Microencapsulated Microbial Seed Coating Could Improve Soil Environment and Maize Grain Yield in Saline Soil
Abstract
1. Introduction
2. Results
2.1. Effect of Different Seed Treatments on Soil Characters and the Inter-Root Microbial Community
2.1.1. Soil Characteristics
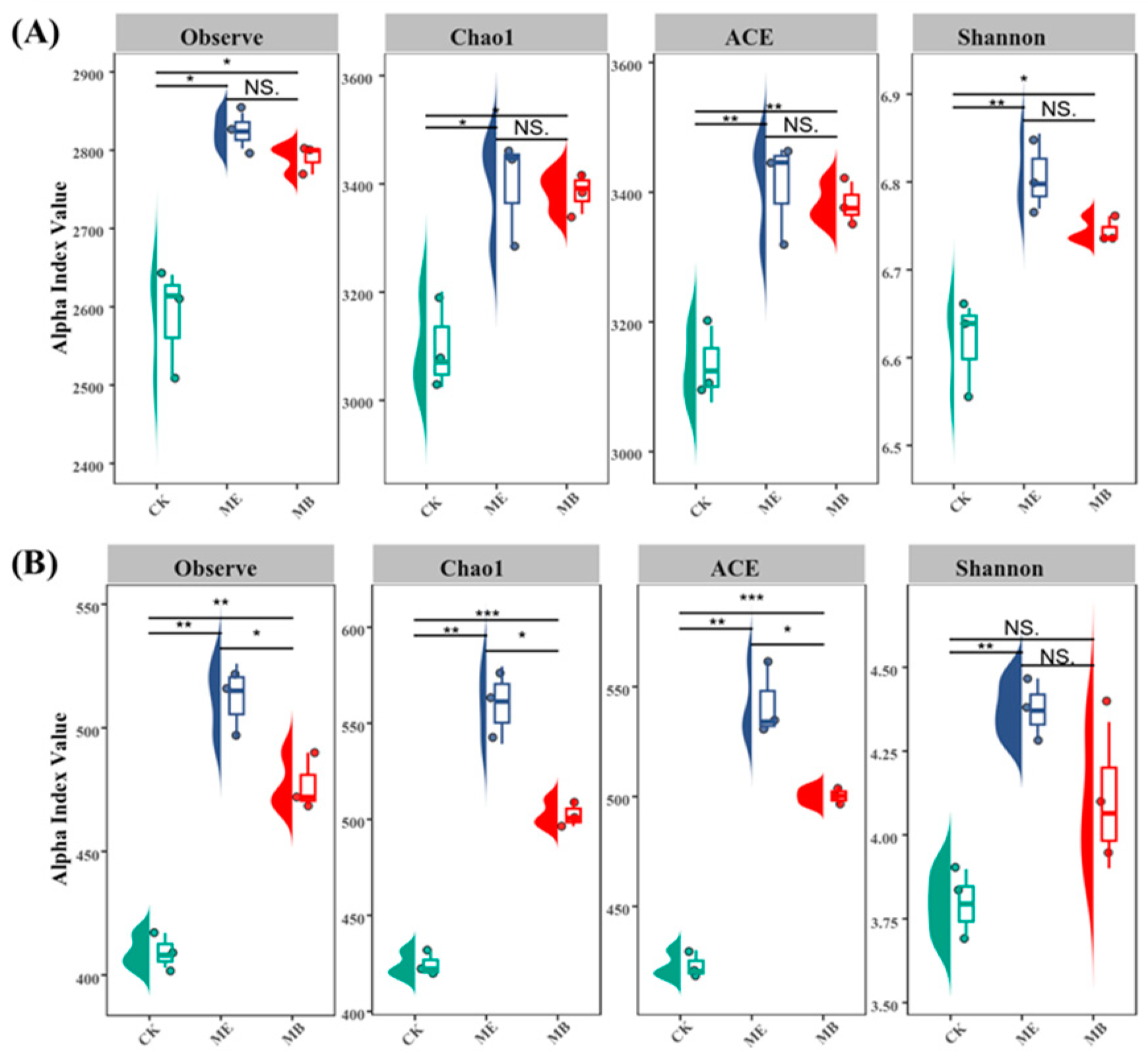
2.1.2. Soil Microbial α-Diversity
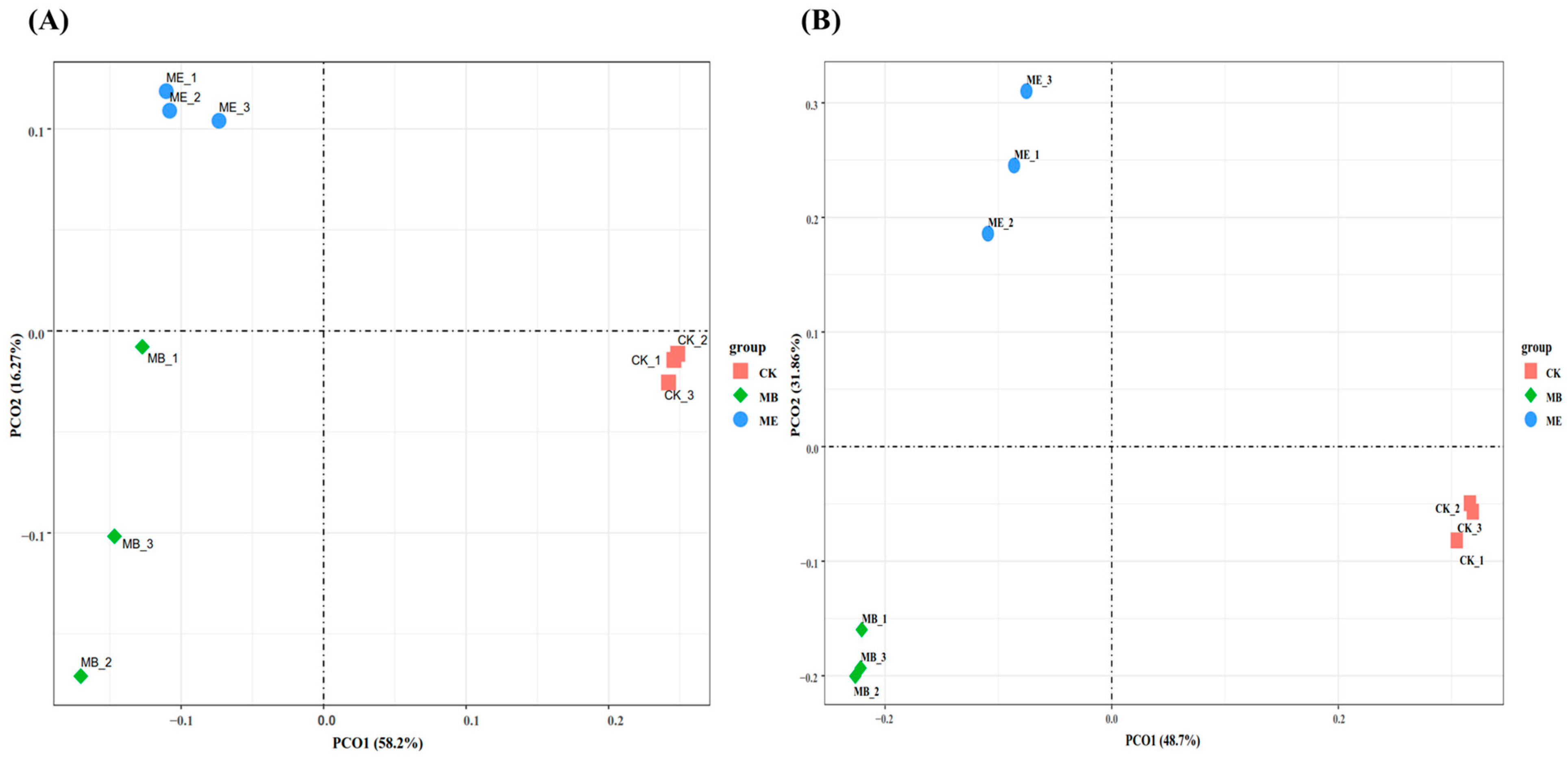
