Photoperiodic Effect on Growth, Photosynthesis, Mineral Elements, and Metabolome of Tomato Seedlings in a Plant Factory
Abstract
:1. Introduction
2. Results
2.1. Effect of Different Photoperiods on Plant Growth
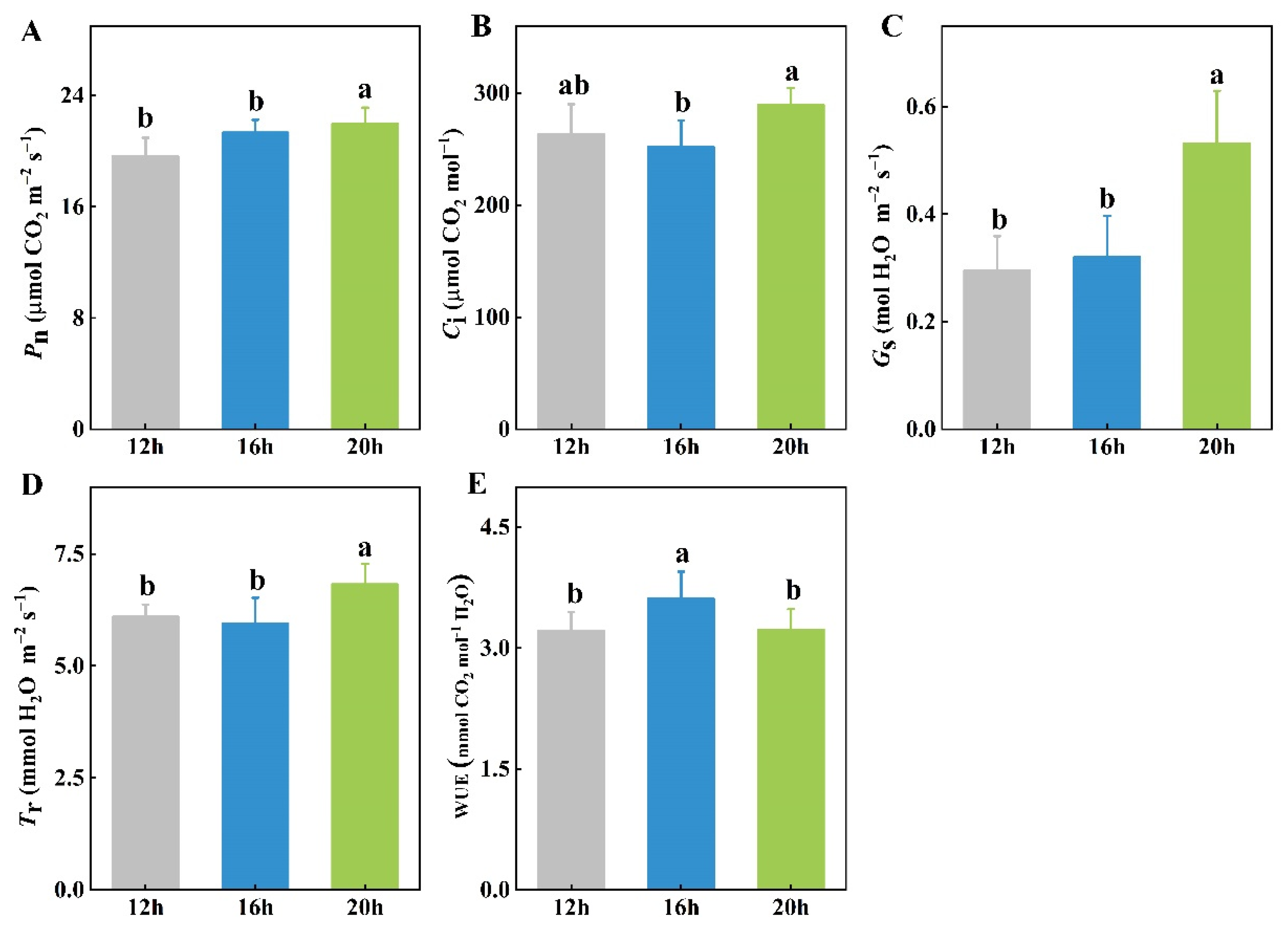
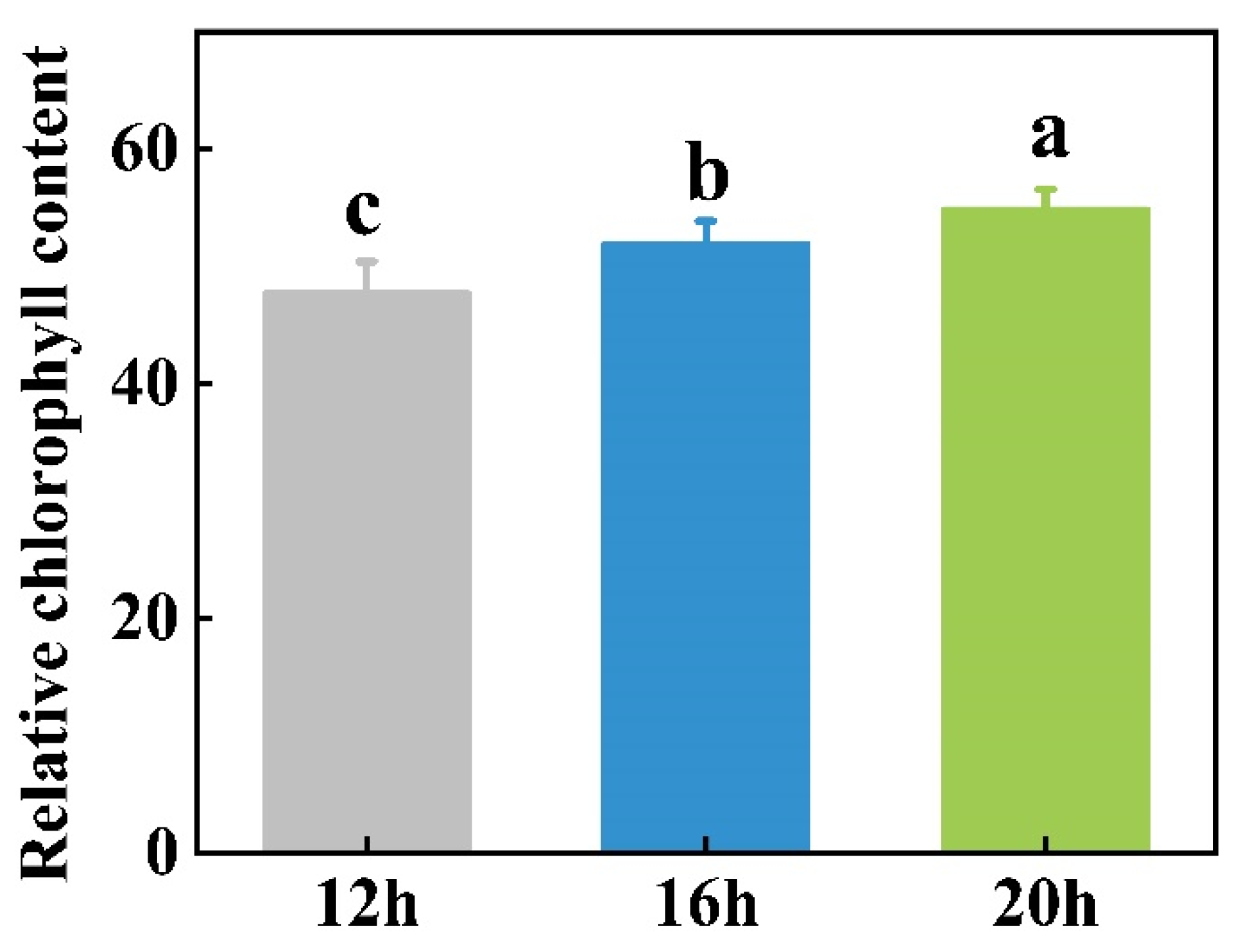
2.2. Effect of Different Photoperiods on Leaf Photosynthesis
2.3. Effect of Different Photoperiods on Mineral Element Contents
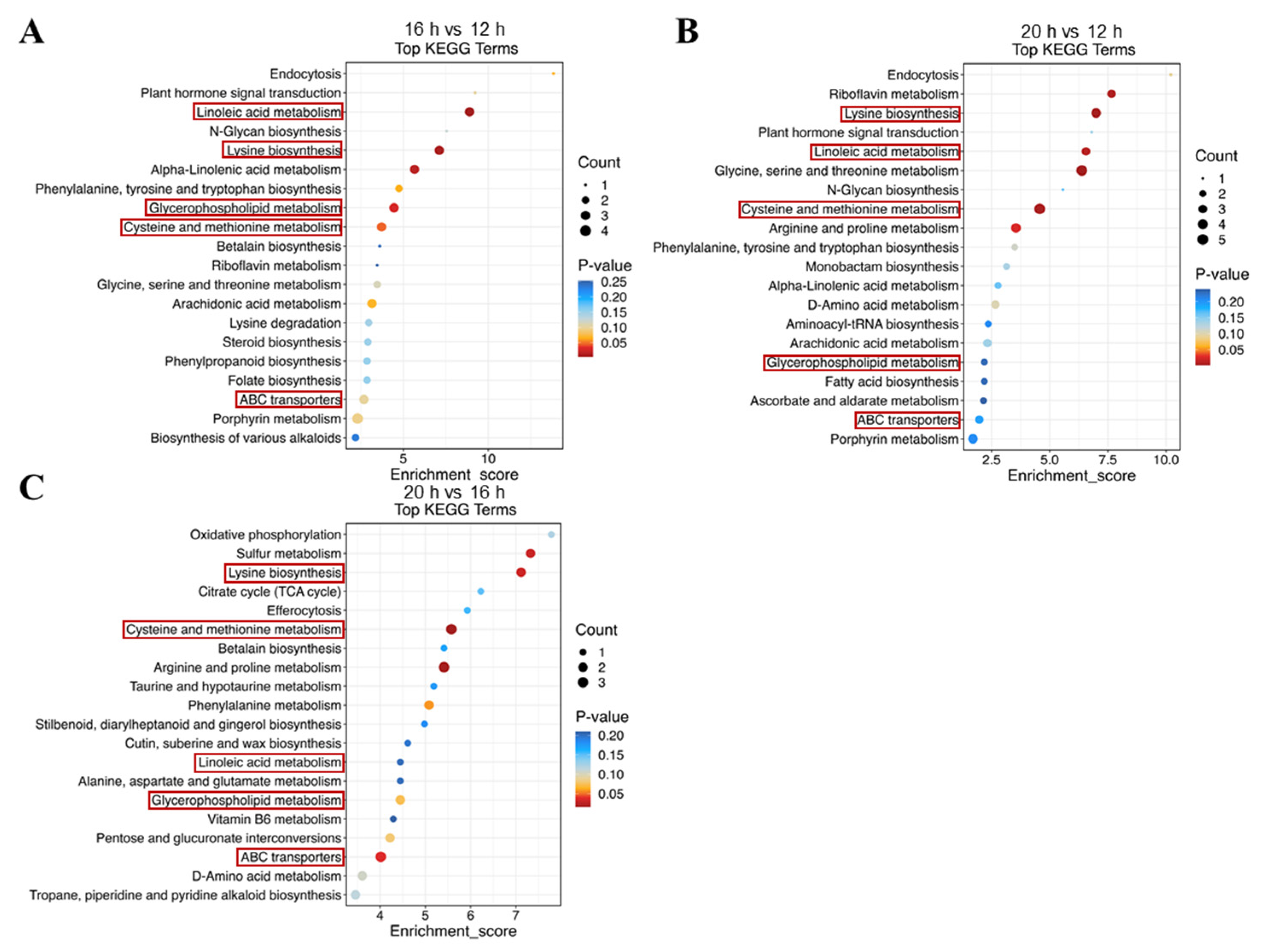
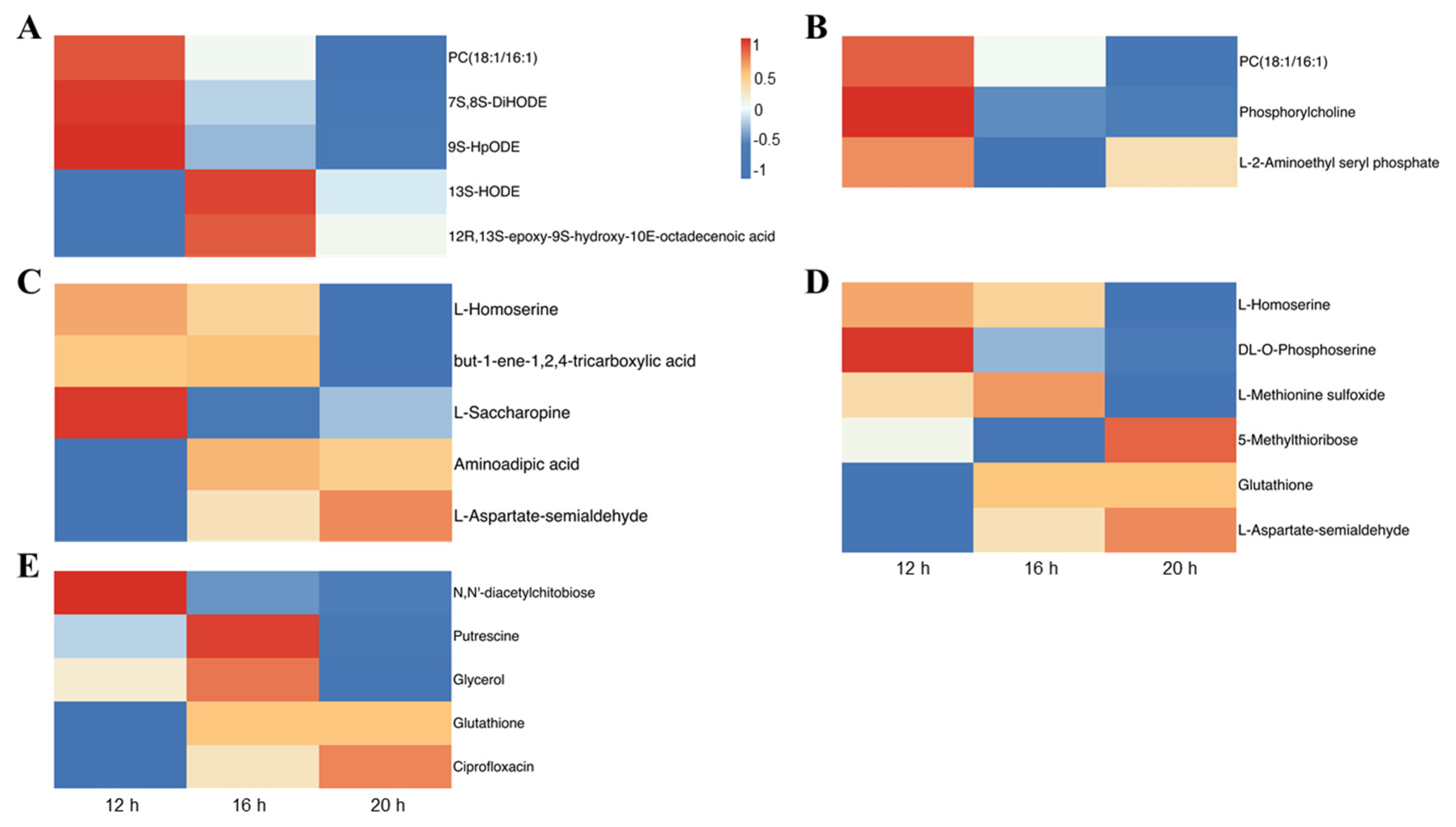
2.4. Effect of Different Photoperiods on Metabolome Profiles in Tomato Leaves
3. Discussion
3.1. Plant Growth and Biomass Are Positively Correlated with Light Duration
3.2. Longer Photoperiod Promotes Photosynthesis of Tomato Seedlings
3.3. Mineral Element Contents of Tomato Plants in Response to Different Photoperiods
3.4. Photoperiodic Regulation of Metabolome in Tomato
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Measurement of Growth Parameters
4.3. Measurement of Relative Chlorophyll Content
4.4. Measurement of Photosynthesis
4.5. Measurement of Mineral Element Contents
4.6. Metabolite Profiling and Data Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Avgoustaki, D.D.; Bartzanas, T.; Xydis, G. Minimising the energy footprint of indoor food production while maintaining a high growth rate: Introducing disruptive cultivation protocols. Food Control 2021, 130, 108290. [Google Scholar] [CrossRef]
- Kochetova, G.V.; Avercheva, O.V.; Bassarskaya, E.M.; Zhigalova, T.V. Light quality as a driver of photosynthetic apparatus development. Biophys. Rev. 2022, 14, 779–803. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.D. Plant responses to photoperiod. New Phytol. 2009, 181, 517–531. [Google Scholar] [CrossRef]
- Casal, J. Signalling for developmental plasticity. Trends Plant Sci. 2004, 9, 309–314. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, M.; Cheng, F.; Liu, S.; Liang, Y. Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of Cunninghamia lanceolata. BMC Plant Biol. 2020, 20, 269. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.; Both, A.J. Evaluating operating characteristics of light sources for horticultural applications. Acta Hortic. 2016, 1134, 435–443. [Google Scholar] [CrossRef]
- Ali, M.B.; Khandaker, L.; Oba, S. Comparative study on functional components, antioxidant activity and color parameters of selected colored leafy vegetables as affected by photoperiods. J. Food Agric. Environ. 2009, 7, 392–398. [Google Scholar]
- Liu, K.; Gao, M.; Jiang, H.; Ou, S.; Li, X.; He, R.; Li, Y.; Liu, H. Light Intensity and Photoperiod Affect Growth and Nutritional Quality of Brassica Microgreens. Molecules 2022, 27, 883. [Google Scholar] [CrossRef]
