Habitats, Plant Diversity, and Molecular Phylogeny of Endemic Relic Species Incarvillea semiretschenskia (Bignoniaceae)
Abstract
1. Introduction
2. Results
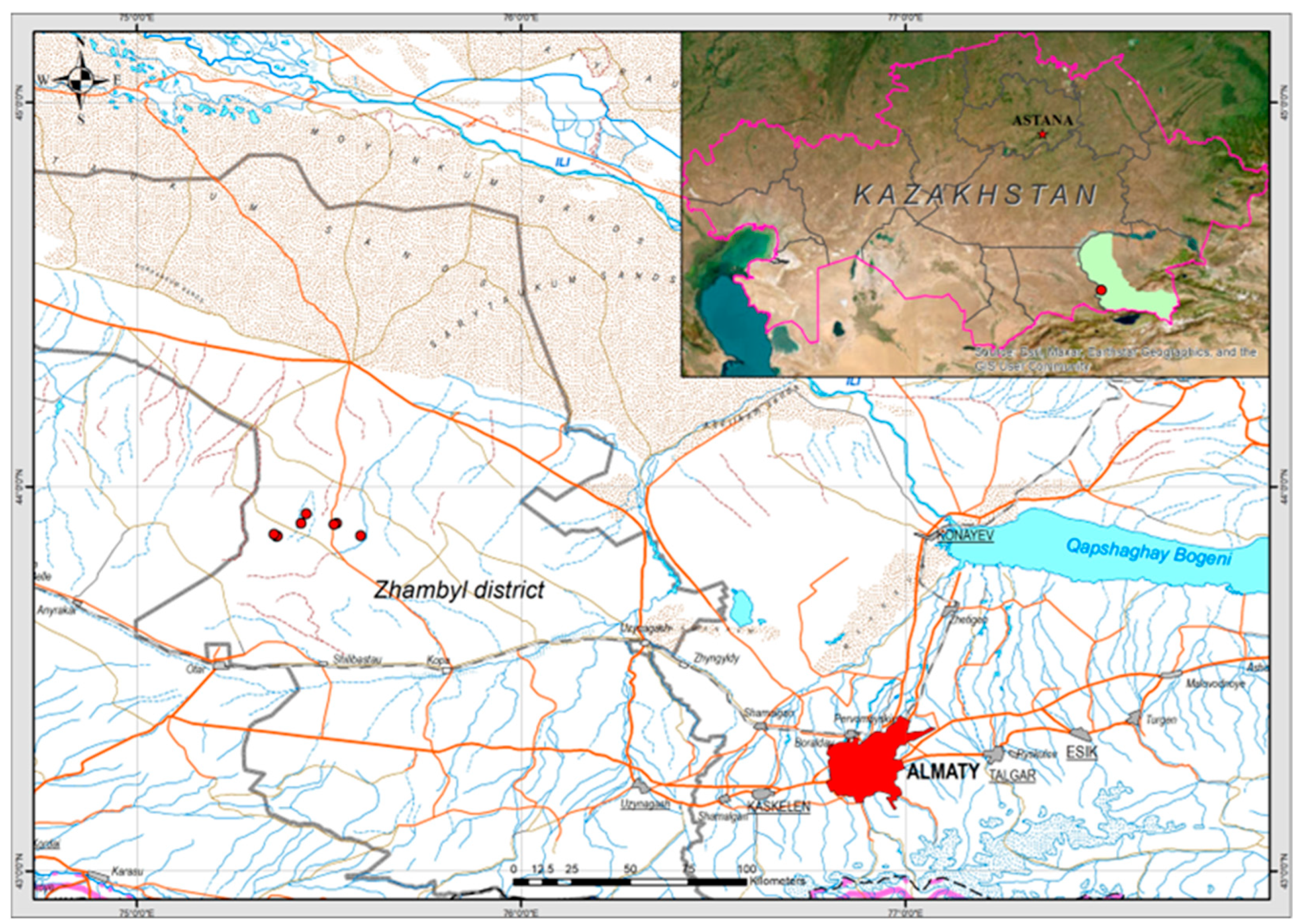
2.1. Ecological Conditions and Vegetation Cover
2.1.1. Tyrnakty Site (B)
Habitats Dominated and Co-Dominated by I. semiretschenskia
Habitats with Sparse Distribution of I. semiretschenskia
2.1.2. Shilozek Site (A)
2.2. Floristic Composition of Plant Communities
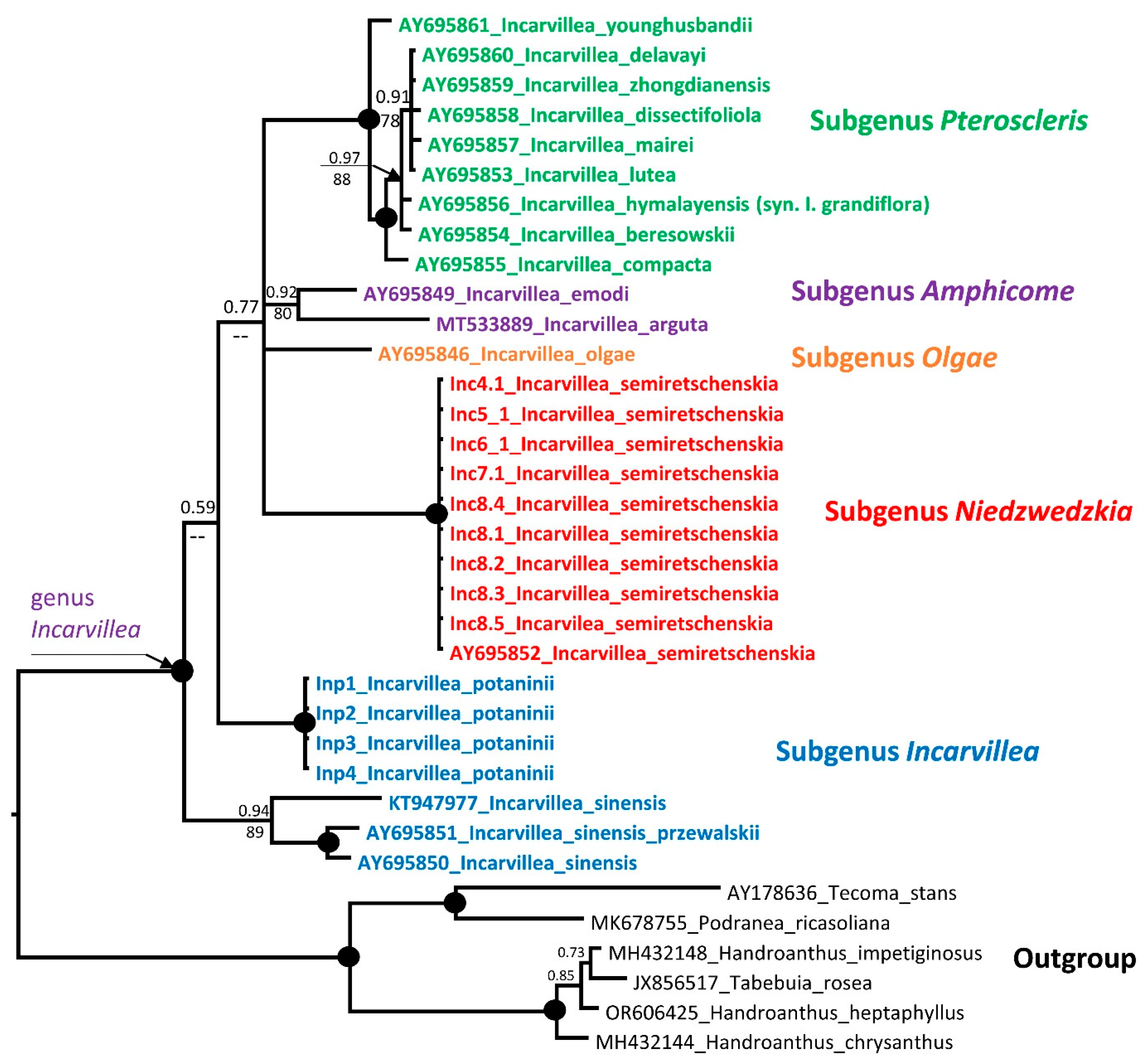
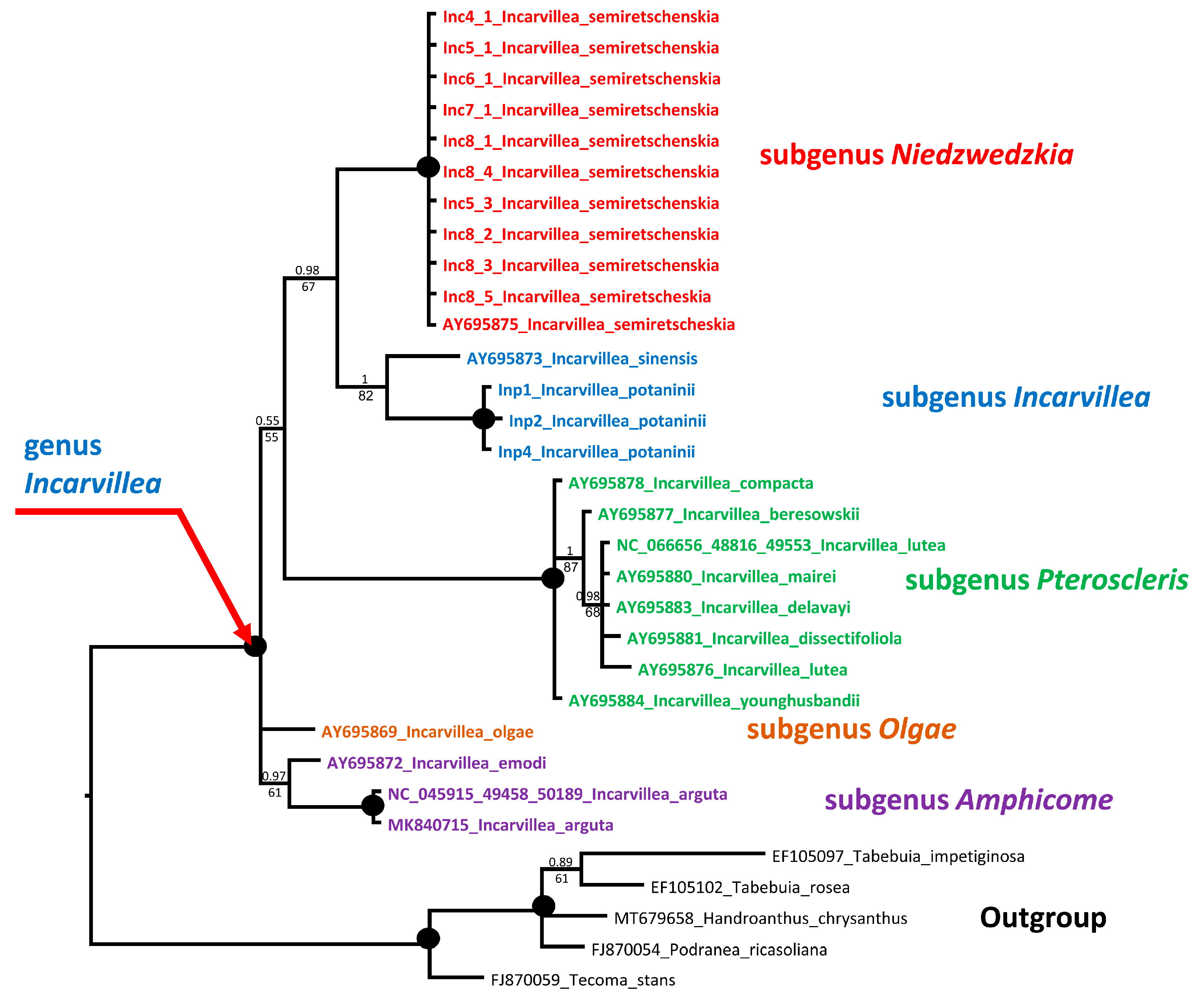
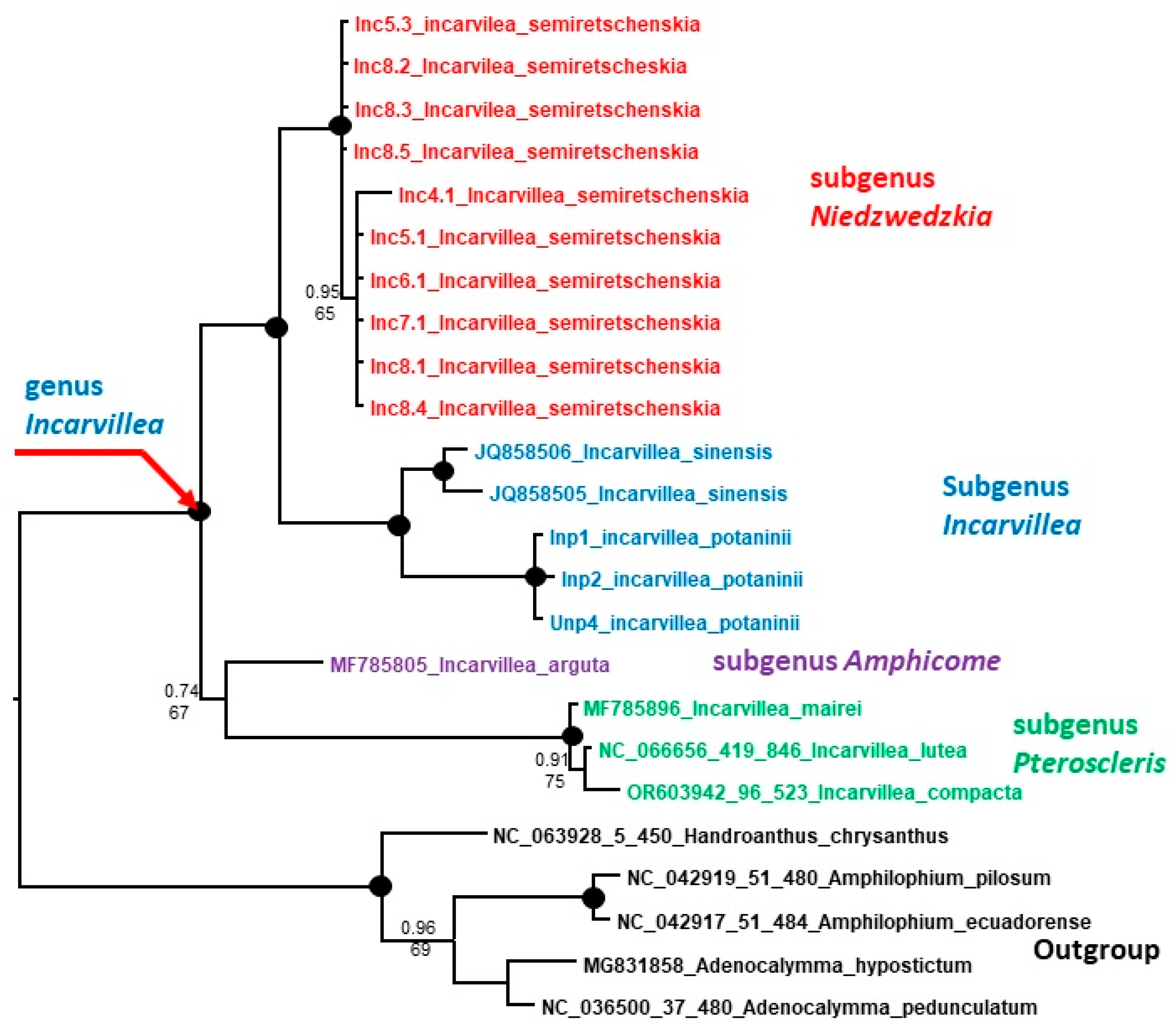
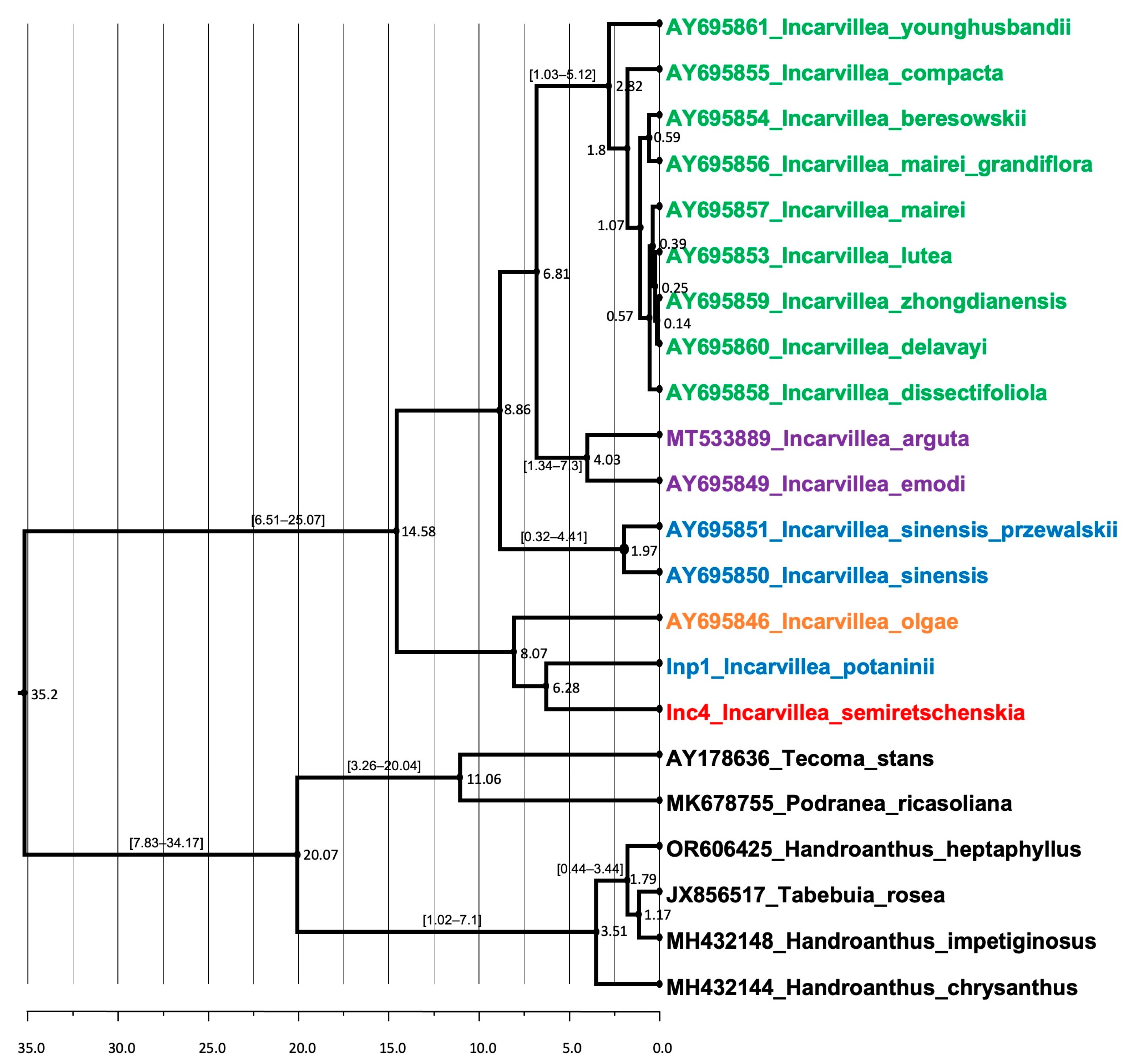
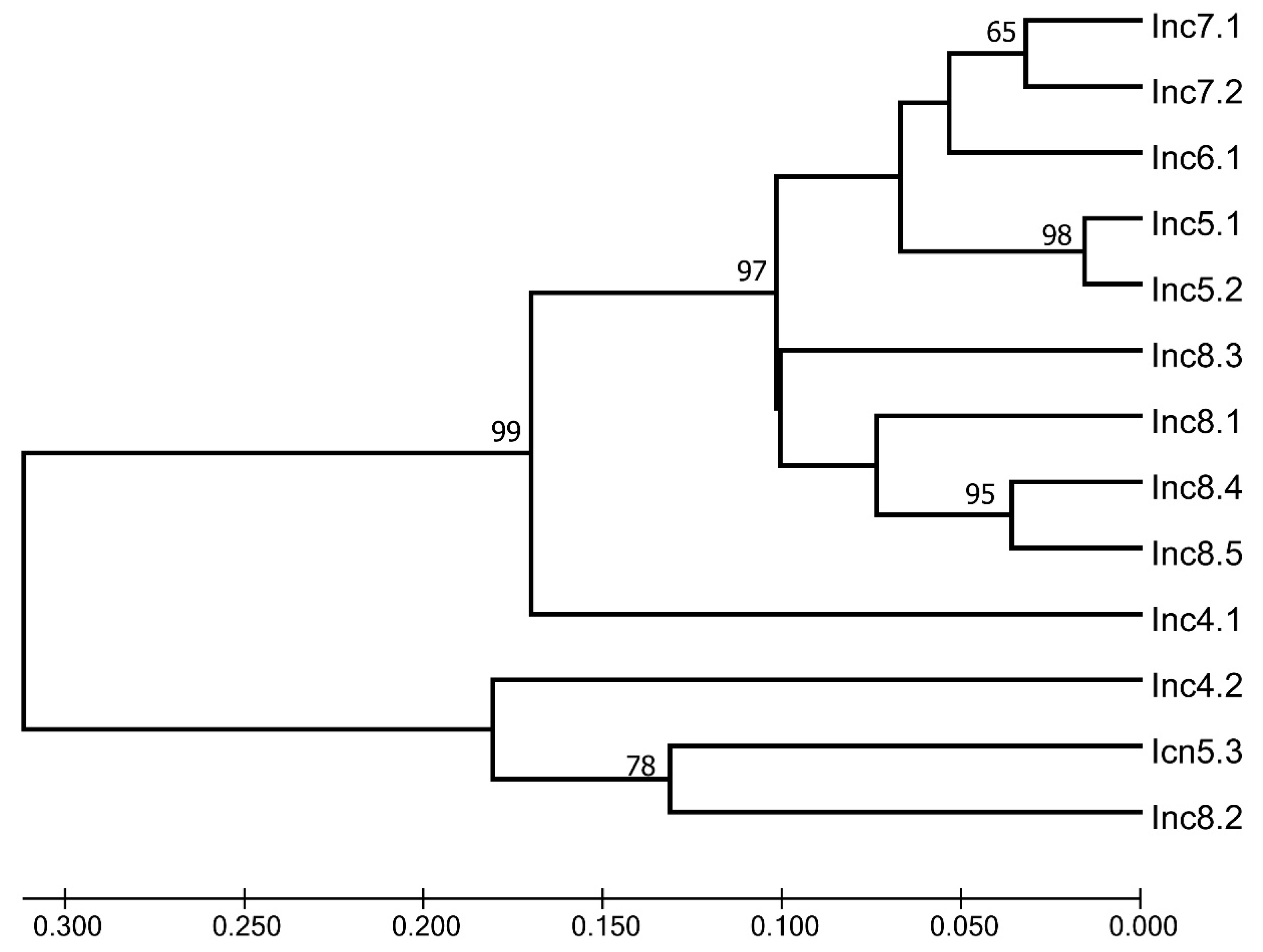
2.3. Molecular Analysis
2.3.1. Phylogenetic Analysis
2.3.2. Divergence Time Estimation
2.3.3. Fingerprint Analysis
3. Discussion
3.1. Ecological Conditions
