Glucosinolates Mediated Regulation of Enzymatic Activity in Response to Oxidative Stress in Brassica spp.
Abstract
1. Introduction
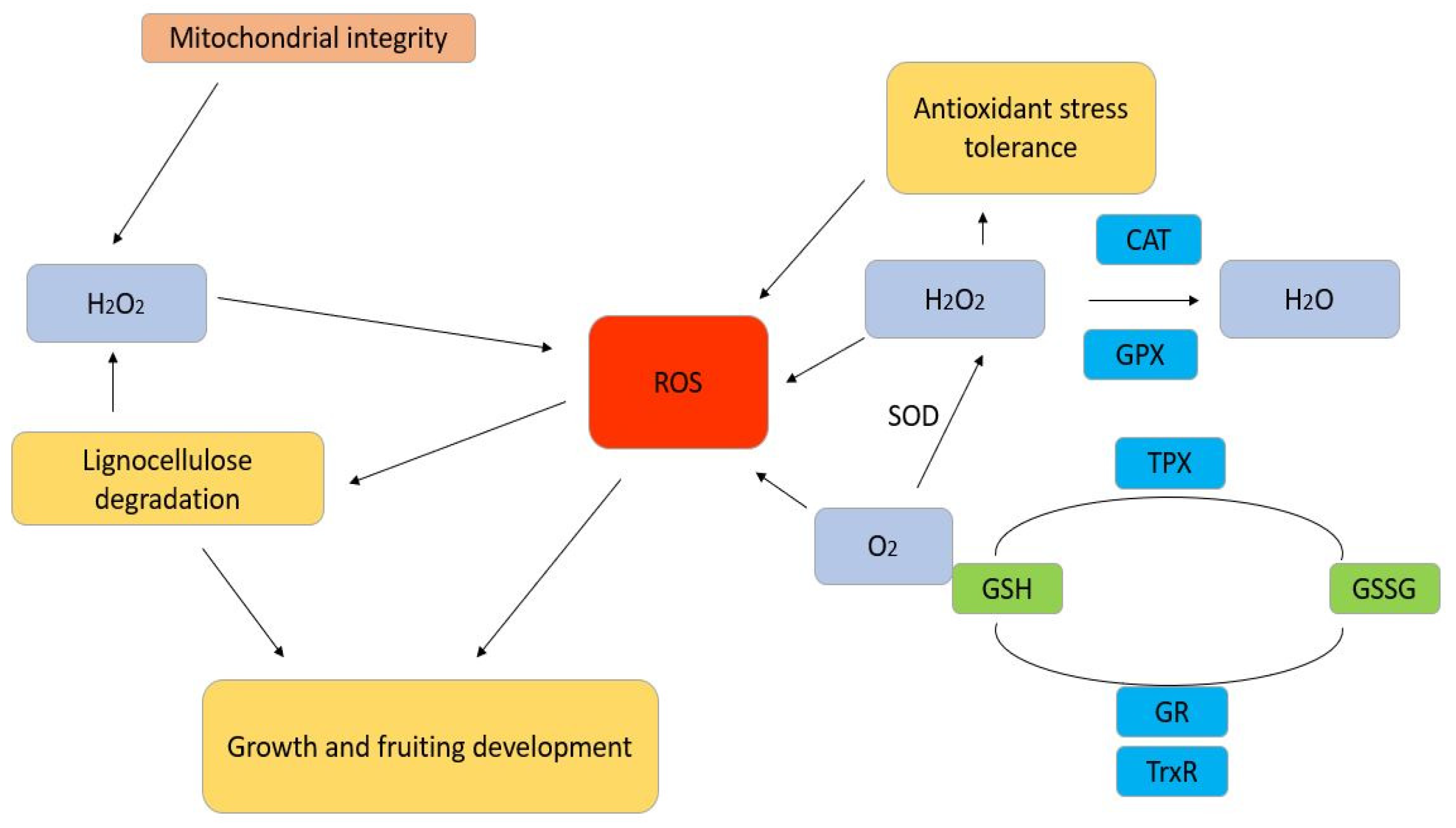
2. Oxidative Stress and Associated Enzymes
3. Enzymatic Defence Mechanism to Prevent Oxidative Stress
- SOD: It neutralizes superoxide radicals (O2•−), a singlet oxygen, and splits it into hydrogen peroxide (H2O2) and molecular oxygen (O2), depending on the presence of metal cofactors, such as Mn, Cu, or Zn, in its active site [33].
- Catalase: It promotes the breakdown of hydrogen peroxide in water and oxygen, which in turn decreases the amount of oxidative injury [34].
- Glutathione Peroxidase: It catalyzes the breakdown of hydrogen peroxide and organic peroxide using glutathione as a substrate [35].
3.1. Superoxide Dismutase (SOD)
3.2. Catalase (CAT)
3.3. Ascorbate Peroxidase (APX)
3.4. Glutathione Reductase (GR)
3.5. Monodehydroascorbate Reductase (MDHAR)
3.6. Dehydroascorbate Reductase (DHAR)
3.7. Glutathione Peroxidase (GPX)
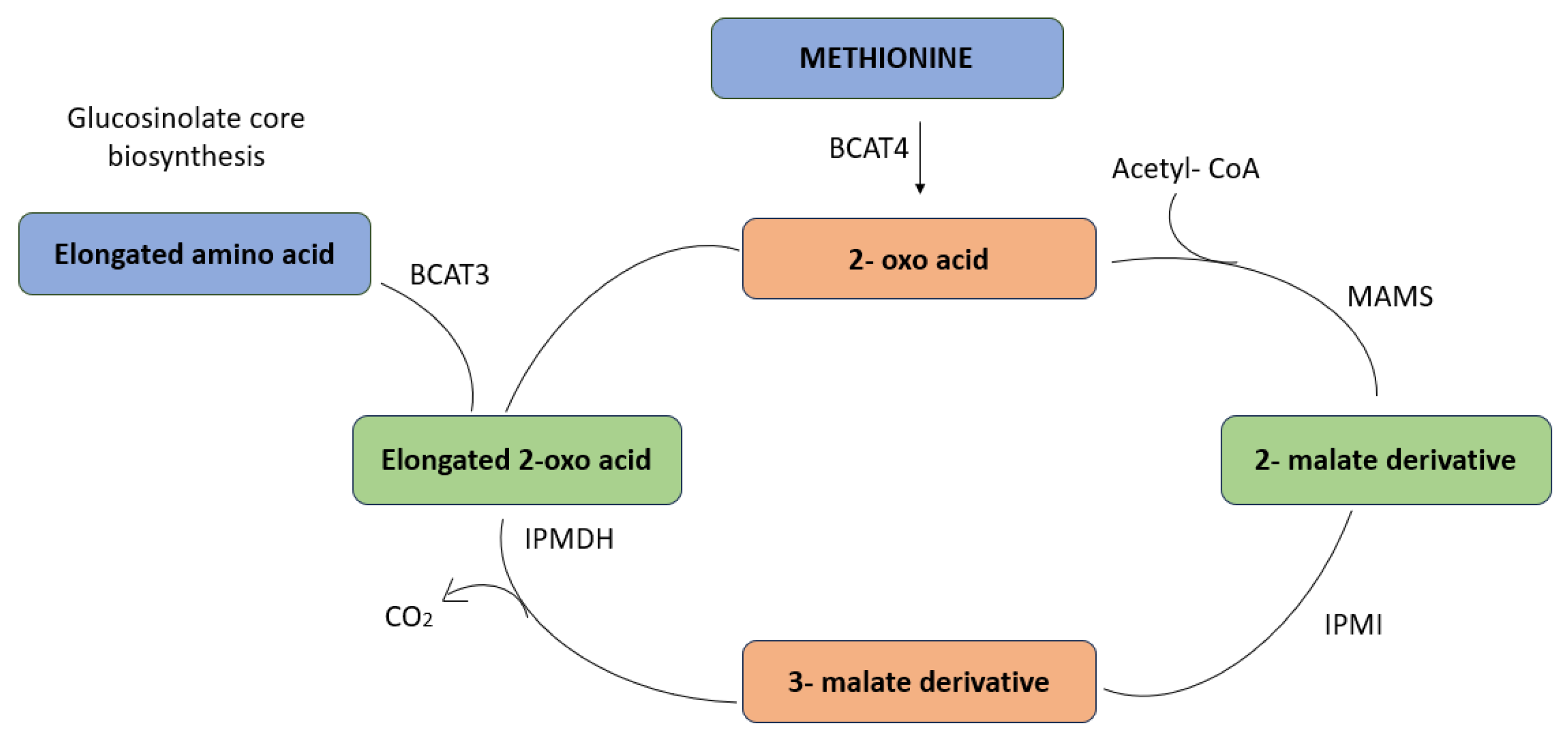
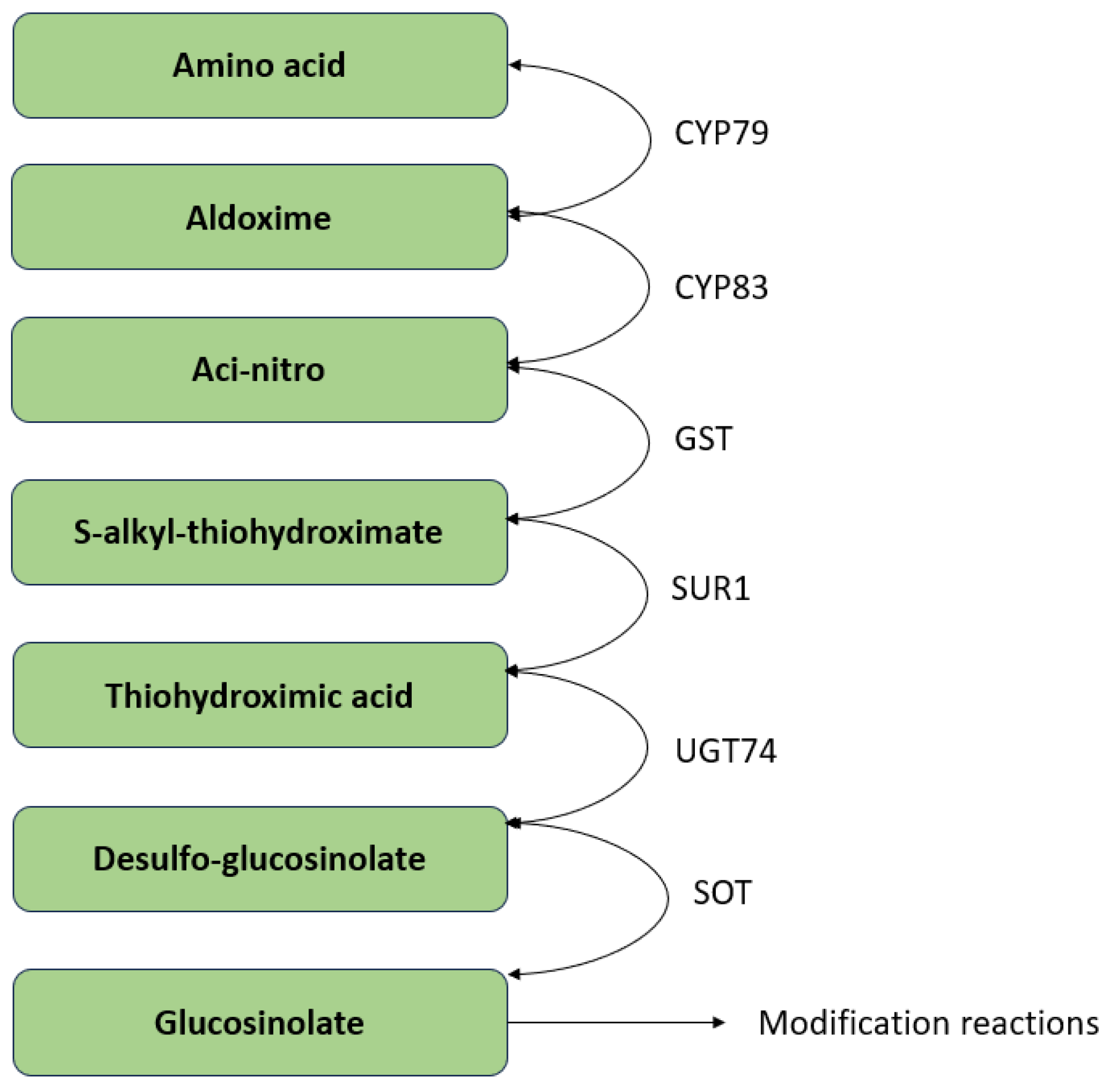
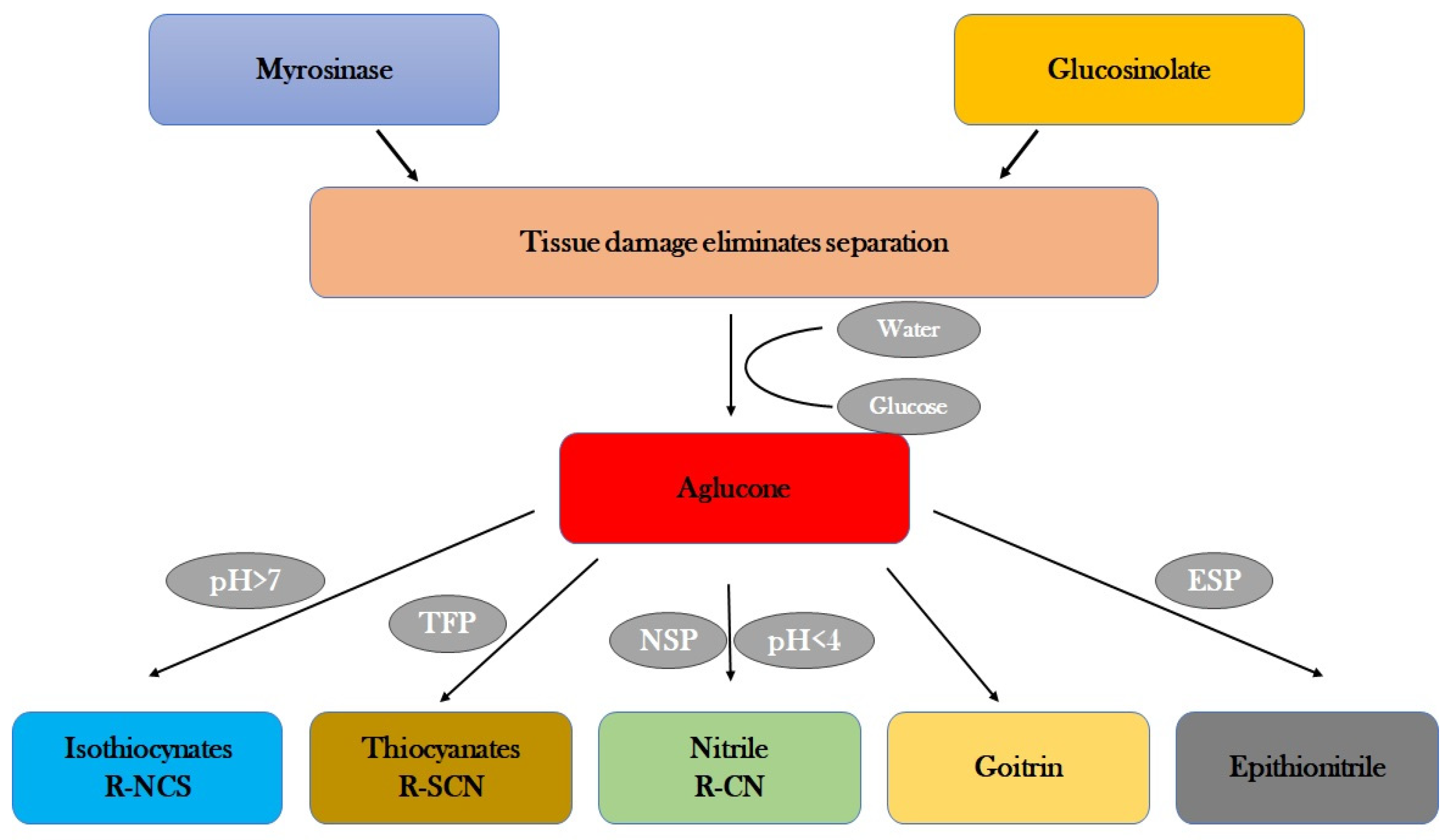
4. Glucosinolates in Brassica spp.
5. Pattern of Activity of Glucosinolates to Regulate Enzymatic Activity
6. Regulation of Enzymatic Activity in Response to Oxidative Stress
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalia, P.; Singh, S.; Selvakumar, R.; Mangal, M.; Nagarathna, T.K. Genome Designing for Nutritional Quality in Vegetable Brassicas. In Compendium of Crop Genome Designing for Nutraceuticals; Kole, C., Ed.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Yadava, D.K.; Yashpal; Saini, N.; Nanjundan, J.; Vasudev, S. Brassica Breeding. In Fundamentals of Field Crop Breeding; Yadava, D.K., Dikshit, H.K., Mishra, G.P., Tripathi, S., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Solanki, N.S.; Nagar, C. Study of Phenological Parameters and Agrometeorological Indices of Indian Mustard (Brassica juncea L. Czern) Varieties under Different Sowing Dates. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 3431–3436. [Google Scholar] [CrossRef]
- Chauhan, J.S.; Choudhury, P.R.; Pal, S.; Singh, K.H. Analysis of seed chain and its implication in rapeseed-mustard (Brassica spp.) production in India. J. Oilseeds Res. 2020, 37, 71–84. [Google Scholar] [CrossRef]
- Sahu, A.; Salam, J.L.; Verma, S.; Samuel, S. Combining Ability and Heterosis for Seed Yield and its Attributing Traits in Indian Mustard (Brassica juncea L. Czern & Coss). Int. J. Curr. Microbiol. App. Sci. 2020, 9, 720–727. [Google Scholar] [CrossRef]
- Hecht, F.; Pessoa, C.F.; Gentile, L.B.; Rosenthal, D.; Carvalho, D.P.; Fortunato, R.S. The role of oxidative stress on breast cancer development and therapy. Tumor Biol. 2016, 37, 4281–4291. [Google Scholar] [CrossRef]
- Bouslimi, H.; Ferreira, R.; Dridi, N.; Brito, P.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Effects of barium stress in Brassica juncea and Cakile maritima: The indicator role of some antioxidant enzymes and secondary metabolites. Phyton-Int. J. Exp. Bot. 2021, 90, 145–158. [Google Scholar] [CrossRef]
- Maina, S.; Ryu, D.H.; Cho, J.Y.; Jung, D.S.; Park, J.E.; Nho, C.W.; Bakari, G.; Misinzo, G.; Jung, J.H.; Yang, S.H.; et al. Exposure to salinity and light spectra regulates glucosinolates, phenolics, and antioxidant capacity of Brassica carinata L. Microgreens. Antioxidants 2021, 10, 1183. [Google Scholar] [CrossRef]
- Calmes, B.; N’Guyen, G.; Dumur, J.; Brisach, C.A.; Campion, C.; Lacomi, B.; Pigné, S.; Dias, E.; Macherel, D.; Guillemette, T.; et al. Glucosinolate-derived isothiocyanates impact mitochondrial function in fungal cells and elicit an oxidative stress response necessary for growth recovery. Front. Plant Sci. 2015, 6, 414. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, V.A.; Tyutereva, E.V.; Voitsekhovskaja, O.V. Singlet oxygen in plants: Generation, detection, and signaling roles. Int. J. Mol. Sci. 2020, 21, 3237. [Google Scholar] [CrossRef]
- Fischer, B.B.; Hideg, E.; Krieger-Liszkay, A. Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxid. Redox Signal. 2013, 18, 2145–2162. [Google Scholar] [CrossRef]