- Dmitry, F. The Photoperiod/Light Intensity Ratio Affects the Yield of Microgreens Within One Daylight Integral. Mod. Concepts Dev. Agron. 2022, 11, 1097–1098. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Vaštakaitė-Kairienė, V.; J, J.; Viršilė, A.; Samuoliene, G.; Miliauskienė, J.; Duchovskis, P. The impact of photoperiod on the growth an internal quality of mustard microgreens. In Proceedings of the 2nd International Conference on the Scientific Actualities and Innovations in Horticulture 2018, Kaunas, Lithuania, 4–6 June 2018. [Google Scholar]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Fang, Y.; Huang, M.; Chen, X.; Zhang, Y.; Zhao, H. The effects of photoperiod and nutrition on duckweed (Landoltia punctata) growth and starch accumulation. Ind. Crops Prod. 2018, 115, 243–249. [Google Scholar] [CrossRef]
- Palmer, S.; van Iersel, M.W. Increasing Growth of Lettuce and Mizuna under Sole-Source LED Lighting Using Longer Photoperiods with the Same Daily Light Integral. Agronomy 2020, 10, 1659. [Google Scholar] [CrossRef]
- Elkins, C.; van Iersel, M.W. Longer Photoperiods with the Same Daily Light Integral Improve Growth of Rudbeckia Seedlings in a Greenhouse. HortScience 2020, 55, 1676–1682. [Google Scholar] [CrossRef]
- Silva, L.M.; Cruz, L.P.; Pacheco, V.S.; Machado, E.C.; Purquerio, L.F.V.; Ribeiro, R.V. Energetic efficiency of biomass production is affected by photoperiod in indoor lettuce cultivation. Theor. Exp. Plant Physiol. 2022, 34, 265–276. [Google Scholar] [CrossRef]
- Galvão, V.C.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef]
- Hernández-Adasme, C.; Palma-Dias, R.; Escalona, V.H. The Effect of Light Intensity and Photoperiod on the Yield and Antioxidant Activity of Beet Microgreens Produced in an Indoor System. Horticulturae 2023, 9, 493. [Google Scholar] [CrossRef]
- Vaštakaitė-Kairienė, V.; Viršilė, A.; Brazaitytė, A.; J, J.; Samuoliene, G.; Novickovas, A.; Sirtautas, R.; Bagdonavičienė, A.; Lekstutytė, B.; Duchovskis, P. The effect of light-emitting diodes (LEDs) photoperiod on nutritional quality of Brassicaceae microgreens. In Proceedings of the 10th Baltic Conference on Food Science and Technology “Future Food: Innovations, Science and Technology”, Kaunas, Lithuania, 21–22 May 2015. [Google Scholar]
