Synergistic Effects of Nitrogen and Zinc Foliar Application on Yield and Nutrient Accumulation in Rice at Various Growth Stages
Abstract
:1. Introduction
2. Results
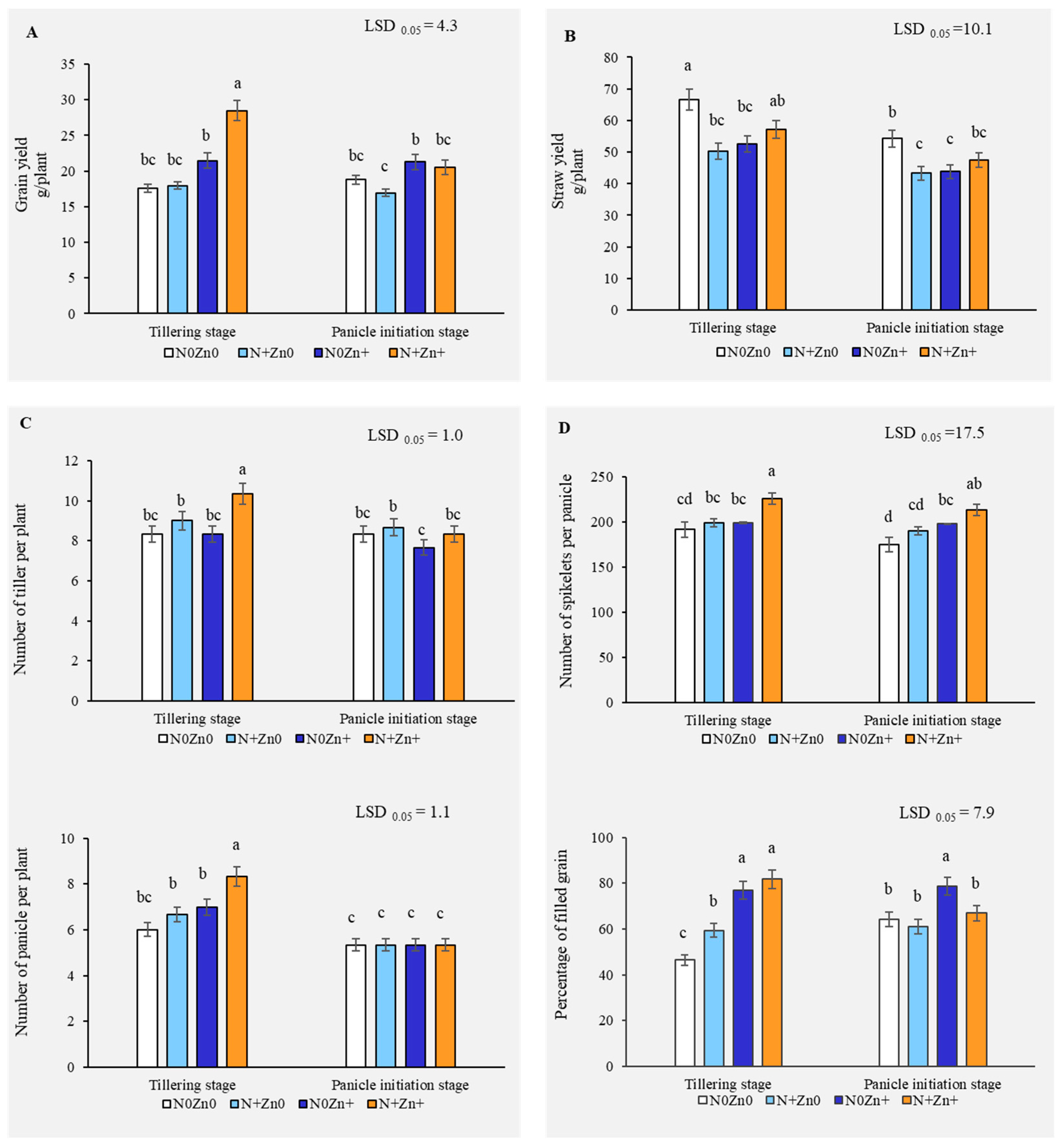
2.1. Enhanced Rice Yield with Combined Foliar N and Zn Application
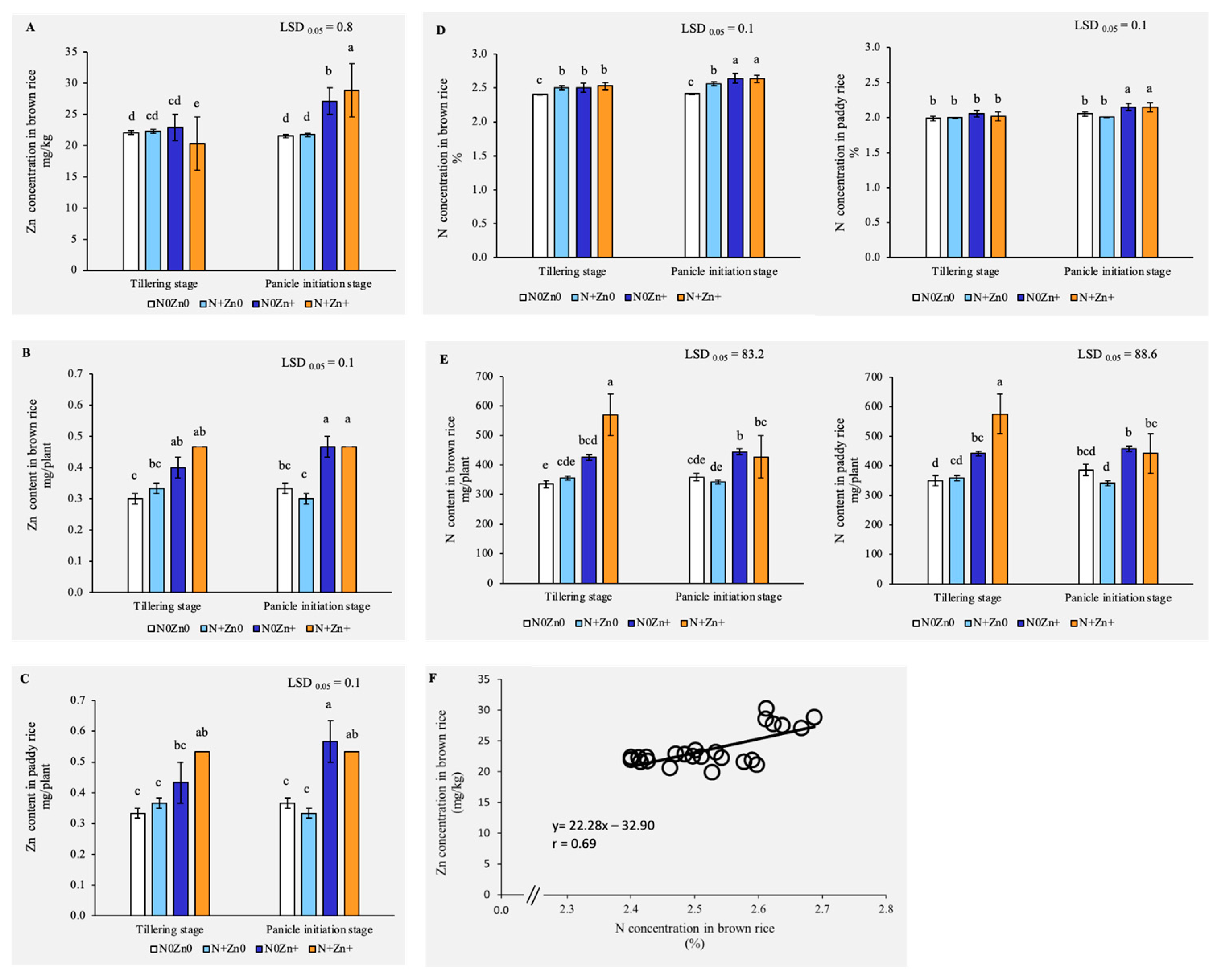
2.2. Foliar N+Zn+ Treatments Significantly Improved Zn and N Accumulation in Rice Grains
2.3. Combined N and Zn Treatment Enhanced Gene Expression for Nutrient Uptake and Metabolism in Rice
3. Discussion
3.1. Foliar Application of Nitrogen and Zinc Fertilizers Improves Yield and Nutrient Accumulation in Rice
3.2. Foliar Application of Nitrogen and Zinc Fertilizers Effects on the Related Genes Expression
4. Materials and Methods
4.1. Experimental Design and Rice Cultivation
4.2. Analytical Procedures
4.3. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cakmak, I.; Kutman, U.B. Agronomic Biofortification of Cereals with Zinc: A Review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Rouached, H. Recent developments in plant zinc homeostasis and the path toward improved biofortification and phytoremediation programs. Plant Signal Behav. 2013, 8, e22681. [Google Scholar] [CrossRef] [PubMed]
- Prom-u-thai, C.; Rashid, A.; Ram, H.; Zou, C.; Guilherme, L.R.G.; Corguinha, A.P.B.; Guo, S.; Kaur, C.; Naeem, A.; Yamuangmorn, S.; et al. Simultaneous biofortification of rice with zinc, iodine, iron and selenium through foliar treatment of a micronutrient cocktail in five countries. Front. Plant Sci. 2020, 11, 589835. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-R.; Kuang, L.; Gao, Y.-Q.; Wang, Y.-L.; Salt, D.E.; Chao, D.-Y. AtHMA4 Drives Natural Variation in Leaf Zn Concentration of Arabidopsis Thaliana. Front. Plant Sci. 2018, 9, 270. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Liu, Z.; Tian, J.; Liang, L.; Qiu, Y.; Wang, G.; Du, Q.; Cheng, D.; Cai, H. A High Activity Zinc Transporter OsZIP9 Mediates Zinc Uptake in Rice. Plant J. 2020, 103, 1695–1709. [Google Scholar] [CrossRef]
- Li, M.; Yang, X.W.; Tian, X.H.; Wang, S.X.; Chen, Y.L. Effect of Nitrogen Fertilizer and Foliar Zinc Application at Different Growth Stages on Zinc Translocation and Utilization Efficiency in Winter Wheat. Cereal Res. Commun. 2014, 42, 81–90. [Google Scholar] [CrossRef]