- Yadav, P.; Kaur, R.; Kohli, S.K.; Sirhindi, G.; Bhardwaj, R. Castasterone assisted accumulation of polyphenols and antioxidant to increase tolerance of B. juncea plants towards copper toxicity. Cogent Food Agric. 2016, 2, 1276821. [Google Scholar] [CrossRef]
- Antoniou, C.; Savvides, A.; Christou, A.; Fotopoulos, V. Unravelling chemical priming machinery in plants: The role of reactive oxygen–nitrogen–sulfur species in abiotic stress tolerance enhancement. Curr. Opin. Plant Biol. 2016, 33, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, K.B.; Benzarti, M.; Debez, A.; Bailly, C.; Savouré, A.; Abdelly, C. NADPH oxidase-dependent H2O2 production is required for salt-induced antioxidant defense in Arabidopsis thaliana. J. Plant Physiol. 2015, 174, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.K.A.; Zia, M.H.; Ahmad, A.; Aref, I.M.; Fatma, T.; Iqbal, M.; Owens, G. Status of antioxidant defense system for detoxification of arsenic in Brassica juncea (L.). Ecoprint Int. J. Ecol. 2016, 22, 7–19. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Ďuračková, Z. Some Current Insights into Oxidative Stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [CrossRef]
- Betteridge, D.J. What Is Oxidative Stress? Metab.-Clin. Exp. 2020, 49, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, J.; Mustafa, G. Mechanism of Reactive Oxygen Species Regulation in Plants. In Reactive Oxygen Species; Faizan, M., Hayat, S., Ahmed, S.M., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I. Oxidative Stress Triggered Damage to Cellular Biomolecules. In Reactive Oxygen Species in Plants; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Sharma, K.; Devi, P.; Kumar, P.; Dey, A.; Dwivedi, P. Hazardous Phytotoxic Nature of Reactive Oxygen Species in Agriculture. In Reactive Oxygen Species; Faizan, M., Hayat, S., Ahmed, S.M., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Borges, C.V.; Orsi, R.O.; Maraschin, M.; Lima, G.P.P. Oxidative stress in plants and the biochemical response mechanisms. In Plant Stress Mitigators: Types, Techniques and Functions; Academic Press: Cambridge, MA, USA, 2023; pp. 455–468. [Google Scholar] [CrossRef]
- Naveen, J.; Hithamani, G.; Pushpalatha, H.G. Stress and its influence on the generation of reactive oxygen species and oxidative damage in plants. In Plant Metabolites Under Environmental Stress; Apple Academic Press: Palm Bay, FL, USA, 2023; pp. 219–248. [Google Scholar]
- Kumari, A.; Singh, B.M.; Sharma, S.; Chitara, K.; Debnath, A.; Maharana, C.; Parihar, M.; Sharma, B. ROS Regulation Mechanism for Mitigation of Abiotic Stress in Plants; IntechOpen: London, UK, 2020. [Google Scholar]
- Fleming, A.M.; Burrows, C.J. Oxidative stress-mediated epigenetic regulation by G-quadruplexes. NAR Cancer 2021, 3, zcab038. [Google Scholar] [CrossRef]
- Muthusamy, M.; Lee, S.I. Abiotic stress-induced secondary metabolite production in Brassica: Opportunities and challenges. Front. Plant Sci. 2023, 14, 1323085. [Google Scholar] [CrossRef]
- Natasha, S.M.; Khalid, S.; Bibi, I.; Khalid, S.; Masood, N.; Qaisrani, S.A.; Niazi, N.K.; Dumat, C. Arsenic-induced oxidative stress in Brassica oleracea: Multivariate and literature data analyses of physiological parameters, applied levels and plant organ type. Environ. Geochem. Health 2022, 44, 1827–1839. [Google Scholar] [CrossRef]
- Tan, Z.; Wu, C.; Xuan, Z.; Cheng, Y.; Xiong, R.; Su, Z.; Wang, D. Lead exposure dose-dependently affects oxidative stress, AsA-GSH, photosynthesis, and mineral content in pakchoi (Brassica chinensis L.). Front. Plant Sci. 2022, 13, 1007276. [Google Scholar] [CrossRef]
- Sharma, P.; Chouhan, R.; Bakshi, P.; Gandhi, S.G.; Kaur, R.; Sharma, A.; Bhardwaj, R. Amelioration of Chromium-Induced Oxidative Stress by Combined Treatment of Selected Plant-Growth-Promoting Rhizobacteria and Earthworms via Modulating the Expression of Genes Related to Reactive Oxygen Species Metabolism in Brassica juncea. Front. Microbiol. 2022, 13, 802512. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I. Antioxidant Defensive Mechanisms to Regulate Cellular Redox Homeostatic Balance. In Reactive Oxygen Species in Plants; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Rajput, V.D.; Singh, H.; Verma, R.K.; Sharma, K.K.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; Mandzhieva, S. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Oliverio, M.; Bulotta, S.; Duarte, N. Editorial: Nature Inspired Protective Agents Against Oxidative Stress. Front. Pharmacol. 2022, 13, 859549. [Google Scholar] [CrossRef] [PubMed]
