Food and Environment During the Late Roman Age at the Site of Alba Fucens (Abruzzi, Italy)
Abstract
1. Introduction
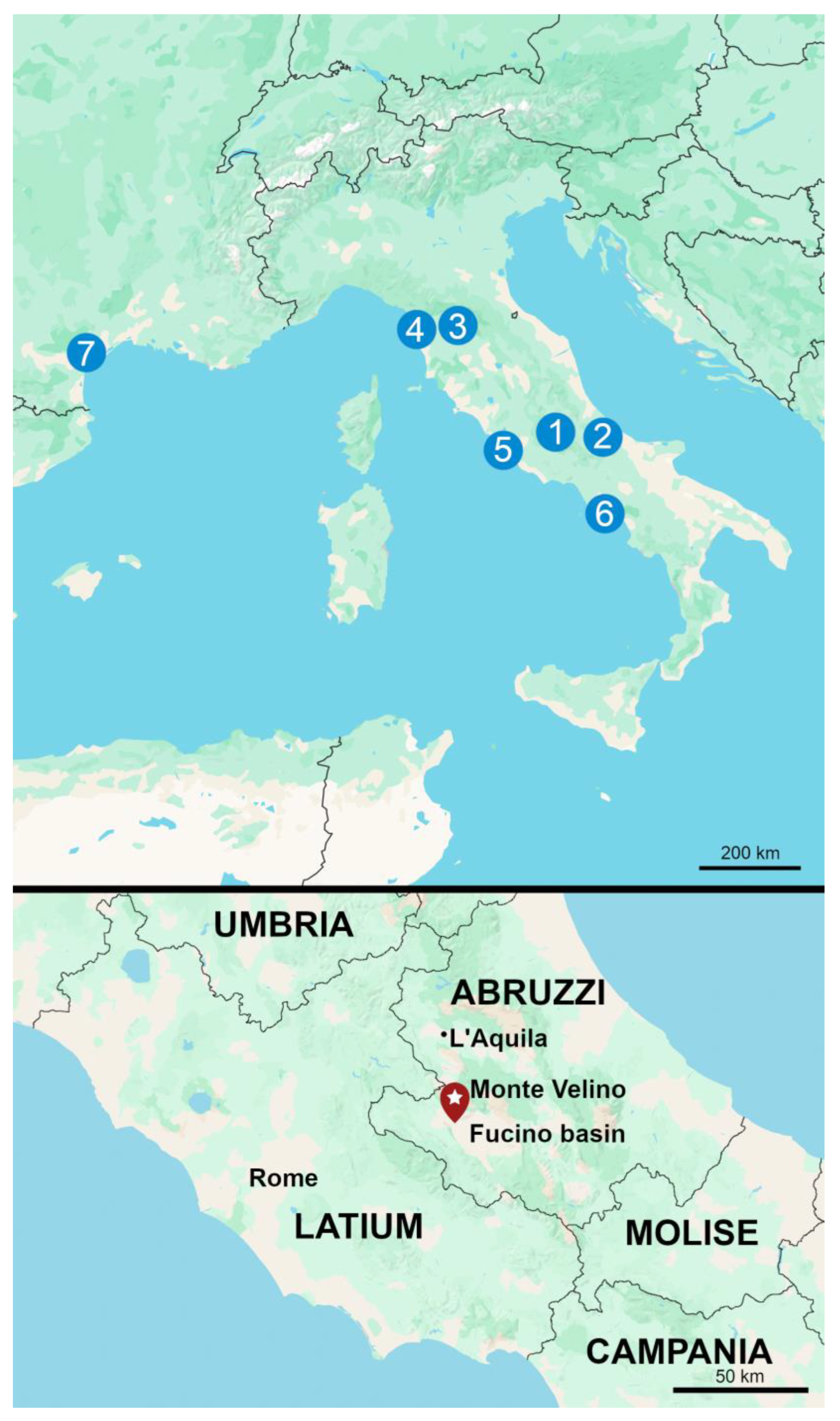
1.1. The Site
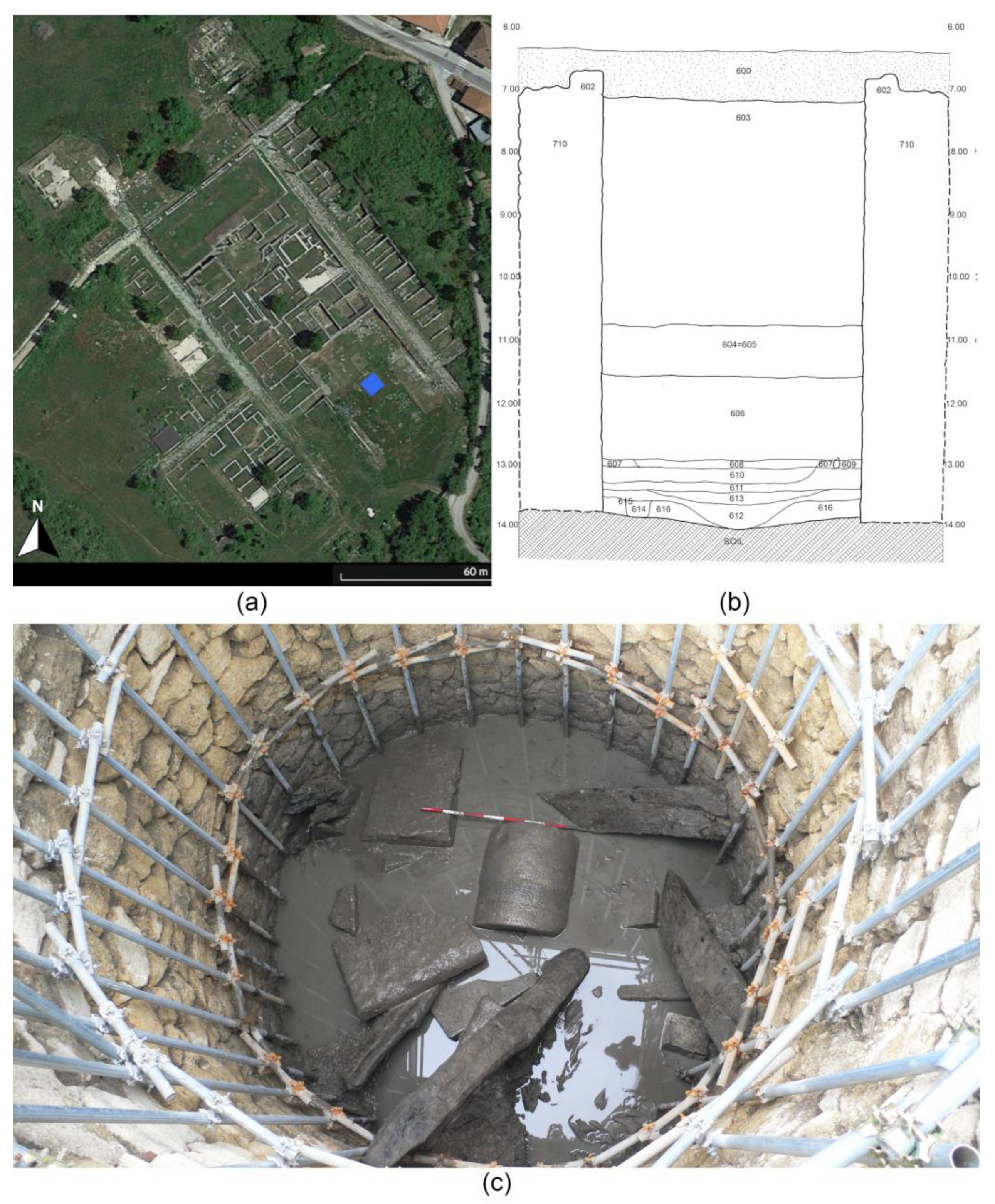
1.2. The Well of the Sanctuary of Hercules
2. Materials and Methods
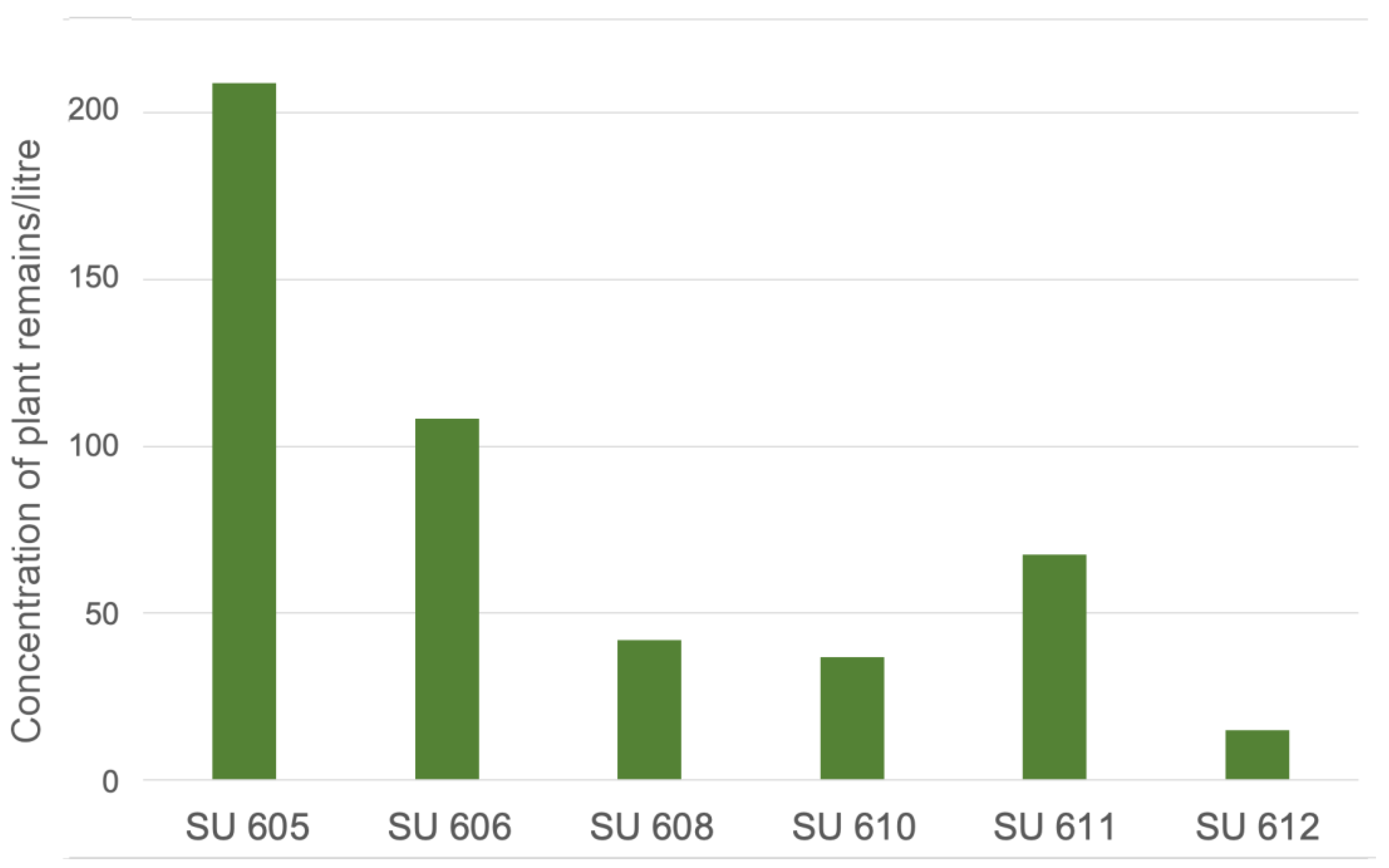
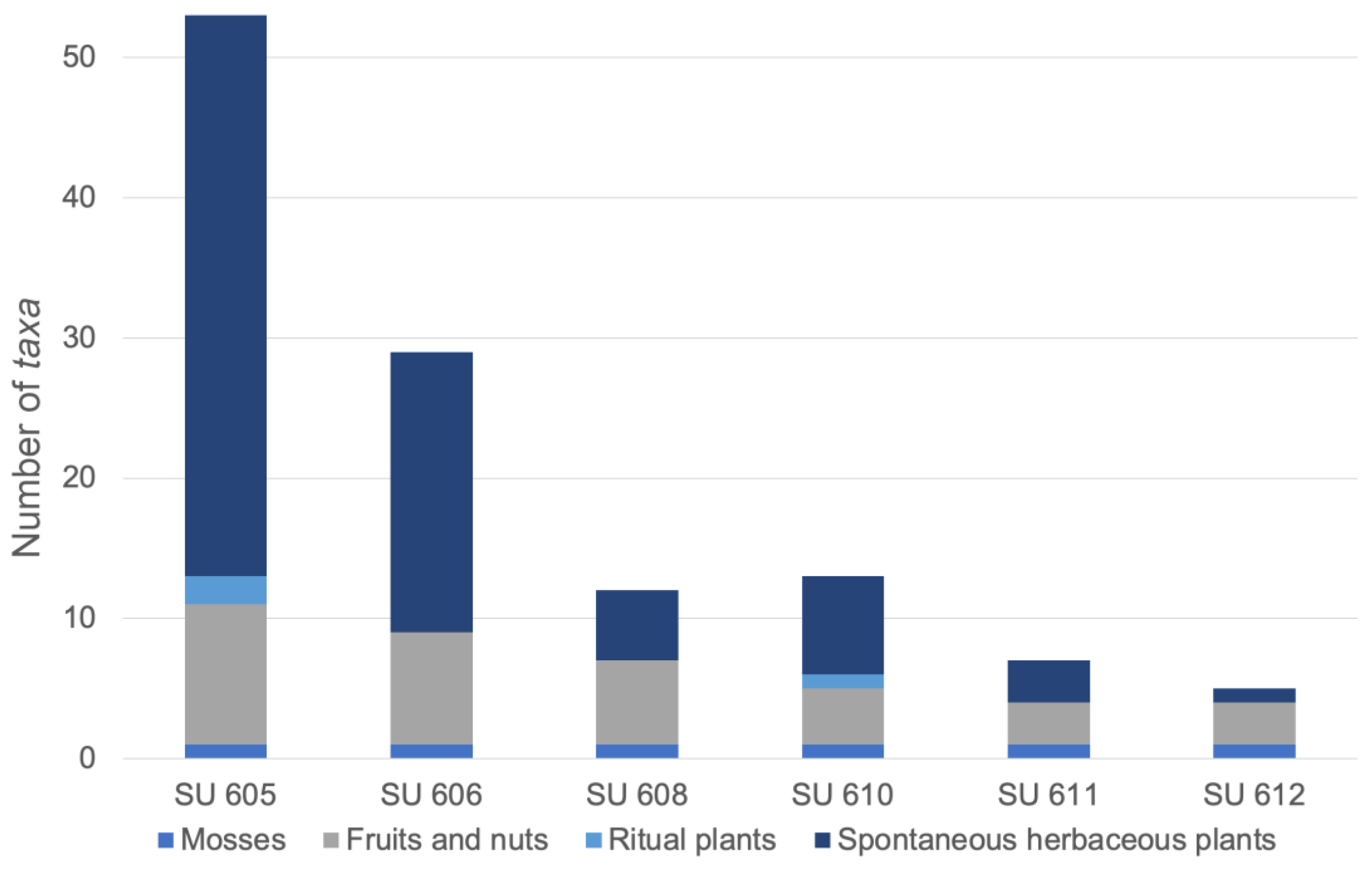
3. Results
4. Discussion
4.1. Subsistence and Dietary Plants
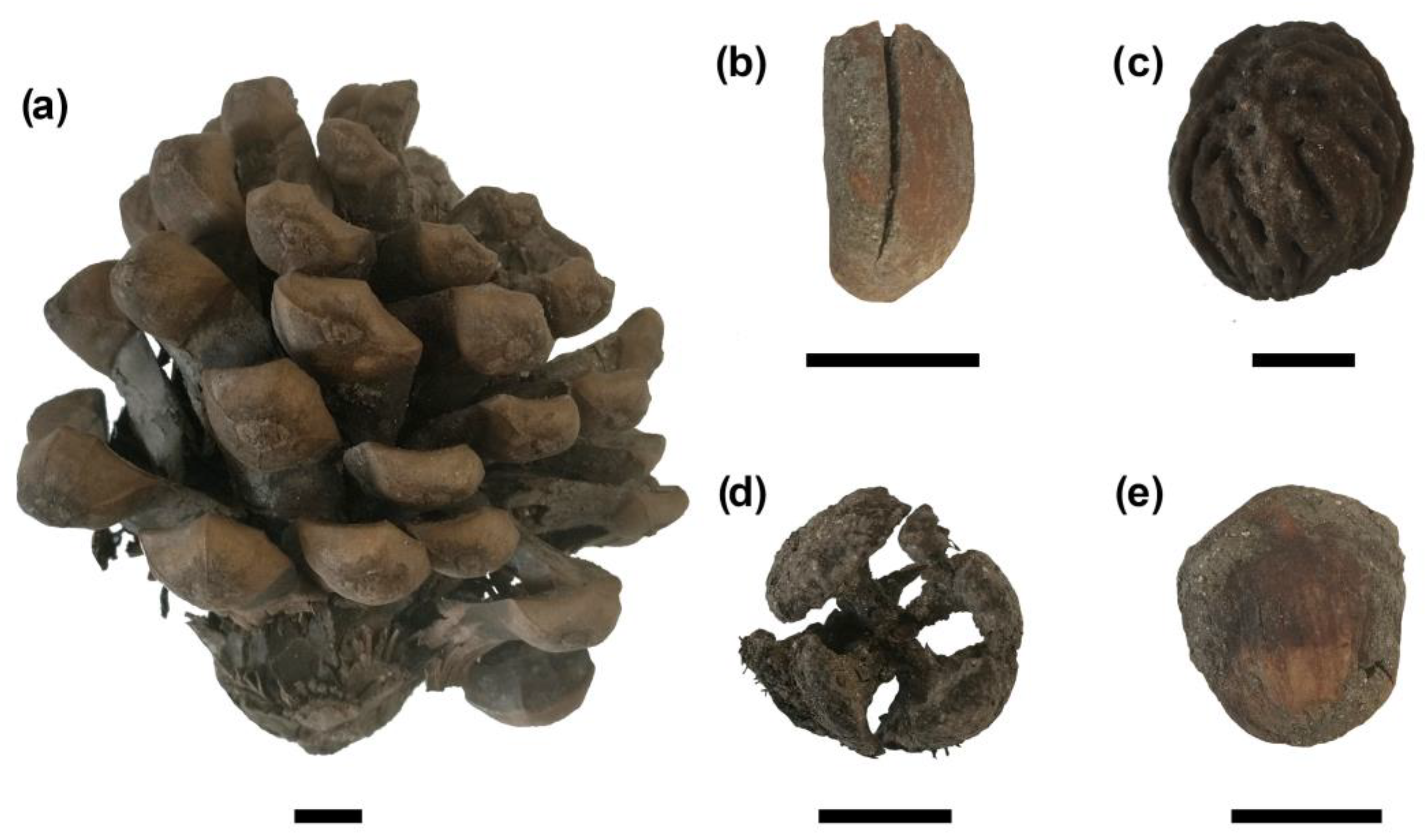
4.2. Ritual Plants
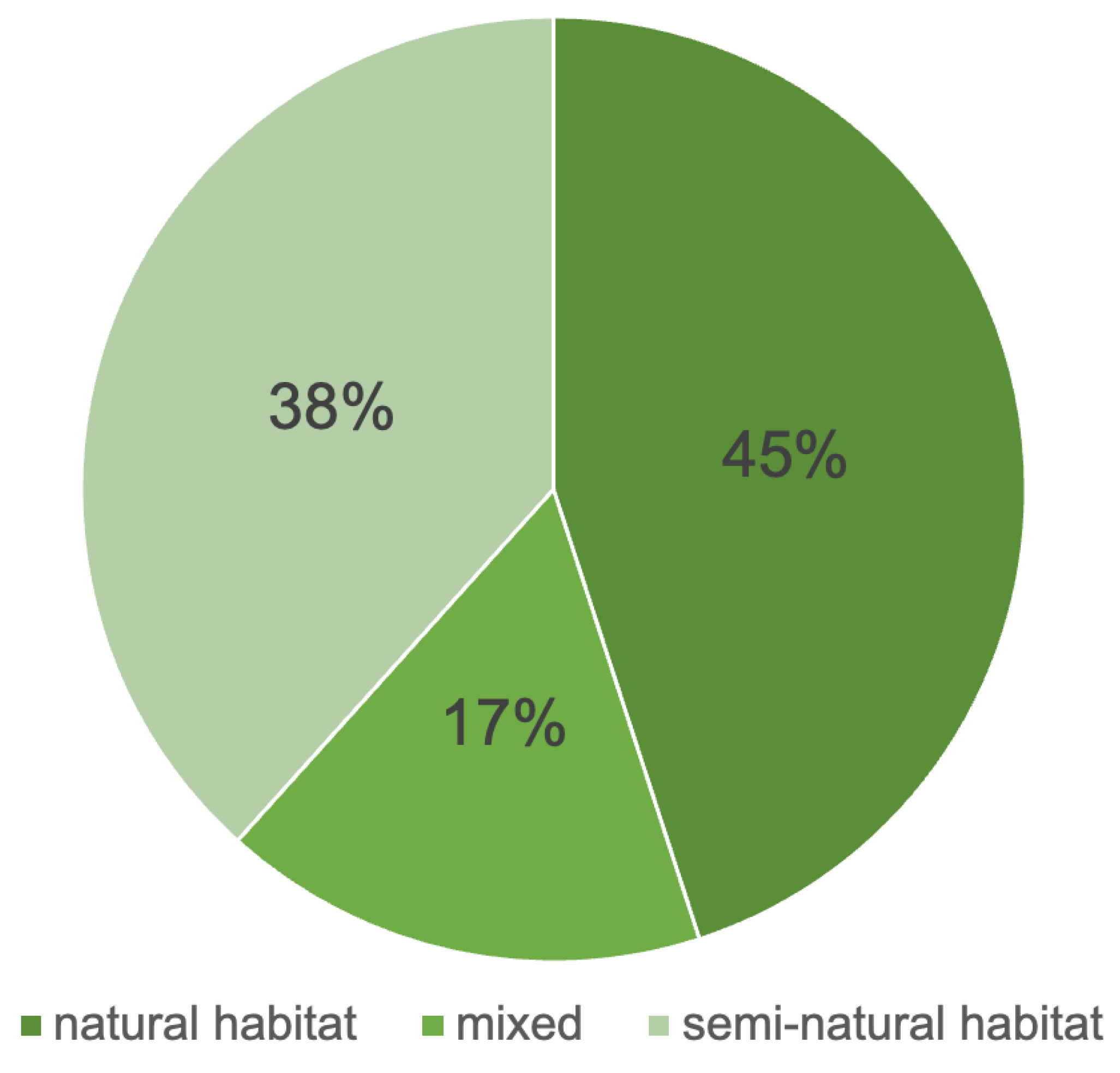
4.3. Environmental Indicators
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galasso, G.; Conti, F.; Peruzzi, L.; Alessandrini, A.; Ardenghi, N.M.G.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D.; et al. A second update to the checklist of the vascular flora alien to Italy. Plant Biosyst. 2024, 158, 297–340. [Google Scholar] [CrossRef]
