Drought Stress, Elevated CO2 and Their Combination Differentially Affect Carbon and Nitrogen in Different Organs of Six Spring Wheat Genotypes
Abstract
:1. Introduction
2. Results
2.1. Threshold Value of Fraction of Transpirable Soil Water (FTSW)
2.2. Carbon, Nitrogen, and CN Ratios in Stem, Leaves, and Grains
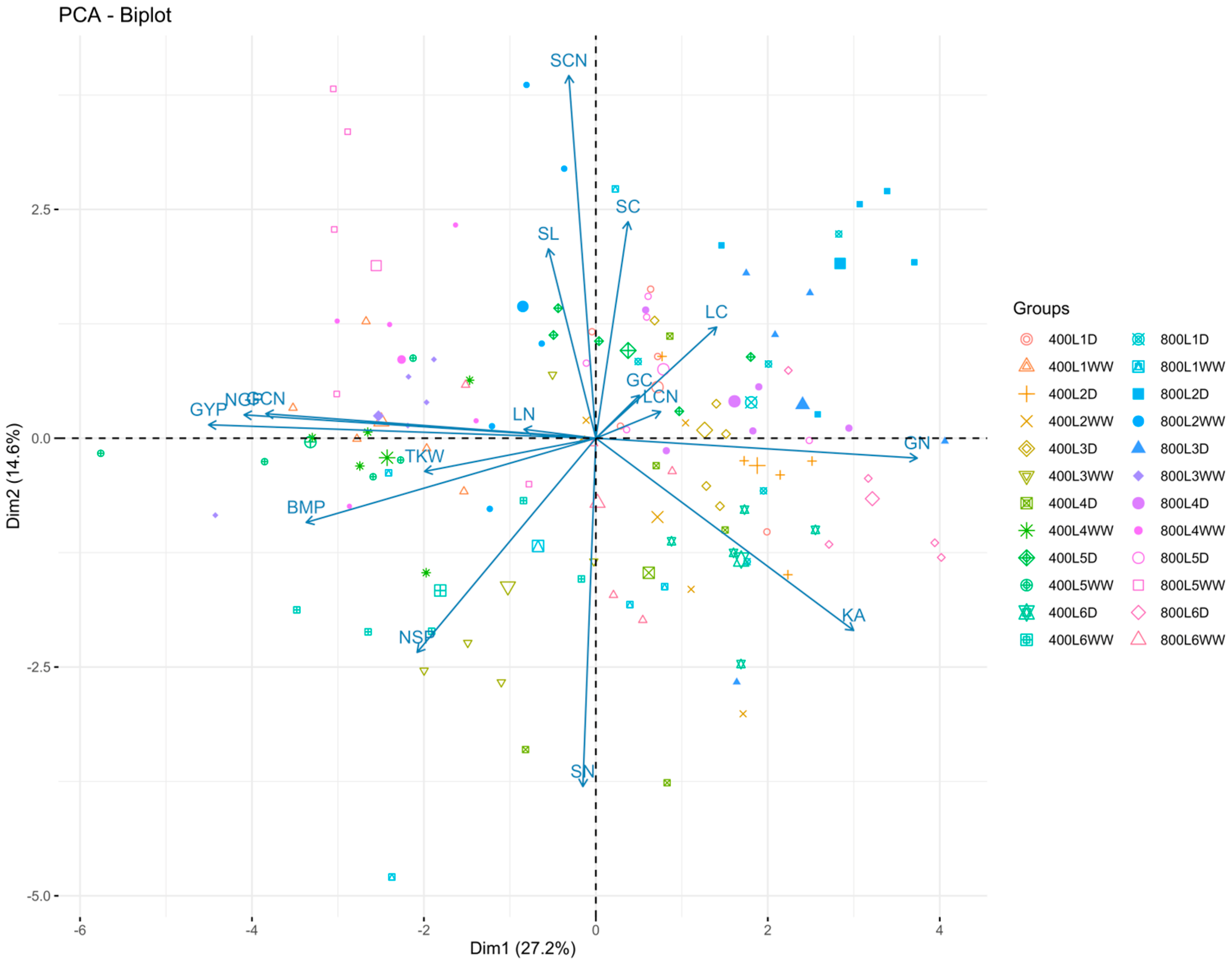
2.3. Combined Correlation and Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Growth Conditions
4.3. Sampling and Measurement of Carbon and Nitrogen of Leaves, Stems, and Grains
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; Li, X.; Wei, Z.; Liu, F. ABA-mediated modulation of elevated CO2 on stomatal response to drought. Curr. Opin. Plant Biol. 2020, 56, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Fang, L.; Li, X.; Liu, J.; Liu, F. Effects of elevated atmospheric CO2 on leaf gas exchange response to progressive drought in barley and tomato plants with different endogenous ABA levels. Plant Soil 2020, 447, 431–446. [Google Scholar] [CrossRef]
- Li, X.; Kristiansen, K.; Rosenqvist, E.; Liu, F. Elevated CO2 modulates the effects of drought and heat stress on plant water relations and grain yield in wheat. J. Agron. Crop Sci. 2019, 205, 362–371. [Google Scholar] [CrossRef]
- Ulfat, A.; Shokat, S.; Li, X.; Fang, L.; Großkinsky, D.K.; Majid, S.A.; Roitsch, T.; Liu, F. Elevated carbon dioxide alleviates the negative impact of drought on wheat by modulating plant metabolism and physiology. Agric. Water Manag. 2021, 250, 106804. [Google Scholar] [CrossRef]
- Shokat, S.; Großkinsky, D.K.; Liu, F. Impact of elevated CO2 on two contrasting wheat genotypes exposed to intermediate drought stress at anthesis. J. Agron. Crop Sci. 2021, 207, 20–33. [Google Scholar] [CrossRef]
- King, C.A.; Purcell, L.C. Evaluation of methods for estimating transpiration response to soil drying for container-grown plants. Crop Sci. 2017, 2148, 2143–2148. [Google Scholar] [CrossRef]
- Xu, Z.; Shimizu, H.; Yagasaki, Y.; Ito, S.; Zheng, Y.; Zhou, G. Interactive Effects of Elevated CO2, Drought, and Warming on Plants. J. Plant Growth Regul. 2013, 32, 692–707. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Li, S.; Li, X.; Liang, K.; Liu, F. Dilemma between yield and quality: Multigenerational effect of elevated CO2 and nitrogen supply on wheat cultivars. J. Agron. Crop Sci. 2023, 209, 887–903. [Google Scholar] [CrossRef]