- Arnab, K.; Das, A.K.; Ghosh, N.; Sil, P.C. Superoxide dismutase. In Antioxidants Effects in Health; Elsevier B.V.: Amsterdam, The Netherlands, 2022; pp. 139–166. [Google Scholar]
- Jomová, K.; Alomar, S.; Alwasel, S.; Nepovimova, E.; Kuča, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Anna, S.; Tolmacheva, G.; Nevinsky, A. Essential Protective Role of Catalytically Active Antibodies (Abzymes) with Redox Antioxidant Functions in Animals and Humans. Int. J. Mol. Sci. 2022, 23, 3898. [Google Scholar] [CrossRef] [PubMed]
- Yuka, I.; Seira, S.; Takamasa, T.; Akiko, M.; Yoshimitsu, K. Crystal structure of Arabidopsis thaliana sulfotransferase SOT16 involved in glucosinolate biosynthesis. Biochem. Biophys. Res. Commun. 2023, 677, 149–154. [Google Scholar] [CrossRef]
- Sharma, M.; Dinesh, R.; Sen, S. Effect of salinity on some enzymatic and non-enzymatic antioxidants of Asada-Halliwell pathway in P. cineraria and P. juliflora. Acta Physiol. Plant. 2023. preprint. [Google Scholar] [CrossRef]
- Ali, S.; Mir, Z.A.; Tyagi, A.; Bhat, J.A.; Chandrashekar, N.; Papolu, P.K.; Rawat, S.; Grover, A. Identification and comparative analysis of Brassica juncea pathogenesis-related genes in response to hormonal, biotic and abiotic stresses. Acta Physiol. Plant. 2017, 39, 268. [Google Scholar] [CrossRef]
- Gusta, L.V.; Benning, N.T.; Wu, G.; Luo, X.; Liu, X.; Gusta, M.L.; McHughen, A. Superoxide dismutase: An all-purpose gene for agri-biotechnology. Mol. Breed. 2009, 24, 103–115. [Google Scholar] [CrossRef]
- Su, W.; Raza, A.; Gao, A.; Jia, Z.; Zhang, Y.; Hussain, M.A.; Mehmood, S.S.; Cheng, Y.; Lv, Y.; Zou, X. Genome-wide analysis and expression profile of superoxide dismutase (Sod) gene family in rapeseed (Brassica napus L.) under different hormones and abiotic stress conditions. Antioxidants 2021, 10, 1182. [Google Scholar] [CrossRef]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2021, 62, 7282–7300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, R.; Yan, X.; Fan, K. Superoxide dismutase nanozymes: An emerging star for anti-oxidation. J. Mater. Chem. B 2021, 9, 6939–6957. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Zaefizadeh, M.; Mohammad, S.; Jalali-E-Emam, S.; Alizadeh, B.; Zakarya, R.A.; Khayatnezhad, M. Superoxide Dismutase (SOD) Activity in Nacl Stress in Salt-Sensitive and Salt-Tolerance Genotypes of Colza (Brassica napus L.). Middle-East J. Sci. Res. 2011, 7, 7–11. [Google Scholar]
- Singh, B.K.; Sharma, S.R.; Singh, B. Heterosis for superoxide dismutase, peroxidase and catalase enzymes in the head of single cross-hybrids of cabbage (Brassica oleracea var. capitata). J. Genet. 2010, 89, 217–221. [Google Scholar] [CrossRef]
- Sami, F.; Siddiqui, H.; Alam, P. Nitric Oxide Mitigates the Salt-Induced Oxidative Damage in Mustard by UpRegulating the Activity of Various Enzymes. J. Plant Growth Regul. 2021, 40, 2409–2432. [Google Scholar] [CrossRef]
- Verma, D.; Lakhanpal, N.; Singh, K. Genome-wide identification and characterization of abiotic-stress responsive SOD (superoxide dismutase) gene family in Brassica juncea and B. rapa. BMC Genom. 2019, 20, 227. [Google Scholar] [CrossRef]
- Khator, K.; Shekhawat, G.S. Nitric oxide mitigates salt-induced oxidative stress in Brassica juncea seedlings by regulating ROS metabolism and antioxidant defense system. 3 Biotech 2020, 10, 499. [Google Scholar] [CrossRef]
- Sirhindi, G.; Kaur, H.; Bhardwaj, R.; Sharma, P.; Mushtaq, R. 28-Homobrassinolide potential for oxidative interface in Brassica juncea under temperature stress. Acta Physiol. Plant. 2017, 39, 228. [Google Scholar] [CrossRef]
- Pan, L.; Luo, Y.; Wang, J.; Li, X.; Tang, B.; Yang, H.; Hou, X.; Liu, F.; Zou, X. Evolution and functional diversification of catalase genes in the green lineage. BMC Genom. 2022, 23, 411. [Google Scholar] [CrossRef]
