Blue Light Enhances the Antioxidant, Antimicrobial, and Antitumor Potential of the Green Microalgae Coelastrella sp. BGV
Abstract
:1. Introduction
2. Results
2.1. Pigment Content of Coelastrella sp. BGV
2.2. Antioxidant Capacity of Coelastrella sp. BGV Ethanol Extracts
2.3. Antimicrobial Activity of Coelastrella sp. BGV Ethanol Extracts
2.4. In Vitro Antitumor Activity of Coelastrella sp. BGV Ethanol Extracts
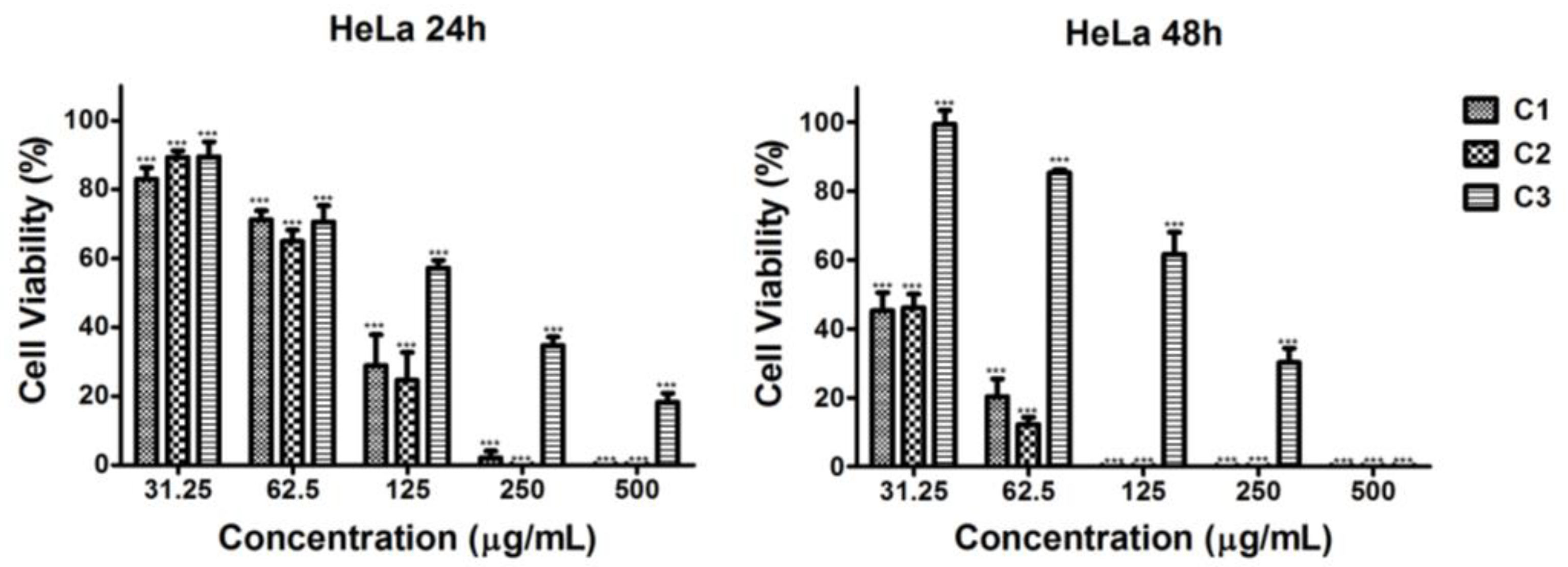
2.4.1. Cytotoxic Effect of Microalgal Extracts Against HeLa Cervical Tumor Cells
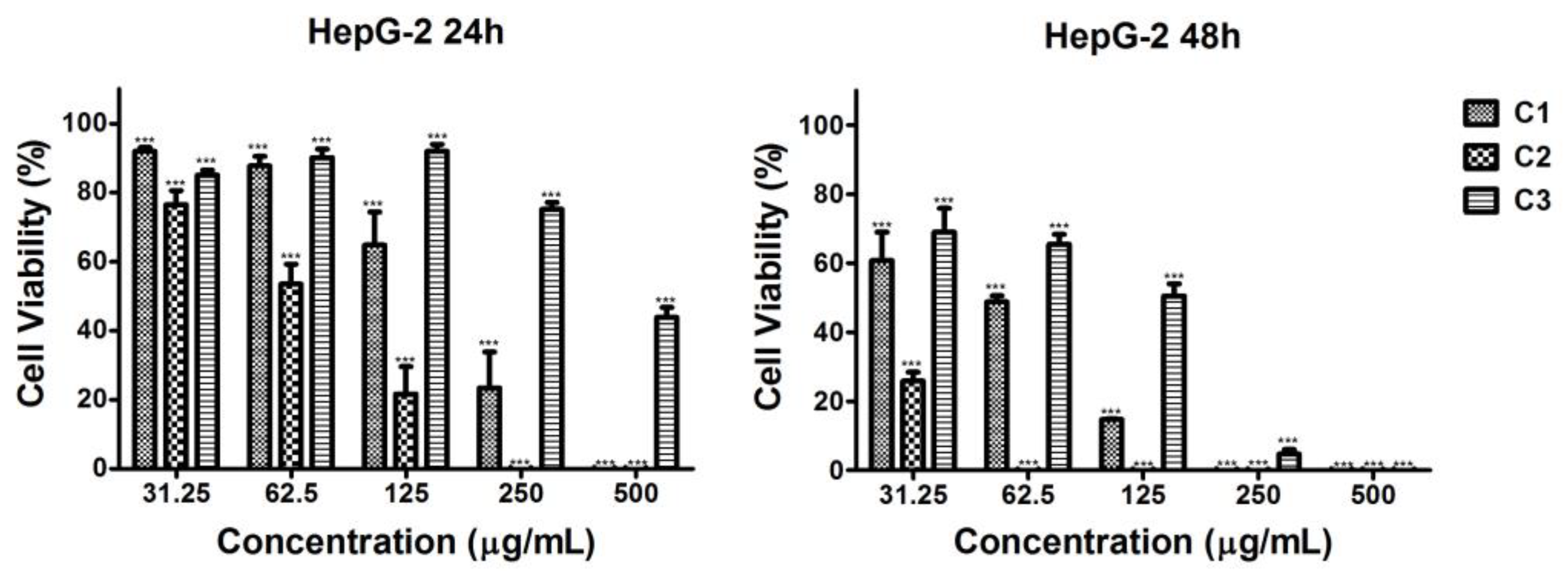
2.4.2. Cytotoxic Effect of the Microalgal Extracts Against HepG2 Hepatocellular Tumor Cells
2.4.3. Cytotoxic Effect of the Microalgal Extracts Against BALB/3T3 Non-Tumorigenic Control Cell Line
2.4.4. Determination of IC50 Concentration and Selectivity Index
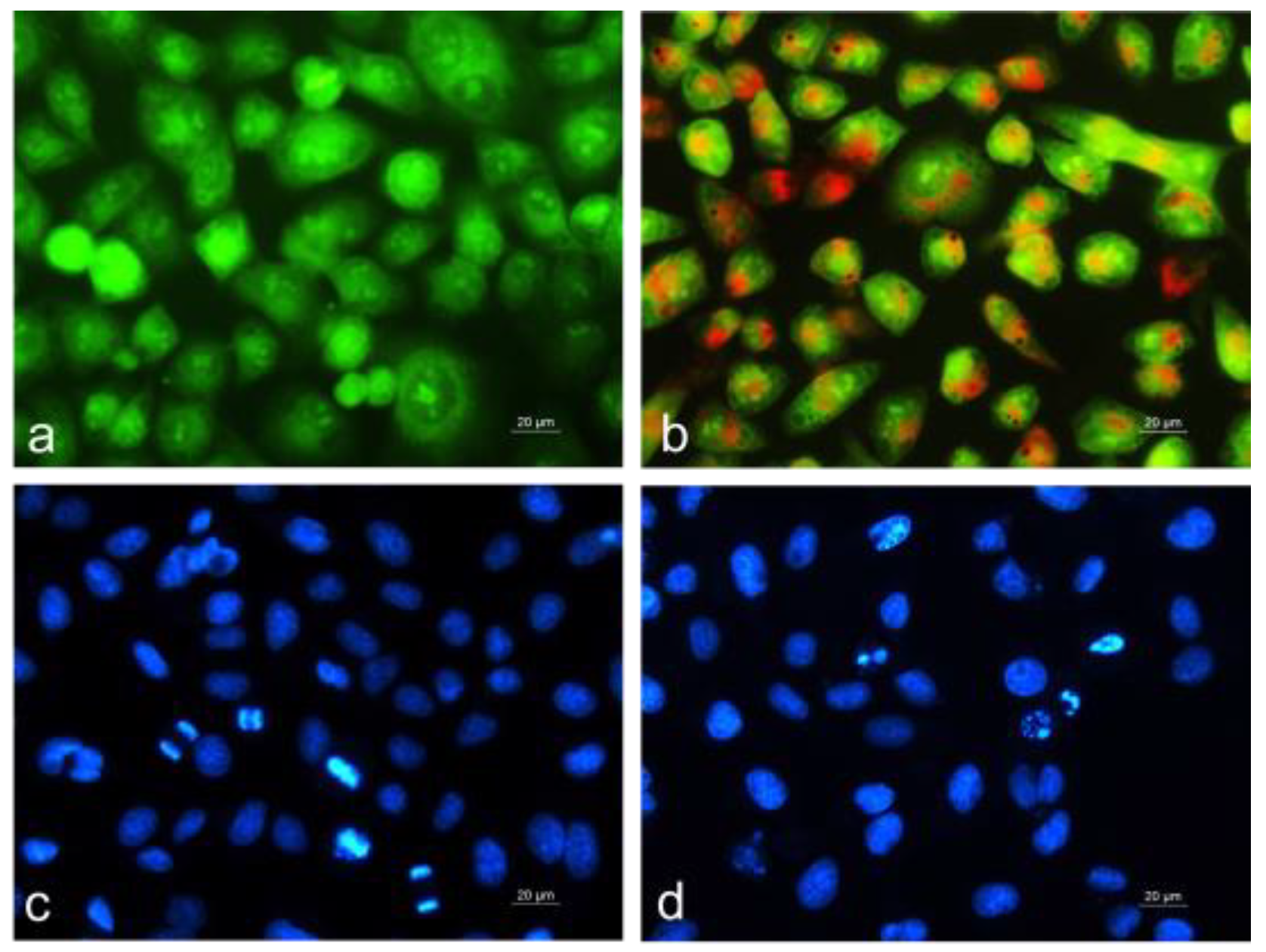
2.4.5. Cytomorphological Analysis of Apoptotic Alterations Induced by Coelastrella sp. BGV Extract in HepG2 Cancer Cells
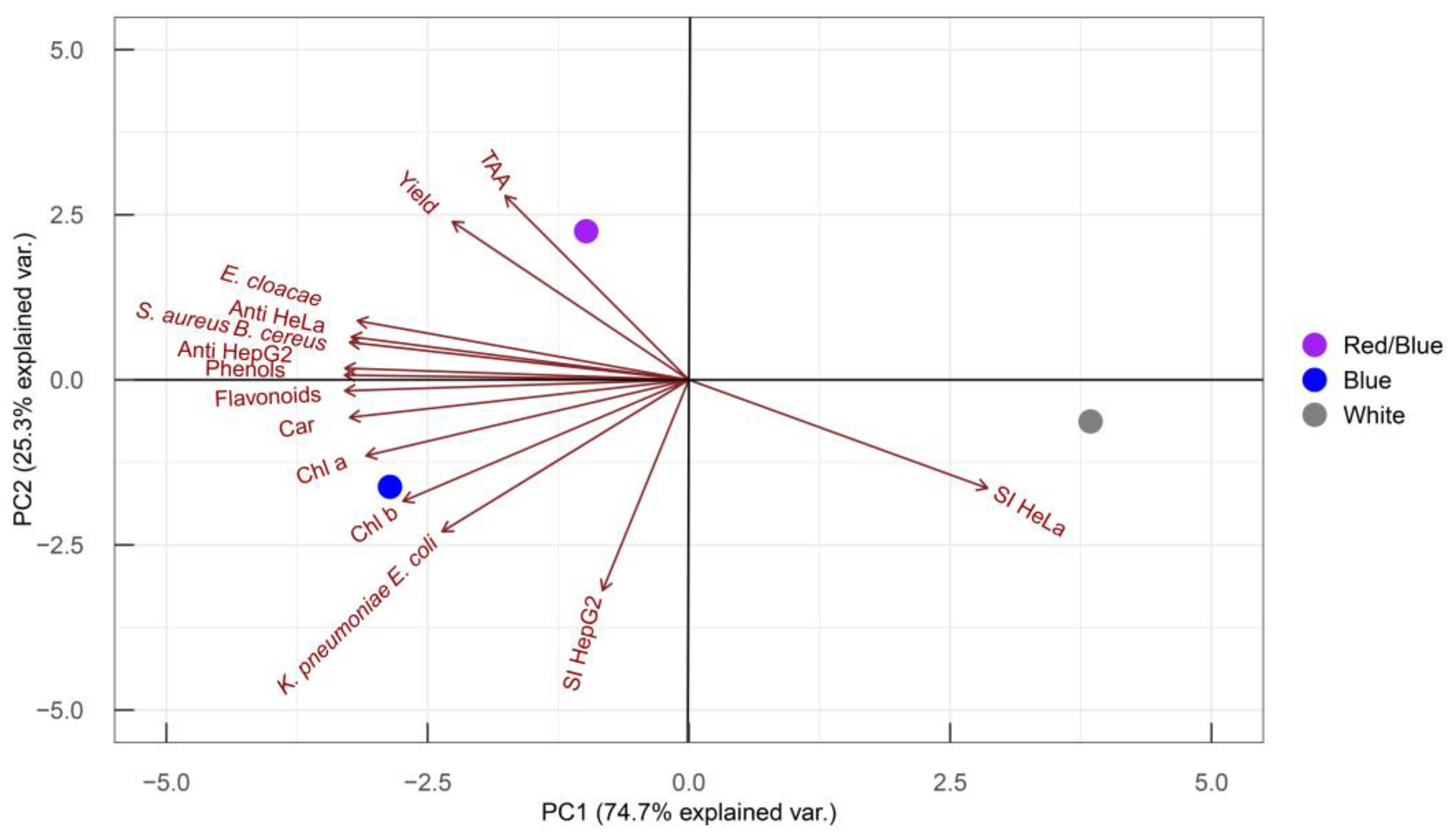
2.5. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Microalgal Cultivation
4.2. Preparation of Algal Extracts
4.3. Pigments
4.4. Antioxidant Activity
4.5. Antimicrobial Activity
4.6. Antitumour Activity
4.6.1. Cell Cultures
4.6.2. Cell Proliferation/Viability Assay
4.6.3. Determination of Half Maximal Inhibitory Concentration and Selectivity Index
4.7. Evaluation of the Morphological Alterations by Fluorescent Tests
4.7.1. Acridine Orange and Ethidium Bromide Fluorescent Staining
4.7.2. DAPI Staining
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laamanen, C.A.; Ross, G.M.; Scott, J.A. Flotation harvesting of microalgae. Renew. Sustain. Energy Rev. 2016, 58, 75–86. [Google Scholar] [CrossRef]
- Navarro, F.; Forján, E.; Vázquez, M.; Montero, Z.; Bermejo, E.; Castaño, M.Á.; Toimil, A.; Chagüaceda, E.; García-Sevillano, M.Á.; Sánchez, M.; et al. Microalgae as a safe food source for animals: Nutritional characteristics of the acidophilic microalga Coccomyxa onubensis. Food Nutr. Res. 2016, 60, 30472. [Google Scholar] [CrossRef]