3.2. Floristic Diversity
3.3. The Influence of Anthropogenic Factors
3.4. DNA Molecular Analysis
3.4.1. Phylogenetic Analysis
3.4.2. Dated Phylogeny of the Genus Incarvillea
3.4.3. Fingerprints Analysis
3.5. In Situ and Ex Situ Conservation
4. Materials and Methods
4.1. Data Collection
4.2. Soil Analysis
4.3. Taxon Sampling and DNA Sequencing
4.3.1. Taxon Sampling
4.3.2. DNA Isolation, Amplification and Sequencing
4.3.3. Phylogenetic Analysis
4.3.4. Divergence Time Estimation
4.3.5. Fingerprints Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Winterholler, B.A. The Genus Incarvillea and the Enigmatic Niedzwedzkia; Super Publishers: St. Petersburg, Russia, 2019. [Google Scholar]
- Grierson, A.J.C. A revision of the genus Incarvillea. Notes R. Bot. Gard. Edinb. 1961, 22, 303–354. [Google Scholar]
- Baitulin, I.O. (Ed.) Red Data Book of Kazakhstan, Plants, 2nd ed.; ArtPrintXXI LLP: Astana, Kazakhstan, 2014. [Google Scholar]
- Fedchenko, B.A. Notes on new and rare plants. Izv. Sib. Bot. Gard. 1915, 15, 1–6. [Google Scholar]
- Kokoreva, I.I.; Otradnykh, I.G.; Sjedina, I.A. Anthropogenic Impact on Natural Populations of Rare Endemic Species of the Northern Tien Shan; Luxe Media Publishing: Almaty, Kazakhstan, 2017. [Google Scholar]
- Rusanov, F.N. New data on Nedzvedskia semirechenskaia. Bull. Main Bot. Gard. Acad. Sci. USSR 1961, 40, 52–57. [Google Scholar]
- Baitulin, I.O.; Sinitsina, V.G. The ecological and morphological features of Niedzwedzkia semiretschenskia (Bignoniaceae). Bot. J. 1991, 76, 265–276. [Google Scholar]
- Kokoreva, I.I.; Otradnykh, I.G.; Sjedina, I.A.; Lyssenko, V.V. Rare Plant Species of the Northern Tien Shan (Populations, Morphology, Ontogeny, Renewal); Luxe Media Publishing: Almaty, Kazakhstan, 2013. [Google Scholar]
- Uvarova, E.V. Morpho-Biological Indices of Herbaceous Species of the Family Bignoniaceae Introduced to Alma-Ata; AISTI: Moscow, USSR, 1983. [Google Scholar]
- Orazova, A. Family Bignoniaceae—Bignoniaceae Pers. In Flora of Kazakhstan; Pavlov, N.V., Bykov, B.A., Goloskokov, V.P., Kubanskaya, Z.V., Eds.; Publishing House of the Academy of Sciences of the Kazakh SSR: Alma-Ata, USSR, 1965; Volume 8, pp. 147–149. [Google Scholar]
- Fungi. Available online: https://www.fungi.su/articles.php?article_id=418 (accessed on 5 April 2024).
- iNaturalist. Available online: https://www.inaturalist.org/taxa/909951 (accessed on 10 June 2024).
- Gbif. Available online: https://www.gbif.org/species/4091313 (accessed on 5 April 2024).
