Abstract
Top grafting is an efficient and practical technique for the renewal and rejuvenation of citrus trees in old orchards. However, root death after top grafting restricts plant growth and canopy reconstruction. Thus, applications of rooting promotion substances before citrus top grafting may increase the amount and activity of roots, thereby enhancing top-grafted plant performance. To test this assumption, four rooting promotion substances, i.e., rooting promotion powder, biochar, organic fertilizer, and potassium fulvic acid, were applied before top grafting, and the effects on biometric and physiological parameters were analyzed after top grafting. The results showed that the application of all rooting promotion substances before top grafting has a positive effect on growth and mineral nutrient acquisition, as well as on foliar C and N assimilates and the activity of anti-oxidative enzymes of top-grafted plants. Rooting promotion powder and biochar had the best effect on top-grafted tree performance in the short term. In conclusion, pre-grafting root promotion reduced root damage, enhanced nutrient acquisition, and improved the physiological performance of top-grafted plants. Therefore, this approach can play a crucial role in accelerating canopy reconstruction in old citrus orchards and in improving citrus plant development.
1. Introduction
Based on production and cultivation area, citrus is one of the most important fruit crops in the world, particularly in tropical and subtropical regions [1,2]. Grafting constitutes an excellent asexual propagation technique that is widely applied by the citrus industry to improve growth [3], resistance [4], and fruit yield and quality [5,6]. In recent years, top grafting has rapidly become a means to adjust the structure of citrus trees and improve market competitiveness. Top-grafting after root grafting combines three different citrus species in one to form a new hybrid with excellent characteristics, such as rapid seeding, crown formation, and high fruit yield and quality [7,8].
However, improper grafting and cultivation management often lead to the slow growth of scions, late crown establishment, and slow yield recovery. For successful grafting and appropriate scion growth, grafting time [9,10] and grafting height are essential [7,11]. In addition, management practices such as fertilization [12], bud blotting [13], and irrigation after top grafting are key factors affecting grafting success [14]. Truncated top grafting mediates an imbalance in the root-to-crown ratio and causes a large area of the root to decline [15]. However, significant root apoptosis restricts mineral nutrient uptake and constrains the growth and development of the aboveground portion of top-grafted plants, which mainly depend on the nutrition status of the root and the trunk [16,17].
In this context, substances that promote root growth, commonly used in horticulture/agriculture, may improve grafted citrus growth. Biochar, as a soil amendment, can regulate the physical structure of the soil and improve the growth environment of roots [18]. Organic fertilizer, which can decompose to produce organic acids, enhances the mineralization of organic nutrients and increases the abundance of beneficial microorganisms in the soil [19]. Potassium fulvic acid can adsorb, exchange, and activate mineral elements in soils, thereby improving soil nutrient availability. As a plant growth regulator, potassium fulvic acid can also stimulate root growth [20].
Previous studies have shown that root growth and performance of fruit trees can be effectively promoted by applying biochar [21], organic fertilizer [22], and several bio-stimulants to grafted plants [23]. Similarly, rooting powder can induce lateral root formation due to its high content of various auxins [24]. In a study with grapes, Zhou et al. [25] showed that treating rootstocks with rooting promotion powder before grafting can inhibit scion formation while promoting tissue healing and rooting, thereby enhancing grafting success. However, similar studies have not been reported for citrus trees. Therefore, we exposed the roots of citrus trees to four rooting promotion substances, namely rooting promotion powder, biochar, organic fertilizer, and potassium fulvic acid, before top grafting and analyzing the performance of the trees after top grafting. We hypothesized that these treatments (a) differently alleviate root apoptosis caused by top grafting and, thus, (b) differently accelerate nutrient uptake as well as the crown reestablishment of top-grafted citrus trees. In addition, we hypothesized that pre-grafting root promotion (c) significantly improves post-grafting tree performance by stimulating C and N assimilation and enhancing the activity of anti-oxidative enzymes in the leaves.
2. Results
2.1. Biometric Parameters
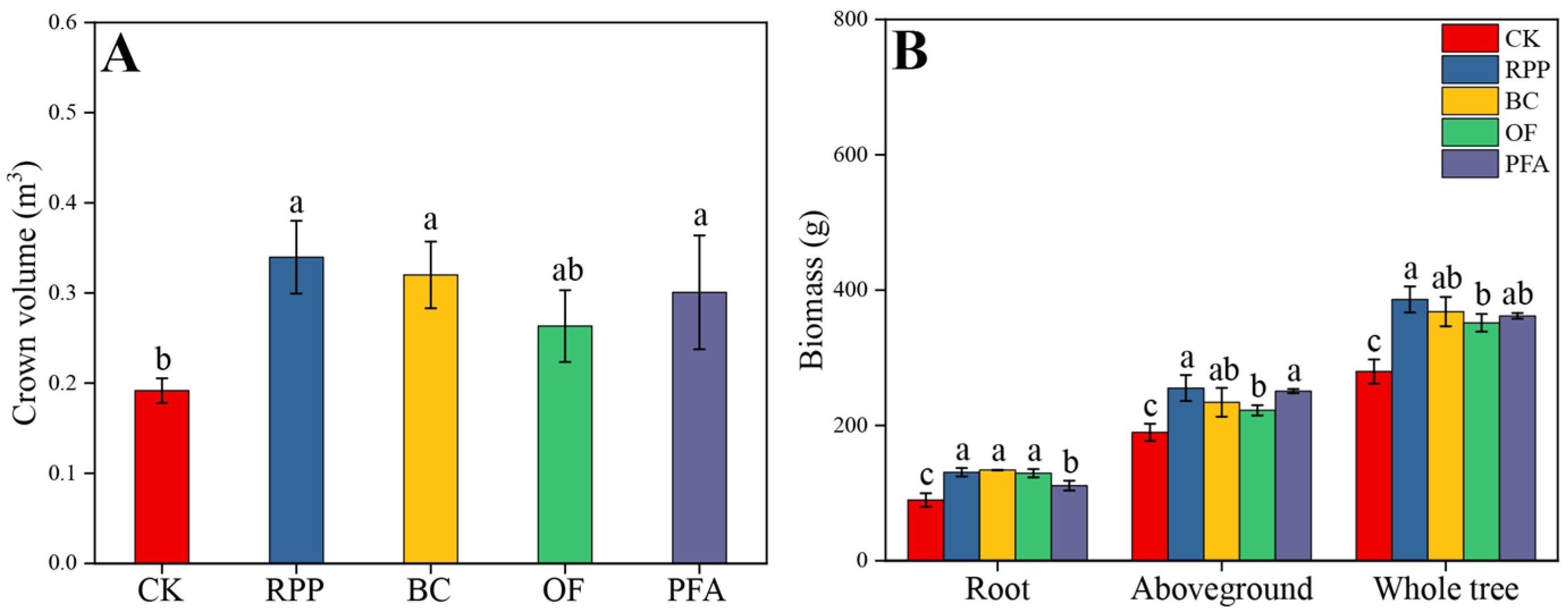
The growth of top-grafted citrus plants was improved by rooting promotion treatments (Figure S1). Except for the organic fertilizer treatment, the other three rooting promotion substances applied before top grafting increased the crown volume of citrus plants after top grafting (Figure 1A). The largest crown volume of 0.34 m3 was observed in the rooting promotion powder treatment, with a significant increase of 79.0%. All rooting promotion treatments significantly increased root biomass, aboveground biomass, and whole tree biomass of citrus (Figure 1B). Biochar treatment showed the highest root biomass (134 g plant−1), with a significant increase of 49.1%. The aboveground biomass and whole tree biomass were highest in the rooting promotion powder treatment, with a significant increase of 34.5% and 38.0%, respectively. These results show that plants treated with rooting promotion powder or biochar had the best growth performance.
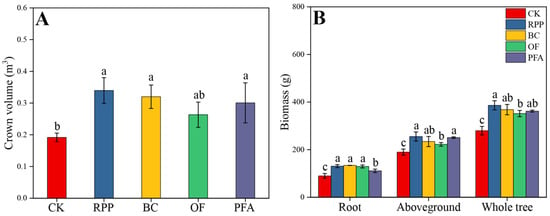
Figure 1.
Crown volume (A) and biomass production (B) were affected by four rooting promotion substances. CK: without rooting promotion substances; RPP: rooting promotion powder; BC: biochar; OF: organic fertilizer; PFA: potassium fulvic acid. Different lowercase letters represent statistically significant differences between treatments (one-way ANOVA (Duncan), p < 0.05, n = 3).
2.2. Root Parameters
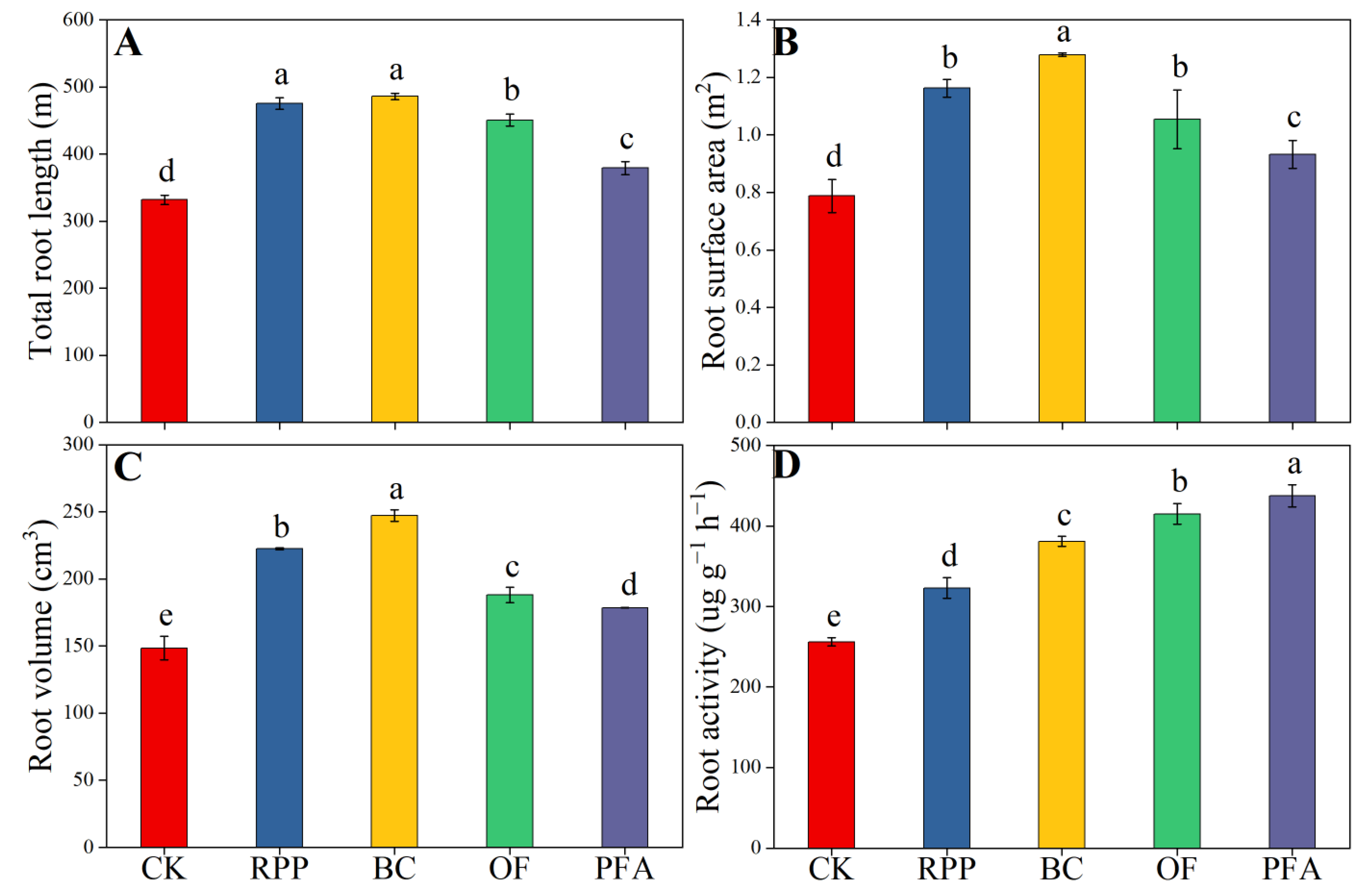
The four rooting promotion substances applied before top grafting significantly promoted total root length, root surface area, root volume, and root activity after top grafting (Figure 2), with the biochar treatment showing the strongest effect. Compared to the control treatment, root growth, total root length, root surface area, root volume, and root activity were significantly improved upon biochar treatment by 46.4%, 62.0%, 66.5%, and 48.7%, respectively. Thus, biochar was the best substance to enhance root growth.