2.1.3. Soil Microbial β-Diversity
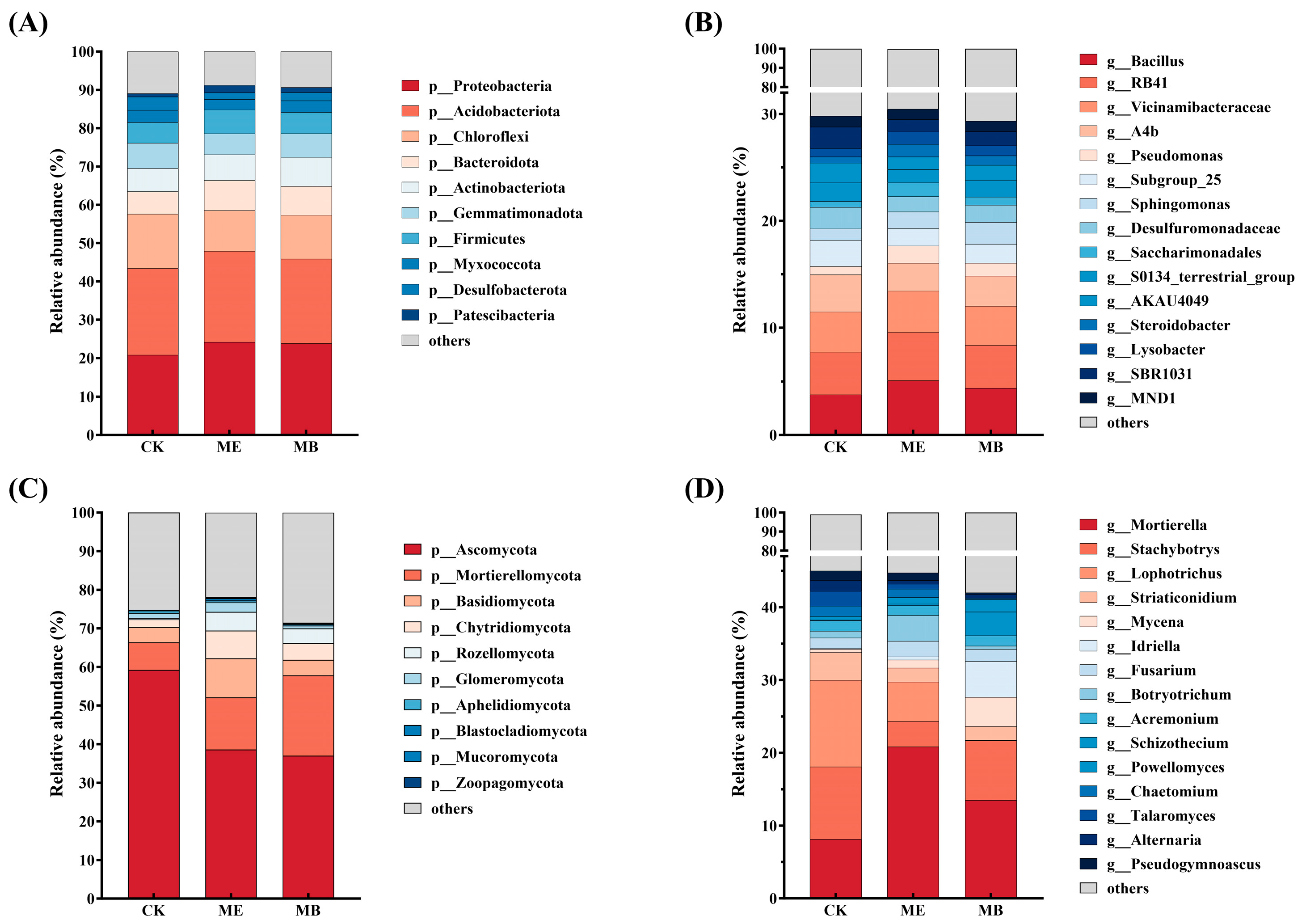
2.1.4. Soil Microbial Community Composition
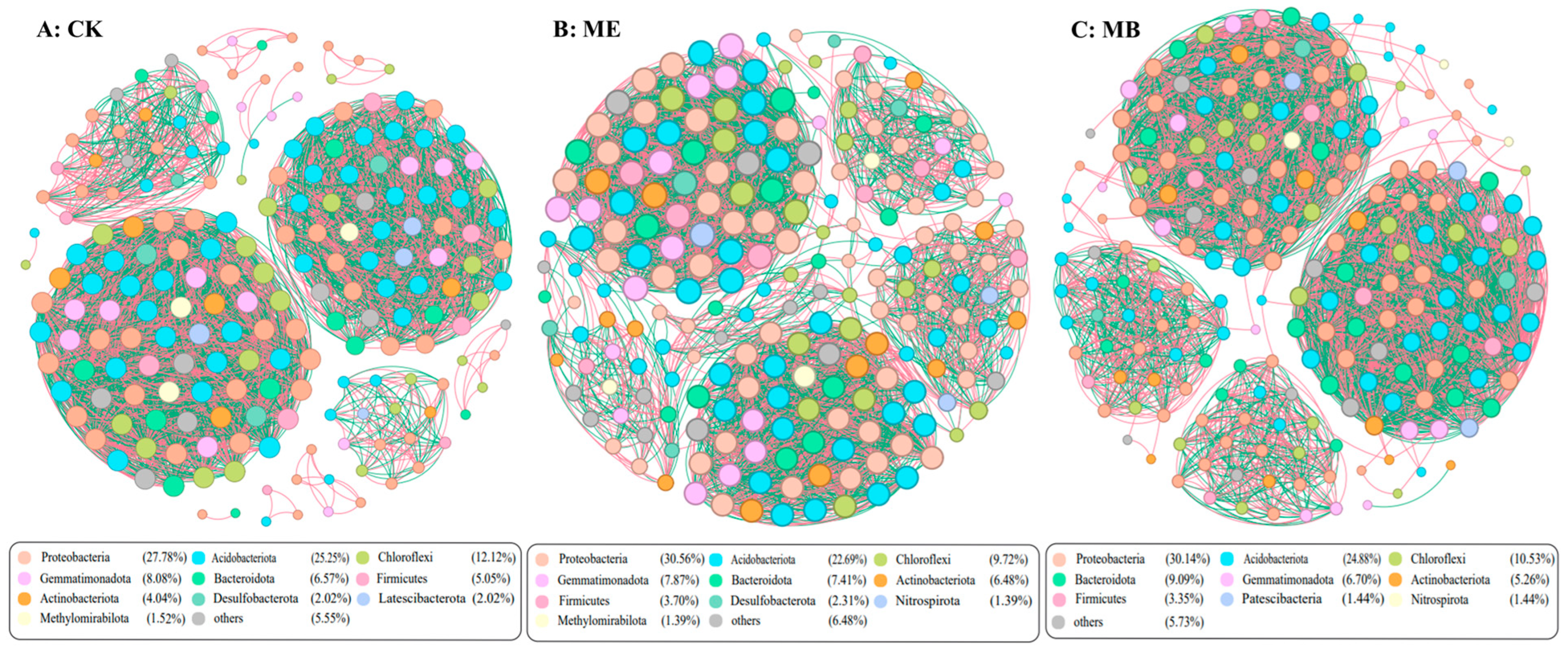
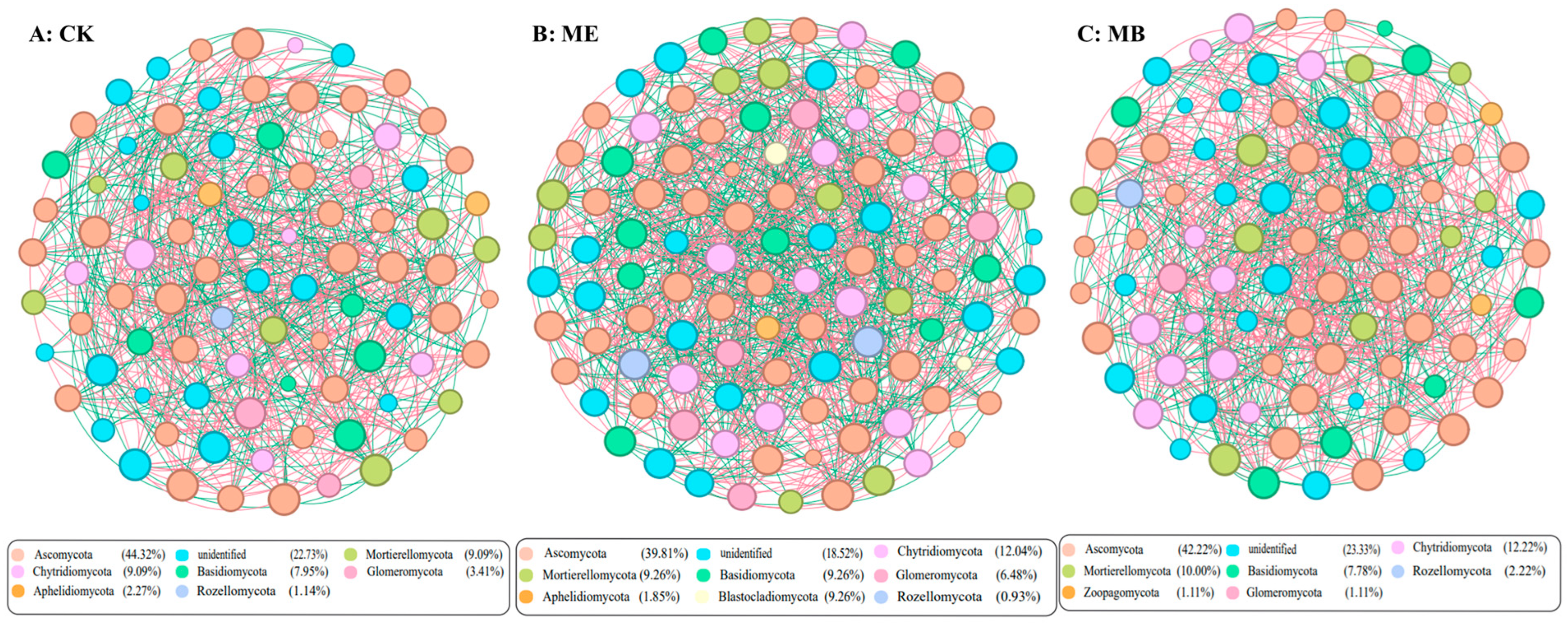
2.1.5. Soil Microbial Symbiotic Network
2.2. Effect of Different Seed Treatments on Maize Growth and Yield
2.2.1. Endogenous Hormones in Maize Seedlings
2.2.2. Maize Growth and Yield