- Sokolov, S.I.; Assing, I.A.; Kurmangaliev, A.B.; Serpikov, S.K. Soils of the Kazakh SSR. Alma-Ata Region; Issue 4; Academy of Sciences of the Kazakh SSR: Alma-Ata, USSR, 1962. [Google Scholar]
- Erokhina, O.G. Soils and Soil Cover of the Chu-Ili Mountains. Ph.D. Thesis, Research Institute of Soil Science and Agrochemistry named after U.U. Uspanov, Almaty, Kazakhstan, 2006. [Google Scholar]
- Rachkovskaya, E.I.; Volkova, V.N.; Khramtsov, V.N. (Eds.) Botanical Geography of Kazakhstan and Central Asia (Within the Desert Region); Boston-Spector LLC: Saint Petersburg, Russia, 2003; pp. 13–17. [Google Scholar]
- Dimeyeva, L.A.; Permitina, V.N.; Kurmantayeva, A.A.; Ussen, K.; Kerdyashkin, A.V.; Islamgulova, A.F.; Imanalinova, A.A.; Govorukhina, S.A.; Dubynin, A.V.; Lyssenko, V.V.; et al. Green Book of Almaty Region: Rare and Needing Protection Plant Communities; Luxe Media Publishing: Almaty, Kazakhstan, 2023. [Google Scholar]
- Kudabayeva, G.M.; Vesselova, P.V.; Danilov, M.P.; Sultanova, B.M. Important Plant Areas of the Peri-North Tien Shan Subprovince as Perspective Protection Areas of Phytobiodiversity. Am. J. Environ. Prot. 2015, 8, 123–129. [Google Scholar] [CrossRef][Green Version]
- Chen, S.T.; Guan, K.Y.; Zhou, Z.K.; Cronk, Q. Molecular phylogeny of Incarvillea (Bignoniaceae) based on ITS and trnL-F sequences. Am. J. Bot. 2005, 92, 625–633. [Google Scholar] [CrossRef]
- Chen, S.T.; Gong, J.; Guan, K.Y.; Zhou, Z. Biodiversity conservation of the genus Incarvillea Juss. (Bignoniaceae) based on molecular diversity and species richness assessment. J. Plant Biol. 2010, 53, 387–394. [Google Scholar]
- Rana, S.K.; Luo, D.; Rana, H.K.; Chen, S.; Sun, H. Molecular phylogeny, biogeography and character evolution of the montane genus Incarvillea Juss. (Bignoniaceae). Plant Divers. 2021, 43, 1–14. [Google Scholar] [CrossRef]
- Kozybayeva, F.E.; Dimeyeva, L.A.; Beiseyeva, G.B.; Toktar, M. Soil and ecological conditions of rare species of tulips in Zhambyl district of Almaty region. Soil Sci. Agrochem. 2024, 3, 45–62. [Google Scholar]
- Raunkiaer, C. Statistik der lebensformen als Grundlage für die biologische Pflanzengeographie. Beih. Bot. Zentralbl. 1910, 27, 171–206. [Google Scholar]
- Korovin, E.P. Vegetation of Central Asia and Southern Kazakhstan; Publishing House Academy of Sciences of the UzSSR: Tashkent, Uzbekistan, 1961. [Google Scholar]
- Suvorov, N.I.; Shilina, Z.A. Ecological features of wild-growing thickets of Nedzvetskii in the Chu-Ili mountains. Sci. Notes Alma-Ata Paedogogical Inst. 1955, 6, 40–45. [Google Scholar]
- Fisyun, V.V. Materials on systematics and ecology of Nedzvetskyia semirechenskaya. Bot. Mater. Herb. Inst. Bot. Acad. Sci. Kaz. SSR 1982, 12, 49–57. [Google Scholar]
- Khramtsov, V.N. Vegetation of the Chu-Ili Mountains (Geography and Cartography). Ph.D. Thesis, Komarov Botanical Institute RAS, Leningrad, Russia, 1983. [Google Scholar]
- Kokoreva, I.I.; Danilov, M.P. Current state of the population of Niedzwedzkia semiretschenskia (Bignoniaceae) in the Shu-Ile Mountains (Kazakhstan). Bot. J. 2006, 91, 1215–1221. [Google Scholar]
- Lavrenko, E.M. Main Features of Botanical Geography of the Deserts of Eurasia and North Africa; Komarov Readings; USSR Academy of Sciences: Leningrad, Moscow, USSR, 1962. [Google Scholar]
- Ivashchenko, A.A. Floristic composition of communities with the participation of the relic endem Niedzwedzkia semiretschenskia B. Fedtsch. In Proceedings of the International Scientific and Practical Conference “Conservation of Ecosystems of Central Asia and Sustainable Development: Principles, Challenges, Prospects”, Bishkek, Kyrgyzstan, 19–20 September 2024. [Google Scholar]
- Wagensommer, R.P.; Venanzoni, R. Geranium lucarinii sp. nov. and re-evaluation of G. kikianum (Geraniaceae). Phytotaxa 2021, 489, 252–262. [Google Scholar] [CrossRef]
- Rana, S.K.; Luo, D.; Rana, H.K.; O’Neill, A.R.; Sun, H. Geoclimatic factors influence the population genetic connectivity of Incarvillea arguta (Bignoniaceae) in the Himalayae Hengduan Mountains biodiversity hotspot. J. Syst. Evol. 2019, 59, 151–168. [Google Scholar] [CrossRef]
- Hurka, H.; Friesen, N.; Bernhardt, K.G.; Neuffer, D.; Smirnov, S.V.; Shmakov, A.I.; Blattner, F.R. The Eurasian steppe belt: Status quo, origin and evolutionary history. Turczaninowia 2019, 22, 5–71. [Google Scholar]
- Winterholler, B.A. Incarvillea semirechenskaia; Nauka: Alma-Ata, USSR, 1976. [Google Scholar]
- Baitulin, I.O.; Winterholler, B. Ecological and cenotic features and protection of Niedzwedzkia semiretschenskia B. Fedtsch. in the Chu-Ili mountains. Bot. Res. Kaz. 1988, 6. [Google Scholar]
- Yang, J.; Sun, W. A new programme for conservation of Plant Species with Extremely Small Populations in south-west China. Oryx 2017, 51, 391–399. [Google Scholar] [CrossRef]
- Rusanov, F.N. A new ornamental plant of Nedzvetskia. Bull. Main Bot. Gard. Acad. Sci. USSR 1949, 2, 97–98. [Google Scholar]
- Belolipov, I.V. Rhythm of development, biology, flowering and fruiting of Incarvillaea semirechenskaya in the botanical garden of the Academy of Sciences of the UzSSR. In Introduction and Acclimatisation of Plants; Fan: Tashkent, Uzbekistan, 1980; pp. 108–115. [Google Scholar]
- Sadybekov, A.; Shakirov, M. Peculiarities of seed germination and development of seedlings of Nedzvetskyia semirechenskaya in culture. Sci. Notes Alma-Ata Paedogogical Inst. 1955, 7, 198–201. [Google Scholar]
- Lyashenko, N.V.; Turdieva, V.M. Morphology of the juvenile period of some endemic species of Kazakhstan. Proc. Bot. Gard. Acad. Sci. Kaz. SSR 1972, 12, 24–32. [Google Scholar]
- Lyashenko, N.V. Experience in the introduction of Nedzvetskia semirechenskaya in the Central Botanical Garden of the Academy of Sciences of the KazSSR. Izv. Acad. Sci. Kaz. SSR 1974, 6, 32–37. [Google Scholar]
- Lyashenko, N.V. Results of introduction of some species of the flora of Kazakhstan. Izv. Acad. Sci. Kaz. SSR 1979, 3, 13–20. [Google Scholar]
- Uvarova, E.V. Biological substantiation of introduction of herbaceous perennials of the Bignoniaceae Juss. Family to Alma-Ata. Ph.D. Thesis, Main Botanical Garden of the Academy of Sciences of the Kazakh SSR, Alma-Ata, USSR, 1984. [Google Scholar]
- Winterholler, B.A. Results of introduction and tasks of development of natural flora of Kazakhstan. Introd. Plants Nat. Flora Kazakhstan 1984, 21–30. [Google Scholar]
- Kupriyanov, A.N.; Mynbayeva, R.O. Nedzvetskyia semirechenskaya in the Karaganda Botanical Garden. Bull. MBG AS USSR 1991, 160, 3–7. [Google Scholar]