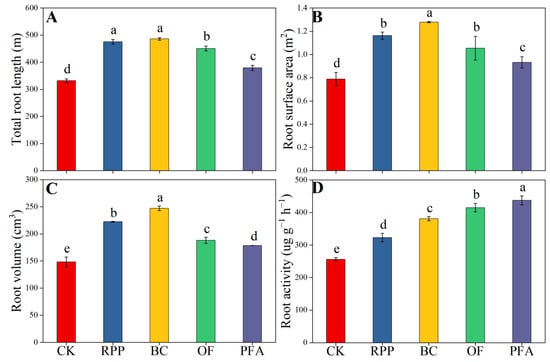
Figure 2.
The total length (A), surface area (B), volume (C), and activity (D) of the root. CK: without rooting promotion substances; RPP: rooting promotion powder; BC: biochar; OF: organic fertilizer; PFA: potassium fulvic acid. Different lowercase letters represent statistically significant differences between treatments (one-way ANOVA (Duncan), p < 0.05, n = 3).
2.3. Leaf Physiological Characteristics
2.3.1. Soluble Sugar and Starch Contents
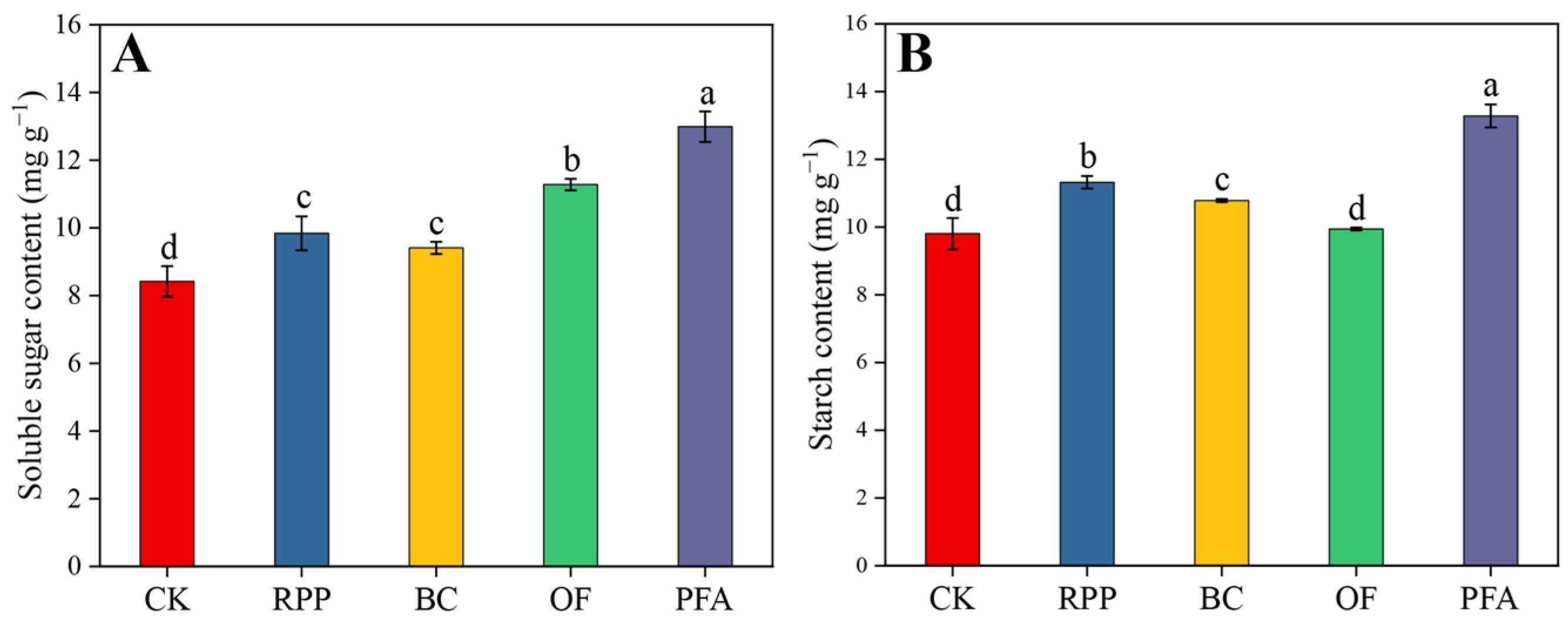
After top grafting, the leaf soluble sugar contents were significantly enhanced by the four rooting promotion treatments (Figure 3A). The leaf soluble sugar contents were highest in plants treated with potassium fulvic acid (13.0 mg g−1), with an increase of 54.3%. Except for the organic fertilizer application, leaf starch contents in plants subjected to the other rooting promotion treatments were significantly increased by 10.0–35.5% (Figure 3B), with the potassium fulvic acid treatment showing the highest effect. Thus, potassium fulvic acid was the best substance to increase the content of non-structural carbohydrates in leaves.
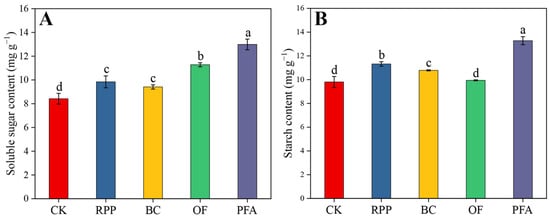
Figure 3.
The contents of soluble sugar (A) and starch (B) in the leaves. CK: without rooting promotion substances; RPP: rooting promotion powder; BC: biochar; OF: organic fertilizer; PFA: potassium fulvic acid. Different lowercase letters represent statistically significant differences between treatments (one-way ANOVA (Duncan), p < 0.05, n = 3).
2.3.2. Soluble Protein and Free Amino Acid Contents
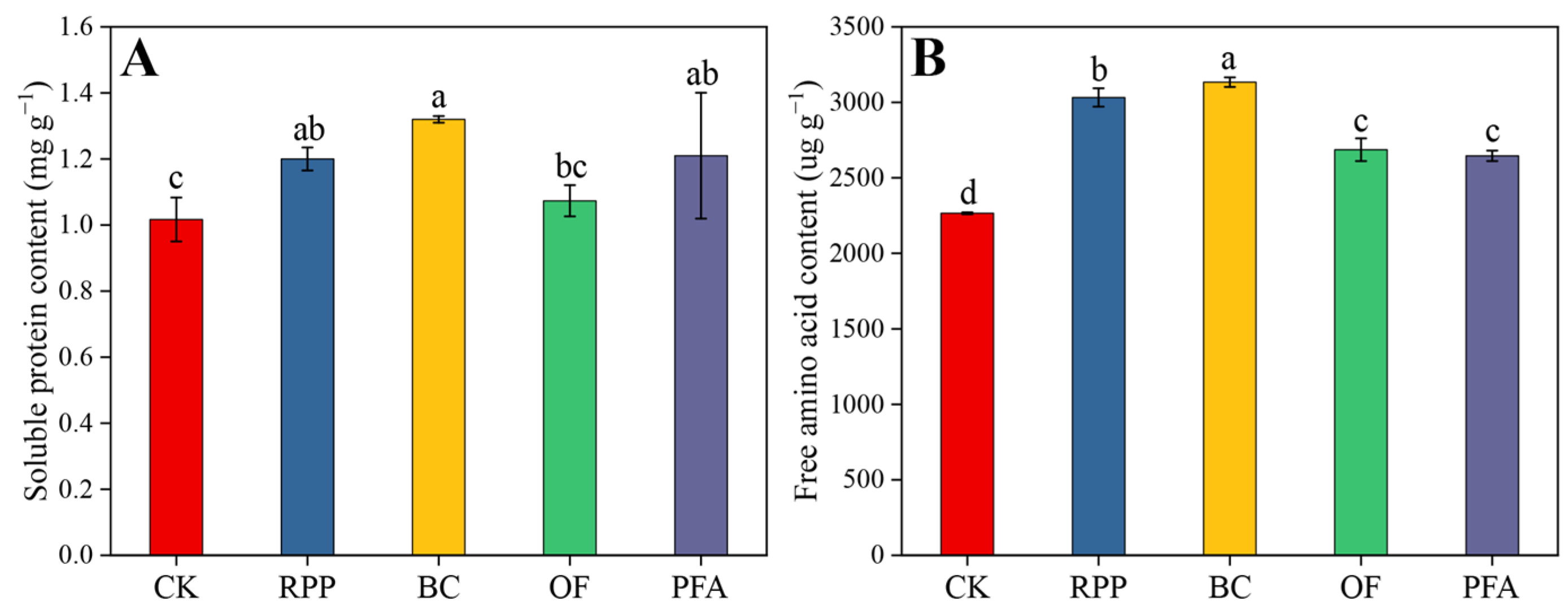
Except for the organic fertilizer treatment, leaf soluble protein of plants subjected to the other rooting promotion treatments was significantly higher than in controls after top grafting. Moreover, the effects of rooting promotion powder, biochar, and potassium fulvic acid did not differ significantly between each other (Figure 4A). All rooting promotion treatments significantly increased the leaf amino acid contents by 16.8–38.3%, with the biochar treatment showing the highest increase (Figure 4B). Thus, biochar was the best substance to increase the content of soluble protein and free amino acids in leaves.
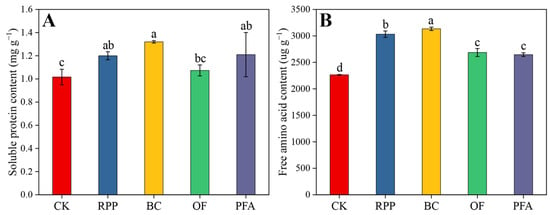
Figure 4.
The contents of soluble protein (A) and free amino acid (B) in the leaves. CK: without rooting promotion substances; RPP: rooting promotion powder; BC: biochar; OF: organic fertilizer; PFA: potassium fulvic acid. Different lowercase letters represent statistically significant differences between treatments (one-way ANOVA (Duncan), p < 0.05, n = 3).