- Raza, A.; Wei, S.; Ang, G.; Mehmood, S.S.; Hussain, M.A.; Nie, W.; Yan, L.; Zou, X.; Zhang, X. Catalase (Cat) gene family in rapeseed (Brassica napus L.): Genome-wide analysis, identification and expression pattern in response to multiple hormones and abiotic stress conditions. Int. J. Mol. Sci. 2021, 22, 4281. [Google Scholar] [CrossRef] [PubMed]
- Addesso, R.; Baldantoni, D.; Cubero, B.; De La Rosa, J.M.; Gutierrez-Patricio, S.; Tiago, I.; Caldeira, A.T.; De Waele, J.; Miller, A.Z. Unveiling the menace of lampenflora to underground tourist environments. Sci. Rep. 2024, 14, 20789. [Google Scholar] [CrossRef] [PubMed]
- Hudek, L.; Enez, A.; Bräu, L. Cyanobacterial catalase activity prevents oxidative stress induced by Pseudomonas fluorescens DUS1-27 from inhibiting Brassica napus L. (canola) growth. Microbes Environ. 2018, 33, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, Z.; Chen, X.; Li, E.; Li, Y.; Zhang, C.; Hou, X. BcWRKY22 Activates BcCAT2 to Enhance Catalase (CAT) Activity and Reduce Hydrogen Peroxide (H2O2) Accumulation, Promoting Thermotolerance in Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis). Antioxidants 2023, 12, 1710. [Google Scholar] [CrossRef]
- Mohamadi, N.; Baghizadeh, A.; Saadatmand, S.; Asrar, Z. Alleviation of oxidative stress induced by drought stress through priming by β-aminobutyric acid (BABA) in Rapeseed (Brassica napus L.) plants. Iran. J. Plant Physiol. 2018, 7, 2203–2210. [Google Scholar]
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 2019, 18, 100173. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, K.; Liang, Z.; Zhu, Z.; Yang, J. Transcriptome Analysis of Glutathione Response: RNA-Seq Provides Insights into Balance between Antioxidant Response and Glucosinolate Metabolism. Antioxidants 2022, 11, 1322. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, 412–419. [Google Scholar] [CrossRef]
- Du, Y.Y.; Wang, P.C.; Chen, J.; Song, C.P. Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J. Integr. Plant Biol. 2008, 50, 1318–1326. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Barcellos Rosa, S.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Gent. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Kumar, P. Measurement of Ascorbate Peroxidase Activity in Sorghum. Bio-Protocol 2022, 12, e4531. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Messeder, D.; Caverzan, A.; Balbinott, N.; Menguer, P.K.; Paiva, A.L.S.; Lemos, M.; Cunha, J.R.; Gaeta, M.L.; Costa, M.; Zamocky, M.; et al. Stromal Ascorbate Peroxidase (OsAPX7) Modulates Drought Stress Tolerance in Rice (Oryza sativa). Antioxidants 2023, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Yoshimura, K.; Sakai, K.; Tamoi, M.; Takeda, T.; Shigeoka, S. Molecular Characterization and Physiological Role of a Glyoxysome-Bound Ascorbate Peroxidase from Spinach. Plant Cell Physiol. 1998, 39, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Novel insight into functions of ascorbate peroxidase in higher plants: More than a simple antioxidant enzyme. Redox Biol. 2023, 64, 102789. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Messeder, D.; Caverzan, A.; Bastos, G.A.; Galhego, V.; de Souza-Vieira, Y.; Lazzarotto, F.; Felix-Mendes, E.; Lavaquial, L.; Junior, J.N.; Margis-Pinheiro, M.; et al. Genome-wide, evolutionary, and functional analyses of ascorbate peroxidase (APX) family in Poaceae species. Genet. Mol. Biol. 2022, 46, e20220153. [Google Scholar] [CrossRef] [PubMed]
- Danna, C.H.; Bartoli, C.G.; Sacco, F.; Ingala, L.R.; Santa-María, G.E.; Guiamet, J.J.; Ugalde, R.A. Thylakoid-bound ascorbate peroxidase mutant exhibits impaired electron transport and photosynthetic activity. Plant Physiol. 2003, 132, 2116–2125. [Google Scholar] [CrossRef]