- de Andrade, M.V.S.; de Castro, R.D.; da Silva Cunha, D.; Gomes Neto, V.; Aparecida Carosio, M.G.; Ferreira, A.G.; de Souza-Neta, L.C.; Fernandez, L.G.; Ribeiro, P.R. Stevia rebaudiana (Bert.) Bertoni cultivated under different photoperiod conditions: Improving physiological and biochemical traits for industrial applications. Ind. Crops Prod. 2021, 168, 113595. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, L.; Dai, J.; Liu, Y.; Lin, D.; Yang, Y. Morphological and Physiological Responses of Cucumber Seedlings to Different Combinations of Light Intensity and Photoperiod with the Same Daily Light Integral. HortScience 2021, 56, 1430–1438. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef]
- Shibaeva, T.G.; Mamaev, A.V.; Titov, A.F. Possible Physiological Mechanisms of Leaf Photodamage in Plants Grown under Continuous Lighting. Russ. J. Plant Physiol. 2023, 70, 15. [Google Scholar] [CrossRef]
- Kawagishi, K.; Abe, T.; Ubukata, M.; Kato, S. Inhibition of flower stalk elongation and abnormal flower development by short-day treatment in a Japanese variety of Chinese chive (Allium tuberosum Rottler ex Sprengel). Sci. Hortic. 2009, 119, 197–202. [Google Scholar] [CrossRef]
- Currey, C.; Erwin, J. Variation amongKalanchoespecies in their flowering responses to photoperiod and short-day cycle number. J. Hortic. Sci. Biotechnol. 2015, 85, 350–354. [Google Scholar] [CrossRef]
- Chu, Q.; Qin, Y.; Li, C.; Cheng, S.; Su, L.; He, Z.; Zhou, X.; Shao, D.; Guo, X. Effects of Different Photoperiods on the Growth and Nutritional Characteristics of Two Celery Cultivars in Plant Factory. Agronomy 2023, 13, 3039. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Yu-Xin, T.; Qi-Chang, Y. Optimal control of environmental conditions affecting lettuce plant growth in a controlled environment with artificial lighting: A review. S. Afr. J. Bot. 2020, 130, 75–89. [Google Scholar] [CrossRef]
- Fukuda, H.; Tanigaki, Y.; Moriyuki, S. Detection and Utilization of Biological Rhythms in Plant Factories. In Smart Plant Factory: The Next Generation Indoor Vertical Farms; Kozai, T., Ed.; Springer: Singapore, 2018; pp. 367–384. [Google Scholar]
- Dannehl, D.; Schwend, T.; Veit, D.; Schmidt, U. Increase of Yield, Lycopene, and Lutein Content in Tomatoes Grown Under Continuous PAR Spectrum LED Lighting. Front. Plant Sci. 2021, 12, 611236. [Google Scholar] [CrossRef]
- Ghate, M.R. An overview of led lighting in agriculture for the growth and development of plants. Int. J. Manag. IT Eng. 2018, 9, 32–39. [Google Scholar]
- Wang, C.; Li, M.; Duan, X.; Abu-Izneid, T.; Rauf, A.; Khan, Z.; Mitra, S.; Emran, T.B.; Aljohani, A.S.M.; Alhumaydhi, F.A.; et al. Phytochemical and Nutritional Profiling of Tomatoes; Impact of Processing on Bioavailability—A Comprehensive Review. Food Rev. Int. 2022, 39, 5986–6010. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Y.; Han, J.; Hu, X.; Li, X.; Zhao, H.; Bai, L.; Shi, Y.; Ahammed, G.J. Interactive Effects of Iron and Photoperiods on Tomato Plant Growth and Fruit Quality. J. Plant Growth Regul. 2022, 42, 376–389. [Google Scholar] [CrossRef]
