Chemical Composition and Biological Activity of Hypericum Species—H. hirsutum, H. barbatum, H. rochelii
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Hypericum Extracts
2.2. Biological Potential of Evaluated Hypericum Species
2.2.1. Antioxidant Potential
2.2.2. Inhibition of Biologically Important Enzymes
Inhibition of Acetylcholinesterase and Monoamine Oxidases A and B
Antihyperglycemic Potential
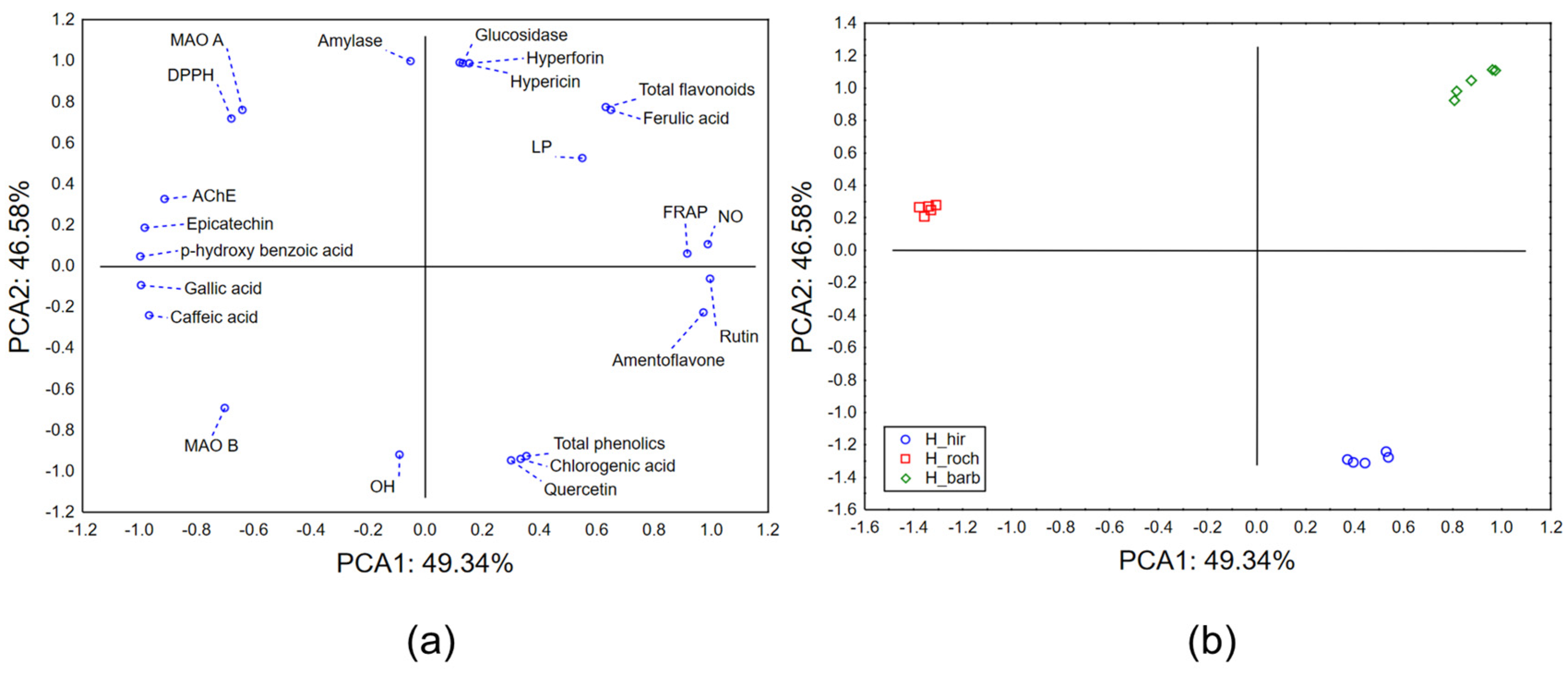
2.2.3. Chemometric Approach—Biological Potential
2.2.4. Antibacterial and Anti-Candida Activity
3. Materials and Methods
3.1. Herbal Material and Preparation of Extracts
3.2. Chemical Profiling of Plant Extracts
3.3. Antioxidant Potential Evaluation
3.3.1. Free Radical Scavenging Capacity (RSC)
3.3.2. Ferric Reduction Antioxidant Potential (FRAP)
3.4. Biologically Important Enzymes Inhibition
3.4.1. Inhibition of Acetylcholinesterase
3.4.2. Monoamine Oxidase A (MAO-A) and Monoamine Oxidase B (MAO-B) Inhibition
3.4.3. Inhibition of α-Amylase and α-Glucosidase
3.5. Antimicrobial Activity
3.6. Data Processing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jahangirova, I.; Zulfugarova, M.; Hajiyeva, E.; Karimova, Z. Chemical Composition and Pharmacological Activity of Plants of the Hypericum L. Genus. Бюллетень Науки И Практики 2023, 9, 60–75. [Google Scholar] [CrossRef]
- Carrubba, A.; Lazzara, S.; Giovino, A.; Ruberto, G.; Napoli, E. Content variability of bioactive secondary metabolites in Hypericum perforatum L. Phytochem. Lett. 2021, 46, 71–78. [Google Scholar] [CrossRef]
- Tocci, N.; Perenzoni, D.; Iamonico, D.; Fava, F.; Weil, T.; Mattivi, F. Extracts from Hypericum hircinum subsp. majus exert antifungal activity against a panel of sensitive and drug-resistant clinical strains. Front. Pharmacol. 2018, 9, 382. [Google Scholar] [CrossRef]
- Nobakht, S.Z.; Akaberi, M.; Mohammadpour, A.H.; Moghadam, A.T.; Emami, S.A. Hypericum perforatum: Traditional uses, clinical trials, and drug interactions. Iran. J. Basic Med. Sci. 2022, 25, 1045. [Google Scholar]
- Ilieva, Y.; Marinov, T.; Trayanov, I.; Kaleva, M.; Zaharieva, M.M.; Yocheva, L.; Kokanova-Nedialkova, Z.; Najdenski, H.; Nedialkov, P. Outstanding Antibacterial Activity of Hypericum rochelii—Comparison of the Antimicrobial Effects of Extracts and Fractions from Four Hypericum Species Growing in Bulgaria with a Focus on Prenylated Phloroglucinols. Life 2023, 13, 274. [Google Scholar] [CrossRef]
- Babotă, M.; Frumuzachi, O.; Mocan, A.; Tămaș, M.; Días, M.I.; Pinela, J.; Stojković, D.S.; Soković, M.D.; Bădărău, A.S.; Crișan, G.; et al. Unravelling Phytochemical and Bioactive Potential of Three Hypericum Species from Romanian Spontaneous Flora: H. alpigenum, H. perforatum and H. rochelii. Plants 2022, 11, 2773. [Google Scholar] [CrossRef]
- Napoli, E.M.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species—A comparative study. Phytochemistry 2018, 152, 162–173. [Google Scholar] [CrossRef]