- Narendra, S.; Venkataramani, S.; Shen, G.; Wang, J.; Pasapula, V.; Lin, Y.; Kornyeyev, D.; Holaday, A.S.; Zhang, H. The Arabidopsis ascorbate peroxidase 3 is a peroxisomal membrane-bound antioxidant enzyme and is dispensable for Arabidopsis growth and development. J. Exp. Bot. 2006, 57, 3033–3042. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Qi, F.; Wang, R.; Jia, Z.; Lin, F.; Yuan, M.; Xin, X.F.; Liang, Y. Ascorbate peroxidase 1 allows monitoring of cytosolic accumulation of effector-triggered reactive oxygen species using a luminol-based assay. Plant Physiol. 2023, 191, 1416–1434. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, L.; Chen, M.; Ruan, Y.; Li, P.; Guo, Z.; Liu, B.; Ruan, Y.; Xiao, M.; Huang, Y. Identification and charactering of APX genes provide new insights in abiotic stresses response in Brassica napus. PeerJ 2022, 10, e13166. [Google Scholar] [CrossRef]
- Saxena, S.C.; Salvi, P.; Kamble, N.U.; Joshy, P.K.; Majee, M.; Arora, S. Ectopic overexpression of cytosolic ascorbate peroxidase gene (Apx1) improves salinity stress tolerance in Brassica juncea by strengthening antioxidative defense mechanism. Acta Physiol. Plant. 2020, 42, 45. [Google Scholar] [CrossRef]
- Jung, H.I.; Lee, B.R.; Chae, M.J.; Lee, E.J.; Lee, T.G.; Jung, G.B.; Kim, M.S.; Lee, J. Ascorbate-Mediated Modulation of Cd Stress Responses: Reactive Oxygen Species and Redox Status in Brassica napus. Front. Plant Sci. 2020, 11, 586547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.G.; Nie, T.T.; Sun, W.C.; Shi, Z.F.; Wang, J. Effects of diverse stresses on gene expression and enzyme activity of glutathione reductase in Brassica campestris. J. Appl. Ecol. 2018, 29, 213–222. [Google Scholar] [CrossRef]
- Wu, X.; Huang, H.; Childs, H.; Wu, Y.; Yu, L.; Pehrsson, P.R. The Annual Review of Food Science and Technology is online at food. Ann. Rev. Food Sci. Technol. 2021, 12, 485–511. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Shin, S.Y.; Kim, Y.S. Glutathione reductase from Brassica rapa affects tolerance and the redox state but not fermentation ability in response to oxidative stress in genetically modified Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2012, 28, 1901–1915. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up-regulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci. 2013, 7, 1053–1063. [Google Scholar]
- Jung, H.I.; Lee, T.G.; Lee, J.; Chae, M.J.; Lee, E.J.; Kim, M.S.; Jung, G.B.; Emmanuel, A.; Jeon, S.; Lee, B.R. Foliar-Applied Glutathione Mitigates Cd-Induced Oxidative Stress by Modulating Antioxidant-Scavenging, Redox-Regulating, and Hormone-Balancing Systems in Brassica napus. Front. Plant Sci. 2021, 12, 700413. [Google Scholar] [CrossRef]
- Małecka, A.; Konkolewska, A.; Hanć, A.; Ciszewska, L.; Staszak, A.M.; Jarmuszkiewicz, W.; Ratajczak, E. Activation of antioxidative and detoxificative systems in Brassica juncea L. plants against the toxicity of heavy metals. Sci. Rep. 2021, 11, 22345. [Google Scholar] [CrossRef]
- Tanaka, M.; Takahashi, R.; Hamada, A.; Terai, Y.; Ogawa, T.; Sawa, Y.; Ishikawa, T.; Maruta, T. Distribution and functions of monodehydroascorbate reductases in plants: Comprehensive reverse genetic analysis of Arabidopsis thaliana enzymes. Antioxidants 2021, 10, 1726. [Google Scholar] [CrossRef]
- Raihan, M.R.H.; Rahman, M.; Mahmud, N.U.; Adak, M.K.; Islam, T.; Fujita, M.; Hasanuzzaman, M. Application of Rhizobacteria, Paraburkholderia fungorum and Delftia sp. Confer Cd Tolerance in Rapeseed (Brassica campestris) through Modulating Antioxidant Defense and Glyoxalase Systems. Plants 2022, 11, 2738. [Google Scholar] [CrossRef] [PubMed]
- Al Mahmud, J.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Fujita, M. Relative tolerance of different species of Brassica to Cd toxicity: Coordinated role of antioxidant defense and glyoxalase systems. Plant Omics 2017, 10, 107–117. [Google Scholar] [CrossRef]
- Park, A.K.; Kim, I.S.; Do, H.; Jeon, B.W.; Lee, C.W.; Roh, S.J.; Shin, S.C.; Park, H.; Kim, Y.S.; Kim, Y.H.; et al. Structure and catalytic mechanism of monodehydroascorbate reductase, MDHAR, from Oryza sativa L. japonica. Sci. Rep. 2016, 22, 33903. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Matin, M.A.; Fardus, J.; Hasanuzzaman, M.; Hossain, M.S.; Parvin, K. Foliar application of salicylic acid improves growth and yield attributes by upregulating the antioxidant defense system in Brassica campestris plants grown in lead-amended soils. Acta Agrobot. 2019, 72, 1765. [Google Scholar] [CrossRef]