- Muller-Feuga, A. Microalgae for aquaculture: The current global situation and future trends. In Handbook of Microalgal Culture; Richmond, A., Hu, Q., Eds.; Willey Blackwell: West Sussex, UK, 2013; pp. 615–627. [Google Scholar] [CrossRef]
- Soontornchaiboon, W.; Joo, S.S.; Kim, S.M. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol. Pharm. Bull. 2012, 35, 1137–1144. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Li, A.; Zhang, L.; Zhao, Z.; Ma, S.; Wang, M.; Liu, P. Prescreening, identification and harvesting of microalgae with antibacterial activity. Biologia 2016, 71, 1111–1118. [Google Scholar] [CrossRef]
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V.; et al. Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Mar. Drugs 2011, 9, 819–831. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; Reina, G.G.B.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Sun, C.Q.; O’Connor, C.J.; Roberton, A.M. Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol. Med. Microbiol. 2003, 36, 9–17. [Google Scholar] [CrossRef]
- Sangeetha, M.; Menakha, M.; Vijayakumar, S. In silico prediction of anticancer cyanobacterial drug from Nostoc. Biomed. Prev. Nutr. 2014, 4, 71–73. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Wu, N.L.; Chiang, Y.C.; Huang, C.C.; Fang, J.Y.; Chen, D.F.; Hung, C.F. Zeaxanthin inhibits PDGF-BB-induced migration in human dermal fibroblasts. Exp. Dermatol. 2010, 19, e173–e181. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of high added-value compounds—A brief review of recent work. Biotechnol. Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Pérez-Sánchez, A.; Valdés, A.; Ibrahim, O.H.M.; Suárez-Alvarez, S.; Ferragut, J.A.; Micol, V.; Cifuentes, A.; Ibáñez, E.; García-Cañas, V. Pressurized liquid extraction of Neochloris oleoabundans for the recovery of bioactive carotenoids with anti-proliferative activity against human colon cancer cells. Food Res. Int. 2017, 99, 1048–1055. [Google Scholar] [CrossRef]