- Dorais, M.; Yelle, S.; Gosselin, A. Influence of extended photoperiod on photosynthate partitioning and export in tomato and pepper plants. N. Z. J. Crop Hortic. Sci. 1996, 24, 29–37. [Google Scholar] [CrossRef]
- Demers, D.-A.; Dorais, M.; Wien, C.H.; Gosselin, A. Effects of supplemental light duration on greenhouse tomato (Lycopersicon esculentum Mill.) plants and fruit yields. Sci. Hortic. 1998, 74, 295–306. [Google Scholar] [CrossRef]
- Rengasamy, N.; Othman, R.Y.; Che, H.S.; Harikrishna, J.A. Artificial Lighting Photoperiod Manipulation Approach to Improve Productivity and Energy Use Efficacies of Plant Factory Cultivated Stevia rebaudiana. Agronomy 2022, 12, 1787. [Google Scholar] [CrossRef]
- Xu, W.; Lu, N.; Kikuchi, M.; Takagaki, M. Continuous Lighting and High Daily Light Integral Enhance Yield and Quality of Mass-Produced Nasturtium (Tropaeolum majus L.) in Plant Factories. Plants 2021, 10, 1203. [Google Scholar] [CrossRef]
- Kozai, T.; Watanabe, K.; Jeong, B.R. Stem elongation and growth of Solanum tuberosum L. in vitro in response to photosynthetic photon flux, photoperiod and difference in photoperiod and dark period temperatures. Sci. Hortic. 1995, 64, 1–9. [Google Scholar] [CrossRef]
- Lefsrud, M.G.; Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Irradiance levels affect growth parameters and carotenoid pigments in kale and spinach grown in a controlled environment. Physiol. Plant. 2006, 127, 624–631. [Google Scholar] [CrossRef]
- Gao, W.; He, D.; Ji, F.; Zhang, S.; Zheng, J. Effects of Daily Light Integral and LED Spectrum on Growth and Nutritional Quality of Hydroponic Spinach. Agronomy 2020, 10, 1082. [Google Scholar] [CrossRef]
- Milford, G.F.J.; Lenton, J.R. Effect of Photoperiod on Growth of Sugar Beet. Ann. Bot. 1976, 40, 1309–1315. [Google Scholar] [CrossRef]
- Soffe, R.W.; Lenton, J.R.; Milford, G.F.J. Effects of photoperiod on some vegetable species. Ann. Appl. Biol. 2008, 85, 411–415. [Google Scholar] [CrossRef]
- Mousseau, M. Effects of Photoperiod on Photosynthesis. In Advances in Photosynthesis Research: Proceedings of the VIth International Congress on Photosynthesis, Brussels, Belgium, August 1–6, 1983. Volume 4; Sybesma, C., Ed.; Springer: Dordrecht, The Netherlands, 1984; pp. 305–308. [Google Scholar]
- Matsuda, R.; Ozawa, N.; Fujiwara, K. Leaf photosynthesis, plant growth, and carbohydrate accumulation of tomato under different photoperiods and diurnal temperature differences. Sci. Hortic. 2014, 170, 150–158. [Google Scholar] [CrossRef]
