Impact of Salinity Gradients on Seed Germination, Establishment, and Growth of Two Dominant Mangrove Species Along the Red Sea Coastline
Abstract
:1. Introduction
2. Results and Discussions
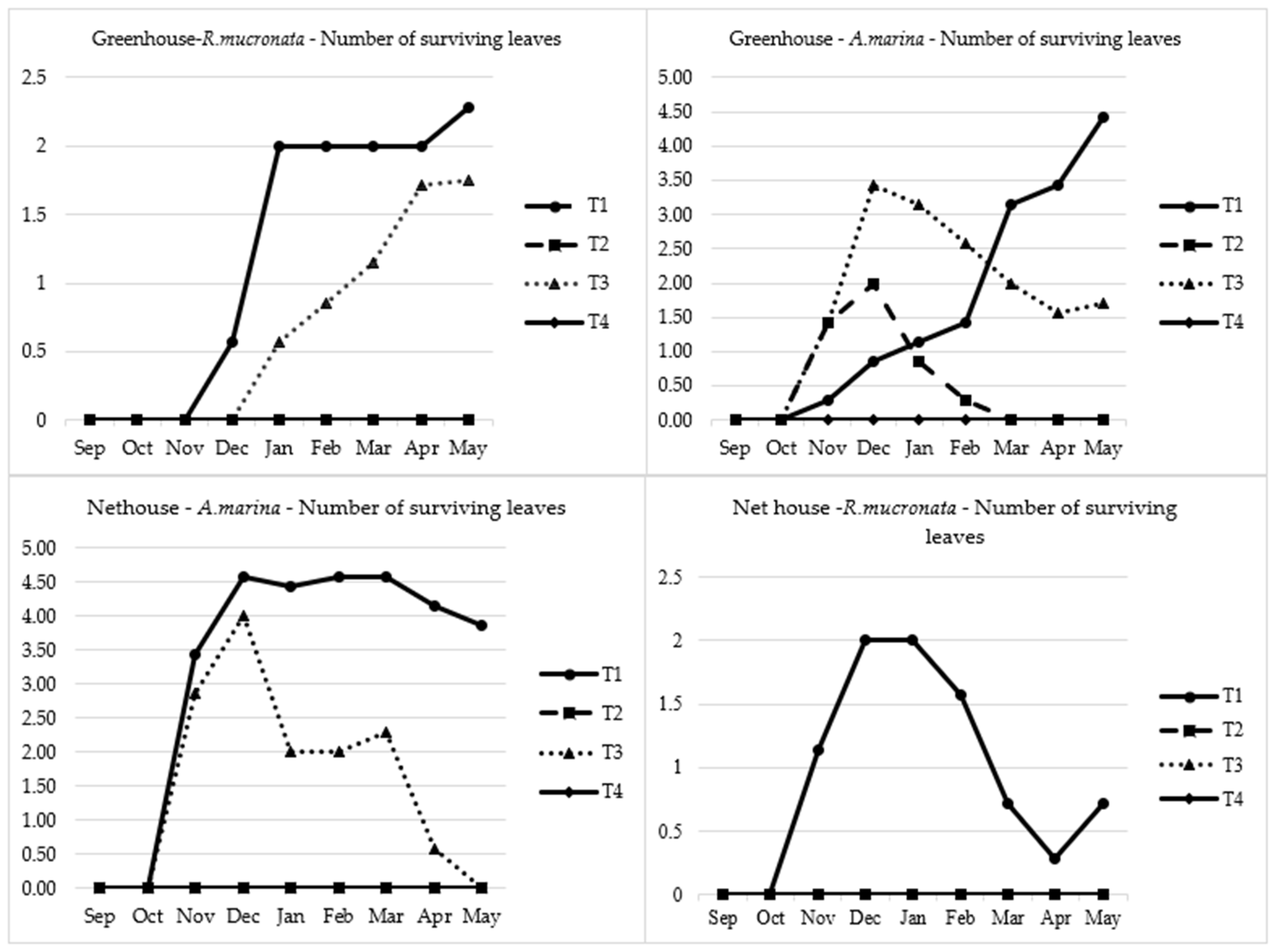
2.1. Seedling Germination, Growth and Survival
2.2. Seedling Establishment and Height
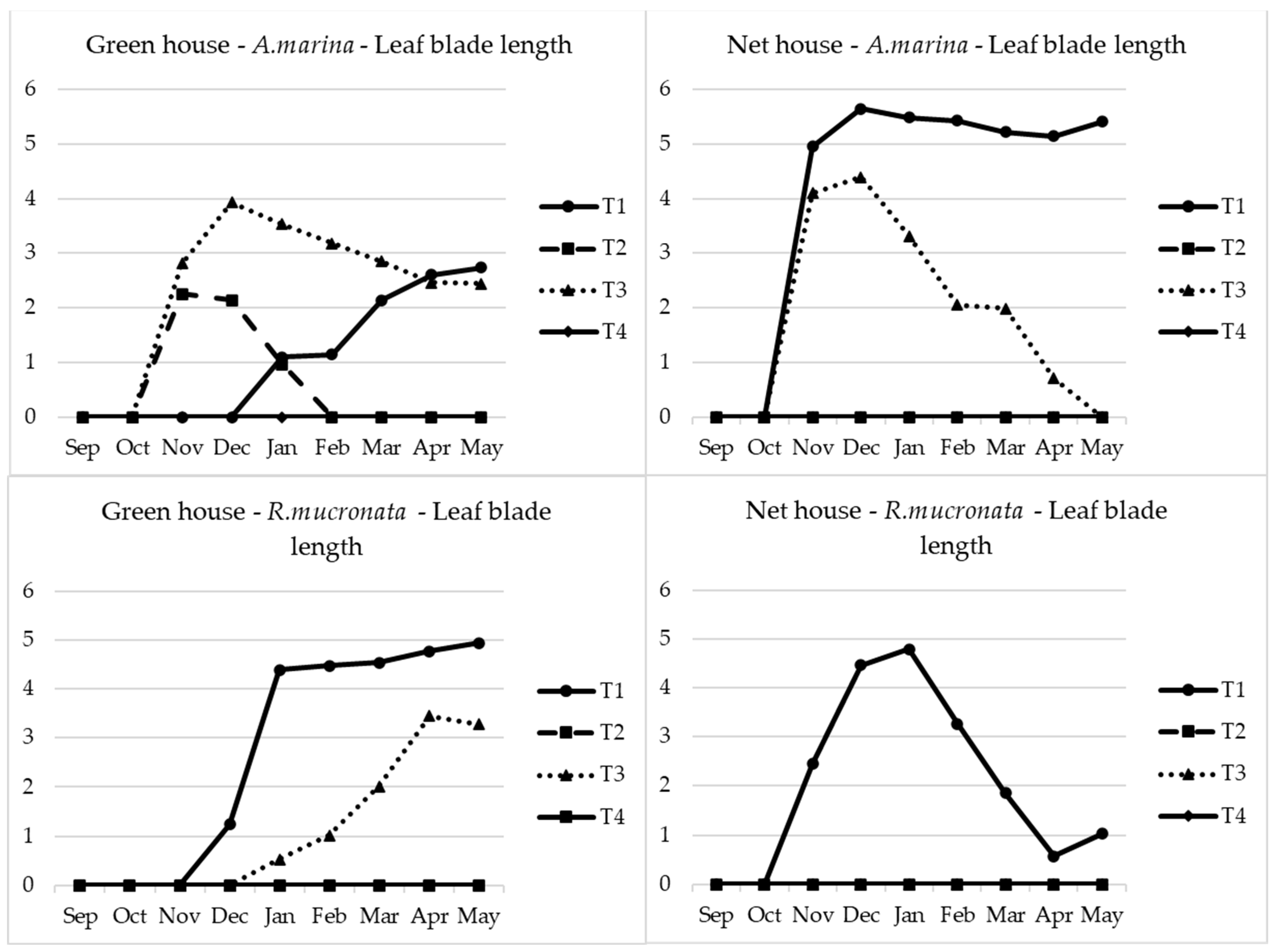
2.3. Seedling Stem Diameter and Leaf Parameters
2.4. Mineral Element Composition
3. Materials and Methods
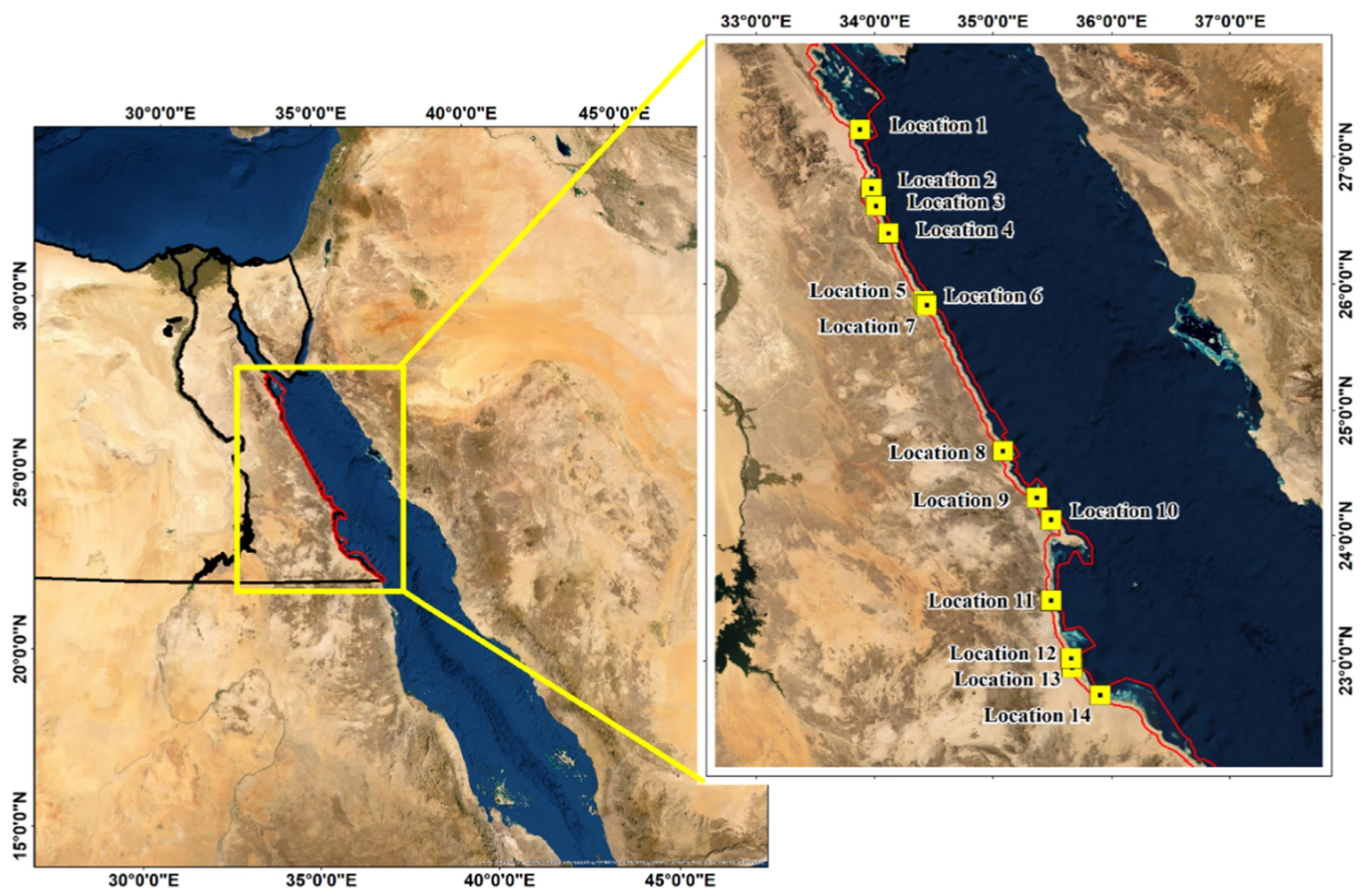
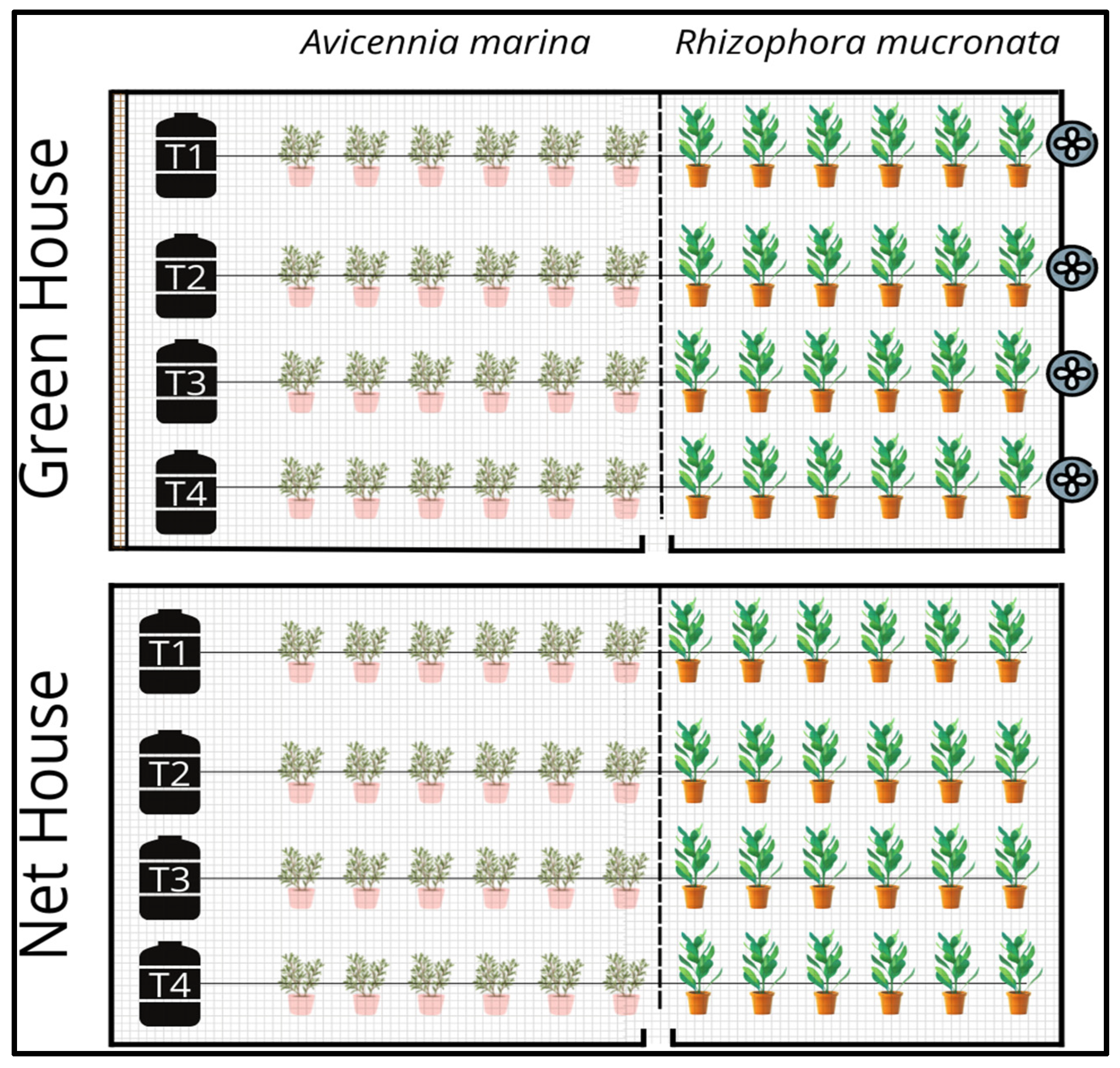
3.1. Experimental Location and Setup
3.2. Data Collection
3.3. Mineral Element Analysis