- Kakouri, E.; Trigas, P.; Daferera, D.; Skotti, E.; Tarantilis, P.A.; Kanakis, C. Chemical characterization and antioxidant activity of nine Hypericum species from Greece. Antioxidants 2023, 12, 899. [Google Scholar] [CrossRef]
- Xiao, C.-Y.; Mu, Q.; Gibbons, S. The phytochemistry and pharmacology of Hypericum. Prog. Chem. Org. Nat. Prod. 2020, 112, 85–182. [Google Scholar]
- Chen, Q.; Di, L.; Zhang, Y.; Li, N. Chemical constituents with cytotoxic and anti-inflammatory activity in Hypericum sampsonii and the antitumor potential under the view of cancer-related inflammation. J. Ethnopharmacol. 2020, 259, 112948. [Google Scholar] [CrossRef]
- Zdunic, G.; Godjevac, D.; Savikin, K.; Petrovic, S. Comparative analysis of phenolic compounds in seven Hypericum species and their antioxidant properties. Nat. Prod. Commun. 2017, 12, 1934578X1701201140. [Google Scholar] [CrossRef]
- Kladar, N.; Božin, B.; Bijelić, K.; Bogavac, M.; Karaman, M.; Srđenović Čonić, B.; Rat, M.; Anačkov, G. Biological Activity of Genus Hypericum Sect. Hypericum Species—H. tetrapterum, H. maculatum subsp. immaculatum, H. triquetrifolium. Molecules 2023, 28, 6218. [Google Scholar] [CrossRef]
- Nürk, N.M.; Crockett, S.L. Morphological and phytochemical diversity among Hypericum species of the Mediterranean Basin. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 14. [Google Scholar]
- Booth, Z.; van Vuuren, S. The combined use of african natural products and conventional antimicrobials: An alternative tool against antimicrobial resistance. In Antimicrobial Research and One Health in Africa; Springer: Berlin/Heidelberg, Germany, 2023; pp. 317–346. [Google Scholar]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Oniga, I.; Toiu, A.; Benedec, D.; Vlase, L. Comparative phytochemical profile of Hypericum perforatum and Hypericum hirsutum (Hypericaceae). Farmacia 2022, 70, 1046–1049. [Google Scholar] [CrossRef]
- Sagratini, G.; Ricciutelli, M.; Vittori, S.; Öztürk, N.; Öztürk, Y.; Maggi, F. Phytochemical and antioxidant analysis of eight Hypericum taxa from Central Italy. Fitoterapia 2008, 79, 210–213. [Google Scholar] [CrossRef]
- Altun, M.L.; Yılmaz, B.S.; Orhan, I.E.; Citoglu, G.S. Assessment of cholinesterase and tyrosinase inhibitory and antioxidant effects of Hypericum perforatum L.(St. John’s wort). Ind. Crops Prod. 2013, 43, 87–92. [Google Scholar] [CrossRef]
- Öztürk, N.; Tunçel, M.; Potoğlu-Erkara, İ. Phenolic compounds and antioxidant activities of some Hypericum species: A comparative study with H. perforatum. Pharm. Biol. 2009, 47, 120–127. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn. Mag. 2010, 6, 74. [Google Scholar] [CrossRef]
- Savikin, K.; Dobrić, S.; Tadić, V.; Zdunić, G. Antiinflammatory activity of ethanol extracts of Hypericum perforatum L., H. barbatum Jacq., H. hirsutum L., H. richeri Vill. and H. androsaemum L. in rats. Phytother. Res. PTR 2007, 21, 176–180. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Verma, V.; Spiteller, M.; Ahmad, S.M.; Puri, S.C.; Qazi, G.N. Phytochemical analysis and genetic characterization of six Hypericum species from Serbia. Phytochemistry 2006, 67, 171–177. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Zuehlke, S.; Spiteller, M.; Raabe, N.; Özen, T. Phenolic constituents of 17 Hypericum species from Turkey. Biochem. Syst. Ecol. 2008, 36, 316–319. [Google Scholar] [CrossRef]