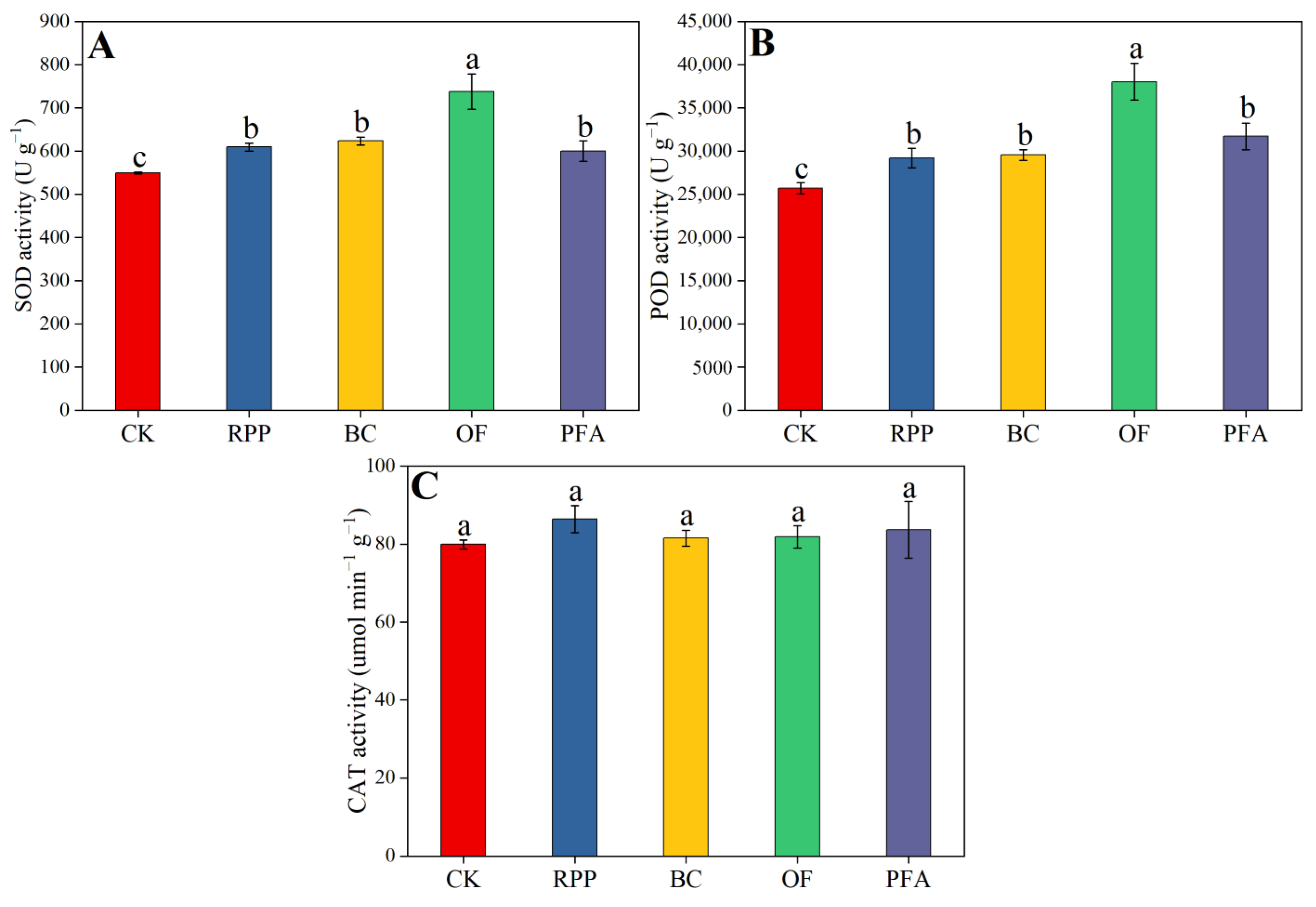
2.3.3. Anti-Oxidative Enzyme Activities
Leaf SOD and POD enzyme activities were enhanced by all rooting promotion treatments, but this increase did not differ between rooting promotion powder, biochar, and potassium fulvic acid and was highest in the organic fertilizer treatment. Relative to the treatments with rooting promotion powder, biochar, and potassium fulvic acid, organic fertilizer significantly improved leaf SOD and POD enzyme activities by 18.4–23.0% (Figure 5A) and 20.0–30.3% (Figure 5B), respectively. The leaf CAT enzyme activity was not affected by the pre-grafting treatments (Figure 5C). Thus, organic fertilizer mediated the strongest increase in the anti-oxidant enzyme activity of citrus leaves.
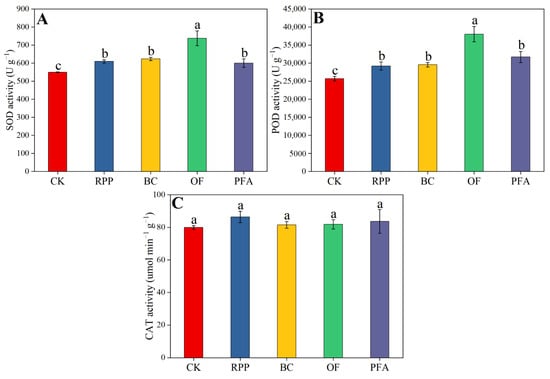
Figure 5.
The activity of SOD (A), POD (B), and CAT (C) in the leaves. CK: without rooting promotion substances; RPP: rooting promotion powder; BC: biochar; OF: organic fertilizer; PFA: potassium fulvic acid. Different lowercase letters represent statistically significant differences between treatments (one-way ANOVA (Duncan), p < 0.05, n = 3).
2.4. Plant Nutrient Composition
2.4.1. Plant Nutrient Concentrations
The effect of root-promoting substances on the mineral nutrient content of the top-grafted citrus tree is shown in Table 1. In roots, biochar treatment significantly enhanced P concentrations of 1.98 g kg−1. Biochar and potassium fulvic acid significantly increased root K concentrations by 77.2% and 68.3%, respectively. In addition, significantly enhanced root Ca concentrations of 27.2% were found in the organic fertilizer treatment. In stems, rooting promotion powder and organic fertilizer significantly increased the K concentrations by 7.62% and 9.93%, respectively, while biochar treatment significantly decreased stem K concentrations by 5.87%. In branches, the P concentrations of branches treated with organic fertilizer were significantly increased by 10.6%. Potassium fulvic acid significantly increased the branch K concentrations by 10.8%. Rooting promotion powder and biochar significantly increased the branch Ca concentrations by 1.21% and 4.59%, respectively. In leaves, total N concentrations of plants treated with rooting promotion powder significantly increased by 7.40%. Except for potassium fulvic acid, the other three rooting promotion treatments markedly improved leaf Ca concentrations by 13.0–28.8%. However, significantly enhanced Mg concentrations of 27.4% were found in leaves with the potassium fulvic acid treatment. Thus, rooting promotion powder can increase the concentration of several nutrients in citrus stems, branches, and leaves; and biochar and potassium humic acid in citrus roots, branches, and leaves. Organic fertilizer increased the concentration of several nutrients in the whole tree.

Table 1.
Effect of different treatments on the nutrient content of the top-grafted citrus. CK: without rooting promotion substances; RPP: rooting promotion powder; BC: biochar; OF: organic fertilizer; PFA: potassium fulvic acid. Data were presented as mean ± standard deviation. Different lowercase letters represent statistically significant differences between treatments (one-way ANOVA (Duncan), p < 0.05, n = 3).
2.4.2. Plant Nutrient Accumulation
Rooting promotion powder, biochar, and organic fertilizer significantly increased the root accumulation of total N by 51.3–62.9%, of P by 59.2–108%, and of Ca by 59.1–84.4% (Figure S2A,D,J). Except for organic fertilizer, there was a significant increase in root K accumulation by the other three rooting promotion treatments by 106–164% (Figure S2G). All four rooting promotion treatments, significantly improved root Mg accumulation by 38.8–59.1% (Figure S2M), as well as aboveground total N, P, K, and Ca accumulation by 24.0–44.8%, 24.5–37.2%, 20.6–45.8%, and 34.6–43.7%, respectively (Figure S2B,E,H,K). Except for organic fertilizer, aboveground Mg accumulation was significantly enhanced by 26.2–47.9% in the other three rooting promotion treatments. All rooting promotion treatments significantly improved the whole tree total N, P, K, Ca, and Mg accumulation by 33.6–49.0%, 33.7–62.6%, 31.1–60.6%, 38.7–55.6%, and 37.1–52.3%, respectively (Figure S2C,F,I,L,O). In summary, the four rooting promotion treatments increase the nutrient accumulation in roots, aboveground biomass, and the whole tree, but at different degrees.
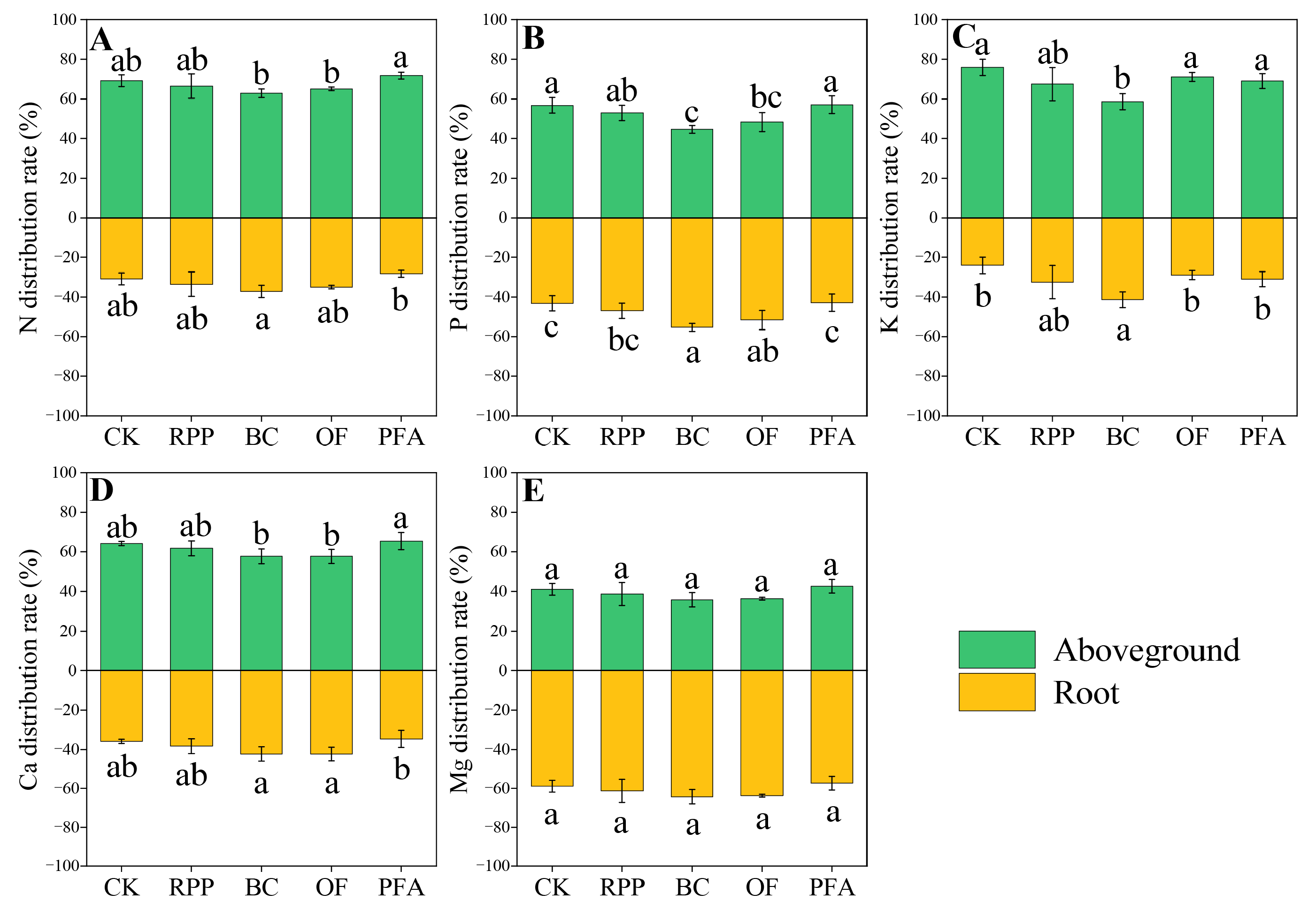
2.4.3. Plant Nutrient Distribution
In citrus trees treated with biochar, total N distribution was increased in favor of the roots by 20.56% compared to the control (Figure 6A). Biochar and organic fertilizer significantly increased the P distribution in favor of the roots by 12.2% and 8.46%, respectively, but there was no significant effect of the other two rooting promotion treatments (Figure 6B). However, the biochar treatment significantly enhanced the K distribution in favor of the roots by 17.3% (Figure 6C). However, in citrus trees treated with biochar and organic fertilizer, higher Ca distribution was increased in favor of the roots by 10.3 to 22.2% and 10.5 to 22.3%, respectively (Figure 6D). Thus, the rooting promotion treatments enhanced the distribution of specific nutrients in favor of the roots but not in favor of aboveground biomass.