3.4. Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kathiresan, K.; Bingham, B.L. Biology of mangroves and mangrove ecosystems. Adv. Mar. Biol. 2001, 40, 81–251. [Google Scholar] [CrossRef]
- Agaton, C.B.; Collera, A.A. Now or later? Optimal timing of mangrove rehabilitation under climate change uncertainty. Ecol. Manag. 2022, 503, 119739. [Google Scholar] [CrossRef]
- Duke, N.C.; Meynecke, J.-O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A world without mangroves? Science 2007, 317, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M. Mangrove forests: Resilience, protection from tsunamis, and responses to global climate change. Estuar. Coast. Shelf Sci. 2008, 76, 1–13. [Google Scholar] [CrossRef]
- Thatoi, H.; Samantaray, D.; Das, S.K. The genus Avicennia, a pioneer group of dominant mangrove plant species with potential medicinal values: A review. Front. Life Sci. 2016, 9, 267–291. [Google Scholar] [CrossRef]
- Sewilam, H.; Hassan, B.T.; Khalil, B.S. Spatiotemporal distribution of mangrove along the Egyptian Red Sea coast and analysis of hydrological impact on growth patterns. Int. J. Environ. Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Karami, L.; Majd, A.; Mehrabian, S.; Nabiuni, M.; Salehi, M.; Irian, S. Antimutagenic and anticancer effects of Avicennia marina leaf extract on Salmonella typhimurium TA100 bacterium and human promyelocytic leukaemia HL-60 cells. ScienceAsia 2012, 38, 349–355. [Google Scholar] [CrossRef]
- Aljaghthmi, O.H.; Heba, H.M.; Zeid, I.M. Antihyperglycemic Properties of Mangrove Plants (Rhizophora mucronata and Avicennia marina): An Overview. Adv. Biol. Res. 2017, 11, 161–170. [Google Scholar]
- Ximenes, A.C. Global Mangrove Mapping: A Critical Tool for Conservation Biodiversity Distribution Modeling View Project. 2015. Available online: https://www.researchgate.net/publication/285593975 (accessed on 11 September 2022).