- Tsuruyama, J.; Shibuya, T. Growth and Flowering Responses of Seed-propagated Strawberry Seedlings to Different Photoperiods in Controlled Environment Chambers. HortTechnology 2018, 28, 453–458. [Google Scholar] [CrossRef]
- Dodd, A.N.; Kusakina, J.; Hall, A.; Gould, P.D.; Hanaoka, M. The circadian regulation of photosynthesis. Photosynth. Res. 2013, 119, 181–190. [Google Scholar] [CrossRef]
- Venkat, A.; Muneer, S. Role of Circadian Rhythms in Major Plant Metabolic and Signaling Pathways. Front. Plant Sci. 2022, 13, 836244. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Munir, M.; Sattar, M.N. Morphological, Biochemical, and Physiological Response of Butterhead Lettuce to Photo-Thermal Environments. Horticulturae 2022, 8, 515. [Google Scholar] [CrossRef]
- Shen, Y.Z.; Guo, S.S.; Ai, W.D.; Tang, Y.K. Effects of illuminants and illumination time on lettuce growth, yield and nutritional quality in a controlled environment. Life Sci. Space Res. 2014, 2, 38–42. [Google Scholar] [CrossRef]
- Xin, Z.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar]
- Ghorbanzadeh, P.; Aliniaeifard, S.; Esmaeili, M.; Mashal, M.; Azadegan, B.; Seif, M. Dependency of Growth, Water Use Efficiency, Chlorophyll Fluorescence, and Stomatal Characteristics of Lettuce Plants to Light Intensity. J. Plant Growth Regul. 2020, 40, 2191–2207. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, W.; Wang, Z.; Qi, K.; Xie, Z.; Zhang, S.; Wu, J.; Wang, P. Diurnal transcriptome dynamics reveal the photoperiod response of Pyrus. Physiol. Plant 2023, 175, e13893. [Google Scholar] [CrossRef]
- Zheng, L.; He, H.; Song, W. Application of Light-emitting Diodes and the Effect of Light Quality on Horticultural Crops: A Review. HortScience 2019, 54, 1656–1661. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Langton, F.A.; Adams, S.R.; Cockshull, K.E. Effects of photoperiod on leaf greenness of four bedding plant species. J. Hortic. Sci. Biotechnol. 2015, 78, 400–404. [Google Scholar] [CrossRef]
- Lefsrud, M.G.; Kopsell, D.A.; Augé, R.M.; Both, A.J. Biomass Production and Pigment Accumulation in Kale Grown Under Increasing Photoperiods. HortScience 2006, 41, 603–606. [Google Scholar] [CrossRef]
- Mao, H.; Hang, T.; Zhang, X.; Lu, N. Both Multi-Segment Light Intensity and Extended Photoperiod Lighting Strategies, with the Same Daily Light Integral, Promoted Lactuca sativa L. Growth and Photosynthesis. Agronomy 2019, 9, 857. [Google Scholar] [CrossRef]
- Hu, G.; Li, X.; Yang, J.; Yuan, Q.; Yang, S.; Fu, W.; Zhang, X.; Li, Y.; Shen, Z.; Jiang, J. Effects of Photoperiod and Light Quality on Germination and Growth of Camellia sinensis ‘HuangKui’. Plants 2024, 13, 1782. [Google Scholar] [CrossRef]