2.3. Relationship of Microbial Communities with Maize Growth and Soil Characteristics
3. Discussion
4. Materials and Methods
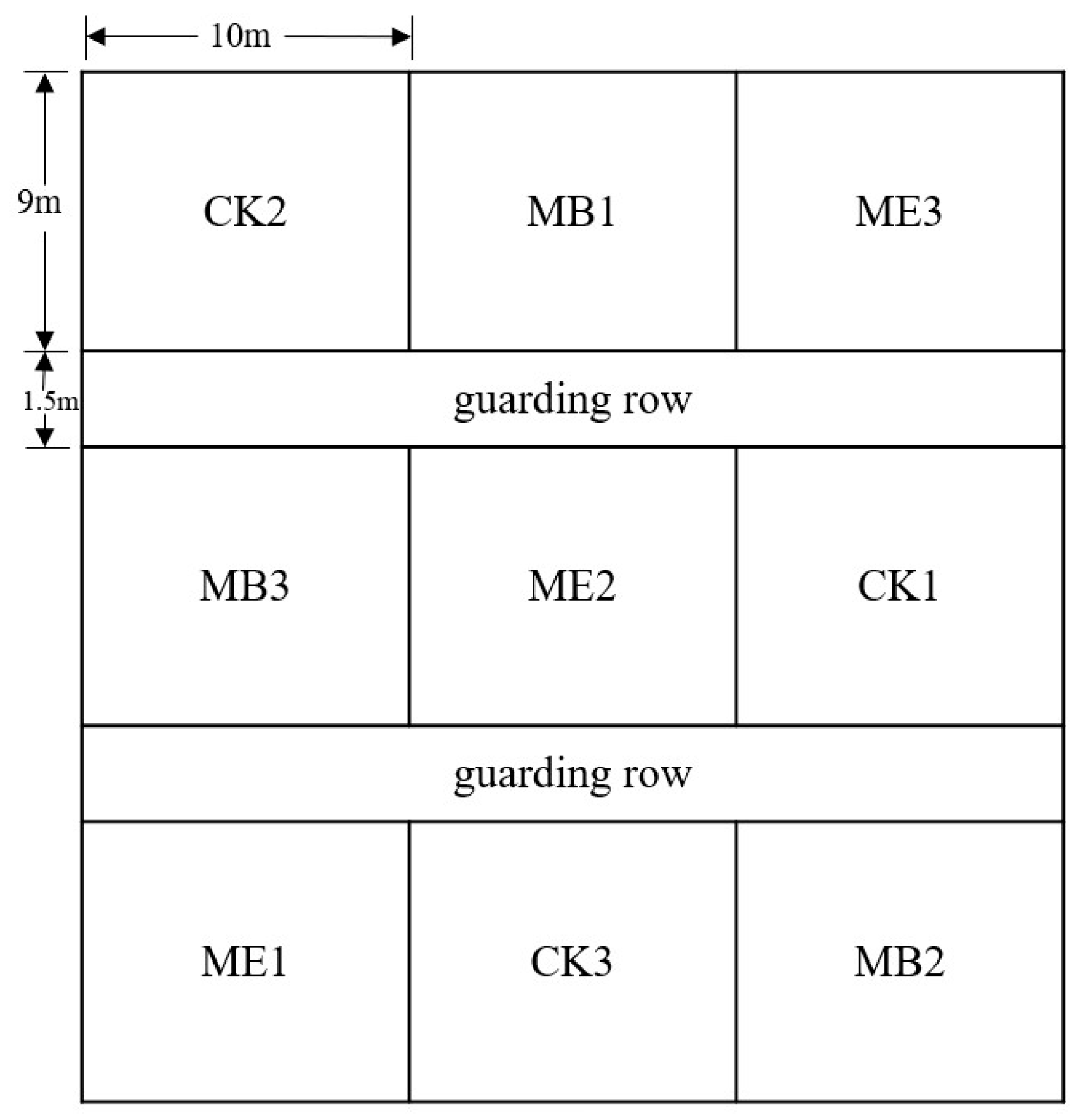
4.1. Indoor and Field Experiment Design
4.2. Pontibacter actiniarum DSM 19842 Features
4.3. Plant Hormone Detection
4.4. Determination of Soil Characteristics and Maize Yield Factors
4.5. Soil Microbial DNA Extraction, PCR Amplification and Sequencing
4.6. Statistical Analyses
5. Conclusions
- (1)
- Improvement of soil environment: Inoculating microencapsulated salt-tolerant bacteria (Pontibacter actiniarum DSM 19842) helped to reduce soil pH and electric conductivity and increase soil available phosphorus. The richness and uniformity of bacterial and fungal communities significantly increased in saline soil. This led to an increase in the complexity of microbial co-occurrence networks, revealing its important potential in improving microbial diversity.
- (2)
- Regulation of endogenous hormones: Inoculation with microencapsulated salt-tolerant bacteria significantly reduced the content of ABA, SA and JA in maize seedlings, while increasing the content of the growth hormone IAA, contributing to improvements in resistance to salt stress.