- Pettersen, E.F.; Bjørløw, I.; Hagland, T.J.; Wergeland, H.I. Effect of seawater temperature on leucocyte populations in Atlantic salmon post-smolts. Vet. Immunol. Immunopathol. 2005, 106, 65–76. [Google Scholar] [CrossRef]
- Morrisey, D.; Swales, A.; Dittmann, S.; Morrison, M.; Lovelock, C.; Beard, C. The Ecology and Management of Temperate Mangroves. In Oceanography and Marine Biology: An Annual Review; Chapman and Hall/CRC: Boca Raton, FL, USA, 2010; pp. 43–160. [Google Scholar] [CrossRef]
- Suárez, N.; Medina, E. Salinity effect on plant growth and leaf demography of the mangrove, Avicennia germinans L. Trees 2005, 19, 722–728. [Google Scholar] [CrossRef]
- Almahasheer, H.; Aljowair, A.; Duarte, C.M.; Irigoien, X. Decadal stability of Red Sea mangroves. Estuar. Coast. Shelf Sci. 2016, 169, 164–172. [Google Scholar] [CrossRef]
- Rastegar, S.; Gozari, M. Effect of mangrove plant extract on growth of four fungal pathogens. J. Paramed. Sci. 2017, 8, 1–6. [Google Scholar]
- Rastogi, R.P.; Phulwaria, M.; Gupta, D.K. Mangroves: Ecology, Biodiversity and Management; Springer: Singapore, 2017. [Google Scholar]
- Horstman, E.M.; Lundquist, C.J.; Bryan, K.R.; Bulmer, R.H.; Mullarney, J.C.; Stokes, D.J. The Dynamics of Expanding Mangroves in New Zealand. 2018. Available online: http://www.springer.com/series/8795 (accessed on 23 August 2022).
- Duke, N.C.; Ball, M.C.; Ellison, J.C. Factors influencing biodiversity and distributional gradients in mangroves. Glob. Ecol. Biogeogr. Lett. 1998, 7, 27. [Google Scholar] [CrossRef]
- Michel, J. Oil Spills in Mangroves; Planning & Response Considerations. 2014. Available online: https://www.researchgate.net/publication/271193539 (accessed on 9 August 2022).
- Saintilan, N.; Wilson, N.C.; Rogers, K.; Rajkaran, A.; Krauss, K.W. Mangrove expansion and salt marsh decline at mangrove poleward limits. Glob. Chang. Biol. 2014, 20, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, C.E.; Feller, I.C.; Reef, R.; Hickey, S.; Ball, M.C. Mangrove dieback during fluctuating sea levels. Sci. Rep. 2017, 7, 1680. [Google Scholar] [CrossRef]
- Clough, B. Growth and salt balance of the mangrove Avicennia marina (Forsk.) Vierh. and Rhizophora stylosa Griff. in relation to salinity. Aust. J. Plant Physiol. 1984, 11, 419–430. [Google Scholar] [CrossRef]
- Khan, M.A.; Aziz, I. Salinity tolerance in some mangrove species from Pakistan. Wetl. Ecol. Manag. 2001, 9, 229–233. [Google Scholar] [CrossRef]
- Cheng, H.; Inyang, A.; Da Li, C.; Fei, J.; Zhou, Y.W.; Wang, Y.S. Salt tolerance and exclusion in the mangrove plant Avicennia marina in relation to root apoplastic barriers. Ecotoxicology 2020, 29, 676–683. [Google Scholar] [CrossRef]
- Medina, E.; Lugo, A.E.; Novelo, A. Mineral content of foliar tissues of mangrove species in Laguna de Sontecomapan (Veracruz, Mexico) and its relation to salinity. Biotropica 1995, 27, 317. [Google Scholar] [CrossRef]
- Khan, M.; Zaheer, M.; Ahmed, A.; Hameed, A. Effect of sea salt and L-ascorbic acid on the seed germination of halophytes. J. Arid. Environ. 2006, 67, 535–540. [Google Scholar] [CrossRef]
