Liao, Y.C. Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol. Bioch. 2016, 100, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Asthir, B. Modulation of polyamines during grain development under different concentrations of nitrogen in wheat. Cereal Res. Commun. 2019, 47, 580–592. [Google Scholar] [CrossRef]
- Chen, M.; Fu, Y.; Mou, Q.; An, J.; Zhu, X.; Ahmed, T.; Zhang, S.; Basit, F.; Hu, J.; Guan, Y. Spermidine induces expression of stress associated proteins (SAPs) genes and protects rice seed from heat stress-induced damage during grain-filling. Antioxidants 2021, 10, 1544. [Google Scholar] [CrossRef]
- Feng, H.Y.; Wang, Z.M.; Kong, F.N.; Zhang, M.J.; Zhou, S.L. Roles of carbohydrate supply and ethylene, polyamines in maize kernel set. J. Integr. Plant Biol. 2011, 53, 388–398. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Simpson, S.J. Nutritional ecology and human health. Annu. Rev. Nutr. 2016, 36, 603–626. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Sharma, D.; Singh, S.; Ramamurthy, P.C.; Verma, T.; Pujari, M.; Singh, J.; Kapoor, D.; Prasad, R. Physiological and molecular insights into the role of silicon in improving plant performance under abiotic stresses. Plant Soil 2023, 486, 25–43. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiong, K.; Ma, B.; Chen, S.Y.; Zhang, J.S. Ethylene biosynthesis, signaling, and crosstalk with other hormones in rice. Small Methods 2020, 4, 8. [Google Scholar] [CrossRef]
- Zhang, X.C.; Lei, J.C.; Zheng, D.Y.; Liu, Z.H.; Li, G.H.; Wang, S.H.; Ding, Y.F. Amino acid composition of leaf, grain and bracts of japonica rice (Oryza sativa ssp. japonica) and its response to nitrogen. Plant Growth Regul. 2017, 82, 1–9. [Google Scholar] [CrossRef]
- Kang, M.; Liu, G.; Zeng, Y.W.; Zhou, J.; Shi, J.Y.; Tang, L.; Liu, L.L.; Cao, W.X.; Zhu, Y.; Liu, B. Extreme low-temperature stress affects nutritional quality of amino acids in rice. Front. Plant Sci. 2022, 13, 905348. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.X.; Chang, R.; Lu, H.; Ma, R.R.; Qiu, L.Z.; Tian, Y.Q. Effect of amino acids composing rice protein on rice starch digestibility. LWT Food Sci. Technol. 2021, 146, 111417. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Yu, J.X.; Xu, Y.J.; Wang, Z.Q.; Liu, L.J.; Zhang, H.; Gu, J.F.; Zhang, J.H.; Yang, J.C. Alternate wetting and drying irrigation combined with the proportion of polymer-coated urea and conventional urea rates increases grain yield, water and nitrogen use efficiencies in rice. Field Crops Res. 2021, 268, 108165. [Google Scholar] [CrossRef]
- Ling, Q.H.; Su, Z.F.; Zhang, H.C.; Cai, J.Z.; He, J.S. The leaf-age-model of development process in different varieties of rice. Chin. J. Rice Sci. 1983, 1, 11–20. (In Chinese) [Google Scholar]
- Flores, H.E.; Galston, A.W. Analysis of polyamine in higher plants by high performance liquid chromatography. Plant Physiol. 1982, 69, 701–706. [Google Scholar] [CrossRef]
- Zhao, H.; Duan, K.X.; Ma, B.; Yin, C.C.; Hu, Y.; Tao, J.J.; Huang, Y.H.; Cao, W.Q.; Chen, H.; Yang, C.; et al. Histidine kinase MHZ1/OsHK1 interacts with ethylene receptors to regulate root growth in rice. Nat. Commun. 2020, 11, 518. [Google Scholar] [CrossRef]
- Faroza, N.; Badar, J.; Sarika, K.; Noushina, I.; Mohammed, A.; Adriano, S.M.; Iqbal, R.K. Brassinosteroid modulates ethylene synthesis and antioxidant metabolism to protect rice (Oryza sativa) against heat stress-induced inhibition of source–sink capacity and photosynthetic and growth attributes. J. Plant Physiol. 2023, 289, 154096. [Google Scholar]
