Stress-Inducible Expression of HvABF2 Transcription Factor Improves Water Deficit Tolerance in Transgenic Barley Plants
Abstract
:1. Introduction
2. Results
2.1. Isolation of ABF2 Orthologous in Barley
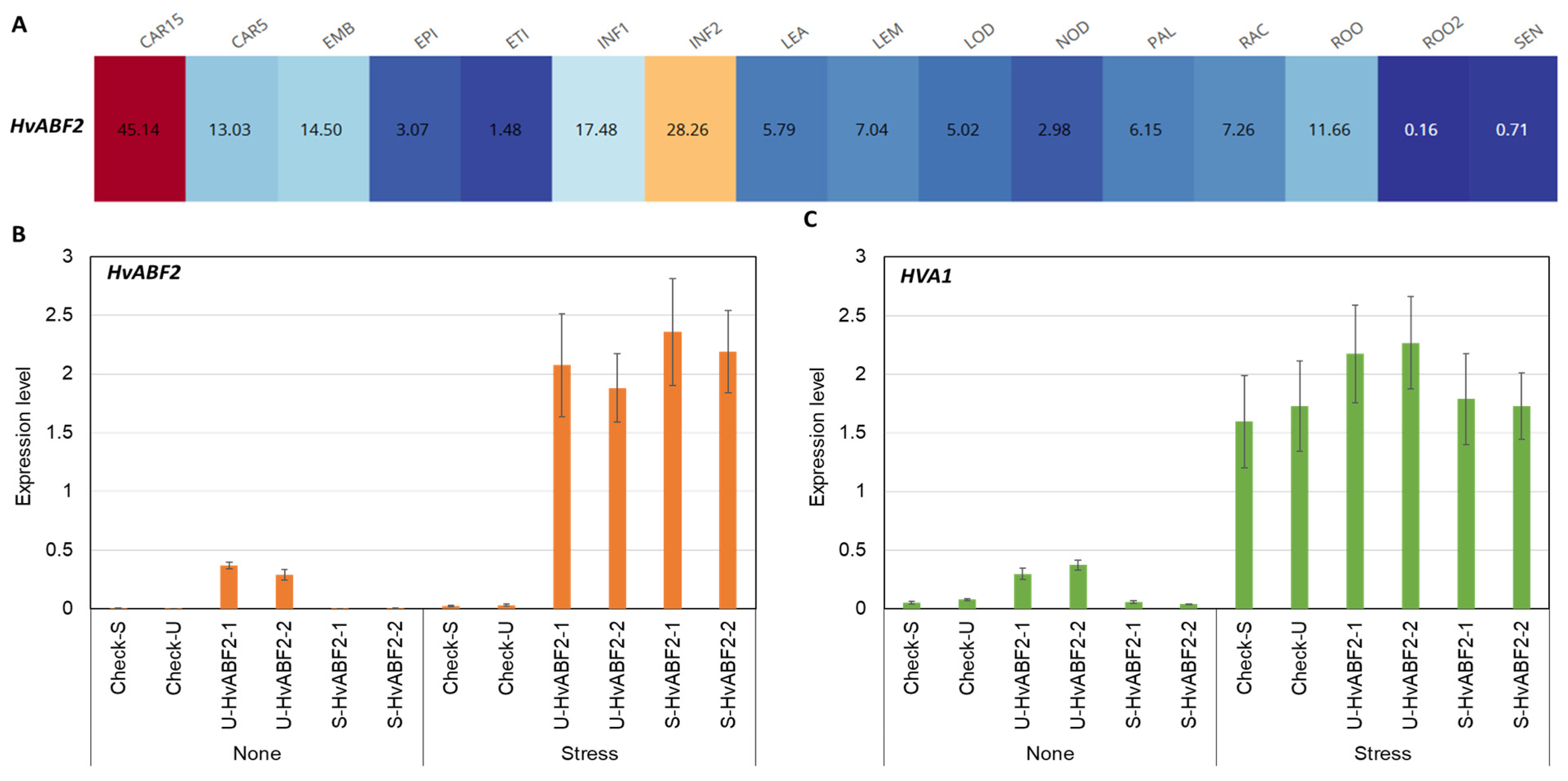
2.2. HvABF2 In Silico Gene Expression Analysis
2.3. Stress-Inducible and Constitutive Expression of HvABF2 in Transgenic Barley
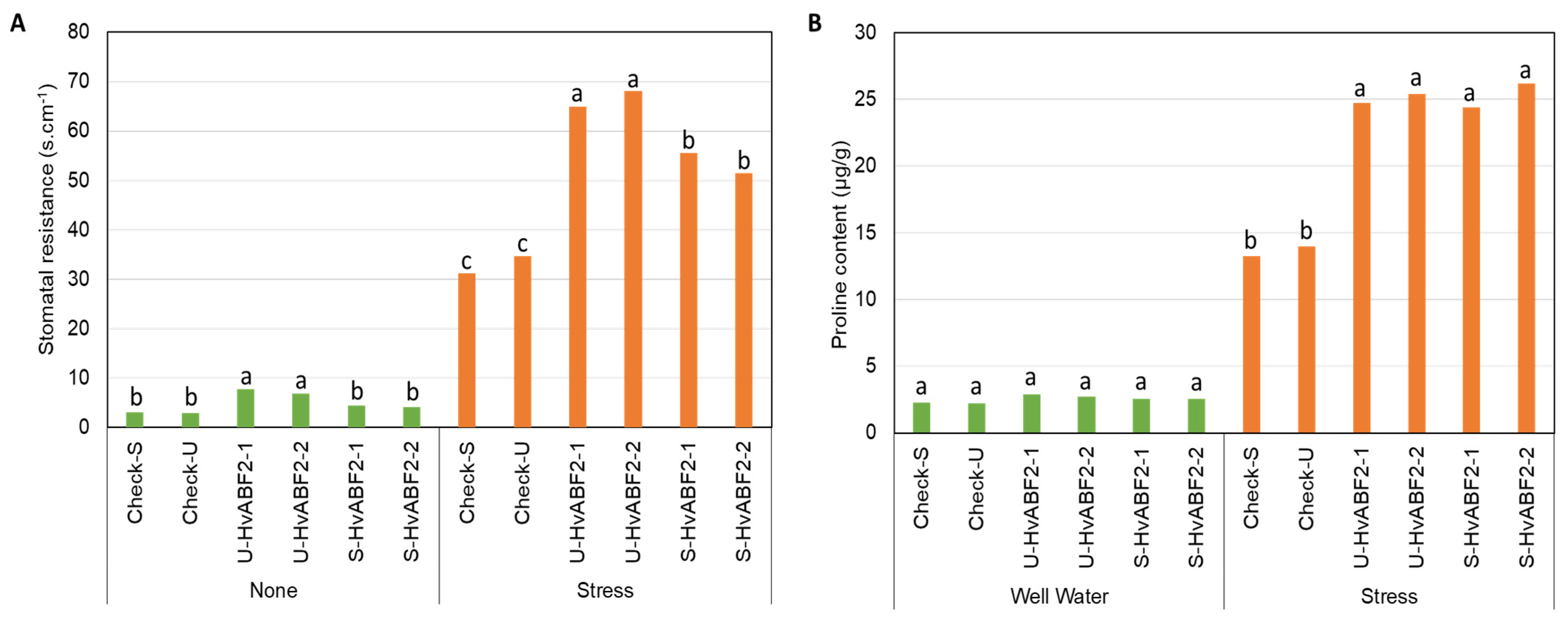
2.4. Stress-Inducible Expression of HvABF2 Improves Drought Tolerance in Barley
3. Discussion
4. Materials and Methods
4.1. Cloning of HvABF2 in Barley Plant and SNAC1 Promoter in Rice
4.2. Bioinformatics Analysis and Gene Expression Analysis
4.3. Plant Material
4.4. Stress Experiments
4.5. Data Collection and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- Achli, S.; Epule, T.E.; Dhiba, D.; Chehbouni, A.; Er-Raki, S. Vulnerability of barley, maize, and wheat yields to variations in growing season precipitation in Morocco. Appl. Sci. 2022, 12, 3407. [Google Scholar] [CrossRef]
- Al-Sayaydeh, R.; Al-Bawalize, A.; Al-Ajlouni, Z.; Akash, M.W.; Abu-Elenein, J.; Al-Abdallat, A.M. Agronomic evaluation and yield performance of selected barley (Hordeum vulgare L.) landraces from Jordan. Int. J. Agron. 2019, 2019, 9575081. [Google Scholar] [CrossRef]
- Rajsekhar, D.; Gorelick, S.M. Increasing drought in Jordan: Climate change and cascading Syrian land-use impacts on reducing transboundary flow. Sci. Adv. 2017, 3, e1700581. [Google Scholar] [CrossRef]
- Havrlentová, M.; Kraic, J.; Gregusová, V.; Kovácsová, B. Drought stress in cereals—A review. Agriculture 2021, 67, 47–60. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Siddique, K.H.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.; Nayyar, H. Drought or/and heat-stress effects on seed filling in food crops: Impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef]
- Abu-Elenein, J.; Al-Sayaydeh, R.; Akkeh, Z.; Al-Ajlouni, Z.; Al-Bawalize, A.A.; Hasan, S.; Alhindi, T.; Albdaiwi, R.N.; Ayad, J.Y.; Al-Abdallat, A.M. Agronomic performance and flowering behavior in response to photoperiod and vernalization in barley (Hordeum vulgare L.) genotypes with contrasting drought tolerance behavior. Environ. Exp. Bot. 2021, 192, 104661. [Google Scholar] [CrossRef]
- Lawas, L.M.F.; Zuther, E.; Jagadish, S.K.; Hincha, D.K. Molecular mechanisms of combined heat and drought stress resilience in cereals. Curr. Opin. Plant Biol. 2018, 45, 212–217. [Google Scholar] [CrossRef]
- Zahra, N.; Wahid, A.; Hafeez, M.B.; Ullah, A.; Siddique, K.H.; Farooq, M. Grain development in wheat under combined heat and drought stress: Plant responses and management. Environ. Exp. Bot. 2021, 188, 104517. [Google Scholar] [CrossRef]