- Hosni, K.; Msaada, K.; Taârit, M.B.; Marzouk, B. Phenological variations of secondary metabolites from Hypericum triquetrifolium Turra. Biochem. Syst. Ecol. 2011, 39, 43–50. [Google Scholar] [CrossRef]
- Cheung, Z.H.; Leung, M.C.; Yip, H.K.; Wu, W.; Siu, F.K.; So, K.-F. A neuroprotective herbal mixture inhibits caspase-3-independent apoptosis in retinal ganglion cells. Cell. Mol. Neurobiol. 2008, 28, 137–155. [Google Scholar] [CrossRef]
- Mártonfi, P.; Repčák, M.; Mártonfiová, L. Secondary metabolites during ontogenetic phase of reproductive structures in Hypericum maculatum. Biologia 2006, 61, 473–478. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal plants of the Russian Pharmacopoeia; their history and applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of antioxidant properties of agents from the perspective of their action mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef]
- Larit, F.; Elokely, K.M.; Chaurasiya, N.D.; Benyahia, S.; Nael, M.A.; León, F.; Abu-Darwish, M.S.; Efferth, T.; Wang, Y.-H.; Belouahem-Abed, D.; et al. Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants. Phytomedicine Int. J. Phytother. Phytopharm. 2017, 40, 27–36. [Google Scholar] [CrossRef]
- Chimenti, F.; Cottiglia, F.; Bonsignore, L.; Casu, L.; Casu, M.; Floris, C.; Secci, D.; Bolasco, A.; Chimenti, P.; Granese, A. Quercetin as the active principle of hypericum h ircinum exerts a selective inhibitory activity against MAO-A: Extraction, Biological analysis, and computational study. J. Nat. Prod. 2006, 69, 945–949. [Google Scholar] [CrossRef]
- Herraiz, T.; Guillén, H. Monoamine Oxidase-A Inhibition and Associated Antioxidant Activity in Plant Extracts with Potential Antidepressant Actions. BioMed Res. Int. 2018, 2018, 4810394. [Google Scholar] [CrossRef]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C.P. Neuroprotective Activity of Hypericum perforatum and Its Major Components. Front. Plant Sci. 2016, 7, 1004. [Google Scholar] [CrossRef]
- Dong, Q.; Hu, N.; Yue, H.; Wang, H.; Wei, Y. Rapid screening of α-glucosidase inhibitors in Hypericum perforatum L. by bio-affinity chromatography coupled with UPLC/MS. Biomed. Chromatogr. 2022, 37, e5536. [Google Scholar] [CrossRef]
- Rafailovska, E.; Tushevski, O.; Gadzovska-Simic, S.; Dinevska-Kjovkarovska, S.; Miova, B. Hypericum perforatum L. Hairy Root Extracts—Regulation of Glycemic, Metabolic, Serum Enzyme and Lipid Profile in STZ—Induced Diabetic Rats. Maced. Vet. Rev. 2021, 45, 5–15. [Google Scholar] [CrossRef]
- Rafailovska, E.; Tushevski, O.; Shijakova, K.; Simic, S.G.; Kjovkarovska, S.D.; Miova, B. Hypericum perforatum L. extract exerts insulinotropic effects and inhibits gluconeogenesis in diabetic rats by regulating AMPK expression and PKCε concentration. J. Ethnopharmacol. 2022, 302, 115899. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Zengin, G.; Lobine, D.; Molina-García, L.; Mollica, A.; Mahomoodally, M.F. Phytochemical characterization, in vitro and in silico approaches for three Hypericum species. New J. Chem. 2018, 42, 5204–5214. [Google Scholar] [CrossRef]