- Al Mahmud, J.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Fujita, M. EDTA reduces Cd toxicity in mustard (Brassica juncea L.) by enhancing metal chelation, antioxidant defense and glyoxalase systems. Acta Agrobot. 2019, 72, 1722. [Google Scholar] [CrossRef]
- Ma, L.; Qi, W.; Bai, J.; Li, H.; Fang, Y.; Xu, J.; Xu, Y.; Zeng, X.; Pu, Y.; Wang, W.; et al. Genome-Wide Identification and Analysis of the Ascorbate Peroxidase (APX) Gene Family of Winter Rapeseed (Brassica rapa L.) Under Abiotic Stress. Front. Genet. 2022, 12, 753624. [Google Scholar] [CrossRef] [PubMed]
- Negi, B.; Salvi, P.; Bhatt, D.; Majee, M.; Arora, S. Molecular cloning, in-silico characterization and functional validation of monodehydroascorbate reductase gene in Eleusine coracana. PLoS ONE 2017, 12, e0187793. [Google Scholar] [CrossRef]
- Shin, S.Y.; Kim, M.H.; Kim, Y.H. Co-expression of monodehydroascorbate reductase and dehydroascorbate reductase from Brassica rapa effectively confers tolerance to freezing-induced oxidative stress. Mol. Cells 2013, 36, 304–315. [Google Scholar] [CrossRef]
- Berwal, M.K.; Ram, C. Superoxide dismutase: A stable biochemical marker for abiotic stress tolerance in higher plants. In Abiotic and Biotic Stress in Plants; De Oliveira, A., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Lin, S.T.; Chiou, C.W.; Chu, Y.L.; Hsiao, Y.; Tseng, Y.F.; Chen, Y.C.; Chen, H.J.; Chang, H.Y.; Lee, T.M. Enhanced ascorbate regeneration via dehydroascorbate reductase confers tolerance to photo-oxidative stress in chlamydomonas reinhardtii. Plant Cell Physiol. 2016, 57, 2104–2121. [Google Scholar] [CrossRef]
- Omar, S.A.; Elsheery, N.I.; Kalaji, H.M.; Xu, Z.F.; Song-Quan, S.; Carpentier, R.; Choon-Hwan, L.; Allakhverdiev, S.I. Dehydroascorbate reductase and glutathione reductase play an important role in scavenging hydrogen peroxide during natural and artificial dehydration of Jatropha curcas seeds. J. Plant Biol. 2012, 55, 469–480. [Google Scholar] [CrossRef][Green Version]
- Fujiwara, A.; Togawa, S.; Hikawa, T.; Matsuura, H.; Masuta, C.; Inukai, T. Ascorbic acid accumulates as a defense response to Turnip mosaic virus in resistant Brassica rapa cultivars. J. Exp. Bot. 2016, 67, 4391–4402. [Google Scholar] [CrossRef]
- Arora, P.; Bhardwaj, R.; Kumar, K. 24-epibrassinolide induced antioxidative defense system of Brassica juncea L. under Zn metal stress. Physiol. Mol. Biol. Plants 2010, 16, 285–293. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Alharby, H.F.; Razafindrabe, B.H.N.; Fujita, M. Hydrogen peroxide pretreatment mitigates Cd-induced oxidative stress in Brassica napus L.: An intrinsic study on antioxidant defense and glyoxalase systems. Front. Plant Sci. 2017, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Duan, W.; Chen, Z.; Zhang, S.; Song, Z.; Liu, T.; Hou, X.; Ying, L. Overexpression of the Monodehydroascorbate Reductase Gene from Non-heading Chinese Cabbage Reduces Ascorbate Level and Growth in Transgenic Tobacco. Plant Mol. Biol. Rep. 2015, 33, 881–892. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, Y.H.; Lee, H.J.; Jung, H.; Hong, J.K. H2O2 production and gene expression of antioxidant enzymes in kimchi cabbage (Brassica rapa var. glabra Regel) seedlings regulated by plant development and nitrosative stress-triggered cell death. Plant Biotechnol. Rep. 2015, 9, 67–78. [Google Scholar] [CrossRef]
- Li, W.; Huai, X.; Li, P.; Raza, A.; Mubarik, M.S.; Habib, M.; Faiz, S.; Zhang, B.; Pan, J.; Khan, R.S.A. Genome-wide characterization of glutathione peroxidase (GPX) gene family in rapeseed (Brassica napus L.) revealed their role in multiple abiotic stress response and hormone signaling. Antioxidants 2021, 10, 1481. [Google Scholar] [CrossRef]
- Datta, R.; Mandal, K.; Boro, P.; Sultana, A.; Chattopadhyay, S. Glutathione imparts stress tolerance against Alternaria brassicicola infection via miRNA mediated gene regulation. Plant Signal. Behav. 2022, 17, 2047352. [Google Scholar] [CrossRef]