- Hocking, P.J. Dry-matter production, mineral nutrient concentrations, and nutrient distribution and redistribution in irrigated spring wheat. J. Plant Nutr. 1994, 17, 1289–1308. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, L.; Shen, Z.; Jing, W.; Ge, H.; Zhao, J.; Zhang, W. The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice. Plant Cell Environ. 2015, 38, 2766–2779. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Zhang, Y.; Li, Z.; Liu, W. Effect of Continuous Red/Blue LED Light and Its Light Intensity on Growth and Mineral Elements Absorption of Lettuce. Spectrosc. Spectr. Anal. 2019, 39, 2474–2480. [Google Scholar]
- Li, X.; Gao, Y.; Wei, H.; Xia, H.; Chen, Q. Growth, biomass accumulation and foliar nutrient status in fragrant rosewood (Dalbergia odorifera T.C. Chen) seedlings cultured with conventional and exponential fertilizations under different photoperiod regimes. Soil Sci. Plant Nutr. 2017, 63, 153–162. [Google Scholar] [CrossRef]
- Mortley, D.G.; Burrell, S.; Bonsi, C.K.; Hill, W.A.; Morris, C.E. Influence of Daily Light Period and Irradiance on Yield and Leaf Elemental Concentration of Hydroponically Grown Sweetpotato. HortScience 2009, 44, 1491–1493. [Google Scholar] [CrossRef]
- Song, J.; Huang, H.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Effects of Photoperiod Interacted with Nutrient Solution Concentration on Nutritional Quality and Antioxidant and Mineral Content in Lettuce. Agronomy 2020, 10, 920. [Google Scholar] [CrossRef]
- An, B.; Wei, H.; Li, L.; Guo, P. Nutrient Uptake and Utilization and Antioxidants of Fruits in Red Raspberry (Rubus idaeus L.) Cultivar ‘Autumn Bliss’ in response to Fertilization under Extended Photoperiod. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 440–448. [Google Scholar] [CrossRef]
- Jones, J.B. Plant Analysis Handbook: A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Pub.: Athens, GA, USA, 1991. [Google Scholar]
- Pichersky, E.; Lewinsohn, E. Convergent evolution in plant specialized metabolism. Annu. Rev. Plant Biol. 2011, 62, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Hornyak, M.; Dziurka, M.; Kula-Maximenko, M.; Pastuszak, J.; Szczerba, A.; Szklarczyk, M.; Plazek, A. Photosynthetic efficiency, growth and secondary metabolism of common buckwheat (Fagopyrum esculentum Moench) in different controlled-environment production systems. Sci. Rep. 2022, 12, 257. [Google Scholar] [CrossRef] [PubMed]