- Hamdan, I.I.; Afifi, F.U. Screening of Jordanian Flora for α-Amylase Inhibitory Activity. Pharm. Biol. 2008, 46, 746–750. [Google Scholar] [CrossRef]
- Shen, H.; Wang, J.; Ao, J.; Hou, Y.; Xi, M.; Cai, Y.; Li, M.; Luo, A. Structure-activity relationships and the underlying mechanism of α-amylase inhibition by hyperoside and quercetin: Multi-spectroscopy and molecular docking analyses. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2022, 285, 121797. [Google Scholar] [CrossRef]
- Mandrone, M.; Scognamiglio, M.; Fiorentino, A.; Sanna, C.; Cornioli, L.; Antognoni, F.; Bonvicini, F.; Poli, F. Phytochemical profile and α-glucosidase inhibitory activity of Sardinian Hypericum scruglii and Hypericum hircinum. Fitoterapia 2017, 120, 184–193. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, L.; Hu, Z.-X.; Yi, P.; Yang, W.-X.; Gu, W.-P.; Huang, L.-j.; Yuan, C.; Hao, X.-J. Chromanopyrones and a flavone from Hypericum monogynum. Fitoterapia 2018, 125, 59–64. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Coelho, N.; Santos-Rufo, A.; Gonçalves, S.; Pérez-Santín, E.; Romano, A. The influence of in vitro gastrointestinal digestion on the chemical composition and antioxidant and enzyme inhibitory capacities of carob liqueurs obtained with different elaboration techniques. Antioxidants 2019, 8, 563. [Google Scholar] [CrossRef]
- Gnanamani, A.; Hariharan, P.; Paul-Satyaseela, M. Staphylococcus aureus: Overview of bacteriology, clinical diseases, epidemiology, antibiotic resistance and therapeutic approach. Front. Staphylococcus Aureus 2017, 4, 10–5772. [Google Scholar]
- Radulović, N.; Stankov-Jovanović, V.; Stojanović, G.; Šmelcerović, A.; Spiteller, M.; Asakawa, Y. Screening of in vitro antimicrobial and antioxidant activity of nine Hypericum species from the Balkans. Food Chem. 2007, 103, 15–21. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Gonzalez-Manzano, S.; Dueñas, M.; Gonzalez-Paramas, A.M. Extraction and isolation of phenolic compounds. Nat. Prod. Isol. 2012, 427–464. [Google Scholar]
- Veiko, A.G.; Olchowik-Grabarek, E.; Sekowski, S.; Roszkowska, A.; Lapshina, E.A.; Dobrzynska, I.; Zamaraeva, M.; Zavodnik, I.B. Antimicrobial activity of quercetin, naringenin and catechin: Flavonoids inhibit Staphylococcus aureus-induced hemolysis and modify membranes of bacteria and erythrocytes. Molecules 2023, 28, 1252. [Google Scholar] [CrossRef]
- Xiong, X.; Tang, N.; Lai, X.; Zhang, J.; Wen, W.; Li, X.; Li, A.; Wu, Y.; Liu, Z. Insights into amentoflavone: A natural multifunctional biflavonoid. Front. Pharmacol. 2021, 12, 768708. [Google Scholar] [CrossRef]
- Feyzioğlu, B.; Demircili, M.E.; Özdemir, M.; Doğan, M.; Baykan, M.; Baysal, B. Antibacterial effect of hypericin. Afr. J. Microbiol. Res. 2013, 7, 979–982. [Google Scholar]