- Tavarez, M.; Grusak, M.A.; Sankaran, R.P. Effects of Zinc Fertilization on Grain Cadmium Accumulation, Gene Expression, and Essential Mineral Partitioning in Rice. Agronomy 2022, 12, 2182. [Google Scholar] [CrossRef]
- Tian, S.; Liang, S.; Qiao, K.; Wang, F.; Zhang, Y.; Chai, T. Co-Expression of Multiple Heavy Metal Transporters Changes the Translocation, Accumulation, and Potential Oxidative Stress of Cd and Zn in Rice (Oryza sativa). J. Hazard. Mater. 2019, 380, 120853. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastava, S. Plant Metal and Metalloid Transporters; Springer Nature: Berlin/Heidelberg, Germany, 2022; ISBN 981-19610-3-4. [Google Scholar]
- Liu, D.-Y.; Liu, Y.-M.; Zhang, W.; Chen, X.-P.; Zou, C.-Q. Zinc Uptake, Translocation, and Remobilization in Winter Wheat as Affected by Soil Application of Zn Fertilizer. Front. Plant Sci. 2019, 10, 426. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Ren, T.; Hussain, S.; Guo, C.; Wang, S.; Cong, R.; Li, X. Effects of Nitrogen and Tiller Type on Grain Yield and Physiological Responses in Rice. AoB Plants 2017, 9, plx012. [Google Scholar] [CrossRef]
- Persson, D.P.; De Bang, T.C.; Pedas, P.R.; Kutman, U.B.; Cakmak, I.; Andersen, B.; Finnie, C.; Schjoerring, J.K.; Husted, S. Molecular Speciation and Tissue Compartmentation of Zinc in Durum Wheat Grains with Contrasting Nutritional Status. New Phytol. 2016, 211, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.J.; Garcia-Ibañez, P.; Carvajal, M. The Use of Biovesicles to Improve the Efficiency of Zn Foliar Fertilization. Colloids Surf. B Biointerfaces 2019, 173, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Kutman, U.B.; Yildiz, B.; Cakmak, I. Effect of Nitrogen on Uptake, Remobilization and Partitioning of Zinc and Iron throughout the Development of Durum Wheat. Plant Soil 2011, 342, 149–164. [Google Scholar] [CrossRef]
- Bala, R.; Kalia, A.; Dhaliwal, S.S. Evaluation of Efficacy of ZnO Nanoparticles as Remedial Zinc Nanofertilizer for Rice. J. Soil Sci. Plant Nutr. 2019, 19, 379–389. [Google Scholar] [CrossRef]
- Khampuang, K.; Rerkasem, B.; Lordkaew, S.; Prom-u-thai, C. Nitrogen Fertilizer Increases Grain Zinc along with Yield in High Yield Rice Varieties Initially Low in Grain Zinc Concentration. Plant Soil 2021, 467, 239–252. [Google Scholar] [CrossRef]
- Zhang, J.; He, N.; Liu, C.; Xu, L.; Chen, Z.; Li, Y.; Wang, R.; Yu, G.; Sun, W.; Xiao, C. Variation and Evolution of C:N Ratio among Different Organs Enable Plants to Adapt to N-limited Environments. Glob. Chang. Biol. 2020, 26, 2534–2543. [Google Scholar] [CrossRef]