- Mellacheruvu, S.; Talakayala, A.; Garladinne, M. Crop improvement of cereals through manipulation of signaling pathways in response to drought stress. In Plant Signaling Molecules, 1st ed.; Khan, M.R.I., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 125–139. [Google Scholar] [CrossRef]
- Batra, N.G.; Sharma, V.; Kumari, N. Drought-induced changes in chlorophyll fluorescence, photosynthetic pigments, and thylakoid membrane proteins of Vigna radiata. J. Plant Interact. 2014, 9, 712–721. [Google Scholar] [CrossRef]
- Mejri, M.; Siddique, K.H.; Saif, T.; Abdelly, C.; Hessini, K. Comparative effect of drought duration on growth, photosynthesis, water relations, and solute accumulation in wild and cultivated barley species. J. Plant Nutr. Soil Sci. 2016, 179, 327–335. [Google Scholar] [CrossRef]
- Collin, A.; Daszkowska-Golec, A.; Kurowska, M.; Szarejko, I. Barley ABI5 (Abscisic Acid INSENSITIVE 5) is involved in abscisic acid-dependent drought response. Front. Plant Sci. 2020, 11, 1138. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.; Sun, M.; Chen, S.; Ma, H.; Lin, J.; Sun, Y.; Zhong, M. Molecular characterization and gene expression analysis of tomato WOX transcription factor family under abiotic stress and phytohormone treatment. J. Plant Biochem. Biotechnol. 2021, 30, 973–986. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Dar, N.A.; Amin, I.; Wani, W.; Wani, S.A.; Shikari, A.B.; Wani, S.H.; Masoodi, K.Z. Abscisic acid: A key regulator of abiotic stress tolerance in plants. Plant Gen. 2017, 11, 106–111. [Google Scholar] [CrossRef]
- Soma, F.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Cellular phosphorylation signaling and gene expression in drought stress responses: ABA-dependent and ABA-independent regulatory systems. Plants 2021, 10, 756. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Sticklen, M. Barley HVA1 gene confers drought and salt tolerance in transgenic maize (Zea mays L.). Adv. Crop Sci. Tech. 2013, 1, 2. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujita, M.; Satoh, R.; Maruyama, K.; Parvez, M.M.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell. 2005, 17, 3470–3488. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, H.; Li, X.; Xiao, J.; Xiong, L. Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiol. 2012, 158, 1755–1768. [Google Scholar] [CrossRef]