- Al-Majmaie, S.; Nahar, L.; Sharples, G.P.; Wadi, K.; Sarker, S.D. Isolation and antimicrobial activity of rutin and its derivatives from Ruta chalepensis (Rutaceae) growing in Iraq. Rec. Nat. Prod. 2019, 13, 64–70. [Google Scholar] [CrossRef]
- Keitel, S. Pharmacopoeial Standards: European Pharmacopoeia. Encycl. Pharm. Sci. Technol. 2013, 6, 2691–2703. [Google Scholar]
- Bijelić, K.; Srdjenović Čonić, B.; Prpa, B.; Pilija, V.; Vukmirović, S.; Kladar, N. The Potential of Hemp Extracts to Modify the Course of Oxidative-Stress Related Conditions. Plants 2024, 13, 1630. [Google Scholar] [CrossRef]
- Bradic, J.; Petrovic, A.; Nikolic, M.; Nedeljkovic, N.; Andjic, M.; Baljak, J.; Jakovljevic, V.; Kocovic, A.; Tadic, V.; Stojanovic, A. Potentilla tormentilla Extract Loaded Gel: Formulation, In Vivo and In Silico Evaluation of Anti-Inflammatory Properties. Int. J. Mol. Sci. 2024, 25, 9389. [Google Scholar] [CrossRef]
- Ziaková, A.; Brandšteterová, E. Validation of HPLC determination of phenolic acids present in some Lamiaceae family plants. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 443–453. [Google Scholar] [CrossRef]
- Samoylenko, V.; Rahman, M.M.; Tekwani, B.L.; Tripathi, L.M.; Wang, Y.-H.; Khan, S.I.; Khan, I.A.; Miller, L.S.; Joshi, V.C.; Muhammad, I. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J. Ethnopharmacol. 2010, 127, 357–367. [Google Scholar] [CrossRef]
- Patel, J.B.; Tenover, F.C.; Turnidge, J.D.; Jorgensen, J.H. Susceptibility test methods: Dilution and disk diffusion methods. In Manual of Clinical Microbiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1122–1143. [Google Scholar]
- Fothergill, A.W. Antifungal susceptibility testing: Clinical laboratory and standards institute (CLSI) methods. In Interactions of Yeasts, Moulds, and Antifungal Agents: How to Detect Resistance; Springer: Berlin/Heidelberg, Germany, 2011; pp. 65–74. [Google Scholar]
| Sample | H. hirsutum | H. rochelii | H. barbatum | |
|---|---|---|---|---|
| Variables | ||||
| Total phenolics (mg GAE/g de) | 195.30 (6.74) a | 137.50 (4.22) b | 139.14 (5.98) b | |
| Total flavonoids (mg QE/g de) | 29.32 (1.35) a | 29.76 (1.54) a | 46.98 (2.11) b | |
| Dry extract yield (%) | 19.76 (1.11) a | 27.66(1.88) b | 17.37 (1.35) a | |
| Class of compounds | Compound | µg/g dry herb | ||
| Naphthodianthrones | Hypericin | 70.02 (3.32) a | 1044.53 (11.53) b | 1838.39 (45.15) c |
| Phloroglucinols | Hyperforin | nd a | 1047.66 (21.76) b | 1993.18 (114.65) c |
| Biflavonoids | Amentoflavone | 295.26 (2.82) a | 51.54 (0.73) b | 280.96 (7.56) c |
| Flavonoids and flavonoid glycosides | Apigenin | nd a | nd a | nd a |
| Naringenin | 682.22 (3.71) a | nd b | nd b | |
| Rutin | 278.12 (6.99) a | 133.72 (0.37) b | 301.61 (12.39) a | |
| Quercetin | 230.47 (2.95) a | 121.14 (5.72) b | 116.64 (4.32) c | |
| Epicatechin | nd a | 386.70 (13.88) b | nd a | |
| Phenolic acids | Ferulic acid | nd a | nd a | 116.49 (0.26) b |
| Gallic acid | 35.59 (1.31) a | 123.99 (1.78) b | nd c | |
| Chlorogenic acid | 31.69 (0.84) a | nd b | nd b | |