- Guedes, A.C.; Meireles, L.A.; Amaro, H.M.; Malcata, F.X. Changes in lipid class and fatty acid composition of cultures of Pavlova lutheri, in response to light intensity. J. Am. Oil Chem. Soc. 2010, 87, 791–801. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Narendran, R.; Jayasingam, P.; Sampathkumar, P. Cultivation and chemical composition of microalgae Chlorella vulgaris and its antibacterial activity against human pathogens. J. Aquac. Mar. Biol. 2017, 5, 00119. [Google Scholar] [CrossRef]
- Hamouda, R.A.E.; Abou-El-Souod, G.W. Influence of various concentrations of phosphorus on the antibacterial, antioxidant and bioactive components of green microalgae Scenedesmus obliquus. Int. J. Pharmacol. 2018, 14, 99–107. [Google Scholar] [CrossRef]
- Martínez Andrade, K.A.; Lauritano, C.; Romano, G.; Ianora, A. Marine microalgae with anti-cancer properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef]
- Fu, W.; Guðmundsson, Ó.; Paglia, G.; Herjólfsson, G.; Andrésson, Ó.S.; Palsson, B.Ø.; Brynjólfsson, S. Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Appl. Microbiol. Biotechnol. 2013, 97, 2395–2403. [Google Scholar] [CrossRef]
- Krzemińska, I.; Pawlik-Skowrońska, B.; Trzcińska, M.; Tys, J. Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioprocess Biosyst. Eng. 2014, 37, 735–741. [Google Scholar] [CrossRef]
- Sharma, N.; Fleurent, G.; Awwad, F.; Cheng, M.; Meddeb-Mouelhi, F.; Budge, S.M.; Germain, H.; Desgagné-Penix, I. Red light variation an effective alternative to regulate biomass and lipid profiles in Phaeodactylum tricornutum. Appl. Sci. 2020, 10, 2531. [Google Scholar] [CrossRef]
- Bialevich, V.; Zachleder, V.; Bišová, K. The effect of variable light source and light intensity on the growth of three algal species. Cells 2022, 11, 1293. [Google Scholar] [CrossRef]
- Grasso, L.L.; Martino, D.C.; Alduina, R. Production of antibacterial compounds from Actinomycetes. In Actinobacteria-Basics and Biotechnological Applications; Dhanasekaran, D., Jiang, Y., Eds.; InTech: London, UK, 2016; Volume 7, pp. 177–198. [Google Scholar] [CrossRef]
- Ruffell, S.E.; Müller, K.M.; McConkey, B.J. Comparative assessment of microalgal fatty acids as topical antibiotics. J. Appl. Phycol. 2016, 28, 1695–1704. [Google Scholar] [CrossRef]