- Jin, X.F.; Xiong, A.S.; Peng, R.H.; Liu, J.G.; Gao, F.; Chen, J.M.; Yao, Q.H. OsAREB1, an ABRE-binding protein responding to ABA and glucose, has multiple functions in Arabidopsis. BMB Rep. 2010, 43, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.; Mao, X.; Li, A.; Jing, R. Wheat transcription factor TaAREB3 participates in drought and freezing tolerances in Arabidopsis. Int. J. Biol. Sci. 2016, 12, 257. [Google Scholar] [CrossRef] [PubMed]
- Schoonheim, P.J.; Sinnige, M.P.; Casaretto, J.A.; Veiga, H.; Bunney, T.D.; Quatrano, R.S.; de Boer, A.H. 14-3-3 adaptor proteins are intermediates in ABA signal transduction during barley seed germination. Plant J. 2007, 49, 289–301. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Meng, H.; Wen, H.; Fan, Y.; Zhao, J. Maize ABP9 enhances tolerance to multiple stresses in transgenic Arabidopsis by modulating ABA signaling and cellular levels of reactive oxygen species. Plant Mol. Biol. 2011, 75, 365–378. [Google Scholar] [CrossRef]
- Morran, S.; Eini, O.; Pyvovarenko, T.; Parent, B.; Singh, R.; Ismagul, A.; Eliby, S.; Shirley, N.; Langridge, P.; Lopato, S. Improvement of stress tolerance of wheat and barley by modulation of expression of DREB/CBF factors. Plant Biotechnol. J. 2011, 9, 230–249. [Google Scholar] [CrossRef]
- Manna, M.; Thakur, T.; Chirom, O.; Mandlik, R.; Deshmukh, R.; Salvi, P. Transcription factors as key molecular target to strengthen the drought stress tolerance in plants. Physiol. Plant. 2021, 172, 847–868. [Google Scholar] [CrossRef]
- Kasuga, M.; Liu, Q.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 1999, 17, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014, 5, 170. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, Y.S.; Redillas, M.C.; Jang, G.; Jung, H.; Bang, S.W.; Choi, Y.D.; Ha, S.H.; Reuzeau, C.; Kim, J.K. OsNAC5 overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field. Plant Biotechnol. J. 2013, 11, 101–114. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A. Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnol. Adv. 2009, 27, 744–752. [Google Scholar] [CrossRef]
- Kaur, B.; Sandhu, K.S.; Kamal, R.; Kaur, K.; Singh, J.; Röder, M.S.; Muqaddasi, Q.H. Omics for the improvement of abiotic, biotic, and agronomic traits in major cereal crops: Applications, challenges, and prospects. Plants 2021, 10, 1989. [Google Scholar] [CrossRef] [PubMed]
- Tambussi, E.A.; Nogues, S.; Ferrio, P.; Voltas, J.; Araus, J.L. Does higher yield potential improve barley performance in Mediterranean conditions: A case study. Field Crops Res. 2005, 91, 149–160. [Google Scholar] [CrossRef]