- Shokat, S.; Novák, O.; Široká, J.; Singh, S.; Gill, K.S.; Roitsch, T.; Großkinsky, D.K.; Liu, F. Elevated CO2 modulates the effect of heat stress responses in Triticum aestivum by differential expression of an isoflavone reductase-like gene. J. Exp. Bot. 2021, 72, 7594–7609. [Google Scholar]
- Kang, H.; Zhu, T.; Zhang, Y.; Ke, X.; Sun, W.; Hu, Z.; Zhu, X.; Shen, H.; Huang, Y.; Tang, Y. Elevated CO2 Enhances Dynamic Photosynthesis in Rice and Wheat. Front. Plant Sci. 2021, 12, 727374. [Google Scholar] [CrossRef]
- Shokat, S.; Großkinsky, D.K.; Singh, S.; Liu, F. The role of genetic diversity and pre-breeding traits to improve drought and heat tolerance of bread wheat at the reproductive stage. Food Energy Secur. 2023, 12, e478. [Google Scholar] [CrossRef]
- Shokat, S.; Arif, M.A.R.; Waheed, M.Q.; Liu, F.; Guzmán, C.; Singh, S. Skipping irrigation at pre- and post-anthesis stages influences grain yield and starch contents of bread wheat derived from synthetic or landraces. Cereal Res. Commun. 2023, 52, 1145–1152. [Google Scholar] [CrossRef]
- Singh, S.; Vikram, P.; Sehgal, D.; Burgueño, J.; Sharma, A.; Singh, S.K.; Sansaloni, C.P.; Joynson, R.; Brabbs, T.; Ortiz, C.; et al. Harnessing genetic potential of wheat germplasm banks through impact-oriented-prebreeding for future food and nutritional security. Sci. Rep. 2018, 8, 12527. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Jighly, A.; Sehgal, D.; Burgueño, J.; Joukhadar, R.; Singh, S.K.; Sharma, A.; Vikram, P.; Sansaloni, C.P.; Govindan, V.; et al. Direct introgression of untapped diversity into elite wheat lines. Nat. Food 2021, 2, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, T.; Fang, L.; Peng, X.; Liu, F. CO2 elevation modulates the response of leaf gas exchange to progressive soil drying in tomato plants. Agric. For. Meteorol. 2019, 268, 181–188. [Google Scholar] [CrossRef]