- Mynbayeva, R.O. Introduction of Rare and Endemic Plants in Central Kazakhstan. Ph.D. Thesis, Institute of Botany and Phytointroduction NAS Republic of Kazakhstan, Almaty, Kazakhstan, 1996. [Google Scholar]
- Nashenov, Z.B.; Selovanova, K.M.; Nashenova, G.Z.; Klimchuk, S.K.; Ivlev, V.I.; Klimchuk, A.T. Assortment of Plants for Gardening and Greening of Zhezkazgan Industrial Region; NCSTE: Zhezkazgan, Kazakhstan, 2017. [Google Scholar]
- Nashenov, Z.B.; Ivlev, V.I.; Nashenova, G.Z. Endemic and Red Book Plants of Kazakhstan in Culture and Wild Flora of Zhezkazgan Botanical Garden; Asar: Zhezkazgan, Kazakhstan, 2020. [Google Scholar]
- Tkachenko, V.I.; Assorina, I.A. Rare and Endangered Plant Species of the Natural Flora of Kyrgyzstan; Ilim: Frunze, USSR, 1978. [Google Scholar]
- Sikura, I.I. Resettlement of Plants of Central Asia to Ukraine; Naukova Dumka: Kiev, USSR, 1982. [Google Scholar]
- Sikura, I.I. Features of ontogenesis of Nedzvetskyia semirechenskaia in conditions of coastal forest-steppe of Ukraine. In Ontogenesis of Higher Flowering Plants; Recommendations; Naukova Dumka: Kiev, USSR, 1989. [Google Scholar]
- Sitpayeva, G.T. (Ed.) Catalog of Collection Fund of Living Plants of the Main Botanical Garden of IBP FWCM EGNR RK; Luxe Media Publishing: Almaty, Kazakhstan, 2021. [Google Scholar]
- Sitpayeva, G.T. (Ed.) Catalog of Collection Fund of Living Plants of Zhezkazgan Botanical Garden IBP FWCM EGNR RK; Luxe Media Publishing: Almaty, Kazakhstan, 2021. [Google Scholar]
- Sitpayeva, G.T.; Murzataeva, T.S.; Aitymbetova, K.S.; Makhmudova, K.H.; Alpysova, A.Z.; Algazy, A.T.; Dukenbaeva, B.S.; Elubaeva, A.S.; Sarzhanova, S.D. Atlas of Plant Seeds of Almaty Region; Luxe Media Publishing: Almaty, Kazakhstan, 2023. [Google Scholar]
- Bykov, B.A. Geobotany, 2nd ed.; Nauka: Alma-Ata, USSR, 1957. [Google Scholar]
- Bykov, B.A. Geobotany, 3rd ed.; Nauka: Alma-Ata, USSR, 1978. [Google Scholar]
- Drude, O. Die Ökologie der Pflanzen; Vieweg: Braunschweig, Germany, 1913. [Google Scholar]
- Pavlov, N.V. (Ed.) Flora of Kazakhstan; Publishing House of the Academy of Sciences of the Kazakh SSR: Alma-Ata, USSR, 1956; Volume 1. [Google Scholar]
- Pavlov, N.V. (Ed.) Flora of Kazakhstan; Publishing House of the Academy of Sciences of the Kazakh SSR: Alma-Ata, USSR, 1958; Volume 2. [Google Scholar]
- Pavlov, N.V. (Ed.) Flora of Kazakhstan; Publishing House of the Academy of Sciences of the Kazakh SSR: Alma-Ata, USSR, 1960; Volume 3. [Google Scholar]
- Pavlov, N.V. (Ed.) Flora of Kazakhstan; Publishing House of the Academy of Sciences of the Kazakh SSR: Alma-Ata, USSR, 1961; Volume 4. [Google Scholar]
- Pavlov, N.V. (Ed.) Flora of Kazakhstan; Publishing House of the Academy of Sciences of the Kazakh SSR: Alma-Ata, USSR, 1961; Volume 5. [Google Scholar]
- Pavlov, N.V. (Ed.) Flora of Kazakhstan; Publishing House of the Academy of Sciences of the Kazakh SSR: Alma-Ata, USSR, 1963; Volume 6. [Google Scholar]
- Pavlov, N.V. (Ed.) Flora of Kazakhstan; Publishing House of the Academy of Sciences of the Kazakh SSR: Alma-Ata, USSR, 1964; Volume 7. [Google Scholar]
- Pavlov, N.V. (Ed.) Flora of Kazakhstan; Nauka: Alma-Ata, USSR, 1965; Volume 8. [Google Scholar]
- Pavlov, N.V. (Ed.) Flora of Kazakhstan; Nauka: Alma-Ata, USSR, 1966; Volume 9. [Google Scholar]
- Goloskokov, V.P. (Ed.) Illustrated Guide for Identification of the Plants of Kazakhstan; Nauka: Alma-Ata, USSR, 1969; Volume 1. [Google Scholar]
- Goloskokov, V.P. (Ed.) Illustrated Guide for Identification of the Plants of Kazakhstan; Nauka: Alma-Ata, USSR, 1972; Volume 2. [Google Scholar]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 19 August 2024).
- Sörensen, T. A Method of Establishing Groups of Equal Amplitude in Plant Sociology Based on Similarity of Species and Its Application to Analyses of the Vegetation on Danish Commons; Munksgaard in Komm; Kongelige Danske Videnskabernes Selskab: København, Denmark, 1948; pp. 1–34. [Google Scholar]
- Soil Survey. Guide to Field Research and Soil Mapping; Publishing House of the Academy of Sciences of the USSR: Moscow, USSR, 1959; 340p. [Google Scholar]
- Arinushkina, E.V. Guide to the Chemical Analysis of Soils; MSU: Moscow, Russia, 1962; 491p. [Google Scholar]
- Interstate Standard GOST:17.4.3.01-83. Nature Protection. Soils. General Requirements for Sampling. Available online: https://files.stroyinf.ru/Data2/1/4294849/4294849589.htm (accessed on 30 June 2024).
- Interstate Standard GOST 26428-85. Soils. Methods for Determination of Calcium and Magnesium in Water Extract. Available online: https://online.zakon.kz/Document/?doc_id=31534834&pos=3;-106#pos=3;-106 (accessed on 30 June 2024).
- Interstate Standard GOST 26423-85. Soils. Methods for Determining Specific Electrical Conductivity, pH and Dense Residual of Aqueous Extract. Available online: https://files.stroyinf.ru/Data2/1/4294828/4294828015.htm (accessed on 30 June 2024).
- ST RK 3477-19. Soils. Determination of Humus by the Method of I.V. Tyurin. Available online: https://online.zakon.kz/Document/?doc_id=39003906 (accessed on 30 June 2024).
- State Standard of the USSR. Soils. Methods for Determination of Organic Matter; GOST.26213-91. Available online: https://files.stroyinf.ru/Data2/1/4294828/4294828267.htm#i87015 (accessed on 30 June 2024).
- Interstate Standard: GOST 12536-2014. Soils. Methods of Laboratory Granulometric (Grain-Size) and Microaggregate Distribution. Available online: https://files.stroyinf.ru/Data2/1/4293766/4293766967.htm (accessed on 30 June 2024).
- Interstate Standard GOST 26423-85-26428-85. Soils. Methods for Determination of Specific Electric Conductivity pH and Solid Residue of Water Extract. Soils. Methods for Determination of Calcium and Magnesium in Water Extract. Available online: https://files.stroyinf.ru/Data/201/20148.pdf (accessed on 30 June 2024).
- Blattner, F.R. Direct amplification of the entire ITS region from poorly preserved plant material using recombinant PCR. Biotechniques 1999, 27, 1180–1186. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Shaw, J.; Lickey, E.B.; Schilling, E.E.; Small, R.L. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: The tortoise and the hare III. Am. J. Bot. 2007, 94, 275–288. [Google Scholar] [CrossRef]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef]
- Microsynth Seqlab. Available online: https://srvweb.microsynth.ch/home/seqlab (accessed on 11 August 2024).