- Zaman, Q.U.; Aslam, Z.; Rashid, M.; Khaliq, A.; Yaseen, M. Influence of Zinc Fertilization on Morpho-Physiological Attributes, Growth, Productivity and Hematic Appraisal of Paddy Rice. JAPS J. Anim. Plant Sci. 2018, 28. [Google Scholar]
- Zhao, A.; Tian, X.; Chen, Y.; Li, S. Application of ZnSO4 or Zn-EDTA Fertilizer to a Calcareous Soil: Zn Diffusion in Soil and Its Uptake by Wheat Plants. J. Sci. Food Agric. 2016, 96, 1484–1491. [Google Scholar] [CrossRef]
- Kutman, U.B.; Yildiz, B.; Cakmak, I. Improved Nitrogen Status Enhances Zinc and Iron Concentrations Both in the Whole Grain and the Endosperm Fraction of Wheat. J. Cereal Sci. 2011, 53, 118–125. [Google Scholar] [CrossRef]
- Hao, H.-L.; Wei, Y.-Z.; Yang, X.-E.; Ying, F.; Wu, C.-Y. Effects of Different Nitrogen Fertilizer Levels on Fe, Mn, Cu and Zn Concentrations in Shoot and Grain Quality in Rice (Oryza sativa). Rice Sci. 2007, 14, 289–294. [Google Scholar] [CrossRef]
- Hussain, S.; Sahar, K.; Naeem, A.; Zafar-ul-Hye, M.; Aon, M. Combined Zinc and Nitrogen Applications at Panicle Initiation for Zinc Biofortification in Rice. Period. Biol. 2018, 120, 105–110. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Lombi, E.; Cheng, M.; Tang, C.; Howard, D.L.; Menzies, N.W.; Kopittke, P.M. Absorption of Foliar-Applied Zn Fertilizers by Trichomes in Soybean and Tomato. J. Exp. Bot. 2018, 69, 2717–2729. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, A.; Pitann, B.; Hossain, S.; Saqib, Z.A.; Nawaz, A.; Mühling, K.H. Zinc and silicon fertilizers in conventional and nano-forms: Mitigating salinity effects in maize (Zea mays L.). J. Plant Nutr. Soil Sci. 2024, 187, 678–689. [Google Scholar] [CrossRef]
- Impa, S.M.; Morete, M.J.; Ismail, A.M.; Schulin, R.; Johnson-Beebout, S.E. Zn Uptake, Translocation and Grain Zn Loading in Rice (Oryza sativa L.) Genotypes Selected for Zn Deficiency Tolerance and High Grain Zn. J. Exp. Bot. 2013, 64, 2739–2751. [Google Scholar] [CrossRef]
- Jaksomsak, P.; Rerkasem, B.; Prom-u-Thai, C. Responses of Grain Zinc and Nitrogen Concentration to Nitrogen Fertilizer Application in Rice Varieties with High-Yielding Low-Grain Zinc and Low-Yielding High Grain Zinc Concentration. Plant Soil 2017, 411, 101–109. [Google Scholar] [CrossRef]