- Cha, K.H.; Kang, S.W.; Kim, C.Y.; Um, B.H.; Na, Y.R.; Pan, C.H. Effect of pressurized liquids on extraction of antioxidants from Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 4756–4761. [Google Scholar] [CrossRef]
- Bidigare, R.R.; Ondrusek, M.E.; Kennicutt, M.C.; Iturriaga, R.; Harvey, H.R.; Hoham, R.W.; Macko, S.A. Evidence a photoprotective for secondary carotenoids of snow algae. J. Phycol. 1993, 29, 427–434. [Google Scholar] [CrossRef]
- Romay, C.H.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Li, H.B.; Cheng, K.W.; Wong, C.C.; Fan, K.W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Kumar, A.; Vajpayee, P.; Ali, M.B.; Tripathi, R.D.; Singh, N.; Rai, U.N.; Singh, S.N. Biochemical responses of Cassia siamea Lamk. grown on coal combustion residue (fly-ash). Bull. Environ. Contam. Toxicol. 2002, 68, 675–683. [Google Scholar] [CrossRef]
- Abe, K.; Hattori, H.; Hirano, M. Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata. Food Chem. 2007, 100, 656–661. [Google Scholar] [CrossRef]
- Hu, C.W.; Chuang, L.T.; Yu, P.C.; Chen, C.N.N. Pigment production by a new thermotolerant microalga Coelastrella sp. F50. Food Chem. 2013, 138, 2071–2078. [Google Scholar] [CrossRef]
- Iyer, G.; Nagle, V.; Gupte, Y.V.; Desai, S.; Iyer, M.; Moramkar, N.; Sawant, V. Characterization of high carotenoid producing Coelastrella oocystiformis and its anti-cancer potential. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 527–536. [Google Scholar]
- Minyuk, G.; Chelebieva, E.; Chubchikova, I.; Dantsyuk, N.; Drobetskaya, I.; Sakhon, E.; Solovchenko, A. Stress-induced secondary carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a producer of value-added keto-carotenoids. Algae 2017, 32, 245–259. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. Two-phase method of cultivating Coelastrella species for increased production of lipids and carotenoids. Bioresour. Technol. Rep. 2020, 9, 100366. [Google Scholar] [CrossRef]
- Schulze, P.S.; Barreira, L.A.; Pereira, H.G.; Perales, J.A.; Varela, J.C. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef]