- Visioni, A.; Al-Abdallat, A.; Elenien, J.A.; Verma, R.P.S.; Gyawali, S.; Baum, M. Genomics and Molecular Breeding for Improving Tolerance to Abiotic Stress in Barley (Hordeum Vulgare L.). In Genomics Assisted Breeding of Crops for Abiotic Stress Tolerance, Volume II; Rajpal, V., Sehgal, D., Kumar, A., Raina, S., Eds.; Sust. Dev. Biodivers.; Springer: Cham, Switzerland, 2019; Volume 21. [Google Scholar] [CrossRef]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef]
- Kumari, A.; Kaur, R.; Kaur, R. An insight into drought stress and signal transduction of abscisic acid. Plant Sci. Today. 2018, 5, 72–80. [Google Scholar] [CrossRef]
- Joo, J.; Lee, Y.H.; Song, S.I. OsbZIP42 is a positive regulator of ABA signaling and confers drought tolerance to rice. Planta 2019, 249, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yoshida, T.; Yamaguchi-Shinozaki, K. Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol. Plant. 2013, 147, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Furihata, T.; Maruyama, K.; Fujita, Y.; Umezawa, T.; Yoshida, R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc. Natl. Acad. Acad. Sci. USA 2006, 103, 1988–1993. [Google Scholar] [CrossRef] [PubMed]
- García, M.N.M.; Stritzler, M.; Capiati, D.A. Heterologous expression of Arabidopsis ABF4 gene in potato enhances tuberization through ABA-GA crosstalk regulation. Planta 2014, 239, 615–631. [Google Scholar] [CrossRef]
- Casaretto, J.; Ho, T.H.D. The transcription factors HvABI5 and HvVP1 are required for the abscisic acid induction of gene expression in barley aleurone cells. Plant Cell. 2003, 15, 271–284. [Google Scholar] [CrossRef]
- Chen, Y.T.; Liu, H.; Stone, S.; Callis, J. ABA and the ubiquitin E3 ligase KEEP ON GOING affect proteolysis of the Arabidopsis thaliana transcription factors ABF1 and ABF3. Plant J. 2013, 75, 965–976. [Google Scholar] [CrossRef]
- Kim, S.Y. The role of ABF family bZIP class transcription factors in stress response. Physiol. Plant. 2006, 126, 519–527. [Google Scholar] [CrossRef]
- Marques, D.N.; dos Reis, S.P.; de Souza, C.R. Plant NAC transcription factors responsive to abiotic stresses. Plant Gene 2017, 11, 170–179. [Google Scholar] [CrossRef]
- Hu, H.; Dai, M.; Yao, J.; Xiao, B.; Li, X.; Zhang, Q.; Xiong, L. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances water deficit resistance and salt tolerance in rice. Proc. Natl. Acad. Acad. Sci. USA 2006, 103, 12987–12992. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, M.; Daszkowska-Golec, A. Molecular mechanisms of SNAC1 (Stress-responsive NAC1) in conferring the abiotic stress tolerance. Plant Sci. 2023, 337, 111894. [Google Scholar] [CrossRef]