- Yamuangmorn, S.; Rinsinjoy, R.; Lordkaew, S.; Dell, B.; Prom-u-thai, C. Responses of Grain Yield and Nutrient Content to Combined Zinc and Nitrogen Fertilizer in Upland and Wetland Rice Varieties Grown in Waterlogged and Well-Drained Condition. J. Soil Sci. Plant Nutr. 2020, 20, 2112–2122. [Google Scholar] [CrossRef]
- Phattarakul, N.; Rerkasem, B.; Li, L.; Wu, L.; Zou, C.; Ram, H.; Sohu, V.S.; Kang, B.S.; Surek, H.; Kalayci, M.; et al. Biofortification of rice grain with zinc through zinc fertilization in different countries. Plant Soil 2012, 361, 131–141. [Google Scholar] [CrossRef]
- Wei, Y.; Shohag, M.J.I.; Yang, X. Biofortification and Bioavailability of Rice Grain Zinc as Affected by Different Forms of Foliar Zinc Fertilization. PLoS ONE 2012, 7, e45428. [Google Scholar] [CrossRef]
- Ma, Y.; Wen, Y.; Wang, C.; Wu, Z.; Yuan, X.; Xiong, Y.; Chen, K.; He, L.; Zhang, Y.; Wang, Z. ZIP Genes Are Involved in the Retransfer of Zinc Ions during the Senescence of Zinc-Deficient Rice Leaves. Int. J. Mol. Sci. 2023, 24, 13989. [Google Scholar] [CrossRef]
- Mu, S.; Yamaji, N.; Sasaki, A.; Luo, L.; Du, B.; Che, J.; Shi, H.; Zhao, H.; Huang, S.; Deng, F. A Transporter for Delivering Zinc to the Developing Tiller Bud and Panicle in Rice. Plant J. 2021, 105, 786–799. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F. The Node, a Hub for Mineral Nutrient Distribution in Graminaceous Plants. Trends Plant Sci. 2014, 19, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhang, S.; Gu, M.; Xu, G. Function, Transport, and Regulation of Amino Acids: What Is Missing in Rice? Crop J. 2021, 9, 530–542. [Google Scholar] [CrossRef]
- Tan, L.; Zhu, Y.; Fan, T.; Peng, C.; Wang, J.; Sun, L.; Chen, C. OsZIP7 Functions in Xylem Loading in Roots and Inter-Vascular Transfer in Nodes to Deliver Zn/Cd to Grain in Rice. Biochem. Biophys. Res. Commun. 2019, 512, 112–118. [Google Scholar] [CrossRef] [PubMed]
- DeLoose, M.; Cho, H.; Bouain, N.; Choi, I.; Prom-U-Thai, C.; Shahzad, Z.; Zheng, L.; Rouached, H. PDR9 allelic variation and MYB63 modulate nutrient-dependent coumarin homeostasis in Arabidopsis. Plant J. 2024, 117, 1716–1727. [Google Scholar] [CrossRef]