- (3)
- Improvement in maize productivity: Due to improvements in the emergence rate and 100-grain weight, the grain yield of maize was significantly increased, demonstrating that the seed coating played a key role in optimizing the growing environment and enhancing maize productivity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Negacz, K.; Malek, Z.; de Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Shukla, P.; Agarwal, P.; Jha, B. Improved Salinity Tolerance of Arachis hypogaea (L.) by the Interaction of Halotolerant Plant-Growth-Promoting Rhizobacteria. J. Plant Growth Regul. 2012, 31, 195–206. [Google Scholar] [CrossRef]
- Li, K.; Li, Q.; Liu, C. Effect of freezing temperature and water content on pore structure characteristics of coastal saline-alkali soil under frost heave. J. Soils Sediments 2022, 22, 1819–1827. [Google Scholar] [CrossRef]
- Delaune, K.; Nesich, D.; Goos, J.; Relyea, R. Impacts of salinization on aquatic communities: Abrupt vs. gradual exposures. Environ. Pollut. 2021, 285, 117636. [Google Scholar] [CrossRef] [PubMed]
- Kulmatov, R.; Mirzaev, J.; Abuduwaili, J.; Karimov, B. Challenges for the sustainable use of water and land resources under a changing climate and increasing salinization in the Jizzakh irrigation zone of Uzbekistan. J. Arid Land 2020, 12, 90–103. [Google Scholar] [CrossRef]
- Veenklaas, R.; Koppenaal, E.; Bakker, J.; Esselink, P. Salinization during salt-marsh restoration after managed realignment. J. Coast. Conserv. 2015, 19, 405–415. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, J.; Yang, H.; Liu, J.; Shao, P. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef]
- Kumawat, C.; Kumar, A.; Parshad, J.; Sharma, S.; Patra, A.; Dogra, P.; Yadav, G.; Dadhich, S.; Verma, R.; Kumawat, G. Microbial Diversity and Adaptation under Salt-Affected Soils: A Review. Sustainability 2022, 14, 9280. [Google Scholar] [CrossRef]
- Liu, K.; Gao, Y.; Gao, Z.; Dai, C. Research progress on soil salinization remediation technology. Heilongjiang Agric. Sci. 2024, 1, 99–107. [Google Scholar]
- Ma, L.; Liu, X.; Lv, W.; Yang, Y. Molecular Mechanisms of Plant Responses to Salt Stress. Front. Plant Sci. 2022, 13, 934877. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, R.; Zhu, H.; Cheng, X.; Shutes, B.; Yan, B. Seed germination and early seedling growth of six wetland plant species in saline-alkaline environment. Int. J. Phytoremediat. 2020, 22, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Bai, X.; Xu, W.; Wang, H.; Bao, L.; Deng, X.; Scriber, K.; Chen, Z.; Zhou, J. Chemical soil disinfestation decreases soil salinisation and the presence of potential pathogens at the cost of higher nitrate leaching. Agric. Ecosyst. Environ. 2024, 366, 108935. [Google Scholar] [CrossRef]
- Yue, Y.; Shao, T.; Long, X.; He, T.; Gao, X.; Zhou, Z.; Liu, Z.; Rengel, Z. Microbiome structure and function in rhizosphere of Jerusalem artichoke grown in saline land. Sci. Total Environ. 2020, 724, 138259. [Google Scholar] [CrossRef]
- Tang, L.; Zhan, L.; Han, Y.; Wang, Z.; Dong, L.; Zhang, Z. Microbial community assembly and functional profiles along the soil-root continuum of salt-tolerant Suaeda glauca and Suaeda salsa. Front. Plant Sci. 2023, 14, 1301117. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Gu, H.; Guo, G.; Hao, X.; Liu, C.; Meng, L. Bioremediation of saline soils. Chin. Agric. Sci. Bull. 2024, 40, 36–42. [Google Scholar]
- Glick, B. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G. Mining Halophytes for Plant Growth-Promoting Halotolerant Bacteria to Enhance the Salinity Tolerance of Non-halophytic Crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.; Pan, X.; Yuan, Z. Microbially Mediated Plant Salt Tolerance and Microbiome-based Solutions for Saline Agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J. Does plant-Microbe interaction confer stress tolerance in plants: A review. Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef]
- Fan, C. Genetic mechanisms of salt stress responses in halophytes. Plant Signal. Behav. 2020, 15, 1704528. [Google Scholar] [CrossRef]