- Fang, H.; Meng, Q.; Xu, J.; Tang, H.; Tang, S.; Zhang, H.; Huang, J. Knock-down of stress inducible OsSRFP1 encoding an E3 ubiquitin ligase with transcriptional activation activity confers abiotic stress tolerance through enhancing antioxidant protection in rice. Plant Mol. Biol. 2015, 87, 441–458. [Google Scholar] [CrossRef]
- Tran, L.S.P.; Nakashima, K.; Sakuma, Y.; Simpson, S.D.; Fujita, Y.; Maruyama, K.; Yamaguchi-Shinozaki, K. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 2004, 16, 2481–2498. [Google Scholar] [CrossRef]
- Nakashima, K.; Tran, L.S.; Van Nguyen, D.; Fujita, M.; Maruyama, K.; Todaka, D.; Ito, Y.; Hayashi, N.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 2007, 51, 617–630. [Google Scholar] [CrossRef]
- Kang, J.Y.; Choi, H.I.; Im, M.Y.; Kim, S.Y. Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. The Plant Cell 2002, 14, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Fujita, Y.; Maruyama, K.; Mogami, J.; Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K.A.Z.U.K.O. Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 2015, 38, 35–49. [Google Scholar] [CrossRef]
- Shah, S.H.; Jan, S.A.; Ahmad, N.; Khan, S.U.; Kumar, T.; Iqbal, A.; Ali, U.A. Use of different promoters in transgenic plant development: Current challenges and future perspectives. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 664. [Google Scholar] [CrossRef]
- Kummari, D.; Palakolanu, S.R.; Kishor, P.K.; Bhatnagar-Mathur, P.; Singam, P.; Vadez, V.; Sharma, K.K. An update and perspectives on the use of promoters in plant genetic engineering. J. Biosci. 2020, 45, 119. [Google Scholar] [CrossRef]
- Misra, S.; Ganesan, M. The impact of inducible promoters in transgenic plant production and crop improvement. Plant Gene 2021, 27, 100300. [Google Scholar] [CrossRef]
- Al Abdallat, A.M.; Ayad, J.Y.; Abu Elenein, J.M.; Al Ajlouni, Z.; Harwood, W.A. Overexpression of the transcription factor HvSNAC1 improves drought tolerance in barley (Hordeum vulgare L.). Mol. Breed. 2014, 33, 401–414. [Google Scholar] [CrossRef]
- Alhindi, T.; Al-Abdallat, A.M. Genome-wide identification and analysis of the MADS-box gene family in American beautyberry (Callicarpa americana). Plants 2021, 10, 1805. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1–research0034.11. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Li, Y.H.; Shangguan, H.B.; Bian, J.X.; Luo, R.H.; Tian, Y.; Li, Z.M.; Nie, X.J.; Cui, L.C. BarleyExpDB: An integrative gene expression database for barley. BMC Plant Biol. 2023, 23, 170. [Google Scholar] [CrossRef]