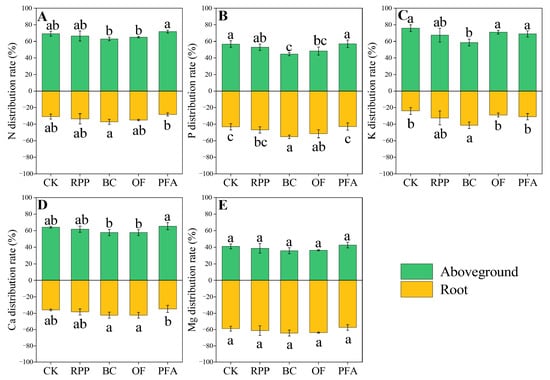
Figure 6.
The N (A), P (B), K (C), Ca (D), and Mg (E) distribution of the top-grafted plant. CK: without rooting promotion substances; RPP: rooting promotion powder; BC: biochar; OF: organic fertilizer; PFA: potassium fulvic acid. Different lowercase letters represent statistically significant differences between treatments (one-way ANOVA (Duncan), p < 0.05, n = 3).
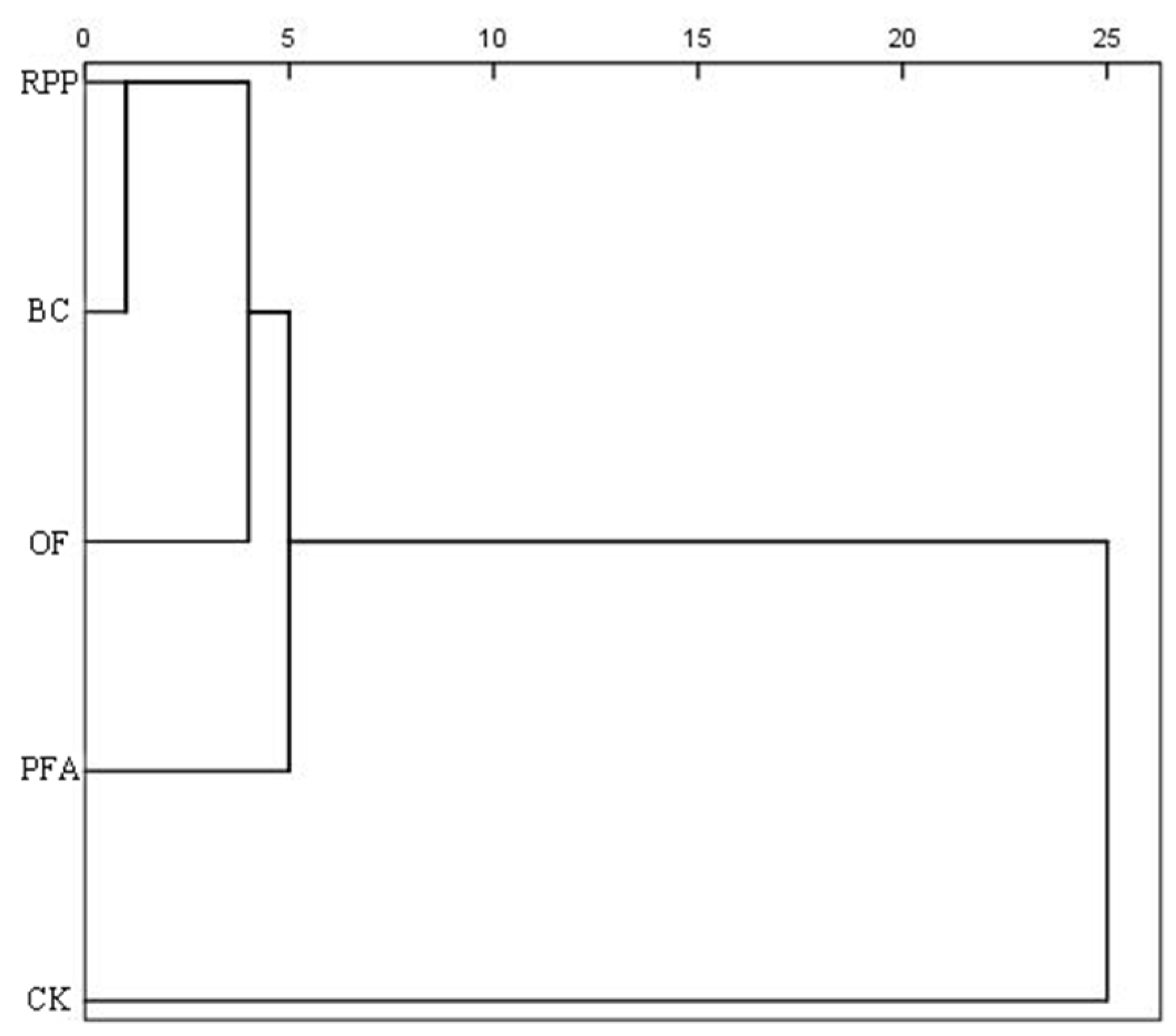
2.5. Cluster and Comprehensive Effects Analyses
Cluster analysis revealed that the five treatments were categorized into two main clusters (Figure 7). The first cluster comprised the four rooting promotion treatments, accounting for 80% of all treatments, while the second cluster represented the control, making up 20%. These results indicated that rooting promotion substances had significant effects on top-grafted plant growth, physiology, and nutrient accumulation.
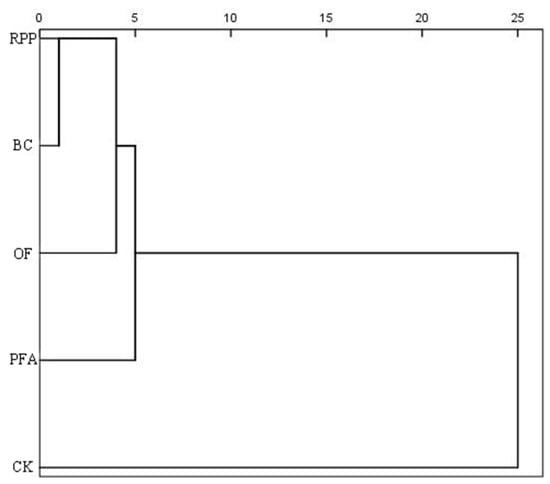
Figure 7.
Cluster analysis of different rooting promotion treatments on the growth, physiology, and nutrient accumulation of citrus after top grafting. CK: without rooting promotion substances; RPP: rooting promotion powder; BC: biochar; OF: organic fertilizer; PFA: potassium fulvic acid.
To further assess the comprehensive effects, a coefficient of variation analysis was performed on the five treatments, and composite scores were ranked (Table 2). The results indicated that treatments with rooting promotion powder and biochar had the most favorable outcomes among the four rooting promotion treatments, with composite scores of 0.65 and 0.64, respectively.

Table 2.
Comprehensive score and ranking of coefficient of variation for various rooting promotion treatments. CK: without rooting promotion substances; RPP: rooting promotion powder; BC: biochar; OF: organic fertilizer; PFA: potassium fulvic acid.
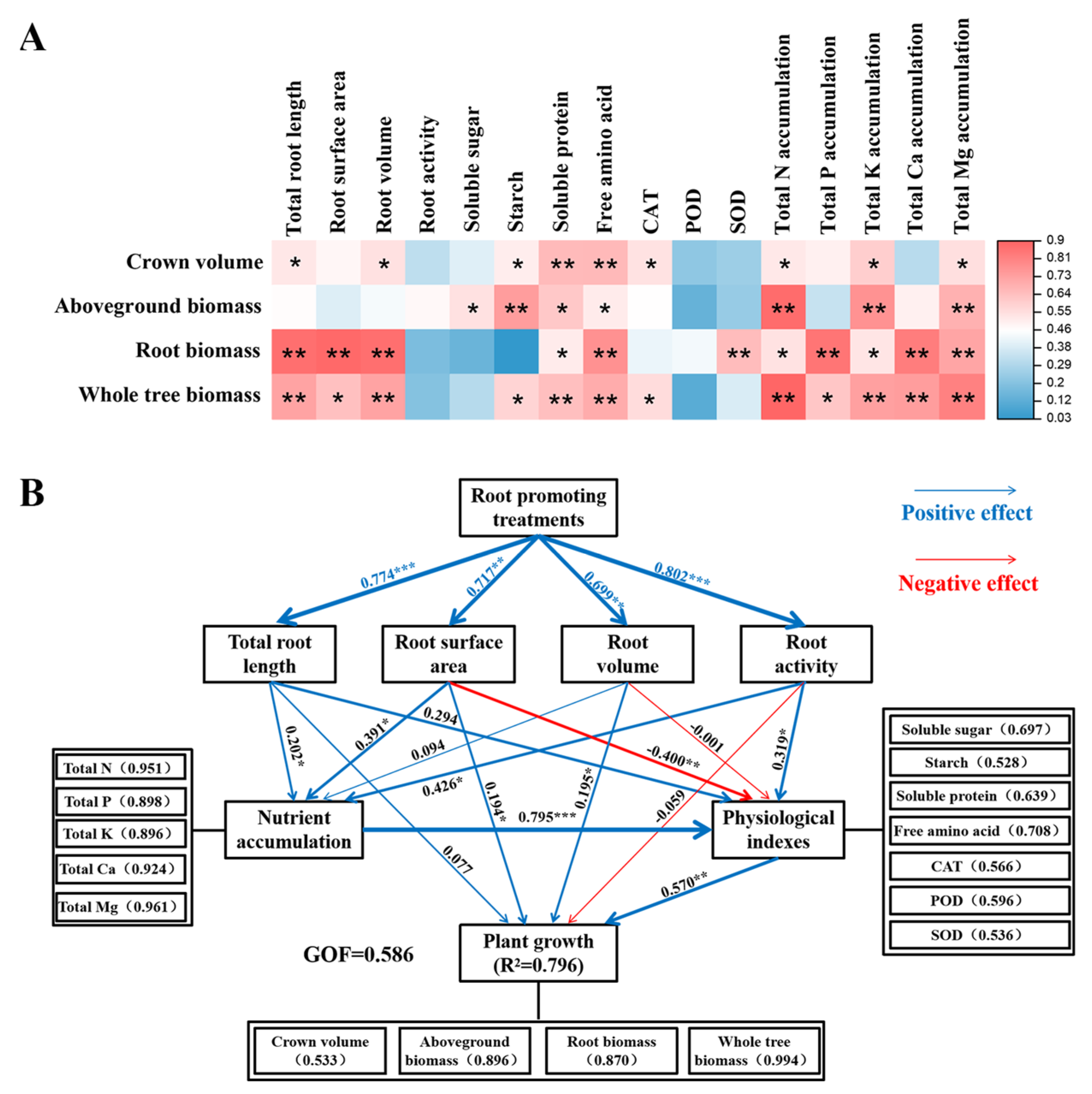
2.6. Correlation Analysis and Partial Least Squares Path Model Analysis
Correlation analysis revealed that canopy size and biomass production were significantly positively correlated with the morphological structure of the root system and most leaf physiological indicators, such as starch, soluble protein, and free amino acids. Moreover, there was a significantly positive correlation between citrus nutrient accumulation and growth indicators (Figure 8A).
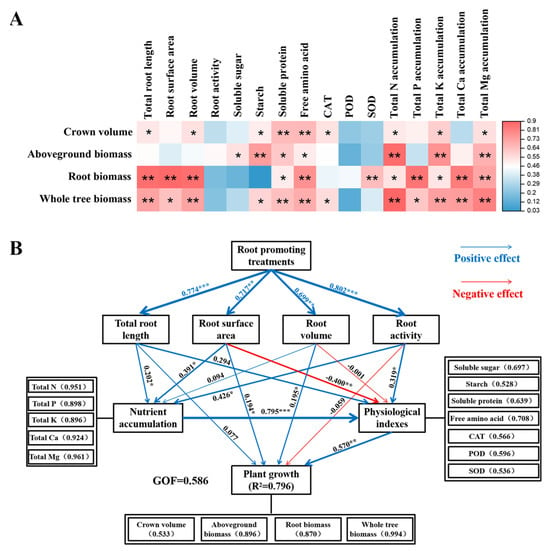
Figure 8.