- Numan, N.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.; Khan, A.; Khan, A.; AL-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Maheshwari, D. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; He, J.; Kong, M.; Huo, Q.; Jiang, Y.; Song, J.; Han, W.; Lv, G. A microencapsulation approach to design microbial seed coatings to boost wheat seed germination and seedling growth under salt stress. Front. Plant Sci. 2023, 14, 1283590. [Google Scholar] [CrossRef]
- Chhetri, G.; Kim, J.; Kim, H.; Kim, I.; Seo, T. Pontibacter oryzae sp. nov., a carotenoid-producing species isolated from a rice paddy field. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2019, 112, 1705–1713. [Google Scholar] [CrossRef]
- Anal, A.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Luo, C.; Lu, F.; Shao, L.; He, P. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 2015, 68, 710–718. [Google Scholar] [CrossRef]
- Katerji, N.; Hoorn, J.; Hamdy, A.; Karam, F.; Mastrorilli, M. Effect of salinity on emergence and on water stress and early seedling growth of sunflower and maize. Agric. Water Manag. 1994, 26, 81–91. [Google Scholar] [CrossRef]
- Wang, M.; Luo, G.; Sun, Y.; Chang, C. Numerical modeling of stress perturbations caused by geometric changes of salt bodies. Pet. Explor. Dev. 2020, 47, 331–342. [Google Scholar] [CrossRef]
- Qi, X.; Chen, T.; Ding, C.; Chen, X.; He, V.; Hu, W. Diversity of fungal communities in the rhizosphere soil of Tamarix chinensis in saline-alkaline wetland. Environ. Dev. Sustain. 2023. [Google Scholar] [CrossRef]
- Bekkaye, M.; Baha, N.; Behairi, S.; MariaPerez-Clemente, R.; Kaci, Y. Impact of Bio-inoculation with Halotolerant Rhizobacteria on Growth, Physiological, and Hormonal Responses of Durum Wheat Under Salt Stress. J. Plant Growth Regul. 2023, 42, 6549–6564. [Google Scholar] [CrossRef]
- Sorty, A.; Meena, K.; Choudhary, K.; Bitla, U.; Minhas, P.; Krishnani, K. Effect of Plant Growth Promoting Bacteria Associated with Halophytic Weed (Psoralea corylifolia L) on Germination and Seedling Growth of Wheat Under Saline Conditions. Appl. Biochem. Biotechnol. 2016, 180, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Khan, M.; Misra, S.; Dixit, V.; Khare, P.; Srivastava, S.; Chauhan, P. Characterisation of Pseudomonas spp. and Ochrobactrum sp. isolated from volcanic soil. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2017, 110, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Ellouzi, H.; Hamed, K.; Hernández, I.; Cela, J.; Müller, M.; Magné, C.; Abdelly, C.; Munné-Bosch, S. A comparative study of the early osmotic, ionic, redox and hormonal signaling response in leaves and roots of two halophytes and a glycophyte to salinity. Planta 2014, 240, 1299–1317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Meng, W.; Wang, Y.; Zhou, Y.; Wang, S.; Qi, F.; Wang, N.; Ma, J. Comparative Analysis of Physiological, Hormonal and Transcriptomic Responses Reveal Mechanisms of Saline-Alkali Tolerance in Autotetraploid Rice (Oryza sativa L.). Int. J. Mol. Sci. 2022, 23, 16146. [Google Scholar] [CrossRef]
- Zvinavashe, A.; Lim, E.; Sun, H.; Marelli, B. A bioinspired approach to engineer seed microenvironment to boost germination and mitigate soil salinity. Proc. Natl. Acad. Sci. USA 2019, 116, 25555–25561. [Google Scholar] [CrossRef]
- Jouanneau, D.; Klau, L.; Larocque, R.; Jaffrennou, A.; Duva, G.; Duff, N.; Roret, T.; Jeudy, A.; Aachmann, F.; Czjzek, M. Structure-function analysis of a new PL17 oligoalginate lyase from the marine bacterium Zobellia galactanivorans DsijT. Glycobiology 2021, 31, 1364–1377. [Google Scholar] [CrossRef]
- Xu, X.; Iwamoto, Y.; Kitamura, Y.; Oda, T.; Muramatsu, T. Root growth-promoting activity of unsaturated oligomeric uronates from alginate on carrot and rice plants. Biosci. Biotechnol. Biochem. 2003, 67, 2022–2025. [Google Scholar] [CrossRef]
- Sá, C.; Cardoso, P.; Figueira, E. Alginate as a feature of osmotolerance differentiation among soil bacteria isolated from wild legumes growing in Portugal. Sci. Total Environ. 2019, 681, 312–319. [Google Scholar] [CrossRef]
- Shu, X.; He, J.; Zhou, Z.; Xia, L.; Hu, Y.; Zhang, Y.; Zhang, Y.; Luo, Y.; Chu, H.; Liu, W.; et al. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [CrossRef]
- O’Callaghan, M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef]