- Pirone, G.; Frattaroli, A.R. Lineamenti della biodiversità vegetale in Abruzzo. Acta Italus Hortus 2011, 1, 9–12. [Google Scholar]
- Mercuri, A.M.; Allevato, E.; Arobba, D.; Mazzanti, M.B.; Bosi, G.; Caramiello, R.; Castiglioni, E.; Carra, M.L.; Celant, A.; Costantini, L.; et al. Pollen and macroremains from Holocene archaeological sites: A dataset for the understanding of the bio-cultural diversity of the Italian landscape. Rev. Palaeobot. Palynol. 2015, 218, 250–266. [Google Scholar] [CrossRef]
- Mariotti Lippi, M.; Florenzano, A.; Rinaldi, R.; Allevato, E.; Arobba, D.; Bacchetta, G.; Bal, M.C.; Bandini Mazzanti, M.; Benatti, A.; Benes, J.; et al. The Botanical Record of Archaeobotany Italian Network—BRAIN: A cooperative network, database and website. Flora Mediterr. 2018, 28, 365–376. [Google Scholar] [CrossRef]
- Shelton, C. Food, Economy and Identity in the Sangro River Valley, Abruzzo, Italy, 650 B.C.—A.D. 150. Ph.D. Dissertation, Boston University, Boston, MA, USA, 2009. [Google Scholar]
- Mariotti Lippi, M.; Mori Secci, M.; Giachi, G.; Bouby, L.; Terral, J.F.; Castiglioni, E.; Cottini, M.; Rottoli, M.; de Grummond, N.T. Plant remains in an Etruscan-Roman well at Cetamura del Chianti, Italy. Archaeol. Anthrol. Sci. 2020, 12, 35. [Google Scholar] [CrossRef]
- Galadini, F.; Ceccaroni, E.; Dixit Dominus, G.; Falcucci, E.; Gori, S.; Maceroni, D.; Bonasera, M.; Di Giulio, G.; Moro, M.; Saroli, M.; et al. Combining earth sciences with archaeology to investigate natural risks related to the cultural heritage of the Marsica region (central Apennines, Italy). Mediterr. Geosci. Rev. 2022, 4, 287–318. [Google Scholar] [CrossRef]
- Galadini, F.; Ceccaroni, E.; Falcucci, E. Archaeoseismological evidence of a disruptive Late Antique earthquake at Alba Fucens (central Italy). Boll. Geofis. Teor. Appl. 2010, 51, 143–161. [Google Scholar]