- Mahalingam, R.; Duhan, N.; Kaundal, R.; Smertenko, A.; Nazarov, T.; Bregitzer, P. Heat and drought induced transcriptomic changes in barley varieties with contrasting stress response phenotypes. Front. Plant Sci. 2022, 13, 1066421. [Google Scholar] [CrossRef]
- Bartlett, J.G.; Alves, S.C.; Smedley, M.; Snape, J.W.; Harwood, W.A. High-throughput Agrobacterium-mediated barley transformation. Plant Methods. 2008, 4, 22. [Google Scholar] [CrossRef]
- Lawrenson, T.; Shorinola, O.; Stacey, N.; Li, C.; Østergaard, L.; Patron, N.; Uauy, C.; Harwood, W. Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease. Genome Biol. 2015, 16, 258. [Google Scholar] [CrossRef]
- Weng, H.; Pan, A.; Yang, L.; Zhang, C.; Liu, Z.; Zhang, D. Estimating number of transgene copies in transgenic rapeseed by real-time PCR assay with HMG I/Y as an endogenous reference gene. Plant Mol. Biol. Rep. 2004, 22, 289–300. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil, 1st ed.; Commonwealth Agriculture Bureaux: Berkeley, UK, 1950; pp. 29–32. [Google Scholar]
- Cassel, D.K.; Nielsen, D.R. Field capacity and available water capacity. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; American Society of Agronomy, Inc.: Fitchburg, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1986; Volume 5, pp. 901–926. [Google Scholar] [CrossRef]
- Mbideen, F.O.; Aburumman, A.; Al-Sayaydeh, R.; Albdaiwi, R.N.; Al-Abdallat, A.M. Effect of Water Deficit Stress on Reproductive Stage of Durum Wheat Near Isogenic Lines Carrying the NAM-B1 Gene. Open Agric. J. 2024, 18, e18743315314060. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sayaydeh, R.; Ayad, J.; Harwood, W.; Al-Abdallat, A.M. Stress-Inducible Expression of HvABF2 Transcription Factor Improves Water Deficit Tolerance in Transgenic Barley Plants. Plants 2024, 13, 3113. https://doi.org/10.3390/plants13223113
Al-Sayaydeh R, Ayad J, Harwood W, Al-Abdallat AM. Stress-Inducible Expression of HvABF2 Transcription Factor Improves Water Deficit Tolerance in Transgenic Barley Plants. Plants. 2024; 13(22):3113. https://doi.org/10.3390/plants13223113
Chicago/Turabian StyleAl-Sayaydeh, Rabea, Jamal Ayad, Wendy Harwood, and Ayed M. Al-Abdallat. 2024. "Stress-Inducible Expression of HvABF2 Transcription Factor Improves Water Deficit Tolerance in Transgenic Barley Plants" Plants 13, no. 22: 3113. https://doi.org/10.3390/plants13223113
APA StyleAl-Sayaydeh, R., Ayad, J., Harwood, W., & Al-Abdallat, A. M. (2024). Stress-Inducible Expression of HvABF2 Transcription Factor Improves Water Deficit Tolerance in Transgenic Barley Plants. Plants, 13(22), 3113. https://doi.org/10.3390/plants13223113