- Florez-Sarasa, I.; Araujo, W.L.; Wallstrom, S.V.; Rasmusson, A.G.; Fernie, A.R.; Ribas-Carbo, M. Light-responsive metabolite and transcript levels are maintained following a dark-adaptation period in leaves of Arabidopsis thaliana. New Phytol. 2012, 195, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-l.; Li, W.-d.; Zheng, Q.-y.; Wang, W.-q.; Xiao, B.; Xing, D. Effect of low light intensity on growth and accumulation of secondary metabolites in roots of Glycyrrhiza uralensis Fisch. Biochem. Syst. Ecol. 2010, 38, 160–168. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Y.; Luo, L.; Feng, F.; Saeed, S.; Luo, J.; Fang, C.; Zhou, J.; Li, K. Photoperiod-Dependent Nutrient Accumulation in Rice Cultivated in Plant Factories: A Comparative Metabolomic Analysis. Foods 2024, 13, 1544. [Google Scholar] [CrossRef]
- Aguirre-Becerra, H.; García-Trejo, J.F.; Vázquez-Hernández, C.; Alvarado, A.M.; Feregrino-Pérez, A.A.; Contreras-Medina, L.M.; Guevara-Gonzalez, R.G. Effect of Extended Photoperiod with a Fixed Mixture of Light Wavelengths on Tomato Seedlings. HortScience 2020, 55, 1832–1839. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.; Cao, J. Pollen wall development: The associated enzymes and metabolic pathways. Plant Biol. 2013, 15, 249–263. [Google Scholar] [CrossRef]
- Harwood, J.L.; Ramli, U.S.; Page, R.A.; Quant, P.A. Modelling lipid metabolism in plants: A slippery problem? Biochem. Soc. Trans. 1999, 27, 285–289. [Google Scholar] [CrossRef]
- Maldini, M.; Natella, F.; Baima, S.; Morelli, G.; Scaccini, C.; Langridge, J.; Astarita, G. Untargeted Metabolomics Reveals Predominant Alterations in Lipid Metabolism Following Light Exposure in Broccoli Sprouts. Int. J. Mol. Sci. 2015, 16, 13678–13691. [Google Scholar] [CrossRef]
- Deng, X.; Fan, X.; Li, P.; Fei, X. A photoperiod-regulating gene CONSTANS is correlated to lipid biosynthesis in Chlamydomonas reinhardtii. Biomed. Res. Int. 2015, 2015, 715020. [Google Scholar] [CrossRef]
- Grimberg, A.; Lager, I.; Street, N.R.; Robinson, K.M.; Marttila, S.; Mahler, N.; Ingvarsson, P.K.; Bhalerao, R.P. Storage lipid accumulation is controlled by photoperiodic signal acting via regulators of growth cessation and dormancy in hybrid aspen. New Phytol. 2018, 219, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Seyfabadi, J.; Ramezanpour, Z.; Amini Khoeyi, Z. Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J. Appl. Phycol. 2010, 23, 721–726. [Google Scholar] [CrossRef]
- Kawade, K.; Tabeta, H.; Ferjani, A.; Hirai, M.Y. The Roles of Functional Amino Acids in Plant Growth and Development. Plant Cell Physiol. 2023, 64, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, N.; Joshi, R. Deciphering the metabolic signatures of Trigonella microgreens as a function of photoperiod and temperature using targeted compound analysis and non-targeted UHPLC-QTOF-IMS based approach. Food Res. Int. 2024, 176, 113834. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Abbasi, B.H.; Fazal, H.; Khan, M.A.; Afridi, M.S. Effect of reverse photoperiod on in vitro regeneration and piperine production in Piper nigrum L. C. R. Biol. 2014, 337, 19–28. [Google Scholar] [CrossRef]