- van Bruggen, A.H.C.; Semenov, A.M. In search of biological indicators for soil health and disease suppression. Appl. Soil Ecol. 2000, 15, 13–24. [Google Scholar] [CrossRef]
- Abdel Latef, A.; Mostofa, M.; Rahman, M.; Abdel-Farid, I.; Tran, L. Extracts from Yeast and Carrot Roots Enhance Maize Performance under Seawater-Induced Salt Stress by Altering Physio-Biochemical Characteristics of Stressed Plants. J. Plant Growth Regul. 2019, 38, 966–979. [Google Scholar] [CrossRef]
- Wang, X.; Tao, M.; Fang, C.; Chen, X. Isolation, identification and characterization of a strain of Sphingomonas sp. strain. J. Dalian Univ. Technol. 2019, 38, 403–407. [Google Scholar]
- Yu, H.; Zhou, F.; Li, F.; Zhang, G.; Zhou, H.; Zhang, X. Research Advances in Phosphorus-solubilizing Microorganisms and Their Applications in Soil Pollution Control. Environ. Sci. Technol. 2020, 43, 44–51. [Google Scholar]
- Nunes, P.; de Medeiros, F.; de Oliveira, T.; Zago, J.; Bettiol, W. Bacillus subtilis and Bacillus licheniformis promote tomato growth. Braz. J. Microbiol. 2023, 54, 397–406. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Kashyap, A.; Kumar, R.; Gujjar, R.; Singh, A.; Manzar, N. Harnessing Rhizospheric Microbes for Eco-friendly and Sustainable Crop Production in Saline Environments. Curr. Microbiol. 2024, 81, 14. [Google Scholar] [CrossRef] [PubMed]
- Le, V.; Nguyen, Q.; Nguyen, N.; Le, T.; Janda, T.; Szalai, G.; RUI, Y. Simultaneous determination of plant endogenous hormones in green mustard by liquid chromatography—Tandem mass spectrometry. Chin. J. Anal. Chem. 2021, 49, 111–117. [Google Scholar] [CrossRef]
- Bao, S. Soil and Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 23–106. [Google Scholar]
- Kuczynski, J.; Lauber, C.; Walters, W.; Parfrey, L.; Clemente, J.; Gevers, D.; Knight, R. Experimental and analytical tools for studying the human microbiome. Nat. Rev. Genet. 2012, 13, 47–58. [Google Scholar] [CrossRef]
- Peiffer, J.; Spor, A.; Koren, O.; Zhao, J.; Tringe, S.; Dangl, J.; Buckler, E.; Ley, R. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6548–6553. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Tang, Z.; Shangguan, Z.; Chang, F.; Jia, F.; Chen, Y.; He, X.; Shi, W.; Deng, L. Effects of grassland afforestation on structure and function of soil bacterial and fungal communities. Sci. Total Environ. 2019, 676, 396–406. [Google Scholar] [CrossRef] [PubMed]




| Treatment | pH | EC dS m−1 | Organic Matter g kg−1 | Available Nitrogen mg kg−1 | Available Phosphorus mg kg−1 | Available Potassium mg kg−1 |
|---|---|---|---|---|---|---|
| CK | 8.3 b | 4.2 b | 15.2 a | 48.5 a | 21.7 b | 117.6 a |
| ME | 8.0 a | 3.3 a | 16.5 a | 49.5 a | 27.3 a | 118.3 a |
| MB | 8.1 b | 3.7 a | 16.2 a | 51.2 a | 23.7 b | 116.1 a |
| Soil Layers cm | pH | EC dS m−1 | Organic Matter g kg−1 | Total Nitrogen g kg−1 | Total Phosphorus g kg−1 | Total Potassium g kg−1 |
|---|---|---|---|---|---|---|
| 0–20 | 8.22 | 4.51 | 15.18 | 1.09 | 1.03 | 18.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, Q.; Gong, M.; Jiang, Y.; Yang, X.; Kong, M.; He, J.; Zhang, Q.; Song, J.; Li, X.; Han, W.; et al. Microencapsulated Microbial Seed Coating Could Improve Soil Environment and Maize Grain Yield in Saline Soil. Plants 2024, 13, 3139. https://doi.org/10.3390/plants13223139
Huo Q, Gong M, Jiang Y, Yang X, Kong M, He J, Zhang Q, Song J, Li X, Han W, et al. Microencapsulated Microbial Seed Coating Could Improve Soil Environment and Maize Grain Yield in Saline Soil. Plants. 2024; 13(22):3139. https://doi.org/10.3390/plants13223139
Chicago/Turabian StyleHuo, Qiuyan, Min Gong, Yawen Jiang, Xi Yang, Meng Kong, Jiuxing He, Qiang Zhang, Jiqing Song, Xinzhu Li, Wei Han, and et al. 2024. "Microencapsulated Microbial Seed Coating Could Improve Soil Environment and Maize Grain Yield in Saline Soil" Plants 13, no. 22: 3139. https://doi.org/10.3390/plants13223139
APA StyleHuo, Q., Gong, M., Jiang, Y., Yang, X., Kong, M., He, J., Zhang, Q., Song, J., Li, X., Han, W., Mei, X., & Lv, G. (2024). Microencapsulated Microbial Seed Coating Could Improve Soil Environment and Maize Grain Yield in Saline Soil. Plants, 13(22), 3139. https://doi.org/10.3390/plants13223139