- Pal, S.; Kisko, M.; Dubos, C.; Lacombe, B.; Berthomieu, P.; Krouk, G.; Rouached, H. TransDetect identifies a new regulatory module controlling phosphate accumulation in Arabidopsis. Plant Physiol. 2017, 175, 916–926. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Mitani-Ueno, N.; Kashino, M.; Ma, J.F. A Node-localized Transporter OsZIP3 Is Responsible for the Preferential Distribution of Zn to Developing Tissues in Rice. Plant J. 2015, 84, 374–384. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Suzuki, M.; Kobayashi, T.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. OsZIP4, a Novel Zinc-Regulated Zinc Transporter in Rice. J. Exp. Bot. 2005, 56, 3207–3214. [Google Scholar] [CrossRef]
- Yang, X.; Huang, J.; Jiang, Y.; Zhang, H.-S. Cloning and Functional Identification of Two Members of the ZIP (Zrt, Irt-like Protein) Gene Family in Rice (Oryza sativa L.). Mol. Biol. Rep. 2009, 36, 281–287. [Google Scholar] [CrossRef]
- Yamaji, N.; Xia, J.; Mitani-Ueno, N.; Yokosho, K.; Feng Ma, J. Preferential Delivery of Zinc to Developing Tissues in Rice Is Mediated by P-Type Heavy Metal ATPase OsHMA2. Plant Physiol. 2013, 162, 927–939. [Google Scholar] [CrossRef]
- Wang, W.-H.; Liu, G.-W.; Cao, F.-Q.; Cheng, X.-Y.; Liu, B.-W.; Liu, L.-H. Inadequate Root Uptake May Represent a Major Component Limiting Rice to Use Urea as Sole Nitrogen Source for Growth. Plant Soil 2013, 363, 191–200. [Google Scholar] [CrossRef]
- Zhu, K.; Zhou, Q.; Shen, Y.; Yan, J.; Xu, Y.; Wang, Z.; Yang, J. Agronomic and Physiological Performance of an Indica–Japonica Rice Variety with a High Yield and High Nitrogen Use Efficiency. Crop Sci. 2020, 60, 1556–1568. [Google Scholar] [CrossRef]
- Tabuchi, M.; Sugiyama, K.; Ishiyama, K.; Inoue, E.; Sato, T.; Takahashi, H.; Yamaya, T. Severe Reduction in Growth Rate and Grain Filling of Rice Mutants Lacking OsGS1; 1, a Cytosolic Glutamine Synthetase1; 1. Plant J. 2005, 42, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Tamura, W.; Hidaka, Y.; Tabuchi, M.; Kojima, S.; Hayakawa, T.; Sato, T.; Obara, M.; Kojima, M.; Sakakibara, H.; Yamaya, T. Reverse Genetics Approach to Characterize a Function of NADH-Glutamate Synthase1 in Rice Plants. Amino Acids 2010, 39, 1003–1012. [Google Scholar] [CrossRef]
- Bashir, K.; Ishimaru, Y.; Shimo, H.; Kakei, Y.; Senoura, T.; Takahashi, R.; Sato, Y.; Sato, Y.; Uozumi, N.; Nakanishi, H. Rice Phenolics Efflux Transporter 2 (PEZ2) Plays an Important Role in Solubilizing Apoplasmic Iron. Soil Sci. Plant Nutr. 2011, 57, 803–812. [Google Scholar] [CrossRef]
- DeLoose, M.; Clúa, J.; Cho, H.; Zheng, L.; Masmoudi, K.; Desnos, T.; Krouk, G.; Nussaume, L.; Poirier, Y.; Rouached, H. Recent advances in unraveling the mystery of combined nutrient stress in plants. Plant J. 2024, 117, 1764–1780. [Google Scholar] [CrossRef]