- Atif, M.J.; Amin, B.; Ghani, M.I.; Ali, M.; Khursheed, S.; Cheng, Z. Transcriptomic analysis of Allium sativum uncovers putative genes involved in photoperiodic pathway and hormone signaling under long day and short day conditions. Plant Sci. 2021, 313, 111095. [Google Scholar] [CrossRef]
- Xiaotao, D.; Yuping, J.; Hong, W.; Haijun, J.; Hongmei, Z.; Chunhong, C.; Jizhu, Y. Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters, antioxidative system and carbohydrate accumulation in cucumber (Cucumis sativus L.) under low light. Acta Physiol. Plant. 2012, 35, 1427–1438. [Google Scholar] [CrossRef]
- Song, J.; Huang, H.; Hao, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Nutritional quality, mineral and antioxidant content in lettuce affected by interaction of light intensity and nutrient solution concentration. Sci. Rep. 2020, 10, 2796. [Google Scholar] [CrossRef]






| Spectral Ranges (nm) | Total (380–780) | UV (380–399) | Blue (400–499) | Green (500–599) | Red (600–700) | Far-Red (701–780) |
|---|---|---|---|---|---|---|
| PPFD (µmol·m−2·s−1) | 242.72 | 0.18 | 63.91 | 51.43 | 123.07 | 4.13 |
| Photoperiod | 12 h | 16 h | 20 h |
|---|---|---|---|
| DLI (mol·m−2·d−1) | 10.49 | 13.98 | 17.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Li, R.; Bu, C.; Zhu, C.; Miao, C.; Zhang, Y.; Cui, J.; Jiang, Y.; Ding, X. Photoperiodic Effect on Growth, Photosynthesis, Mineral Elements, and Metabolome of Tomato Seedlings in a Plant Factory. Plants 2024, 13, 3119. https://doi.org/10.3390/plants13223119
Wu S, Li R, Bu C, Zhu C, Miao C, Zhang Y, Cui J, Jiang Y, Ding X. Photoperiodic Effect on Growth, Photosynthesis, Mineral Elements, and Metabolome of Tomato Seedlings in a Plant Factory. Plants. 2024; 13(22):3119. https://doi.org/10.3390/plants13223119
Chicago/Turabian StyleWu, Shaofang, Rongguang Li, Chongxing Bu, Cuifang Zhu, Chen Miao, Yongxue Zhang, Jiawei Cui, Yuping Jiang, and Xiaotao Ding. 2024. "Photoperiodic Effect on Growth, Photosynthesis, Mineral Elements, and Metabolome of Tomato Seedlings in a Plant Factory" Plants 13, no. 22: 3119. https://doi.org/10.3390/plants13223119
APA StyleWu, S., Li, R., Bu, C., Zhu, C., Miao, C., Zhang, Y., Cui, J., Jiang, Y., & Ding, X. (2024). Photoperiodic Effect on Growth, Photosynthesis, Mineral Elements, and Metabolome of Tomato Seedlings in a Plant Factory. Plants, 13(22), 3119. https://doi.org/10.3390/plants13223119