- Mirtaleb, S.H.; Niknejad, Y.; Fallah, H. Foliar spray of amino acids and potassic fertilizer improves the nutritional quality of rice. J. Plant Nutr. 2021, 44, 2029–2041. [Google Scholar] [CrossRef]
- Mossé, J.; Huet, J.C.; Baudet, J. The amino acid composition of rice grain as a function of nitrogen content as compared with other cereals: A reappraisal of rice chemical scores. J. Cereal Sci. 1988, 8, 165–175. [Google Scholar] [CrossRef]
- Wu, G.Y. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Forno, D.; Cock, J.; Gomez, K. Laboratory Manual for Physiological Studies of Rice; International Rice Research Institute: Manila, Philippines, 1976. [Google Scholar]
- Huang, H.H.; Xu, R.Y.; Yu, J.X.; Zhang, W.Y.; Gu, J.F.; Zhu, K.Y.; Zhang, J.H.; Yang, J.C. A moderate wetting and drying regime combined with appropriate nitrogen application increases grain yield and nitrogen use efficiency in rice. Agronomy 2023, 13, 1729. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Huang, H.H.; Zhou, Y.J.; Zhu, K.Y.; Wu, Y.F.; Xu, Y.J.; Wang, W.L.; Zhang, H.; Gu, J.F.; Xiong, F.; et al. Brassinosteroids mediate moderate soil-drying to alleviate spikelet degeneration under high temperature during meiosis of rice. Plant Cell Environ. 2023, 46, 1340–1362. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef] [PubMed]
- Valdineia, S.F.; Rafael, D.S.M.; José, H.C.; Daniel, F.D.O.; Stelamaris, D.O.P.; Emilio, D.C.M.; Rosemayre, S.F.; José, T.P.; Enéas, G.F. Ethylene and polyamines are biochemically related, since they share a common precursor in biosynthetic pathways, suggesting that alteration in ethylene production can affect polyamine homeostasis. Environ. Exp. Bot. 2018, 145, 75–86. [Google Scholar]
- Pál, M.; Szalai, G.; Gondor, O.K.; Janda, T. Unfinished story of polyamines: Role of conjugation, transport and light-related regulation in the polyamine metabolism in plants. Plant Sci. 2021, 308, 110923. [Google Scholar] [CrossRef]
- Wang, W.; Konstantinos, A.P.; Feng, J.C.; Song, J.; Liu, J.H. Polyamine catabolism in plants: A universal process with diverse functions. Front. Plant Sci. 2019, 10, 561. [Google Scholar] [CrossRef]
- Chen, B.X.; Li, W.Y.; Gao, Y.T.; Chen, Z.J.; Zhang, W.N.; Liu, Q.J.; Chen, Z. Involvement of polyamine oxidase-produced hydrogen peroxide during coleorhiza-limited germination of rice seeds. Front. Plant Sci. 2016, 7, 1219. [Google Scholar] [CrossRef]


| Cultivar | Chemical Treatment | Panicle Number (m−2) | Spikelet Number Per Panicle | Fully Filled Grain (%) | 1000–Grain Weight (g) | Grain Yield (g m−2) |
|---|---|---|---|---|---|---|
| YD–6 | CK | 218 a | 143 a | 62.9 b | 30.1 b | 619 b |
| 1 mmol L−1 Spd | 220 a | 145 a | 67.9 a | 32.8 a | 689 a | |
| 50 μmol L−1 AVG | 219 a | 145 a | 68.2 a | 32.5 a | 694 a | |
| JXY–1 | CK | 276 a | 131 a | 62.1 b | 26.2 b | 610 b |
| 1 mmol L−1 Spd | 277 a | 131 a | 66.8 a | 29.3 a | 683 a | |
| 50 μmol L−1 AVG | 279 a | 132 a | 67.1 a | 28.9 a | 678 a |
| Cultivar | Chemical Treatment | 10 Days after Spraying | 20 Days after Spraying | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SG | IG | SG | IG | ||||||||||
| Spm | Spd | Put | Spm | Spd | Put | Spm | Spd | Put | Spm | Spd | Put | ||
| YD-6 | CK | 1.20 b | 1.54 b | 6.81 a | 1.03 b | 1.14 b | 9.28 a | 3.25 b | 3.19 b | 4.43 a | 2.13 b | 2.83 b | 4.99 a |
| 1 mmol L−1 Spd | 1.50 a | 2.26 a | 6.72 a | 1.43 a | 1.66 a | 9.18 a | 4.15 a | 4.16 a | 4.32 a | 3.11 a | 3.51 a | 4.96 a | |