- Pieczul, K.; Dobrzycka, A.; Wolko, J.; Perek, A.; Zielezińska, M.; Bocianowski, J.; Rybus-Zając, M. The activity of β-glucosidase and guaiacol peroxidase in different genotypes of winter oilseed rape (Brassica napus L.) infected by Alternaria black spot fungi. Acta Physiol. Plant. 2020, 42, 142. [Google Scholar] [CrossRef]
- Srivastava, A. Rapid call us induction and culturing of Allium sativum using different phyto-hormonal combinations. In Proceedings of the 6th World Congress on Biotechnology, New Delhi, India, 5–7 October 2015; Volume 2. [Google Scholar]
- Chen, L.; Guo, Y.; Yang, L.; Wang, Q.Q. Synergistic defensive mechanism of phytochelatins and antioxidative enzymes in Brassica chinensis L. against Cd stress. Chin. Sci. Bull. 2008, 53, 1503–1511. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P. Glucosinolates in Brassica foods: Bioavailability in food and significance for human health. Phytochem. Rev. 2008, 7, 213–229. [Google Scholar] [CrossRef]
- Li, Y.; Huang, F.; Tao, Y.; Zhou, Y.; Bai, A.; Yu, Z.; Xiao, D.; Zhang, C.; Liu, T.; Hou, X.; et al. BcGR1.1, a Cytoplasmic Localized Glutathione Reductase, Enhanced Tolerance to Copper Stress in Arabidopsis thaliana. Antioxidants 2022, 11, 389. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Sun, S.; Liu, B.; Cheng, F.; Sun, R.; Wang, X. Glucosinolate biosynthetic genes in Brassica rapa. Gene 2011, 487, 135–142. [Google Scholar] [CrossRef]
- Harun, S.; Abdullah-Zawawi, M.R.; Goh, H.H.; Mohamed-Hussein, Z.A. A Comprehensive Gene Inventory for Glucosinolate Biosynthetic Pathway in Arabidopsis thaliana. J. Agric. Food Chem. 2020, 68, 7281–7297. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Daun, J.K.; DeClercq, D.R. Glucosinolates in Brassica oilseeds: Processing effects and extraction. In Antinutrients and Phytochemicals in Food; ACS Symposium Series; Oxford University Press: Oxford, UK, 1997; pp. 152–170. [Google Scholar] [CrossRef]
- Neugart, S.; Hanschem, F.S.; Schreiner, M. Glucosinolates in Brassica. In The Physiology of Vegetable Crops; CABI: Wallingford, UK, 2020; pp. 389–398. [Google Scholar] [CrossRef]
- O’Grady, E.; Pileckaite, G.; Gilheany, A.; Kucana, E.; Jaiswal, S.; Jaiswal, A.K. Health-promoting effects of glucosinolates and their breakdown products. In Understanding and Optimising the Nutraceutical Properties of Fruit and Vegetables; Burleigh Dodds Science Publishing: Cambridge, UK, 2022. [Google Scholar]
- Merinas-Amo, T.; Lozano-Baena, M.D.; Obregón-Cano, S.; Alonso-Moraga, Á.; de Haro-Bailón, A. Role of glucosinolates in the nutraceutical potential of selected cultivars of Brassica rapa. Foods 2021, 10, 2720. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Han, S.; Kim, H.; Won, S.Y.; Park, H.W.; Choi, H.; Choi, M.; Lee, M.Y.; Ha, I.J.; Lee, S.G. Anticancer Effects of High Glucosinolate Synthesis Lines of Brassica rapa on Colorectal Cancer Cells. Antioxidants 2022, 11, 2463. [Google Scholar] [CrossRef] [PubMed]
- Oloyede, O.O.; Wagstaff, C.; Methven, L. Influence of cabbage (Brassica oleracea) accession and growing conditions on myrosinase activity, glucosinolates and their hydrolysis products. Foods 2021, 10, 2903. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Zeng, W.; Wang, J.; Zhang, F.; Sun, B.; Wang, Q. Improvement of glucosinolates by metabolic engineering in Brassica crops. Abiotech 2021, 2, 314–329. [Google Scholar] [CrossRef]
- Das, B. Glucosinolate biosynthesis: Role of MAM synthase and its perspectives. Biosci. Rep. 2021, 41, BSR20211634. [Google Scholar] [CrossRef]
- Sikorska-Zimny, K.; Beneduce, L. The metabolism of glucosinolates by gut microbiota. Nutrients 2021, 13, 2750. [Google Scholar] [CrossRef]
- Becker, T.; Juvik, J. The Role of Glucosinolate Hydrolysis Products from Brassica Vegetable Consumption in Inducing Antioxidant Activity and Reducing Cancer Incidence. Diseases 2016, 4, 22. [Google Scholar] [CrossRef]
- Li, X.; Cai, F.; Kuerban, G.; Zhang, S.; Li, C.; Zhao, Y.; Jin, L.; Ma, X. The Effect of Glucosinolates on the Growth and Development of Helicoverpa armigera Larvae and the Expression of Midgut Sulfatase Genes. Agronomy 2022, 12, 306. [Google Scholar] [CrossRef]
- Frerigmann, H. Glucosinolate regulation in a complex relationship–MYC and MYB–no one can act without each other. Adv. Bot. Res. 2016, 80, 57–97. [Google Scholar] [CrossRef]