- Wong, Y.K.; Ho, Y.H.; Ho, K.C.; Leung, H.M.; Chow, K.P.; Yung, K.K.L. Effect of different light sources on algal biomass and lipid production in internal leds-illuminated photobioreactor. J. Mar. Biol. Aquac. 2016, 2, 2381-0750. [Google Scholar] [CrossRef]
- Syarifah, M.S.; Nurhanan, M.; Haffiz, J.M.; Ilham, A.M.; Getha, K.; Asiah, O.; Norhayati, I.; Sahira, H.L.; Suryani, S.A. Potential anticancer compound from Cerbera odollam. J. Trop. For. Sci. 2011, 23, 89–96. [Google Scholar]
- Dimitrova, P.; Marinova, G.; Alexandrov, S.; Iliev, I.; Pilarski, P. Biochemical characteristics of a newly isolated strain Coelastrella sp. BGV cultivated at different temperatures and light intensities. Annu. Univ. Sofía St. Kliment Ohridski Fac. Biol. 2017, 102, 139–146. [Google Scholar]
- Toshkova-Yotova, T.; Pilarski, P.; Yocheva, L.; Petrova, D.; Chaneva, G. Screening of antimicrobial and antioxidant properties of green microalga Coelastrella sp. BGV. Oxid. Commun. 2020, 43, 265–279. [Google Scholar]
- Sharif, N.; Munir, N.; Saleem, F.; Aslam, F.; Naz, S. Prolific anticancer bioactivity of algal extracts. Cell 2014, 3, 8–21. [Google Scholar]
- Cha, K.H.; Koo, S.Y.; Lee, D.U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Saraiva, J.A.; Martins, A.P.; Pinto, C.A.; Prieto, M.A.; Simal-Gandara, J.; Cao, H.; Xiao, J.; Barba, F.J. Extraction of lipids from microalgae using classical and innovative approaches. Food Chem. 2022, 384, 132236. [Google Scholar] [CrossRef] [PubMed]
- European Union. Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients. Off. J. Eur. Union 2009, L141, 3–11. [Google Scholar]
- Wang, X.; Wang, L.; Fekrazad, R.; Zhang, L.; Jiang, X.; He, G.; Wen, X. Polyphenolic natural products as photosensitizers for antimicrobial photodynamic therapy: Recent advances and future prospects. Front. Immunol. 2023, 14, 1275859. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, N.; Pogorilyy, V.; Diachkova, E.; Vasil’ev, Y.; Mironov, A.; Grin, M. Derivatives of natural chlorophylls as agents for antimicrobial photodynamic therapy. Int. J. Mol. Sci. 2021, 22, 6392. [Google Scholar] [CrossRef]