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (* and Other Methods); Version 4; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Ronquist, R.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef]
- Kay, K.M.; Whittall, J.B.; Hodges, S.A. A survey of nuclear ribosomal internal transcribed spacer substitution rates across angiosperms: An approximate molecular clock with life history effects. BMC Evol. Biol. 2006, 6, 36. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Mackill, D.J. Start Codon Targeted (SCoT) Polymorphism: A Simple, Novel DNA Marker Technique for Generating Gene-Targeted Markers in Plants. Plant Mol. Biol. Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Gupta, M.; Chyi, Y.-S.; Romero-Severson, J.; Owen, J.L. Amplification of DNA markers from evolutionarily diverse genomes using single primers of simple-sequence repeats. Theor. Appl. Genet. 1994, 89, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Volis, S. How to conserve threatened Chinese plant species with extremely small populations? Plant Diver. 2016, 38, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Perrino, E.V.; Mahmoud, Z.N.A.; Valerio, F.; Tomaselli, V.; Wagensommer, R.P.; Trani, A. Synecology of Lagoecia cuminoides L. in Italy and evaluation of functional compounds presence in its water or hydroalcoholic extracts. Sci. Rep. 2023, 13, 20906. [Google Scholar] [CrossRef] [PubMed]
- Adilet.zan. Legal Information System of Regulatory Legal Acts of the Republic of Kazakhstan. Available online: https://adilet.zan.kz/eng/docs/Z060000175_ (accessed on 18 August 2024).
- Adilet.zan. Legal Information System of Regulatory Legal Acts of the Republic of Kazakhstan. Available online: https://adilet.zan.kz/eng/docs/Z2300000183 (accessed on 18 August 2024).
- Dubynin, A.V.; Epiktetov, V. Incarvillea semiretschenskia. The IUCN Red List of Threatened Species 2024: E.T257422099A265145821. Available online: https://www.iucnredlist.org/species/257422099/265145821 (accessed on 8 November 2024).
- Dubynin, A.V.; Epiktetov, V.G. Assessment of the threat of extinction of the Kazakh paleoendemic Incarvillea semiretschenskia and measures for its protection. Probl. Bot. South. Sib. Mong. 2024, 23, 76–85. [Google Scholar]



| Species | Abundance by Drude Scale | |
|---|---|---|
| Tyrnakty (B) | Shilozek (A) | |
| 1063–1075 m asl. | 812 m asl. | |
| TPC: 35–40% | TPC: 25–30% | |
| Shrubs and dwarf shrubs | ||
| Atraphaxis compacta Ledeb. | sol | - |
| A. pyrifolia Bunge | sol | - |
| A. spinosa L. | - | sol |
| Ephedra intermedia Schrenk & C.A.Mey. | sol | un-sol |
| Helianthemum songaricum Schrenk ex Fisch. & C.A.Mey. | sp | - |
| Prunus griffithii var. tianshanica (Pojark.) Ingram | sol | sol |
| Spiraea hypericifolia (Pojark.) Ingram | sp | sp |
| Semishrubs and dwarf semishrubs | ||
| Artemisia juncea Kar. & Kir. | sol | sp |
| A. sublessingiana (B.Keller) Krasch. ex Poljakov | sol | sol |
| Bassia prostrata (L.) Beck | sol | sol |
| Incarvillea semiretschenskia (B.Fedtsch.) Grierson | sp-cop1 | sp |
| Krascheninnikovia ceratoides (L.) Gueldenst. | - | sol |
| Perennial herbs | ||
| Artemisia turanica Krasch. | sol-sp | - |
| Allium petraeum Kar. & Kir. | sol | sol-sp |
| A. trachyscordum Vved. | sol | - |
| Astragalus chaetodon Bunge | sol | sol |
| A. schrenkianus Fisch. & C.A. Mey. | sol | - |
| A. sieversianus Pall. | sol | - |
| Biebersteinia multifida DC. | sol | - |
| Bothriochloa ischaemum (L.) Keng | - | un-sol |
| Brassica elongata subsp. integrifolia (Boiss.) Breistr. | - | un-sol |
| Carex pachystylis J.Gay | sp-sol | - |
| Centaurea virgata subsp. squarrosa (Boiss.) Gugler | sol | - |
| Cousinia affinis Schrenk ex Fisch. & C.A. Mey. | sol | sol |
| Eremurus cristatus Vved. | sol | sol |
| Euphorbia rapulum Kar. & Kir. | sol | sol |
| Festuca valesiaca Schleich. ex Gaudin | sp | sp |
| Ferula ovina Boiss. | un-sol | - |
| F. tschuiliensis Bajtenov | sol | - |
| Gelasia circumflexa (Krasch. & Lipsch.) Zaika, Sukhor. & N. Kilian | sol | sol |
| Gentiana olivieri Griseb. | sol | - |
| Goniolimon cuspidatum Gamajun. | sol | - |
| G. speciosum (L.) Boiss. | sol | - |
| Haplophyllum acutifolium (DC.) G. Don | sol | sol |
| H. latifolium Kar. & Kir. | sol-sp | - |
| Iris albomarginata R.C. Foster | sol | - |
| I. halophila var. sogdiana (Bunge) Skeels | sol | - |
| I. kuschakewiczii B. Fedtsch. | sol-sp | sol |
| I. songarica Schrenk ex Fisch. & C.A. Mey. | - | sol |
| Ixiolirion tataricum (Pall.) Schult. & Schult. f. | sol | - |
| Jurinea adenocarpa Schrenk ex Fisch. & C.A. Mey. | sol | sol |
| Lagochilus platycalyx Schrenk ex Fisch. & C.A. Mey. | sol | sol |
| Linum perenne L. | sol | - |
| Marrubium anisodon K. Koch | - | un-sol |
| Oedibasis apiculata (Kar. & Kir.) Koso-Pol. | un-sol | - |
| Phlomoides molucelloides (Bunge) Salmaki | un-sol | - |
| P. septentrionalis (Popov) Adylov, Kamelin & Makhm. | - | sol |
| Piptatherum songaricum (Trin. & Rupr.) Roshev. | sol-sp | - |
| Poa bulbosa L. | sp | sp |
| Potentilla multicaulis Bunge | sol | - |
| P. soongorica Bunge | - | sol |
| Prangos cachroides (Schrenk) Pimenov & V.N.Tikhom. | sol | - |
| Pseudosedum affine (Schrenk) A. Berger | sol | - |
| Ranunculus platyspermus Fisch. ex DC. | sol | - |
| Rindera tetraspis Pall. | sol | sol |
| Rosularia glabra (Regel & C.Winkl.) A. Berger | sol | - |