Correlation analysis (A) and partial least squares path model (PLS-PM) analysis (B) of citrus growth under different treatments before top grafting. Colors and asterisks represent the magnitude of correlation (* indicates significance at p < 0.05, ** indicates significance at p < 0.01, *** indicates significance at p < 0.001). Numbers within parentheses correspond to specific loading values, and GOF represents the overall fit of the model.
To further explore the mechanism by which root-promoting substances regulate the growth of top-grafted citrus trees, after top grafting, we used the partial least squares path model (PLS-PM) to dissect the relationships among root traits, nutrient acquisition, leaf physiological indicators, and the growth of citrus plants under different rooting promotion treatments. The goodness of fit under the model was 0.586, and the PLS-PM explained 79.6% of the variation in plant growth. Rooting treatments significantly influenced root-related morphological traits, such as total root length, root surface area, root activity, and nutrient acquisition. Root activity and nutrient acquisition significantly affected leaf physiological and biochemical characteristics. Moreover, root surface area, root volume, and leaf physiological and biochemical traits directly affected plant growth. Thus, PLS-PM analyses indicated that rooting promotion treatments had a direct impact on root traits. Root development modified leaf physiological and biochemical characteristics through influencing nutrient acquisition, thereby improving plant growth (Figure 8B).
3. Discussion
3.1. Root Promotion Before Top Grafting Supports Root Growth of Top-Grafted Citrus Trees
Consistent with our hypothesis (a), the present research results indicate that pre-grafting root promotion can improve the post-grafting growth of roots. This suggests that rooting-promoting substances may effectively alleviate root apoptosis caused by top grafting through different root-promoting mechanisms. Rooting promotion powder significantly affected root morphology after top grafting (Figure 2). Previous studies showed that rooting promotion powder contains various growth-promoting hormones such as IAA, NAA, and IBA [26]. Apparently, the auxins present in rooting promotion powder affect root morphology by inducing lateral root production and adventitious root formation [24,27,28,29], thereby promoting root growth and significantly increasing root length, surface area, and volume [30,31,32]. However, the root activity in the rooting promotion powder treatment was significantly lower than in the other rooting promotion treatments of this study (Figure 2D). This might be due to the time-limited efficacy of the rooting promotion powder, with the root-promoting effect diminishing over time. In the present study, the most noticeable improvement in root traits was observed by the application of biochar. This effect can be attributed to the porous nature of biochar, which reduces substrate bulk density, increases porosity, and enhances substrate permeability [18], thereby improving the growth environment of roots and boosting root growth and vitality. Organic fertilizer treatment exhibited weaker effects on root traits compared to rooting promotion powder and biochar treatments (Figure 2A–C). This could be due to the fact that organic fertilizer constitutes a slow-release fertilizer [19], and its maximum root-promoting effect might only be realized in the long run. Still, organic fertilizer significantly increased root activity (Figure 2D), which is consistent with previous observations in sugar beet [33]. This result can be attributed to the presence of active substances, such as amino acids, humic acids, and beneficial microorganisms, that stimulate root physiological characteristics. In the present study, the increase in root activity by the application of potassium fulvic acid was significantly higher than in the other treatments (Figure 2D), possibly due to the influence of this compound on plant hormone metabolism and the concomitant regulation of root growth [34].
3.2. Rooting Promotion Before Top Grafting Enhances Nutrient Acquisition of Top-Grafted Citrus Trees
Consistent with our hypothesis (b), pre-grafting root promotion effectively improved post-grafting nutrient acquisition and the crown reconstruction of citrus trees. PLS-PM analysis indicated a significantly positive correlation between root traits and plant nutrient acquisition, suggesting that rooting promotion treatments enhanced nutrient accumulation by improving the root development of top-grafted citrus (Figure 8B). Overall, rooting promotion substances increased the acquisition of N, P, K, Ca, and Mg (Figure S2). This may be attributed to hormone regulation [26], soil adsorption [35,36,37,38], and soil fertilization effects of the root-promoting substances. Rooting promotion powder had the most significant effects on total N accumulation (Figure S2A–C). Du et al. [39] suggested that growth hormones could positively regulate N remobilization in cucumber. The application of rooting promotion powder might elevate plant hormone levels, thereby enhancing the efficiency of N accumulation and utilization in plants. The increased K distribution in favor of the root part (Figure 6C) confirmed that biochar could serve as a valuable potassium source, providing plants with a high concentration of exchangeable K [40]. Furthermore, biochar application promoted P acquisition by the roots and significantly increased P distribution (Figure 6B) in favor of the roots and P accumulation in both aboveground biomass and roots (Figure S2). From previous studies in herbaceous plants, it was concluded that the porosity and surface characteristics of biochar provided a favorable habitat for the growth and reproduction of soil microorganisms, reducing competition among microorganisms and protecting beneficial soil microorganisms, especially mycorrhizal fungi [41,42,43]. This is of particular significance since numerous nutrients, such as P, N, and Zn, are not only directly absorbed by roots but also indirectly through mycorrhizal fungi. Nutrients absorbed and accumulated by plants through acquisition by mycorrhizal fungi can account for 30% to 70% of the total nutrient accumulation [44,45]. According to the results of grain crop studies, the decomposition of organic fertilizer produces organic acids that enhance the mineralization of organic nutrients in the soil, thereby promoting the decomposition of insoluble nutrients, increasing plant-available nutrients, and effectively improving mineral nutrition [46,47]. The application of organic fertilizer particularly increased the content of plant-available phosphorus in the soil [48], thereby enhancing P acquisition by the roots and increasing P distribution in favor of the roots (Figure 6B). The application of potassium fulvic acid increased the absorption and accumulation of nutrients, especially of K, by citrus plants in the present study (Figure S2G–I). This effect can be attributed to the interaction of potassium fulvic acid with organic matter, oxides, hydroxides, minerals, and metal ions in the soil, effectively enhancing the availability of soil nutrients and promoting plant nutrient accumulation and utilization [20,49,50].
3.3. Rooting Promotion Before Top Grafting Affects Leaf Physiology of Top-Grafted Citrus Trees
The application of rooting promotion powder effectively stimulated C and N assimilation in top-grafted citrus leaves (Figure 3 and Figure 4). In previous studies of Tamarix chinensis and tobacco, this effect was attributed to increased chlorophyll contents [51,52], thereby improving photosynthesis and positively affecting sugar production in leaves [53]. This is due to auxin’s regulation of chlorophyll synthesis and the formation of chloroplasts [54,55]. Additionally, IBA and IAA in rooting powder can regulate nitrogen metabolism and protein synthesis [56]. In the present study, biochar treatment significantly increased the level of carbohydrates and nitrogen-containing compounds, such as soluble proteins and free amino acids, in the leaves of top-grafted citrus trees (Figure 4). Also, previous studies showed that biochar can increase soil C and N content [57] and improve the availability of nutrients in the soil [58]. In addition, our study demonstrated that organic fertilizer significantly increased the soluble sugar content in leaves (Figure 3), consistent with previous results [59]. Particularly, the combined use of organic and inorganic fertilizers can increase the photosynthetic rate and sucrose phosphate synthase (SPS) activity in leaves, leading to enhanced sucrose accumulation [60]. In the present potassium fulvic acid treatment, leaf soluble sugar and starch contents were significantly higher than in other treatments (Figure 3). A previous study in maize indicated that potassium fulvic acid directly affects CO2 assimilation and promotes carbohydrate accumulation by increasing phosphoenolpyruvate carboxylase (PEPC) and pyruvate orthophosphate dikinase (PPDK) activity at the transcriptional and translational levels [61].
As observed in the present study (Figure 5), a moderate rooting promotion powder treatment in Tamarix chinensis was previously shown to increase the activity of superoxide dismutase and peroxidase in leaves [51]. This effect can be interpreted either as oxidative stress by the rooting promotion powder treatment or as the induction of improved stress resistance. However, due to the time-limited nature of a single application of rooting promotion powder, its effect was significantly lower than in the organic fertilizer treatment. Also, biochar increased the activity of leaf superoxide dismutase and peroxidase in the present study. Zhang et al. [62] reported that the addition of biochar to the soil helps to alleviate the toxicity of organic and inorganic pollutants and enhances the anti-oxidant activity of maize. However, the organic fertilizer treatment exhibited the highest activity of superoxide dismutase and peroxidase in the leaves of top-grafted citrus trees (Figure 5), suggesting that organic fertilizer either posed particularly high oxidative stress to the plants or provided plants with particularly high stress resistance. The latter can be explained by beneficial microorganisms contained in organic fertilizers that enhance plant resistance to biotic or abiotic stress [63,64]. In the present study, anti-oxidative enzyme activities were significantly increased in the potassium fulvic acid treatment as compared to the control (Figure 5). Several studies previously showed that the application of potassium fulvic acid is a promising approach to mitigating abiotic stress in plants [65,66].
Consistent with our hypothesis (c), pre-grafting rooting promotion effectively stimulated C and N assimilation and enhanced the activity of anti-oxidative enzymes in the leaves. PLS-PM analysis of the present results indicates a significant positive correlation between nutrient acquisition and leaf physiological indicators in top-grafted citrus trees (Figure 8B). Numerous studies suggest that the balance of essential nutrients is closely related to plant carbon [67,68] and nitrogen metabolism [69] and participates in the regulation of anti-oxidant enzyme activity [70]. Therefore, improved mineral nutrition by the pre-grafting application of rooting promotion substances may have improved C and N metabolism and strengthened the anti-oxidative capacity of top-grafted citrus trees in the present study.
3.4. Rooting Promotion Before Top Grafting Improves Growth of Top-Grafted Citrus Trees
The application of rooting promotion substances significantly increased both aboveground and root biomass. The application of rooting promotion powder particularly promoted crown volume and aboveground biomass (Figure 1). Also, Parađiković et al. [71] reported that the application of rooting promotion powder significantly increased the plant height, leaf number, fresh weight, and dry weight of medicinal plants. Moreover, naphthylacetic acid, a major component of rooting promotion powder, was shown to significantly promote the plant height, crown volume, and stem and leaf biomass of strawberry [72]. In the present study, the effect of the pre-grafting promotion of rooting on aboveground biomass after top grafting was most significant in the rooting promotion powder treatment (Figure 1), indicating a superior influence of growth hormones in rooting promotion powder for the regulation of the growth and development of aboveground biomass compared to the other rooting promotion treatments.
De Souza Laurentino et al. [73] determined that the optimal dose of biochar for melon seedling development is 12 t ha−1 by the measurement of seedling biomass and root length. In the present study, the effect of biochar treatment on root biomass was most significant among the four rooting promotion treatments (Figure 1). This can be attributed to the influence of biochar application on the growth and distribution of roots in the soil, which enhances soil nutrient availability and fertility [74] and improves nutrient accumulation efficiency. Consistent with the results of the present study (Figure 1), studies on maize and citrus indicated that the application of appropriate amounts of organic fertilizer and potassium fulvic acid can increase both aboveground and root biomass [75,76]. Correlation analysis of the present data indicated that leaf physiology and nutrient acquisition are significantly positively correlated with citrus growth (Figure 8A). PLS-PM analysis further suggested that enhanced nutrient acquisition improves plant growth by affecting leaf physiology (Figure 8B). Thus, rooting promotion substances improve plant nutrient acquisition, thereby affecting not only root but also aboveground biomass traits that regulate plant growth after top grafting.