| 50 μmol L−1 AVG | 1.56 a | 2.20 a | 4.37 b | 1.46 a | 1.69 a | 7.60 b | 4.21 a | 4.19 a | 3.17 b | 3.20 a | 3.78 a | 3.73 b | |
| JXY-1 | CK | 1.13 b | 1.43 b | 6.39 a | 1.03 b | 1.05 b | 9.30 a | 3.13 b | 2.89 b | 4.01 a | 2.14 b | 2.67 b | 4.62 a |
| 1 mmol L−1 Spd | 1.51 a | 2.19 a | 6.33 a | 1.38 a | 1.53 a | 9.14 a | 4.18 a | 4.04 a | 3.96 a | 3.37 a | 3.82 a | 4.60 a | |
| 50 μmol L−1 AVG | 1.40 a | 2.20 a | 3.80 b | 1.41 a | 1.58 a | 7.43 b | 4.22 a | 4.10 a | 2.72 b | 3.28 a | 3.90 a | 3.28 b | |
| Cultivar | Chemical Treatment | 10 Days after Spraying | 20 Days after Spraying | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SG | IG | SG | IG | ||||||
| ACC | ERR | ACC | ERR | ACC | ERR | ACC | ERR | ||
| YD–6 | CK | 132 a | 4.47 a | 149 a | 6.75 a | 120 a | 3.10 a | 132 a | 3.50 a |
| 1 mmol L−1 Spd | 97.4 b | 3.89 b | 134 b | 4.35 b | 102 b | 2.23 b | 120 b | 2.73 b | |
| 50 μmol L−1 AVG | 83.5 c | 3.10 c | 122 c | 4.16 c | 80.2 c | 1.82 c | 98.9 c | 1.88 c | |
| JXY–1 | CK | 104 a | 4.06 a | 118 a | 4.53 a | 94.0 a | 2.44 a | 115 a | 2.74 a |
| 1 mmol L−1 Spd | 66.7 b | 2.08 b | 99.1 b | 3.84 b | 71.0 b | 1.65 b | 100 b | 1.85 b | |
| 50 μmol L−1 AVG | 53.3 c | 1.29 c | 83.6 c | 3.01 c | 49.1 c | 0.85 c | 77.9 c | 1.05 c | |
| Cultivar | Chemical Treatment | 10 Days after Spraying | 20 Days after Spraying | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SG | IG | SG | IG | ||||||||||
| ADC | SAMDC | PAO | ADC | SAMDC | PAO | ADC | SAMDC | PAO | ADC | SAMDC | PAO | ||
| YD-6 | CK | 6.23 b | 8.13 b | 9.38 b | 7.23 b | 5.47 b | 16.6 b | 4.22 b | 5.48 b | 7.07 b | 5.19 b | 4.27 b | 13.8 b |
| 1 mmol L−1 Spd | 8.29 a | 8.98 a | 11.1 a | 9.31 a | 6.66 a | 18.7 a | 6.24 a | 6.44 a | 9.53 a | 7.34 a | 5.71 a | 14.6 a | |
| 50 μmol L−1 AVG | 8.48 a | 9.32 a | 11.5 a | 9.53 a | 6.95 a | 19.0 a | 6.51 a | 6.56 a | 9.61 a | 7.62 a | 5.85 a | 14.9 a | |
| JXY-1 | CK | 6.19 b | 7.02 b | 9.27 b | 6.76 b | 4.02 b | 16.7 b | 4.79 b | 5.11 b | 7.95 b | 4.45 b | 4.05 b | 13.9 b |
| 1 mmol L−1 Spd | 8.18 a | 8.85 a | 11.0 a | 9.70 a | 5.85 a | 18.7 a | 6.54 a | 6.63 a | 9.00 a | 7.16 a | 5.90 a | 15.8 a | |
| 50 μmol L−1 AVG | 8.34 a | 9.02 a | 11.3 a | 9.43 a | 5.92 a | 19.1 a | 6.58 a | 6.66 a | 9.35 a | 7.22 a | 6.12 a | 16.1 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jiang, Y.; Zhong, X.; Li, C.; Xu, Y.; Zhu, K.; Wang, W.; Gu, J.; Zhang, H.; Wang, Z.; et al. Exogenous Spermidine and Amino-Ethoxyvinylglycine Improve Nutritional Quality via Increasing Amino Acids in Rice Grains. Plants 2024, 13, 316. https://doi.org/10.3390/plants13020316
Liu Y, Jiang Y, Zhong X, Li C, Xu Y, Zhu K, Wang W, Gu J, Zhang H, Wang Z, et al. Exogenous Spermidine and Amino-Ethoxyvinylglycine Improve Nutritional Quality via Increasing Amino Acids in Rice Grains. Plants. 2024; 13(2):316. https://doi.org/10.3390/plants13020316
Chicago/Turabian StyleLiu, Ying, Yi Jiang, Xiaohan Zhong, Chaoqing Li, Yunji Xu, Kuanyu Zhu, Weilu Wang, Junfei Gu, Hao Zhang, Zhiqin Wang, and et al. 2024. "Exogenous Spermidine and Amino-Ethoxyvinylglycine Improve Nutritional Quality via Increasing Amino Acids in Rice Grains" Plants 13, no. 2: 316. https://doi.org/10.3390/plants13020316
APA StyleLiu, Y., Jiang, Y., Zhong, X., Li, C., Xu, Y., Zhu, K., Wang, W., Gu, J., Zhang, H., Wang, Z., Liu, L., Zhang, J., Zhang, W., & Yang, J. (2024). Exogenous Spermidine and Amino-Ethoxyvinylglycine Improve Nutritional Quality via Increasing Amino Acids in Rice Grains. Plants, 13(2), 316. https://doi.org/10.3390/plants13020316