| Caffeic acid | 37.10 (1.47) a | 66.04 (1.43) b | 17.00 (0.75) c | |
| p-hydroxybenzoic acid | 48.63 (1.97) a | 327,31 (10.37) b | nd c | |
| Sample | H. hirsutum | H. rochelii | H. barbatum | Positive Control |
|---|---|---|---|---|
| Variable | RSC50 (µg/mL) | |||
| DPPH | 2.81 (0.03) a | 3.63 (0.01) b | 3.20 (0.09) c | QDH, RSC50 = 1.08 (0.10) PG, RSC50 = 0.59 (0.02) |
| NO | 29.90 (1.85) a | 21.69 (1.79) b | 33.64 (1.65) a | PG, RSC50 = 8.90 (0.75) |
| OH, carbohydrate substrate * | 59.29 (4.26) a | 53.25 (1.51) b | 49.77 (3.00) b | BHT, IC50 = 0.04 (0.00) AA, IC50 = 2.26 (0.19) PG, IC50 = 10.15 (0.65) |
| OH, lipid substrate ** | 384.97 (2.36) a | 383.76 (7.40) a | 409.61 (4.80) b | BHT, IC50 = 7.92 (0.66) |
| FRAP (mg AAE/g de) | 155.82 (10.34) a | 142.31 (6.84) a,b | 160.89 (5.79) a | / |
| Enzyme inhibition | IC50 (µg/mL) | Positive control | ||
| AChE | 715.49 (38.44) a | 947.77 (49.17) b | 756.57 (26.54) a | Galantamine IC50 = 9.11 (0.64) |
| MAO-A | 5.11 (0.11) a | 8.69 (0.21) b | 7.51 (0.22) c | Moclobemide IC50 = 0.71 (0.08) |
| MAO-B | 60.18 (3.88) a | 61.76 (4.11) a | 40.50 (2.45) b | Selegiline IC50 = 0.22 (0.02) |
| α-amylase | 80.45 (3.65) a | 977.93 (38.99) b | 1343.55 (48.55) c | Acarbose IC50 = 5.35 (0.72) |
| α-glucosidase | 13.08 (0.25) a | 17.10 (0.39) b | 20.03 (0.42) c | Acarbose IC50 = 48.76 (3.45) |
| Agent | H. hirsutum | H. rochelii | H. barbatum | |||
|---|---|---|---|---|---|---|
| Microbe | MIC | MBC | MIC | MBC | MIC | MBC |
| S. aureus H MRSA | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| E. coli L | 50 | 50 | 50 | 100 | 25 | 50 |
| P. mirabilis H | 12.5 | 12.5 | 25 | 25 | 12.5 | 12.5 |
| P. aeruginosa H | 12.5 | 12.5 | 25 | 25 | 12.5 | 12.5 |
| Enterococcus sp. L | 25 | 25 | 25 | 25 | 25 | 25 |
| P. vulgaris L | 50 | 50 | 25 | 50 | 12.5 | 12.5 |
| Candida L | / | / | / | / | / | / |
| Candida H | / | / | / | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baljak, J.; Bogavac, M.; Karaman, M.; Srđenović Čonić, B.; Vučković, B.; Anačkov, G.; Kladar, N. Chemical Composition and Biological Activity of Hypericum Species—H. hirsutum, H. barbatum, H. rochelii. Plants 2024, 13, 2905. https://doi.org/10.3390/plants13202905
Baljak J, Bogavac M, Karaman M, Srđenović Čonić B, Vučković B, Anačkov G, Kladar N. Chemical Composition and Biological Activity of Hypericum Species—H. hirsutum, H. barbatum, H. rochelii. Plants. 2024; 13(20):2905. https://doi.org/10.3390/plants13202905
Chicago/Turabian StyleBaljak, Jovan, Mirjana Bogavac, Maja Karaman, Branislava Srđenović Čonić, Biljana Vučković, Goran Anačkov, and Nebojša Kladar. 2024. "Chemical Composition and Biological Activity of Hypericum Species—H. hirsutum, H. barbatum, H. rochelii" Plants 13, no. 20: 2905. https://doi.org/10.3390/plants13202905
APA StyleBaljak, J., Bogavac, M., Karaman, M., Srđenović Čonić, B., Vučković, B., Anačkov, G., & Kladar, N. (2024). Chemical Composition and Biological Activity of Hypericum Species—H. hirsutum, H. barbatum, H. rochelii. Plants, 13(20), 2905. https://doi.org/10.3390/plants13202905