- Yamali, C.; Nenni, M.; Sakarya, M.T.; Sakagami, H.; Gul, H.I. Biological activities and drug-likeness properties of phenol-based heterocyclic compounds. Pharm. Chem. J. 2024, 57, 1754–1760. [Google Scholar] [CrossRef]
- Staron, J.; Boron, B.; Karcz, D.; Szczygieł, M.; Fiedor, L. Recent progress in chemical modifications of chlorophylls and bacteriochlrophylls for the applications in photodynamic therapy. Curr. Med. Chem. 2015, 22, 3054–3074. [Google Scholar] [CrossRef]
- Toshkova-Yotova, T.; Georgieva, A.; Iliev, I.; Alexandrov, S.; Ivanova, A.; Pilarski, P.; Toshkova, R. Antitumor and antimicrobial activity of fatty acids from green microalga Coelastrella sp. BGV. S. Afr. J. Bot. 2022, 151, 394–402. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Toshkova-Yotova, T.A.; Georgieva, A.; Pilarski, P.; Toshkova, R. Aqueous extracts, green microalga Coelastrella sp. BGV displays antiproliferative, proapoptotic activity in vitro against HeLa tumor cells. Compt. Rend. Acad. Bulg. Sci. 2021, 74, 696–705. [Google Scholar] [CrossRef]
- Toshkova-Yotova, T.; Georgieva, A.; Todorova, K.; Pilarski, P.; Toshkova, R. Antitumor properties of vegetable oil extract from green microalga Coelastrella sp. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e2744. [Google Scholar] [CrossRef]
- Lee, J.C.; Hou, M.F.; Huang, H.W.; Chang, F.R.; Yeh, C.C.; Tang, J.Y.; Chang, H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013, 13, 55. [Google Scholar] [CrossRef]
- Amaro, H.M.; Barros, R.; Tavares, T.; Almeida, R.; Pinto, I.S.; Malcata, F.X.; Guedes, A.C. Gloeothece sp.—Exploiting a new source of antioxidant, anti-inflammatory, and antitumor agents. Mar. Drugs 2021, 19, 623. [Google Scholar] [CrossRef]
- Schuelter, A.R.; Kroumov, A.D.; Hinterholz, C.L.; Fiorini, A.; Trigueros, D.E.G.; Vendruscolo, E.G.; Zaharieva, M.M.; Módenes, A.N. Isolation and identification of new microalgae strains with antibacterial activity on food-borne pathogens. Engineering approach to optimize synthesis of desired metabolites. Biochem. Eng. J. 2019, 144, 28–39. [Google Scholar] [CrossRef]