The application of top grafting has rapidly become a means of adjusting the structure of citrus varieties and improving their market competitiveness [8]. This technology causes, however, an imbalanced root-to-crown ratio and a significant decline in root biomass [15]. More seriously, a large number of citrus orchards did not pay attention to root strengthening management before top grafting, making it difficult for roots to recover. Using biochar [21], organic fertilizer [22], and some biostimulants constitutes an effective way to improve the growth of top-grafted plants [23]. In our study, all four rooting promotion substances increased the growth and mineral nutrient acquisition, as well as the foliar C and N assimilation and activity, of anti-oxidative enzymes of top-grafted plants. However, the degree of rooting promotion depended on the properties and mechanisms of different rooting promotion substances. Therefore, based on the present results, we recommend using rooting promotion powder and biochar before top grafting as an effective strategy to maintain the root-to-crown balance and, hence, the performance of citrus trees.
4. Materials and Methods
4.1. Site Description and Experimental Setup
The experiment was conducted in Shuangbai Village (107.6694° E, 30.2847° N, 465.7 m asl), Xinli Town, Zhong County, Chongqing, southwest China, from July 2020 to October 2021. This region has a subtropical monsoon climate with a mean annual temperature of 17 °C, a mean annual sunshine of 1327 h, and a mean annual precipitation of 1279 mm. For potted plant growth, an acidic purple soil with pH 5.04, organic matter 12.7 g kg−1, total nitrogen 0.5 g kg−1, available phosphorus 50.5 mg kg−1, available potassium 115 mg kg−1, exchangeable calcium 2311 mg kg−1, exchangeable magnesium 402 mg kg−1, available copper 1.80 mg kg−1, available iron 49.8 mg kg−1, available manganese 50.9 mg kg−1, and available Zn 3.19 mg kg−1 was used.
4.2. Experimental Treatments
Two-year-old citrus seedlings of Citrus reticulate L. grafted on C. junos (Sieb.) Tanaka rootstocks were planted in July 2020 in plastic pots with a top and bottom size of 38 and 30 cm diameter, respectively, and 40 cm height; 35 kg soil was used for each pot. Plants were treated with one of four rooting promotion substances: 2000 mg L−1 rooting promotion powder (2 g rooting promotion powder per plant; refer to Pirlak and Çinar [77] and product description), 2% biochar (0.7 kg biochar per plant; refer to Zhang et al. [78] and product description), 0.7 kg organic fertilizer per plant (refer to Zhang et al. [79] and make adjustments), and 44.6 g potassium fulvic acid (refer to product description), and without any of the above-mentioned rooting promotion substances for the control (CK). Rooting promotion powder was provided by Hebei Dewodo Biotechnology Co., Ltd. (Hengshui, Hebei, China). Biochar was provided by Zhengzhou Haosen Environmental Protection Technology Co., Ltd. (Zhengzhou, Henan, China). Organic fertilizer was provided by Beijing Goldenway Biology Tech Co., Ltd. (Beijing, China). Potassium fulvic acid was provided by Yinhai Chemical Co., Ltd. (Zhengzhou, Henan, China). Each of these treatments was replicated three times. All citrus trees were top grafted in March 2021 with scions of Citrus reticulata ‘Ai Yuan’ and sampled in October 2021 (Figure 9). Fertilizer applications were 150 mg N kg−1, 75 mg P2O5 kg−1, and 150 mg K2O kg−1 in 2020 and 180 mg N kg−1, 75 mg P2O5 kg−1, and 150 mg K2O kg−1 in 2021. The fertilizers used for these amendments were urea, superphosphate, and potassium sulfate.
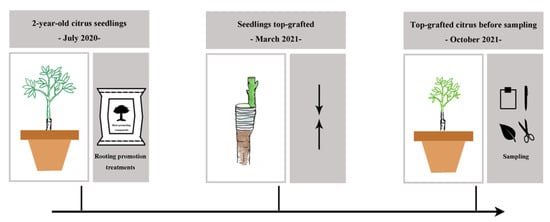
Figure 9.
Experimental timeline. In July 2020, two-year-old citrus seedlings of Citrus reticulate L. grafted on C. junos (Sieb.) Tanaka rootstocks were planted in plastic pots. Then, plants were treated with four rooting promotion substances. In March 2021, all citrus trees were top grafted with scions of Citrus reticulata ‘Ai Yuan’. In October 2021, top-grafted plants were sampled.
4.3. Growth Parameters
The longitudinal and lateral diameters of the canopy, as well as the crown height, were separately measured with a ruler after 7 months of citrus transplanting. The canopy volume (m3) was calculated as follows:
where V, r, and h represented the canopy volume, the canopy radius, and the canopy height, respectively.
V = 2/3 π r2 h,
Citrus saplings were subsequently dug out and cleaned of soil. Then, each plant was divided into the root, stem, branch, and leaf. All samples were dried at 105 °C for 30 min and then at 65 °C to constant weight for biomass determination. The aboveground biomass is the sum of stem, branch, and leaf biomass. The whole tree biomass is the sum of aboveground and root biomass.
4.4. Root Parameters
Newly developed white root tips were taken, and root activity was determined by the triphenyltetrazolium chloride (TTC) method [80]. Root activity primarily indicates the ability of root tissues to undergo cellular respiration and engage in active metabolic processes. Dehydrogenase in plant roots can reduce TTC to triphenylhydrazone (TTF). We recorded TTF production by the absorbance of root extracts at 485 nm to estimate dehydrogenase activity as an indicator of plant root activity. After cleaning soil from the roots, the roots were scanned with an EPSON scanner (Perfection C700, Epson Inc., Tokyo, Japan), and root morphology-related indicators (total root length, root surface area, root volume, and number of root tips) were obtained with the WinRHIZP root analysis system (Pro 2009, Rcgcnt Instrum cnt Inc., Québec, QC, Canada). Samples collected from individual plants were treated as one biological replicate, and three biological replicates were collected for each treatment.
4.5. Physiological Analyses
Mature leaves from spring branches were collected, snap-frozen in liquid N, and stored at −80 °C until further analyses. Total soluble sugars and starch, total soluble protein, and total free amino acids were determined by colorimetric measurements by the anthrone [81], Coomassie Brilliant Blue [82], and ninhydrin methods [83], respectively. The activities of CAT, SOD, and POD were determined with commercial test kits following the instructions of the manufacturer (Suzhou Kemin Biotechnology Co., Ltd., Suzhou, Jiangsu, China). Samples collected from individual plants were treated as one biological replicate, and three biological replicates were collected for each treatment.
4.6. Nutrient Content Analyses
The Kjeldahl method [84], the vanadium–molybdenum yellow colorimetric method [85], and the flame photometric method were applied to determine total N, total phosphorus, and total potassium, respectively [86], in the dried material of roots, stems, branches, and leaves. The calcium, magnesium, and other trace elements were determined using ICP-OES after the microwave digestion of tissue samples with HNO3-H2O2 [87].
4.7. Data Analyses
The following indicators were subjected to hierarchical cluster analysis: canopy volume; aboveground biomass; root biomass; whole tree biomass; total root length; root surface area, volume, and activity; leaf soluble sugar, starch, soluble protein, and amino acid contents; leaf POD, SOD, and CAT enzyme activities; and plant elemental accumulation [88]. The weight of each parameter was evaluated using the coefficient of variation method, and the comprehensive score for each treatment was calculated. To analyze the impact of different dimensions and magnitudes of indicators, the original data were subjected to min-max normalization [89].
The coefficient of variation was calculated for each indicator as shown in Equation (2):
where represents the standard deviation of the jth indicator, and represents the mean of the jth indicator.
The weight of each indicator was calculated as shown in Equation (3):
where n is the number of evaluation indicators.
The comprehensive evaluation score was calculated as shown in Equation (4):
where represents the normalized result of the jth indicator for the ith treatment.
One-way analysis of variance (ANOVA) was used to test the effect of each treatment on plant parameters. Differences between treatments’ means were tested with Duncan’s test (p < 0.05). All statistical analyses were performed using SPSS version 16.0. Graphical analysis was performed using the office 2016 and origin 2018 software. Partial least squares path modeling (PLS-PM) analysis was conducted using the “plspm” package in R (v 4.0.5) [90]. The correlation heat map between influencing factors and growth indicators was constructed using the “ggcorrplot” package [91].
5. Conclusions
The present results demonstrate that the application of rooting promotion substances before top grafting can enhance not only root morphology, root growth, and nutrient acquisition but can also improve the physiological and biochemical traits of aboveground biomass. The underlying mechanism by which rooting promotion treatments affect citrus growth is likely to involve the improvement in the nutrition of aboveground and root biomass, which ultimately regulates top-grafted plant growth. All four rooting promotion treatments that were studied exhibited positive effects on the traits and growth of top-grafted citrus trees, but among these treatments, rooting promotion powder and biochar application achieved the highest overall scores. With these results, the present study provides fundamental support for citrus production practices. Future studies have to elucidate whether the mixed application of several rooting promotion substances on top-grafted trees can further improve citrus growth and development and fruit production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13223159/s1, Figure S1: Top-grafted plant growth as affected by four rooting promotion substances. Figure S2: The N, P, K, Ca, and Mg accumulation of the top-grafted plant.
Author Contributions
Conceptualization, methodology, formal analysis, writing, J.X. and Z.C.; investigation, M.N.L. and J.Z.; formal analysis, Y.W. and R.N.; writing—review and editing, H.X., X.H., and H.R.; writing—review and editing, supervision, Y.Z. and X.S. All authors have read and agreed to the published version of the manuscript.