- Shahzad, Z.; Rouached, H. Protecting plant nutrition from the effects of climate change. Curr. Biol. 2022, 32, R725–R727. [Google Scholar] [CrossRef]


| Gene Name | Primer Sequence (5′ → 3′) | Reference |
|---|---|---|
| OsZIP3 | forward AAAAAGCAGGCTTCTCATCATCTTATTCCCTTCTAC | [37] |
| reverse AGAAAGCTGGGTCTGTGGTGTTAGCACAGTCGC | ||
| OsZIP4 | forward CACCATGGACGCCATGAGGCAGAGCACGCG | [38] |
| reverse TCATGCCCATATGGCAAGCAGAGACATCAT | ||
| OsZIP5 | forward CATGAAGACCAAGGTGCAGAGAAGG | [37] |
| reverse TCACGCCCAGATGGCGATCA | ||
| OsZIP7 | forward TGTCCGATGGAGCGGTTCG | [39] |
| reverse CCTCTACATTAGTCCCTGAG | ||
| OsZIP9 | forward ATCTTCTTCTCGCTAACCACAC | [37] |
| reverse GCAGCCGCTGCGTCGAGAAT | ||
| OsHMA2 | forward CATAGTGAAGCTGCCTGAGATC | [40] |
| reverse GATCAAACGCATAGCAGCATCG | ||
| OsDUR3 | forward CCTTGGCTACTTCACGCTGT | [41] |
| reverse TGCATCTCCGTCTCGTGTAG | ||
| OsAAP1 | forward CCCATTACCACCTCCACCTC | [42] |
| reverse ACCTTCTCTTGCGGCCTCTC | ||
| OsGS1;1 | forward ACCTCCTCCAGAAGGACAT | [43] |
| reverse GTGCCTGAGCTTGAGCTTCT | ||
| OsFd-GOGAT | forward GCATACTTGTGAAGCACCGAAGTG | [44] |
| reverse CTGCAAATAGCAACCTAGCGTCAG | ||
| α-tubulin | forward TCTTCCACCCTGAGCAGCTC | [45] |
| reverse AACCTTGGAGACCAGTGCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuiwong, P.; Cho, H.-K.; Rouached, H.; Prom-U-Thai, C. Synergistic Effects of Nitrogen and Zinc Foliar Application on Yield and Nutrient Accumulation in Rice at Various Growth Stages. Plants 2024, 13, 3274. https://doi.org/10.3390/plants13233274
Tuiwong P, Cho H-K, Rouached H, Prom-U-Thai C. Synergistic Effects of Nitrogen and Zinc Foliar Application on Yield and Nutrient Accumulation in Rice at Various Growth Stages. Plants. 2024; 13(23):3274. https://doi.org/10.3390/plants13233274
Chicago/Turabian StyleTuiwong, Patcharin, Hui-Kyong Cho, Hatem Rouached, and Chanakan Prom-U-Thai. 2024. "Synergistic Effects of Nitrogen and Zinc Foliar Application on Yield and Nutrient Accumulation in Rice at Various Growth Stages" Plants 13, no. 23: 3274. https://doi.org/10.3390/plants13233274
APA StyleTuiwong, P., Cho, H. -K., Rouached, H., & Prom-U-Thai, C. (2024). Synergistic Effects of Nitrogen and Zinc Foliar Application on Yield and Nutrient Accumulation in Rice at Various Growth Stages. Plants, 13(23), 3274. https://doi.org/10.3390/plants13233274