- Georgiev, D.; Dilov, C.; Avramova, S. Buffer nutrient medium and a method for intensive cultivation of green microalgae. Hydrobiologia 1978, 7, 14–24. [Google Scholar]
- Mackinney, G. Criteria for purity of chlorophyll preparations. J. Biol. Chem. 1941, 132, 91–96. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Essawi, T.; Srour, M. Screening of some Palestinian medicinal plants for antibacterial activity. J. Ethnopharmacol. 2000, 70, 343–349. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Abdel Wahab, S.I.; Abdul, A.B.; Alzubairi, A.S.; Mohamed Elhassan, M.; Mohan, S. In vitro ultramorphological assessment of apoptosis induced by zerumbone on (HeLa). Biomed Res. Int. 2009, 2009, 769568. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shevyrin, V.A.; Kovaleva, E.G.; Flisyuk, E.V.; Shikov, A.N. Optimization of extraction of phlorotannins from the arctic Fucus vesiculosus using natural deep eutectic solvents and their HPLC profiling with tandem high-resolution mass spectrometry. Mar. Drugs. 2023, 21, 263. [Google Scholar] [CrossRef]
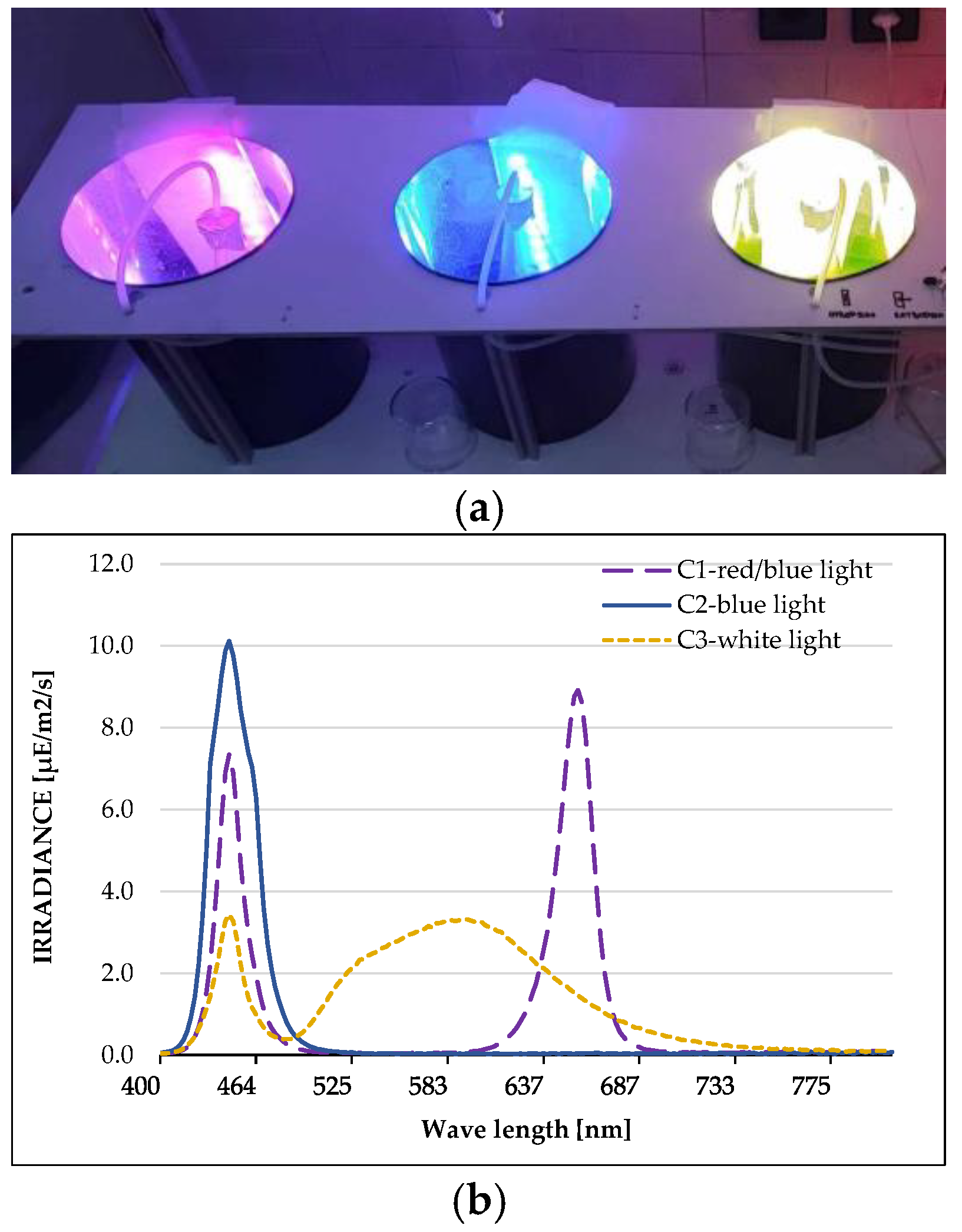

| Extracts | Chlorophyll a, | Chlorophyll b, | Carotenoids, |
|---|---|---|---|
| %DW | %DW | %DW | |
| C1 | 0.25 ± 0.004 b | 0.02 ± 0.003 b | 0.20 ± 0.001 b |
| C2 | 0.38 ± 0.002 a | 0.04 ± 0.004 a | 0.24 ± 0.005 a |
| C3 | 0.16 ± 0.004 c | 0.02 ± 0.006 c | 0.15 ± 0.006 c |
| Extracts | Yield | Phenols | Flavonoids | TAA |
|---|---|---|---|---|
| %DW | mg/g DW | mg/g DW | mM/g DW | |
| C1 | 27.31 ± 0.197 a | 52.56 ± 0.001 b | 18.35 ± 0.002 b | 484.15 ± 0.003 a |
| C2 | 25.00 ± 0.158 b | 55.89 ± 0.001 a | 23.09 ± 0.006 a | 378.39 ± 0.013 b |
| C3 | 22.81 ± 0.258 c | 43.08 ± 0.001 c | 8.55 ± 0.0005 c | 329.60 ± 0.002 c |
| Zone of Inhibition (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Extracts | Gram-Negative Bacterial Strains | Gram-Positive Bacterial Strains | Fungal Strain | |||||||
| Enterobacter cloacae | Escherichia coli | Proteus mirabilis | Pseudomonas aeruginosa | Klebsiella pneumoniae | Enterococcus faecalis | Bacillus subtilis | Bacillus cereus | Staphylococcus aureus | Candida albicans | |
| C1 | 8 ± 0.5 | - | - | - | - | - | - | 8 ± 0.5 | 8 ± 0.5 | - |