Funding
We are thankful for the support from the National Natural Science Foundation of China (32172676), the Natural Science Foundation of Chongqing, China (CSTB2022NSCQ-MSX1094), the Citrus Key Project of the Chongqing Agricultural Technology Extension Station, and the Beijing Changping Soil Quality National Observation and Research Station.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (Yueqiang Zhang) upon reasonable request.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Conti, G.; Xoconostle-Cázares, B.; Marcelino-Pérez, G.; Hopp, H.E.; Reyes, C.A. Citrus Genetic Transformation: An Overview of the Current Strategies and Insights on the New Emerging Technologies. Front. Plant Sci. 2021, 12, 768197. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.P.; Yang, C.; Zhang, L.N.; Feng, J.; Xi, W.P. Effect of Light-Emitting Diodes and Ultraviolet Irradiation on the Soluble Sugar, Organic Acid, and Carotenoid Content of Postharvest Sweet Oranges (Citrus sinensis (L.) Osbeck). Molecules 2019, 24, 3440. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, H.W.L.; De Carvalho, L.M.; De Barros, I.; Teodoro, A.V.; Girardi, E.A.; Passos, O.S. Dos Santos Soares Filho, W., 2022. Productive performance of ‘Pera’ sweet orange grafted onto 37 rootstocks in tropical cohesive soils under rainfed condition. Sci. Hortic. 2022, 303, 111229. [Google Scholar] [CrossRef]
- Primo-Capella, A.; Forner-Giner, M.Á.; Martínez-Cuenca, M.R.; Terol, J. Comparative transcriptomic analyses of citrus cold-resistant vs. sensitive rootstocks might suggest a relevant role of ABA signaling in triggering cold scion adaption. BMC Plant Biol. 2022, 22, 209. [Google Scholar] [CrossRef] [PubMed]
- Balfagón, D.; Rambla, J.L.; Granell, A.; Arbona, V.; Gomez-Cadenas, A. Grafting improves tolerance to combined drought and heat stresses by modifying metabolism in citrus scion. Environ. Exp. Bot. 2022, 195, 104793. [Google Scholar] [CrossRef]
- Chen, Z.; Deng, H.; Xiong, B.; Li, S.; Yang, L.; Yang, Y.; Huang, S.; Tan, L.; Sun, G.; Wang, Z. Rootstock Effects on Anthocyanin Accumulation and Associated Biosynthetic Gene Expression and Enzyme Activity during Fruit Development and Ripening of Blood Oranges. Agriculture 2022, 12, 342. [Google Scholar] [CrossRef]
- Calderón, F.J.; Weibel, A.M.; Trentacoste, E.R. Effects of different interstock length on vegetative growth and flowering in peach cv. Pavie Catherine. Sci. Hortic. 2021, 285, 110174. [Google Scholar] [CrossRef]
- Wang, T.; Xiong, B.; Tan, L.; Yang, Y.; Zhang, Y.; Ma, M.; Xu, Y.; Liao, L.; Sun, G.; Liang, D.; et al. Effects of interstocks on growth and photosynthetic characteristics in ‘Yuanxiaochun’ Citrus seedlings. Funct. Plant Biol. 2020, 47, 977–987. [Google Scholar] [CrossRef]
- Bhandari, N.; Basnet, M.; Khanal, S. Standardization of Grafting Time of Mandarin (Citrus reticulata Blanco) in Central Mid Hill of Nepal. Int. J. Fruit Sci. 2021, 21, 599–608. [Google Scholar] [CrossRef]
- Karunakaran, R.; Ilango, R.V.J. Graft success, growth, and early flowering onset, as affected by grafting time in topworked tea plants. J. Crop Improv. 2020, 34, 397–403. [Google Scholar] [CrossRef]
- Safari, M.; Rezaei, M. Grafting Success of Berberis Integerrima Cv. Bidaneh on Wild Type Barberries. Int. J. Fruit Sci. 2021, 21, 1030–1039. [Google Scholar] [CrossRef]
- Casamali, B.; Van Iersel, M.W.; Chavez, D.J. Plant Growth and Physiological Responses to Improved Irrigation and Fertilization Management for Young Peach Trees in the Southeastern United States. HortScience 2021, 56, 336–346. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, C.; Li, Y.; Jang, C.; Chun, C.; Peng, L. Effects and cost comparison of different interstock sprout inhibition and removal treatments in top grafted citrus trees. J. Fruit Sci. 2018, 35, 711–717. [Google Scholar]
- Espinoza-Núñez, E.; Mourão, F.D.A.; Stuchi, E.S.; Cantuarias-Avilés, T.; Dos Santos Dias, C.T. Performance of ‘Tahiti’ lime on twelve rootstocks under irrigated and non-irrigated conditions. Sci. Hortic. 2011, 129, 227–231. [Google Scholar] [CrossRef]
- Shi, X.G.; Shen, G.X. Fertilization and ground management of renewal citrus orchards by top grafting. Zhejiang Ganju 2000, 2, 27–28. (In Chinese) [Google Scholar]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Cheng, F.; Ahmed, W.; Huang, Y.; Bie, Z. Grafting: A technique to modify ion accumulation in horticultural crops. Front. Plant Sci. 2016, 7, 1457. [Google Scholar] [CrossRef]
- Pokhrel, S.; Meyering, B.; Bowman, K.D.; Albrecht, U. Horticultural attributes and root architectures of field-grown ‘Valencia’trees grafted on different rootstocks propagated by seed, cuttings, and tissue culture. HortScience 2021, 56, 163–172. [Google Scholar] [CrossRef]
- Lal, R.; Mukherjee, A. Biochar Impacts on Soil Physical Properties and Greenhouse Gas Emissions. Agronomy 2013, 3, 313–339. [Google Scholar] [CrossRef]
- Xie, J.; Shi, X.J.; Zhang, Y.; Wan, Y.; Hu, Q.J.; Zhang, Y.Q.; Wang, J.; He, X.H.; Evgenia, B. Improved nitrogen use efficiency, carbon sequestration and reduced environmental contamination under a gradient of manure application. Soil Tillage Res. 2022, 220, 105386. [Google Scholar] [CrossRef]
- Brazien, Z.; Paltanaviius, V.; Aviienyt, D. The influence offulvic acid on spring cereals and sugar beets seed germination and plant productivity. Environ. Res. 2021, 195, 110824. [Google Scholar] [CrossRef]
- Sorrenti, G.; Muzzi, E.; Toselli, M. Root growth dynamic and plant performance of nectarine trees amended with biochar and compost. Sci. Hortic. 2019, 257, 108710. [Google Scholar] [CrossRef]
- Głuszek, S.; Derkowska, E.; Paszt, L.S.; Sitarek, M.; Sumorok, B. Influence of bioproducts and mycorrhizal fungi on the growth and yielding of sweet cherry trees. Hortic. Sci. 2020, 47, 122–129. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.; Meyer, A.S.; Gonçalves, B. Biostimulants to Improved Tree Physiology and Fruit Quality: A Review with Special Focus on Sweet Cherry. Agronomy 2022, 12, 659. [Google Scholar] [CrossRef]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin control of root development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001537. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gao, B.; Li, W.F.; Mao, J.; Yang, S.J.; Li, W.; Ma, Z.H.; Zhao, X.; Chen, B.H. Effects of exogenous growth regulators and bud picking on grafting of grapevine hard branches. Sci. Hortic. 2020, 264, 109186. [Google Scholar] [CrossRef]
- Chen, H.L.; Lei, Y.Z.; Sun, J.J.; Ma, M.Y.; Deng, P.; Quan, J.E.; Bi, H.T. Effects of Different Growth Hormones on Rooting and Endogenous Hormone Content of Two Morus alba L. Cuttings. Horticulturae 2023, 9, 552. [Google Scholar] [CrossRef]
- Dubrovsky, J.G.; Sauer, M.; Napsucialy-Mendivil, S.; Ivanchenko, M.G.; Friml, J.; Shishkova, S.; Celenza, J.; Benková, E. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl. Acad. Sci. USA 2008, 105, 8790–8794. [Google Scholar] [CrossRef]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Yang, J.M.; Ye, T.; Liu, G.H.; Xu, X.T.; Zheng, Y.X.; Wang, W.K. Synthesis and bioactivity of indoleacetic acid-carbendazim and its effects on Cylindrocladium parasiticum. Pestic. Biochem. Physiol. 2019, 158, 128–134. [Google Scholar] [CrossRef]
- Tang, F.D.; Liang, Y.J.; Han, S.J.; Gong, W.G.; Ding, B.Y. Effect of biological agents on survival rate and root growth of Scots Pine seedlings. J. For. Res. 2004, 15, 124–126. [Google Scholar]
- Mao, J.P.; Zhang, D.; Zhang, X.; Li, K.; Liu, Z.; Meng, Y.; Lei, C.; Han, M.Y. Effect of exogenous indole-3-butanoic acid (IBA) application on the morphology, hormone status, and gene expression of developing lateral roots in Malus hupehensis. Sci. Hortic. 2018, 232, 112–120. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G. Exogenous auxin improves root morphology and restores growth of grafted cucumber seedlings. Hortic. Sci. 2017, 44, 82–90. [Google Scholar] [CrossRef]
- Chen, J.T.; Wang, X.R.; Liu, X.Y.; Wang, S.F.; Zhao, J.N.; Zhang, H.; Wang, Y.B.; Li, C.F. Beneficial Effects of Biochar-Based Organic Fertilizers on Nitrogen Assimilation, Photosynthesis, and Sucrose Synthesis of Sugar Beet (Beta vulgaris L.). Int. J. Plant Prod. 2022, 16, 755–768. [Google Scholar] [CrossRef]
- García, A.C.; Olaetxea, M.; Santos, L.A.; Mora, V.; Baigorri, R.; Fuentes, M.; Zamarreño, A.M.; Berbara, R.L.L.; Garcia-Mina, J.M. Involvement of hormone and ROS signaling pathways in the beneficial action of humic substances on plants growing under normal and stressing conditions. BioMed Res. Int. 2016, 2016, 3747501. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Sadaf, J.; Shah, G.A.; Shahzad, K.; Ali, N.; Shahid, M.; Safdar Ali, S.; Hussain, R.A.; Ahmed, Z.I.; Traore, B.; Ismail, I.M.I.; et al. Improvements in wheat productivity and soil quality can accomplish by co-application of biochars and chemical fertilizers. Sci. Total Environ. 2017, 607, 715–724. [Google Scholar] [CrossRef]
- Van der Heijden, G.; Dambrine, E.; Pollier, B.; Zeller, B.; Ranger, J.; Legout, A. Mg and Ca uptake by roots in relation to depth and allocation to aboveground tissues: Results from an isotopic labeling study in a beech forest on base-poor soil. Biogeochemistry 2015, 122, 375–393. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.W.; Wang, S.Q.; Xing, G.X. Successive straw biochar application as a strategy to sequester carbon and improve fertility: A pot experiment with two rice/wheat rotations in paddy soil. Plant Soil 2014, 378, 279–294. [Google Scholar] [CrossRef]
- Du, Y.L.; Fan, L.X.; Tian, C.Y.; Wu, T. Auxin positively regulates nitrogen remobilization in cucumber leaves. Hortic. Environ. Biotechnol. 2018, 59, 189–198. [Google Scholar] [CrossRef]
- Sorrenti, G.; Ventura, M.; Toselli, M. Effect of biochar on nutrient retention and nectarine tree performance: A three-year field trial. J. Plant Nutr. Soil Sci. 2016, 179, 336–346. [Google Scholar] [CrossRef]
- Warnock, D.D.; Mummey, D.L.; McBride, B.; Major, J.; Lehmann, J.; Rillig, M.C. Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: Results from growth-chamber and field experiments. Appl. Soil Ecol. 2010, 46, 450–456. [Google Scholar] [CrossRef]
- Shen, H.J.; Zhang, Q.Q.; Zhang, X.; Jiang, X.Y.; Zhu, S.G.; Chen, A.F.; Wu, Z.; Xiong, Z.Q. In situ effects of biochar field-aged for six years on net N mineralization in paddy soil. Soil Tillage Res. 2021, 205, 104766. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Song, Y.F.; Wu, Z.; Yan, X.Y.; Gunina, A.; Kuzyakov, Y.; Xiong, Z.Q. Effects of six-year biochar amendment on soil aggregation, crop growth, and nitrogen and phosphorus use efficiencies in a rice-wheat rotation. J. Clean. Prod. 2020, 242, 118435. [Google Scholar] [CrossRef]
- Yu, B.; Chen, X.; Cao, W.; Liu, Y.; Zou, C. Responses in Zinc Uptake of Different Mycorrhizal and Non-mycorrhizal Crops to Varied Levels of Phosphorus and Zinc Applications. Front. Plant Sci. 2020, 11, 606472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, X.; Liu, Y.; Liu, D.; Chen, X.; Zou, C. Zinc uptake by roots and accumulation in maize plants as affected by phosphorus application and arbuscular mycorrhizal colonization. Plant Soil 2017, 413, 59–71. [Google Scholar] [CrossRef]