- Galadini, F.; Ceccaroni, E.; Falcucci, E.; Gori, S. Le fasi di colluviamento tardo antiche nel Piano della Civita e la fine della frequentazione dell’abitato di Alba Fucens. In Belgica et Italica. Joseph Mertens: Une vie pour L’archéologie; Balty, C., Ed.; Academia Belgica: Rome, Italy, 2012; pp. 187–199. [Google Scholar]
- Petriccione, B. Flora e Vegetazione del Massiccio del Monte Velino; Ministero delle Risorse Agricole e Forestali: Collana Verde, Italy, 1993; Volume 92, p. 261. [Google Scholar]
- Theurillat, J.P.; Iocchi, M.; Cutini, M.; De Marco, G. Vascular plant richness along an elevation gradient at Monte Velino (Central Apennines, Italy). Biogeographia 2007, 28, 149–166. [Google Scholar] [CrossRef]
- Campanelli, A. Poco Grano Molti Frutti. 50 Anni di Archeologia ad ALBA Fucens; Synapsi: Sulmona, Italy, 2006. [Google Scholar]
- Ceccaroni, E. Alba Fucens (Massa d’Albe AQ), piazzale del Santuario di Ercole. La conclusione delle indagini nel 2013. In Quaderni di Archeologia d’Abruzzo. Notiziario Della Soprintendenza per i Beni Archeologici dell’Abruzzo, 5/2013–2015; All’Insegna del Giglio: Firenze, Italy, 2022; pp. 88–91. [Google Scholar]
- Ceccaroni, E. Gli Interventi Della Soprintendenza per i Beni Archeologici dell’Abruzzo ad Alba Fucens: L’isolato di via del Miliario e il Piazzale del Santuario di Ercole. Rendiconti della Pontificia Accademia di Archeologia Romana. 2014, Volume LXXXV, 2012–2013. pp. 245–277. Available online: https://www.academia.edu/36192896/Alba_Fucens_gli_interventi_della_Soprintendenza_per_i_Beni_Archeologici_dell_Abruzzo_nell_isolato_di_via_del_Miliario_e_nel_piazzale_del_santuario_di_Ercole (accessed on 12 September 2024).