- Arumugam, A.; Razis, A.F.A. Apoptosis as a mechanism of the cancer chemopreventive activity of glucosinolates: A review. Asian Pac. J. Cancer Prev. 2018, 19, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, G.; Jiang, X.; Shen, S.; Guan, M.; Tang, Y.; Sun, F.; Hu, R.; Chen, S.; Zhao, H.; et al. Genome-Wide Association Study of Glucosinolate Metabolites (mGWAS) in Brassica napus L. Plants 2023, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Coves, S.; Soengas, P.; Velasco, P.; Fernández, J.C.; Cartea, M.E. New vegetable varieties of Brassica rapa and Brassica napus with modified glucosinolate content obtained by mass selection approach. Front. Nutr. 2023, 10, 1198121. [Google Scholar] [CrossRef] [PubMed]
- Andini, S.; Dekker, P.; Gruppen, H.; Araya-Cloutier, C.; Vincken, J.P. Modulation of Glucosinolate Composition in Brassicaceae Seeds by Germination and Fungal Elicitation. J. Agric. Food Chem. 2019, 67, 12770–12779. [Google Scholar] [CrossRef]
- Choi, D.; Kim, S.H.; Choi, D.M.; Moon, H.; Kim, J.; Huq, E.; Kim, D.H. Elongated Hypocotyl 5 interacts with Histone Deacetylase 9 to suppress glucosinolate biosynthesis in Arabidopsis. Plant Physiol. 2024, 196, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. The Cellular and Subcellular Organization of the Glucosinolate–Myrosinase System against Herbivores and Pathogens. Int. J. Mol. Sci. 2022, 23, 1577. [Google Scholar] [CrossRef]
- Jewell, J.B.; Berim, A.; Tripathi, D.; Gleason, C.; Olaya, C.; Pappu, H.R.; Gang, D.R.; Tanaka, K. Activation of indolic glucosinolate pathway by extracellular ATP in Arabidopsis. Plant Physiol. 2022, 190, 1574–1578. [Google Scholar] [CrossRef]
- Han, D.; Tan, J.; Yue, Z.; Tao, P.; Lei, J.; Zang, Y.; Hu, Q.; Wang, H.; Zhang, S.; Li, B.; et al. Genome-Wide Identification and Expression Analysis of ESPs and NSPs Involved in Glucosinolate Hydrolysis and Insect Attack Defense in Chinese Cabbage (Brassica rapa subsp. pekinensis). Plants 2023, 12, 1123. [Google Scholar] [CrossRef]
- Srikanth, P.; Maxton, A.; Masih, S.A.; Sofo, A.; Khan, N.A. Isoprene: An Antioxidant to Guard Plants against Stress. Int. J. Plant Biol. 2024, 15, 161–174. [Google Scholar] [CrossRef]
- Addesso, R.; Sofo, A.; Amato, M. Rhizosheath: Roles, Formation Processes and Investigation Methods. Soil Syst. 2023, 7, 106. [Google Scholar] [CrossRef]
- Hurbain, J.; Thommen, Q.; Anquez, F.; Pfeuty, B. Quantitative modeling of pentose phosphate pathway response to oxidative stress reveals a cooperative regulatory strategy. iScience 2022, 25, 104681. [Google Scholar] [CrossRef] [PubMed]
- Muronetz, V.I.; Melnikova, A.K.; Saso, L.; Schmalhausen, E.V. Influence of oxidative stress on catalytic and non-glycolytic functions of glyceraldehyde-3-phosphate dehydrogenase. Curr. Med. Chem. 2020, 27, 2040–2058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, M.; Lin, H. A regulatory cysteine residue mediates reversible inactivation of NADH+-dependent aldehyde dehydrogenases to promote oxidative stress response. ACS Chem. Biol. 2019, 15, 28–32. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gantait, A.; Masih, S.A.; Addesso, R.; Maxton, A.; Sofo, A. Glucosinolates Mediated Regulation of Enzymatic Activity in Response to Oxidative Stress in Brassica spp. Plants 2024, 13, 3422. https://doi.org/10.3390/plants13233422
Gantait A, Masih SA, Addesso R, Maxton A, Sofo A. Glucosinolates Mediated Regulation of Enzymatic Activity in Response to Oxidative Stress in Brassica spp. Plants. 2024; 13(23):3422. https://doi.org/10.3390/plants13233422
Chicago/Turabian StyleGantait, Aishmita, Sam A. Masih, Rosangela Addesso, Ann Maxton, and Adriano Sofo. 2024. "Glucosinolates Mediated Regulation of Enzymatic Activity in Response to Oxidative Stress in Brassica spp." Plants 13, no. 23: 3422. https://doi.org/10.3390/plants13233422
APA StyleGantait, A., Masih, S. A., Addesso, R., Maxton, A., & Sofo, A. (2024). Glucosinolates Mediated Regulation of Enzymatic Activity in Response to Oxidative Stress in Brassica spp. Plants, 13(23), 3422. https://doi.org/10.3390/plants13233422