- Xu, X.; Yang, G.; Yang, X.; Li, Z.; Feng, H.; Xu, B.; Zhao, X. Monitoring ratio of carbon to nitrogen (C/N) in wheat and barley leaves by using spectral slope features with branch-and-bound algorithm. Sci. Rep. 2018, 8, 10034. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Kim, D.G.; Li, J.; Liu, Y.; Hai, X.; Liu, Q.; Huang, C.; Shangguan, Z.; Kuzyakov, Y. Drought effects on soil carbon and nitrogen dynamics in global natural ecosystems. Earth-Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- Schaeffer, S.M.; Homyak, P.M.; Boot, C.M.; Roux-Michollet, D.; Schimel, J.P. Soil carbon and nitrogen dynamics throughout the summer drought in a California annual grassland. Soil Biol. Biochem. 2017, 115, 54–62. [Google Scholar] [CrossRef]
- Sanaullah, M.; Chabbi, A.; Rumpel, C.; Kuzyakov, Y. Carbon allocation in grassland communities under drought stress followed by 14C pulse labeling. Soil Biol. Biochem. 2012, 55, 132–139. [Google Scholar] [CrossRef]
- Cui, J.; Zheng, M.; Bian, Z.; Pan, N.; Tian, H.; Zhang, X.; Qiu, Z.; Xu, J.; Gu, B. Elevated CO2 levels promote both carbon and nitrogen cycling in global forests. Nat. Clim. Chang. 2024, 14, 511–517. [Google Scholar] [CrossRef]
- Fangmeier, L.; De Temmerman, L.; Mortensen, L.; Kemp, K.; Burke, J.; Mitchell, R.; van Oijen, M.; Weigel, H.-J. Effects on nutrients and on grain quality in spring wheat crops grown under elevated CO2 concentrations and stress conditions in the European, multiple-site experiment ‘ESPACE-wheat’. Eur. J. Agron. 1999, 10, 215–229. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, D.; Wang, J.; Ding, Y.; Song, X. Effects of elevated CO2 and drought on plant physiology, soil carbon. Pedosphere 2017, 27, 846–855. [Google Scholar] [CrossRef]
- Feng, Y.; Alam, M.S.; Yan, F.; Frei, M. Alteration of carbon and nitrogen allocation in winter wheat under elevated ozone. Plant Sci. 2024, 338, 111924. [Google Scholar] [CrossRef] [PubMed]

| Genotypes | CO2 Level | Conditions | SC (mg g−1) | SN (mg g−1) | LC (mg g−1) | LN (mg g−1) | GC (mg g−1) | GN (mg g−1) | SCN (%) | LCN (%) | GCN (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | 400 | WW | 43.55 ± 0.22 | 1.16 ± 0.07 | 39.63 ± 0.82 | 2.32 ± 0.09 | 44.87 ± 0.11 | 2.78 ± 0.08 | 38.26 ± 2.51 | 17.12 ± 0.45 | 16.17 ± 0.44 |
| D | 43.39 ± 0.3 | 0.98 ± 0.06 | 41.12 ± 0.36 | 2.38 ± 0.06 | 43.96 ± 0.85 | 3.23 ± 0.09 | 45.1 ± 2.75 | 17.3 ± 0.52 | 13.63 ± 0.36 | ||
| 800 | WW | 43.62 ± 0.19 | 1.89 ± 0.61 | 40.82 ± 0.55 | 3.1 ± 0.27 | 45.15 ± 0.06 | 3.28 ± 0.03 | 35.18 ± 12.36 | 13.49 ± 0.92 | 13.76 ± 0.12 | |