- Cerilli, E.; Ceccaroni, E. Resti faunistici dalla cisterna rinvenuta presso il Santuario di Ercole ad Alba Fucens (Massa d’Albe, Aquila). Ann. Univ. Ferrara 2016, 12, 183–190. [Google Scholar] [CrossRef]
- Ceccaroni, E.; Dal Rì, C.; Labriola, M. Il restauro di 400 reperti lignei imbibiti di Alba Fucens (Massa d’Albe AQ). Scavo, liofilizzazione e messa in deposito. Kermes 2024, 37–44, 133–134, in press. [Google Scholar]
- Russo, G.; Ceccaroni, E.; Conte, A.M.; Medeghini, L.; De Vito, C.; Mignardi, S. Archaeometric study on Roman painted terracottas from the Sanctuary of Hercules in Alba Fucens (Abruzzo, Italy). Minerals 2022, 12, 346. [Google Scholar] [CrossRef]
- Bruni, V. Alba Fucens e il Santuario di Ercole: Una rilettura della città antica attraverso i materiali lapidei del pozzo. Mediterranea 2022, 19, 73–111. [Google Scholar]
- Pearsall, D.M. Curating palaeoethnobotanical specimens and botanical reference collections. In Curating Biocultural Collections, A Handbook; Salick, J., Konchar, K., Nesbitt, M., Eds.; Royal Botanic Gardens: Kew, UK, 2014; pp. 67–83. [Google Scholar]
- Neef, R.; Cappers, R.T.; Bekker, R.M. Digital Atlas of Economic Plants in Archaeology; Barkhuis: Groningen, The Netherlands, 2012; Volume 17. [Google Scholar]
- Sabato, D.; Peña-Chocarro, L. Maris Nostri Novus Atlas. Seeds and Fruits from the Mediterranean Basin; Ediciones Doce Calles: Aranjuez, Spain, 2021. [Google Scholar]
- Portal to the Flora of Italy. Available online: https://dryades.units.it/floritaly/index.php (accessed on 12 September 2024).
- Sadori, L.; Allevato, E.; Bellini, C.; Bertacchi, A.; Boetto, G.; Di Pasquale, G.; Giachi, G.; Giardini, M.; Masi, A.; Pepe, A.; et al. Archaeobotany in Italian ancient Roman harbours. Rev. Palaeobot. Palynol. 2015, 218, 217–230. [Google Scholar] [CrossRef]
- Murphy, C.; Thompson, G.; Fuller, D.Q. Roman food refuse: Urban archaeobotany in Pompeii, Regio VI, Insula 1. Veg. Hist. Archaeobotany 2013, 22, 409–419. [Google Scholar] [CrossRef]
- Figueiral, I.; Bouby, L.; Buffat, L.; Petitot, H.; Terral, J.F. Archaeobotany, vine growing and wine producing in Roman Southern France: The site of Gasquinoy (Béziers, Hérault). J. Archaeol. Sci. 2010, 37, 139–149. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole: Bologna, Italy, 2017. [Google Scholar]
- Bosi, G.; Castiglioni, E.; Rinaldi, R.; Mazzanti, M.; Marchesini, M.; Rottoli, M. Archaeobotanical evidence of food plants in Northern Italy during the Roman period. Veg. Hist. Archaeobotany 2020, 29, 681–697. [Google Scholar] [CrossRef]
- Mariotti Lippi, M.; Bellini, C.; Mori Secci, M.; Gonnelli, T.; Pallecchi, P. Archaeobotany in Florence (Italy): Landscape and urban development from the late Roman to the Middle Ages. Plant Biosyst. 2015, 149, 216–227. [Google Scholar] [CrossRef]
- Bertacchi, A.; Lombardi, T.; Sani, A.; Tomei, P.E. Plant macroremains from the Roman harbour of Pisa (Italy). Environ. Archaeol. 2008, 13, 181–188. [Google Scholar] [CrossRef]
- Robinson, M. Domestic burnt offerings and sacrifices at Roman and pre-Roman Pompeii, Italy. Veg. Hist. Archaeobotany 2002, 11, 93–100. [Google Scholar] [CrossRef]
- Sala, G.; Pasta, S.; Maggio, A.; La Mantia, T. Sambucus nigra L. (fam. Viburnaceae) in Sicily: Distribution, Ecology, Traditional Use and Therapeutic Properties. Plants 2023, 12, 3457. [Google Scholar] [CrossRef]
- Ercole, T. La costruzione del paesaggio di Alba Fucens sulla lunga durata. Riflessioni sull’occupazione dello spazio rurale tra lago, montagne e acquitrini. In The State of the Samnite; Stek, T.D., Ed.; Edizioni Quasar: Rome, Italy, 2021. [Google Scholar]
- Apicius, C. Celio Apicio - Delle vivande e condimenti ovvero dell’arte della cucina - Volgarizzamento con annotazioni di Giambattista Baseggio / Apicii Coelii - De obsoniis et condimentis sive arte coquinaria - Libri decem; Nel Privil. Stabilimento Nazionale di G.Antonelli, "Biblioteca degli Scrittori Latini": Venice, Italy, 1852; pp. 78–80. [Google Scholar]
- Kaya, Y.; Haji, E.K.; Arvas, Y.E.; Aksoy, H.M. Sambucus ebulus L.: Past, present and future. AIP Conf. Proc. 2019, 2155, 020030-2. [Google Scholar] [CrossRef]