- Liang, B.; Yang, X.Y.; He, X.H.; Murphy, D.V.; Zhou, J.B. Long-term combined application of manure and NPK fertilizers influenced nitrogen retention and stabilization of organic C in Loess soil. Plant Soil 2012, 353, 249–260. [Google Scholar] [CrossRef]
- Liu, C.A.; Li, F.R.; Zhou, L.M.; Zhang, R.H.; Yu, J.; Lin, S.L.; Wang, L.J.; Siddique, K.H.M.; Li, F.M. Effect of organic Organic Fertilizer and fertilizer on soil water and crop yields in newly-built terraces with loess soils in a semiarid environment. Agric. Water Manag. 2013, 117, 123–132. [Google Scholar] [CrossRef]
- Gupta, A.K.; Patra, P.K.; Tripathi, L.K. Silicon and organic manure influence on phosphorus dynamics in Alfisols of West Bengal, India. J. Plant Nutr. 2022, 45, 3118–3128. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Tao, J.; Yao, R. Integrated application of inorganic fertilizer with fulvic acid for improving soil nutrient supply and nutrient use efficiency of winter wheat in a salt-affected soil. Appl. Soil Ecol. 2022, 170, 104255. [Google Scholar] [CrossRef]
- Qiao, J.A.; Zhang, Y.; Wang, Q.; Li, M.; Sun, H.; Liu, N.; Zhang, L.L.; Zhang, Y.; Liu, Z.B. Effects of potassium fulvic acid and potassium humate on microbial biodiversity in bulk soil and rhizosphere soil of Panax ginseng. Microbiol. Res. 2022, 254, 126914. [Google Scholar] [CrossRef]
- Sun, J.; Xia, J.B.; Zhao, X.M.; Su, L.; Li, C.R.; Liu, P. Effects of 1-aminobenzotriazole on the growth and physiological characteristics of Tamarix chinensis cuttings under salt stress. J. For. Res. 2021, 32, 1641–1651. [Google Scholar] [CrossRef]
- Chen, P.P.; Song, H.Y.; Zhou, Y.Z.; Yang, M.H.; Pei, X.D.; Yi, Z.X.; Tu, N.M. Effect of Water Control Before Transplanting and ABT Treatment on Tobacco Seedling Quality and Physiological Properties at Green Stage. Agric. Sci. Technol. 2016, 17, 2283–2286+2368. [Google Scholar]
- Pacholczak, A.; Nowakowska, K. The Effect of Biostimulators and Indole-3-Butyric Acid on Rooting of Stem Cuttings of Two Ground Cover Roses. Acta Agrobot. 2020, 73, JM09557. [Google Scholar] [CrossRef]
- Tamizhselvan, P.; Madhavan, S.; Constan-Aguilar, C.; Elrefaay, E.R.; Liu, J.; Pencík, A.; Novák, O.; Cairó, A.; Hrtyan, M.; Geisler, M. Chloroplast Auxin Efflux Mediated by ABCB28 and ABCB29 Fine-Tunes Salt and Drought Stress Responses in Arabidopsis. Plants 2024, 13, 7. [Google Scholar] [CrossRef]
- Sosnowski, J.; Truba, M.; Vasileva, V. The Impact of Auxin and Cytokinin on the Growth and Development of Selected Crops. Agriculture 2023, 13, 724. [Google Scholar] [CrossRef]
- Damodaran, S.; Strader, L.C. Indole 3-Butyric Acid Metabolism and Transport in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 851. [Google Scholar] [CrossRef]
- Shaaban, M.; Van Zwieten, L.; Bashir, S.; Younas, A.; Núñez-Delgado, A.; Chhajro, M.A.; Kubar, K.A.; Ali, U.; Rana, M.S.; Mehmood, M.A.; et al. A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J. Environ. Manag. 2018, 228, 429–440. [Google Scholar] [CrossRef]
- Pandey, D.; Daverey, A.; Arunachalam, K. Biochar: Production, properties and emerging role as a support for enzyme immobilization. J. Clean. Prod. 2020, 255, 120267. [Google Scholar] [CrossRef]
- Martínez-Alcántara, B.; Martínez-Cuenca, M.R.; Bermejo, A.; Legaz, F.; Quiñones, A. Liquid Organic Fertilizers for Sustainable Agriculture: Nutrient Uptake of Organic versus Mineral Fertilizers in Citrus Trees. PLoS ONE 2017, 11, e0161619. [Google Scholar] [CrossRef]
- Bharali, A.; Baruah, K.K. Effects of integrated nutrient management on sucrose phosphate synthase enzyme activity and grain quality traits in rice. Physiol. Mol. Biol. Plants 2022, 28, 383–389. [Google Scholar] [CrossRef]
- Gao, F.; Li, Z.L.; Du, Y.P.; Duan, J.H.; Zhang, T.J.; Wei, Z.B.; Guo, L.; Gong, W.N.; Liu, Z.G.; Zhang, M. The Combined Application of Urea and Fulvic Acid Solution Improved Maize Carbon and Nitrogen Metabolism. Agronomy 2022, 12, 1400. [Google Scholar] [CrossRef]
- Zhang, F.G.; Liu, M.H.; Li, Y.; Che, Y.Y.; Xiao, Y. Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Zhang, Z.C.; Guo, P.R.; Wang, R.; Liu, T.; Luo, J.Q.; Hao, B.H.; Wang, Y.C.; Guo, W. Synergistic mechanisms of bioorganic fertilizer and AMF driving rhizosphere bacterial community to improve phytoremediation efficiency of multiple HMs-contaminated saline soil. Sci. Total Environ. 2023, 883, 163708. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Shen, Z.Z.; Zhang, F.G.; Waseem, R.Z.; Yuan, J.; Huang, R.; Ruan, Y.Z.; Li, R.; Shen, Q.R. Bacillus amyloliquefaciens Strain W19 can Promote Growth and Yield and Suppress Fusarium Wilt in Banana Under Greenhouse and Field Conditions. Pedosphere 2016, 26, 733–744. [Google Scholar] [CrossRef]
- Elrys, A.S.; Abdo, A.I.E.; Abdel-Hamed, E.M.W.; Desoky, E.S.M. Integrative application of licorice root extract or lipoic acid with fulvic acid improves wheat production and defenses under salt stress conditions. Ecotoxicol. Environ. Saf. 2020, 190, 110144. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.Y.; Lin, W.Z.; Jahan, M.S.; Wang, J.; Sun, J.; Jiang, J.K.; Gu, W.Y.; Zou, J.R.; Shu, S.; et al. Foliar application of a mixture of putrescine, melatonin, proline, and potassium fulvic acid alleviates high temperature stress of cucumber plants grown in the greenhouse. Technol. Hortic. 2022, 2, 6. [Google Scholar] [CrossRef]
- Cheng, B.X.; Wang, C.X.; Yue, L.; Chen, F.R.; Cao, X.S.; Lan, Q.Q.; Liu, T.X.; Wang, Z.Y. Selenium nanomaterials improve the quality of lettuce (Lactuca sativa L.) by modulating root growth, nutrient availability, and photosynthesis. NanoImpact 2023, 29, 100449. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Rodrigues, M.; de Oliveira, K.M.; Furlanetto, R.H.; dos Reis, A.S.; dos Santos, G.L.A.A.; Mendonça, W.A.; Crusiol, L.G.T.; Gonçalves, J.V.F.; et al. Nutrient Deficiency Lowers Photochemical and Carboxylation Efficiency in Tobacco. Theor. Exp. Plant Physiol. 2023, 35, 81–97. [Google Scholar] [CrossRef]
- Xue, Y.B.; Zhu, S.N.; Schultze-Kraft, R.; Liu, G.D.; Chen, Z.J. Dissection of Crop Metabolome Responses to Nitrogen, Phosphorus, Potassium, and Other Nutrient Deficiencies. Int. J. Mol. Sci. 2022, 23, 9079. [Google Scholar] [CrossRef]
- Islam, M.M.; Jahan, K.; Sen, A.; Urmi, T.A.; Haque, M.M.; Ali, H.M.; Siddiqui, M.H.; Murata, Y. Exogenous application of calcium ameliorates salinity stress tolerance of tomato (Solanum lycopersicum L.) and enhances fruit quality. Antioxidants 2023, 12, 558. [Google Scholar] [CrossRef]
- Parađiković, N.; Zeljković, S.; Tkalec, M.; Vinković, T.; Dervić, I.; Marić, M. Influence of rooting powder on propagation of sage (Salvia officinalis L.) and rosemary (Rosmarinus officinalis L.) with green cuttings. Poljoprivreda 2013, 19, 10–15. [Google Scholar]
- Katel, S.; Mandal, H.R.; Kattel, S.; Yadav, S.P.S.; Lamshal, B.S. Impacts of plant growth regulators in strawberry plant: A review. Heliyon 2022, 8, e11959. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.L.G.; Chaves, L.H.G.; Cavalcante, A.R.; Guimarães, J.P.; De Souza, F.G.; De Lima, W.B.; Guerra, H.O.C.; Fernandes, J.D. Melon Seedlings Phytomass under Poultry Litter Biochar Doses. Agric. Sci. 2021, 12, 181–197. [Google Scholar]
- Reyes-Cabrera, J.; Leon, R.G.; Erickson, J.E.; Silveira, M.L.; Rowland, D.L.; Morgan, K.T. Biochar Changes Shoot Growth and Root Distribution of Soybean during Early Vegetative Stages. Crop Sci. 2017, 57, 454–461. [Google Scholar] [CrossRef]
- Raza, S.T.; Wu, J.P.; Ali, Z.; Anjum, R.; Bazai, N.A.; Feyissa, A.; Chen, Z. Differential Effects of Organic Amendments on Maize Biomass and Nutrient Availability in Upland Calcareous Soil. Atmosphere 2021, 12, 1034. [Google Scholar] [CrossRef]
- Zhang, M.M.; Li, X.Y.; Wang, X.L.; Feng, J.P.; Zhu, S.P. Potassium fulvic acid alleviates salt stress of citrus by regulating rhizosphere microbial community, osmotic substances and enzyme activities. Front. Plant Sci. 2023, 14, 1161469. [Google Scholar] [CrossRef]
- Pirlak, L.; Çinar, M. Effects of Bacteria and Iba on the Rooting of Citrange Citrus Rootstocks Cuttings. Alinteri J. Agric. Sci. 2020, 35, 1. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Riaz, M.; Xia, H.; Li, Y.X.; Wang, X.L.; Jiang, C.C. Four-year biochar study: Positive response of acidic soil microenvironment and citrus growth to biochar under potassium deficiency conditions. Sci. Total Environ. 2022, 813, 152515. [Google Scholar] [CrossRef]
- Zhang, F.S.; Chen, X.P.; Chen, Q. Fertilization Guide for Major Crops in China; China Agricultural University Press: Beijing, China, 2009; pp. 92–96. [Google Scholar]
- Li, B.; Wang, Y.; Hu, T.; Qiu, D.; Francis, F.; Wang, S.; Wang, S. Root-Associated Microbiota Response to Ecological Factors: Role of Soil Acidity in Enhancing Citrus Tolerance to Huanglongbing. Front. Plant Sci. 2022, 13, 937414. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The Determination of Amino Acids with Ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Ates, F.; Kaya, O. The Relationship Between Iron and Nitrogen Concentrations Based On Kjeldahl Method and SPAD-502 Readings in Grapevine (Vitis vinifera L. cv. ‘Sultana Seedless’). Erwerbs-Obstbau 2021, 63, 53–59. [Google Scholar] [CrossRef]
- Pestana, M.; Beja, P.; Correia, P.J.; De Varennes, A.; Faria, E.A. Relationships between nutrient composition of flowers and fruit quality in orange trees grown in calcareous soil. Tree Physiol. 2005, 25, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.L. Flame photometric determination of potassium in unashed plant leaves. Anal. Chem. 2002, 35, 874–875. [Google Scholar] [CrossRef]
- Wang, T.W.; Tan, J.; Li, L.Y.; Yang, Y.; Zhang, X.M.; Wang, J.R. Combined analysis of inorganic elements and flavonoid metabolites reveals the relationship between flower quality and maturity of Sophora japonica L. Front. Plant Sci. 2023, 14, 1255637. [Google Scholar] [CrossRef]
- Benitez-Altuna, F.; Trienekens, J.; Gaitán-Cremaschi, D. Categorizing the sustainability of vegetable production in Chile: A farming typology approach. Int. J. Agric. Sustain. 2023, 21, 2202538. [Google Scholar] [CrossRef]
- Tan, W.B.; Li, W.S.; Li, J.J.; Liu, D.L.; Xing, W. Drought resistance evaluation of sugar beet germplasms by response of phenotypic indicators. Plant Signal. Behav. 2023, 18, 2192570. [Google Scholar] [CrossRef]
- Bertrand, F.; Gaston Sanchez, G.; Trinchera, L.; Russolillo, G. Plspm: Partial Least Squares Path Modeling (PLS-PM). R Package Version 0.5.1. Available online: http://cran.r-project.org/package=plspm (accessed on 10 February 2024).
- Zhang, Y.; Cheng, D.M.; Xie, J.; Hu, Q.J.; Xie, J.W.; Shi, X.J. Long-term field application of manure induces deep selection of antibiotic resistomes in leaf endophytes of Chinese cabbage. Sci. Total Environ. 2023, 882, 163334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).