| D | 42.63 ± 0.58 | 1.06 ± 0.2 | 41.87 ± 0.25 | 2.78 ± 0.26 | 45.02 ± 0.14 | 3.47 ± 0.1 | 46.67 ± 9.39 | 15.69 ± 1.63 | 13 ± 0.35 | ||
| L2 | 400 | WW | 42.42 ± 0.86 | 1.41 ± 0.27 | 41.09 ± 0.67 | 2.52 ± 0.21 | 44.80 ± 0.15 | 3.63 ± 0.12 | 34.9 ± 6.35 | 16.71 ± 1.24 | 12.39 ± 0.37 |
| D | 42.97 ± 0.25 | 1.09 ± 0.13 | 42.15 ± 0.33 | 2.33 ± 0.1 | 45.1 ± 0.11 | 3.83 ± 0.12 | 41.34 ± 4.28 | 18.19 ± 0.78 | 11.83 ± 0.39 | ||
| 800 | WW | 43.26 ± 0.59 | 0.85 ± 0.22 | 42.64 ± 0.27 | 2.38 ± 0.22 | 44.91 ± 0.04 | 3.36 ± 0.07 | 64.03 ± 13.65 | 18.55 ± 1.68 | 13.4 ± 0.26 | |
| D | 45.34 ± 0.37 | 0.78 ± 0.15 | 42.5 ± 0.45 | 2.79 ± 0.15 | 45.29 ± 0.08 | 3.91 ± 0.15 | 64.99 ± 9.23 | 15.42 ± 0.9 | 11.65 ± 0.46 | ||
| L3 | 400 | WW | 43.22 ± 0.45 | 1.43 ± 0.19 | 40.84 ± 0.31 | 2.14 ± 0.07 | 45.03 ± 0.08 | 3.32 ± 0.11 | 33.25 ± 5.9 | 19.2 ± 0.54 | 13.61 ± 0.41 |
| D | 43.61 ± 0.18 | 1.02 ± 0.08 | 41.31 ± 0.56 | 2.25 ± 0.07 | 44.9 ± 0.09 | 3.24 ± 0.14 | 43.84 ± 3.77 | 18.44 ± 0.61 | 13.95 ± 0.57 | ||
| 800 | WW | 45.22 ± 0.33 | 0.9 ± 0.12 | 40.27 ± 0.67 | 4.37 ± 0.51 | 44.93 ± 0.08 | 3.13 ± 0.05 | 53.06 ± 5.8 | 9.82 ± 1.35 | 14.38 ± 0.21 | |
| D | 43.6 ± 0.63 | 0.99 ± 0.24 | 40.88 ± 0.71 | 2.29 ± 0.15 | 45.21 ± 0.18 | 3.58 ± 0.11 | 54.38 ± 11.87 | 18.27 ± 1.53 | 12.66 ± 0.33 | ||
| L4 | 400 | WW | 42.11 ± 0.4 | 0.86 ± 0.07 | 40.3 ± 0.45 | 2.98 ± 0.29 | 44.53 ± 0.19 | 2.92 ± 0.09 | 50.32 ± 4.57 | 14.02 ± 1.28 | 15.33 ± 0.49 |
| D | 42.61 ± 1.88 | 1.03 ± 0.2 | 40.31 ± 0.38 | 2.83 ± 0.21 | 44.69 ± 0.86 | 3.50 ± 0.22 | 47.37 ± 8.01 | 14.68 ± 1.39 | 12.91 ± 0.56 | ||
| 800 | WW | 44.44 ± 0.5 | 1.06 ± 0.26 | 40.86 ± 0.2 | 3.49 ± 0.77 | 45.06 ± 0.04 | 2.86 ± 0.05 | 52.24 ± 11.05 | 14.08 ± 2.78 | 15.75 ± 0.25 | |
| D | 43.99 ± 0.4 | 1.06 ± 0.14 | 43.05 ± 1.26 | 2.23 ± 0.19 | 45.09 ± 0.07 | 3.37 ± 0.08 | 44.96 ± 6.85 | 19.91 ± 2 | 13.42 ± 0.29 | ||
| L5 | 400 | WW | 43.09 ± 0.38 | 1.07 ± 0.11 | 40.64 ± 0.12 | 2.16 ± 0.07 | 44.85 ± 0.05 | 2.75 ± 0.12 | 42.98 ± 6.14 | 18.91 ± 0.58 | 16.46 ± 0.72 |
| D | 43.64 ± 0.23 | 0.85 ± 0.02 | 41.68 ± 0.2 | 2.44 ± 0.09 | 44.83 ± 0.19 | 3.11 ± 0.15 | 51.33 ± 1.77 | 17.21 ± 0.7 | 14.52 ± 0.58 | ||
| 800 | WW | 42.56 ± 0.76 | 0.61 ± 0.14 | 40.32 ± 0.46 | 2.57 ± 0.33 | 45.22 ± 0.08 | 2.89 ± 0.07 | 87.05 ± 18.66 | 17.08 ± 2.82 | 15.68 ± 0.4 | |