- Tasinov, O.; Kiselova-Kaneva, Y.; Ivanova, D. Sambucus ebulus—From traditional medicine to recent studies. Script. Sci. Med. 2013, 45, 36–42. [Google Scholar] [CrossRef][Green Version]
- Shokrzadeh, M.; Saravi, S.S. The chemistry, pharmacology and clinical properties of Sambucus ebulus: A review. J. Med. Plants Res. 2010, 4, 95–103. [Google Scholar]
- Dayioglu, H.; Kut, D.; Merdan, N.; Canbolat, S. The effect of dyeing properties of fixing agent and plasma treatment on silk fabric dyed with natural dye extract obtained from Sambucus ebulus L. plant. Procedia Soc. Behav. Sci. 2015, 195, 1609–1617. [Google Scholar] [CrossRef]
- Sadori, L.; Allevato, E.; Bosi, G.; Caneva, G.; Castiglioni, E.; Celant, A.; Di Pasquale, G.; Giardini, M.; Mazzanti, M.; Rinaldi, R.; et al. The introduction and diffusion of peach in ancient Italy. In Plants and Culture: Seeds of the Cultural Heritage of Europe; Centro Europeo per i Beni Culturali Ravello: Ravello, Italy, 2009; pp. 45–61. [Google Scholar]
- Follieri, M. Macroscopic plant remains in the west sewer of the Colosseum [Italy]. Ann. Bot. 1975, 34, 123–141. [Google Scholar]
- Mutke, S.; Vendramin, G.G.; Fady, B.; Bagnoli, F.; González-Martínez, S.C. Molecular and quantitative genetics of stone pine (Pinus pinea). In Genetic Diversity in Horticultural Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 61–84. [Google Scholar] [CrossRef]
- Sabato, D.; Peña-Chocarro, L.; Ucchesu, M.; Sarigu, M.; Del Vais, C.; Sanna, I.; Bacchetta, G. New insights about economic plants during the 6th–2nd centuries BC in Sardinia, Italy. Veg. Hist. Archaeobotany 2019, 28, 9–16. [Google Scholar] [CrossRef]
- Moricca, C.; Nigro, L.; Masci, L.; Pasta, S.; Cappella, F.; Spagnoli, F.; Sadori, L. Cultural landscape and plant use at the Phoenician site of Motya (Western Sicily, Italy) inferred from a disposal pit. Veg. Hist. Archaeobotany 2021, 30, 815–829. [Google Scholar] [CrossRef]
- Mercuri, A.M.; Mazzanti, M.B.; Florenzano, A.; Montecchi, M.C.; Rattighieri, E. Olea, Juglans and Castanea: The OJC group as pollen evidence of the development of human-induced environments in the Italian peninsula. Quatern. Int. 2013, 303, 24–42. [Google Scholar] [CrossRef]
- Willcox, G.H. Exotic plants from Roman waterlogged sites in London. J. Archaeol. Sci. 1977, 4, 269–282. [Google Scholar] [CrossRef]
- Kokoszko, M.; Jagusiak, K.; Rzeźnicka, Z. Cereals of Antiquity and Early Byzantine Times. Wheat and Barley in Medical Sources (Second to Seventh Centuries AD); Łódz University Press & Jagiellonian University Press: Łódz-Cracow, Poland, 2014. [Google Scholar] [CrossRef]
- Letta, C. I Marsi e il Fucino nell’Antichità; Cisalpino-Goliardica: Milano, Italy, 1972; p. 14. [Google Scholar]
- Burri, E. Il Fucino e il suo collettore sotterraneo. Opera Ipogea 2005, 1, 56–74. [Google Scholar]
- Toulotte, J.M.; Pantazopoulou, C.K.; Sanclemente, M.A.; Voesenek, L.A.; Sasidharan, R. Water stress resilient cereal crops: Lessons from wild relatives. J. Integr. Plant Biol. 2022, 64, 412–430. [Google Scholar] [CrossRef]
- Lodwick, L. Identifying ritual deposition of plant remains: A case study of stone pine cones in Roman Britain. In TRAC 2014: Proceedings of the Twenty-Fourth Annual Theoretical Roman Archaeology Conference; Brindle, T., Allen, M., Durham, E., Smith, A., Eds.; Oxford: Oxbow, OR, USA, 2015; pp. 54–69. [Google Scholar]
- Wagner Weick, C.; Aamir, N.; Reichart, J. The ethnobotanical evolution of the Mediterranean Cypress (Cupressus sempervirens). Econ. Bot. 2013, 77, 203–221. [Google Scholar] [CrossRef]
- Moricca, C.; Nigro, L.; Spagnoli, F.; Sabatini, S.; Sadori, L. Plant assemblage of the Phoenician sacrificial pit by the Temple of Melqart/Herakles (Motya, Sicily, Italy). Environ. Archaeol. 2023, 28, 383–395. [Google Scholar] [CrossRef]
- D’Auria, A.; Teobaldelli, M.; Di Pasquale, G. The late Holocene history of cypress (Cupressus sempervirens L.) in the Italian peninsula: New perspectives from archaeobotanical data. Holocene 2020, 30, 210–217. [Google Scholar] [CrossRef]