| Scutellaria titovii Juz. | un-sol | - |
| Schrenkia involucrata Regel & Schmalh. | sol | - |
| Seseli sessiliflorum Schrenk | sol | sol |
| Sibbaldianthe bifurca (L.) Kurtto & T. Erikss. | sol | - |
| Stipa capillata L. | sp-cop1 | - |
| S. caucasica Schmalh. | sol | sol |
| S. conferta Poir. | - | sol |
| S. hohenackeriana Trin. & Rupr. | - | sol |
| S. kirghisorum P.A. Smirn. | sol-sp | - |
| S. lessingiana Trin. & Rupr. | sp-cop1 | - |
| S. orientalis Trin. | sp-cop1 | sol |
| S. richteriana Kar. & Kir. | sol | - |
| S. sareptana A.K. Becker | sol | - |
| Tulipa biflora Pall. | sol | - |
| Tragopogon marginifolius Pavlov | sol | - |
| T. ruber S.G. Gmel. | sol | sol |
| Ziziphora clinopodioides Lam. | sol | un-sol |
| Annual herbs | ||
| Alyssum desertorum Stapf | sol | sol |
| Bromus tectorum L. | sp | sp |
| Ceratocarpus arenarius L. | - | sol |
| Eragrostis minor Host | sol | - |
| Eremopyrum orientale (L.) Jaub. & Spach | sol | sol |
| Lappula microcarpa (Ledeb.) Gürke | sol | sol |
| Meniocus linifolius (Stephan ex Willd.) DC. | sol | - |
| Minuartia meyeri (Boiss.) Bornm. | sol | - |
| Ranunculus testiculatus Crantz | sol | - |
| Secale sylvestre Host | sol | - |
| Strigosella africana (L.) Botsch. | sol | - |
| Ziziphora tenuior L. | sol | - |
| Number of species | 74 | 40 |
| Code | Origin | Coordinates | Herbarium | ITS | trnL-trnF | psbA-trnH |
|---|---|---|---|---|---|---|
| Inkarvillea semiretschskia | Kazakhstan | |||||
| Inc4-1 | Shu-Ile Mountains, Tyrnakty tract | 43.872642 N, 75.361853 E | AA | PP864255 | PP869653 | PP869666 |
| Inc4-2 | ||||||
| Inc5-1 | Shu-Ile Mountains, Tyrnakty tract | 43.871202 N, 75.363378 E | AA | PP864256 | PP869654 | PP869667 |
| Inc5-2 | ||||||
| Inc5-3 | PP869655 | PP869668 | ||||
| Inc6-1 | Shu-Ile Mountains, Tyrnakty tract | 43.869667 N, 75.359 E | AA | PP864257 | PP869656 | PP869669 |
| Inc7-1 | Shu-Ile Mountains, Tyrnakty tract | 43.876648 N, 75.35617 E | AA | PP864258 | PP869657 | PP869670 |
| Inc7-2 | ||||||
| Inc8-1 | Spurs of the Shu-Ile Mountains, Shilozek tract | 43.87278 N, 75.581902 E | AA | PP864259 | PP869658 | PP869671 |
| Inc8-2 | PP864260 | PP869659 | PP869672 | |||
| Inc8-3 | PP864261 | PP869660 | PP869673 | |||
| Inc8-4 | PP864262 | PP869661 | PP869674 | |||
| Inc8-5 | PP864263 | PP869662 | PP869675 | |||
| Incarvillea potaninii | Mongolia | |||||
| Inp1 | Bayankhongor prov. Shinejinst sum, Jinst mountain | 44.559183 N, 99.292440 E | UBA, 5 September 1980, Ch. Sanchir | PP864264 | PP869663 | PP869676 |
| Inp2 | Umnogobi prov. Bayandalai sum, Zuramtai mountai | 43.488454 N, 103.773991 E | UBA, 5 September 1976, Ch. Sanchir, Ts. Tseplev | PP864265 | PP869664 | PP869677 |
| Inp3 | Gobi-Altai aimak, Khuren Khana mountain, Muu sar valley | 44.409609 N, 97.346536 E | UBA, 7 September 1979, V.I.Grubov, A. Muldashaev | PP864266 | ||
| Inp4 | Umnogobi prov. Takhilgyn khyr, 21 km south Noyon sum | 43.117826 N, 102.132012 E | UBA, 8 September 1979, V.I.Grubov, A. Muldashaev | PP864267 | PP869665 | PP869678 |
| Primer | Primer Sequence (5′-3′) | Number of Fragments | Polymorphic Fragments | Polymorphism |
|---|---|---|---|---|
| ISSR UBC841 | (GA)8YC | 11 | 9 | 81.8% |
| ISSR UBC884 | HBH(AG)7 | 10 | 10 | 100% |
| ISSR HB12 | (CAC)3 GC | 10 | 5 | 50% |
| ISSR HB13 | (GAG)3GC | 11 | 8 | 72.7% |
| ISSR HB14 | (CTC)3GC | 8 | 4 | 50% |
| Ʃ ISSR | 50 | 36 | 72% | |
| SCoT 2 | CAACAATGGCTACCACCC | 11 | 9 | 81.8% |
| SCoT 7 | CAACAATGGCTACCACGG | 9 | 8 | 88.9% |
| SCoT 8 | CAACAATGGCTACCACGT | 12 | 11 | 91.7% |
| SCoT 12 | ACGACATGGCGACCAACG | 16 | 12 | 75% |
| SCoT 13 | ACGACATGGCGACCATCG | 8 | 7 | 87.5% |
| SCoT 14 | ACGACATGGCGACCACGC | 13 | 9 | 69.2% |
| SCoT 16 | ACCATGGCTACCACCGAC | 12 | 9 | 75% |
| Ʃ SCoT | 81 | 65 | 80.2% | |
| Average level of polymorphism | 77.1% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimeyeva, L.; Permitina, V.; Kurmantayeva, A.; Imanalinova, A.; Osmonali, B.; Kozybayeva, F.; Beiseyeva, G.; Ussen, K.; Iskakov, R.; Oyuntsetseg, B.; et al. Habitats, Plant Diversity, and Molecular Phylogeny of Endemic Relic Species Incarvillea semiretschenskia (Bignoniaceae). Plants 2024, 13, 3299. https://doi.org/10.3390/plants13233299
Dimeyeva L, Permitina V, Kurmantayeva A, Imanalinova A, Osmonali B, Kozybayeva F, Beiseyeva G, Ussen K, Iskakov R, Oyuntsetseg B, et al. Habitats, Plant Diversity, and Molecular Phylogeny of Endemic Relic Species Incarvillea semiretschenskia (Bignoniaceae). Plants. 2024; 13(23):3299. https://doi.org/10.3390/plants13233299
Chicago/Turabian StyleDimeyeva, Liliya, Valeriya Permitina, Alfiya Kurmantayeva, Azhar Imanalinova, Bektemir Osmonali, Farida Kozybayeva, Gulzhan Beiseyeva, Kapar Ussen, Rashid Iskakov, Batlai Oyuntsetseg, and et al. 2024. "Habitats, Plant Diversity, and Molecular Phylogeny of Endemic Relic Species Incarvillea semiretschenskia (Bignoniaceae)" Plants 13, no. 23: 3299. https://doi.org/10.3390/plants13233299
APA StyleDimeyeva, L., Permitina, V., Kurmantayeva, A., Imanalinova, A., Osmonali, B., Kozybayeva, F., Beiseyeva, G., Ussen, K., Iskakov, R., Oyuntsetseg, B., & Friesen, N. (2024). Habitats, Plant Diversity, and Molecular Phylogeny of Endemic Relic Species Incarvillea semiretschenskia (Bignoniaceae). Plants, 13(23), 3299. https://doi.org/10.3390/plants13233299