| D | 44.68 ± 0.12 | 1.19 ± 0.13 | 42.22 ± 0.5 | 1.96 ± 0.21 | 45.42 ± 0.14 | 3.37 ± 0.16 | 39.54 ± 4.97 | 22.67 ± 2.62 | 13.58 ± 0.6 | ||
| L6 | 400 | WW | 42.81 ± 0.82 | 1.44 ± 0.1 | 40.16 ± 0.58 | 2.51 ± 0.16 | 44.3 ± 0.09 | 2.68 ± 0.11 | 30.46 ± 2.73 | 16.18 ± 0.87 | 16.63 ± 0.74 |
| D | 42.23 ± 0.15 | 1.25 ± 0.08 | 40.95 ± 0.32 | 2.23 ± 0.03 | 44.16 ± 0.17 | 3.17 ± 0.09 | 34.5 ± 2.28 | 18.38 ± 0.19 | 13.98 ± 0.41 | ||
| 800 | WW | 43.93 ± 0.52 | 1.09 ± 0.2 | 41.36 ± 0.66 | 3 ± 0.21 | 45.23 ± 0.11 | 3.4 ± 0.11 | 45.8 ± 8.09 | 14.13 ± 1.31 | 13.37 ± 0.4 | |
| D | 43.79 ± 0.62 | 1.14 ± 0.14 | 39.67 ± 0.84 | 2.28 ± 0.21 | 44.29 ± 1.13 | 3.8 ± 0.18 | 40.73 ± 4.67 | 17.95 ± 1.53 | 11.72 ± 0.35 | ||
| p-Value of F-test | Pw = NS | Pw = NS | Pw < 0.001 | Pw < 0.01 | Pw = NS | Pw < 0.001 | Pw < 0.01 | Pw < 0.01 | Pw < 0.001 | ||
| PCO2 < 0.001 | PCO2 = NS | PCO2 = NS | PCO2 < 0.01 | PCO2 < 0.001 | PCO2 < 0.01 | PCO2 = NS | PCO2 = NS | PCO2 < 0.01 | |||
| PG = NS | PG = NS | PG < 0.05 | PG = NS | PG = NS | PG < 0.01 | PG = NS | PG = NS | PG < 0.01 | |||
| Pw × G < 0.05 | Pw × CO2 < 0.01 | Pw × CO2 < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokat, S.; Liu, F.; Großkinsky, D.K. Drought Stress, Elevated CO2 and Their Combination Differentially Affect Carbon and Nitrogen in Different Organs of Six Spring Wheat Genotypes. Plants 2024, 13, 2942. https://doi.org/10.3390/plants13202942
Shokat S, Liu F, Großkinsky DK. Drought Stress, Elevated CO2 and Their Combination Differentially Affect Carbon and Nitrogen in Different Organs of Six Spring Wheat Genotypes. Plants. 2024; 13(20):2942. https://doi.org/10.3390/plants13202942
Chicago/Turabian StyleShokat, Sajid, Fulai Liu, and Dominik K. Großkinsky. 2024. "Drought Stress, Elevated CO2 and Their Combination Differentially Affect Carbon and Nitrogen in Different Organs of Six Spring Wheat Genotypes" Plants 13, no. 20: 2942. https://doi.org/10.3390/plants13202942
APA StyleShokat, S., Liu, F., & Großkinsky, D. K. (2024). Drought Stress, Elevated CO2 and Their Combination Differentially Affect Carbon and Nitrogen in Different Organs of Six Spring Wheat Genotypes. Plants, 13(20), 2942. https://doi.org/10.3390/plants13202942