- Lodwick, L.A. Evergreen plants in Roman Britain and beyond: Movement, meaning and materiality. Britannia 2017, 48, 135–173. [Google Scholar] [CrossRef]
- Rottoli, M.; Castiglioni, E. Plant offerings from Roman cremations in northern Italy: A review. Veg. Hist. Archaeobotany 2011, 20, 495–506. [Google Scholar] [CrossRef]
- Matterne, V.; Derreumaux, M. A Franco-Italian investigation of funerary rituals in the Roman world: “les rites et la mort à Pompéi”, the plant part: A preliminary report. Veg. Hist. Archaeobotany 2008, 17, 105–112. [Google Scholar] [CrossRef]
- Marchesini, M.; Marvelli, S. L’alimentazione nell’oltretomba: Le offerte votive vegetali nelle necropoli romane dell’Emilia Romagna. Atti Soc. Nat. Mat. Modena 2007, 137, 331–342. [Google Scholar]
- Figueiral, I.; Pomarèdes, H.; Court-Picon, M.; Bouby, L.; Tardy, C.; Terral, J.F. New insights into Mediterranean Gallo-Roman farming: A closer look at archaeological wells in Southern France. Archaeol. Anthropol. Sci. 2015, 7, 201–233. [Google Scholar] [CrossRef]
- Caneva, G.; De Marco, G.; Dinelli, A.; Vinci, M. The Wall Vegetation of the Roman Archaeological Areas. Sci. Tech. Cult. Herit. 1992, 1, 217–226. [Google Scholar]
- Hruška, K. Syntaxonomical study of Italian wall vegetation. Vegetatio 1987, 73, 13–20. [Google Scholar] [CrossRef]
- Assini, S.; Brugellis, I.; Nascimbene, J.; Barcella, M.; Gressani, A.; Gheza, G. Dry grasslands of central-western Po Plain (Italy): Implications under Council Directive 92/43/EEC. Plant Sociol. 2024, 61, 1–20. [Google Scholar] [CrossRef]
- Midolo, G.; Axmanová, I.; Divíšek, J.; Dřevojan, P.; Lososová, Z.; Večeřa, M.; Karger, D.N.; Thuiller, W.; Bruelheide, H.; Aćić, S.; et al. Diversity and distribution of Raunkiær’s life forms in European vegetation. J. Veg. Sci. 2024, 35, e13229. [Google Scholar] [CrossRef]
- Dufour, S.; Rodríguez-González, P.M. Riparian Zone/Riparian Vegetation Definition: Principles and Recommendations. 2019. Available online: https://converges.eu/wp-content/uploads/2019/04/Report_definitions_Riparian_V1-2.pdf (accessed on 9 October 2024). Report, Cost Action CA16208 Converges.
- Iamonico, D.; Iberite, M.; Capotorti, G. Alien flora in freshwater ecosystem: Basic knowledge for mitigating threats to native biodiversity in Lazio region (Central Italy). Ann. Bot. 2024, in press. [Google Scholar]
- Galadini, F.; Ceccaroni, E.; Falcucci, E.; Gori, S.; Pagliaroli, A. Ambiente Naturale, Interventi Antropici e Modifiche del Paesaggio ad Alba Fucens (IV sec. a.C.-XXI secolo d.C.). Il Fucino e le Aree Limitrofe Nell’antichità: Archeologia e Rinascita Culturale dopo il Sisma del 1915. 2015, pp. 399–411. Available online: https://www.researchgate.net/publication/316788914_Ambiente_naturale_interventi_antropici_e_modifiche_del_paesaggio_ad_Alba_Fucens_IV_sec_aC-XXI_sec_dC (accessed on 12 September 2024).
- Acta Plantarum. Lista Delle Schede Botaniche. Available online: https://www.actaplantarum.org/schede/schede.php (accessed on 11 August 2023).
- Raunkier, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: London, UK, 1934. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moricca, C.; Russo, G.; Iamonico, D.; Ceccaroni, E.; Favero, G.; Sadori, L. Food and Environment During the Late Roman Age at the Site of Alba Fucens (Abruzzi, Italy). Plants 2024, 13, 2930. https://doi.org/10.3390/plants13202930
Moricca C, Russo G, Iamonico D, Ceccaroni E, Favero G, Sadori L. Food and Environment During the Late Roman Age at the Site of Alba Fucens (Abruzzi, Italy). Plants. 2024; 13(20):2930. https://doi.org/10.3390/plants13202930
Chicago/Turabian StyleMoricca, Claudia, Gilda Russo, Duilio Iamonico, Emanuela Ceccaroni, Gabriele Favero, and Laura Sadori. 2024. "Food and Environment During the Late Roman Age at the Site of Alba Fucens (Abruzzi, Italy)" Plants 13, no. 20: 2930. https://doi.org/10.3390/plants13202930
APA StyleMoricca, C., Russo, G., Iamonico, D., Ceccaroni, E., Favero, G., & Sadori, L. (2024). Food and Environment During the Late Roman Age at the Site of Alba Fucens (Abruzzi, Italy). Plants, 13(20), 2930. https://doi.org/10.3390/plants13202930