| C2 | 8 ± 0.5 | 9 ± 0.5 | - | - | 8 ± 0.5 | - | - | 9 ± 0.5 | 9 ± 0.5 | - |
| C3 | - | - | - | - | - | - | - | - | - | - |
| Antibiotic * | 28 ± 2 | 20 ± 1 | 15 ± 0.5 | 25 ± 2 | 30 ± 2 | 12 ± 0.5 | 30 ± 2 | 15 ± 0.5 | 25 ± 2 | 15 ± 0.5 |
| Extracts | IC50 HeLa | IC50 HepG2 | IC50 BALB/3T3 | |||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| C1 | 84.50 ± 6.68 b (0.72) | 29.05 ± 2.45 a (0.48) | 153.80 ± 16.35 b (0.40) | 48.18 ± 6.34 b (0.29) | 61.01 ± 4.47 a | 14.01 ± 1.65 a |
| C2 | 78.58 ± 7.82 a (1.39) | 29.60 ± 1.64 a (1.03) | 63.10 ± 9.28 a (1.73) | 29.59 ± 0.18 a (1.03) | 109.10 ± 9.96 b | 30.39 ± 2.29 b |
| C3 | 148.80 ± 12.29 c (>3.36) | 155.90 ± 15.06 b (1.08) | 457.20 ± 33.00 c (›1.09) | 85.86 ± 11.79 c (1.96) | >500.00 c | 168.30 ± 10.31 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgieva, Z.; Karcheva, Z.; Toshkova-Yotova, T.; Georgieva, A.; Toshkova, R.; Petrova, D.; Zhiponova, M.; Chaneva, G. Blue Light Enhances the Antioxidant, Antimicrobial, and Antitumor Potential of the Green Microalgae Coelastrella sp. BGV. Plants 2024, 13, 3295. https://doi.org/10.3390/plants13233295
Georgieva Z, Karcheva Z, Toshkova-Yotova T, Georgieva A, Toshkova R, Petrova D, Zhiponova M, Chaneva G. Blue Light Enhances the Antioxidant, Antimicrobial, and Antitumor Potential of the Green Microalgae Coelastrella sp. BGV. Plants. 2024; 13(23):3295. https://doi.org/10.3390/plants13233295
Chicago/Turabian StyleGeorgieva, Zhaneta, Zornitsa Karcheva, Tanya Toshkova-Yotova, Ani Georgieva, Reneta Toshkova, Detelina Petrova, Miroslava Zhiponova, and Ganka Chaneva. 2024. "Blue Light Enhances the Antioxidant, Antimicrobial, and Antitumor Potential of the Green Microalgae Coelastrella sp. BGV" Plants 13, no. 23: 3295. https://doi.org/10.3390/plants13233295
APA StyleGeorgieva, Z., Karcheva, Z., Toshkova-Yotova, T., Georgieva, A., Toshkova, R., Petrova, D., Zhiponova, M., & Chaneva, G. (2024). Blue Light Enhances the Antioxidant, Antimicrobial, and Antitumor Potential of the Green Microalgae Coelastrella sp. BGV. Plants, 13(23), 3295. https://doi.org/10.3390/plants13233295