- Patel, N.; Gupta, A.; Pandey, A. Salinity tolerance of Avicennia marina (Forssk.) Vierh. from Gujarat coasts of India. Aquat. Bot. 2010, 93, 9–16. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Stanton, D.E.; Schmitz, N.; Farquhar, G.D.; Ball, M.C. Growth responses of the mangrove Avicennia marina to salinity: Development and function of shoot hydraulic systems require saline conditions. Ann. Bot. 2015, 115, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Hoppe-Speer, S.; Adams, J.; Rajkaran, A.; Bailey, D. The response of the red mangrove Rhizophora mucronata Lam. to salinity and inundation in South Africa. Aquat. Bot. 2011, 95, 71–76. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Zheng, W.-J.; Wang, W.-Q.; Lin, P. Dynamics of element contents during the development of hypocotyles and leaves of certain mangrove species. J. Exp. Mar. Biol. Ecol. 1999, 233, 247–257. [Google Scholar] [CrossRef]
- Suárez, N.; Sobrado, M.A.; Medina, E. Salinity effects on the leaf water relations components and ion accumulation patterns in Avicennia germinans (L.) L. seedlings. Oecologia 1998, 114, 299–304. [Google Scholar] [CrossRef]
- Suárez, N.; Sobrado, M.A. Adjustments in leaf water relations of mangrove (Avicennia germinans) seedlings grown in a salinity gradient. Tree Physiol. 2000, 20, 277–282. [Google Scholar] [CrossRef]
- Abbas, M.; Soliman, A.; Afefe, A. Physical and Chemical Characteristics of Mangrove Soil under Marine Influence. A Case Study on the Mangrove Forests at Egyptian - African Red Sea Coast. Egypt. J. Aquat. Biol. Fish. 2019, 23, 385–399. [Google Scholar] [CrossRef]
- Seedo, K.A.; Abido, M.S.; Salih, A.; Abahussain, A. Morphophysiological Traits of Gray Mangrove (Avicennia marina (Forsk.) Vierh.) at Different Levels of Soil Salinity. Int. J. For. Res. 2018, 2018, 7404907. [Google Scholar] [CrossRef]
- Ye, Y.; Tam, N.; Lu, C.; Wong, Y. Effects of salinity on germination, seedling growth and physiology of three salt-secreting mangrove species. Aquat. Bot. 2005, 83, 193–205. [Google Scholar] [CrossRef]
- Afefe, A.A.; Khedr, A.H.A.; Abbas, M.S.; Soliman, A.S. Responses and Tolerance Mechanisms of Mangrove Trees to the Ambient Salinity along the Egyptian Red Sea Coast. Limnol. Rev. 2021, 21, 3–13. [Google Scholar] [CrossRef]
- Jayatissa, L.P.; Wickramasinghe, W.A.A.D.L.; Dahdouh-Guebas, F.; Huxham, M. Interspecific variations in responses of mangrove seedlings to two contrasting salinities. Int. Rev. Hydrobiol. 2008, 93, 700–710. [Google Scholar] [CrossRef]




| Treatment | Greenhouse Stem Diameter (cm) for A. marina | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sep | Oct | Nov | Dec | Jan | Feb | Mar | Apr | May | |
| T1 | 0.00 | 0.00 | 0.00 | 0.45 ± 1.11 | 1.025 ± 1.59 | 0.85 ± 1.45 | 0.90 ± 1.52 | 1.88 ± 1.16 | 2.30 ± 1.34 |
| T2 | 0.00 | 0.00 | 0.62 ± 1.15 | 1.28 ± 1.38 | 0.73 ± 1.73 | 0.00 | 0.00 | 0.00 | 0.00 |
| T3 | 0.00 | 0.00 | 2.20 ± 1.58 | 2.96 ± 0.41 | 2.95 ± 0.39 | 1.88 ± 1.30 | 1.64 ± 1.60 | 1.18 ± 1.49 | 1.16 ± 1.51 |
| T4 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Net House Stem Diameter (cm) for A. marina | |||||||||
| T1 | 0.00 | 0.00 | 2.81 ± 0.31 | 2.79 ± 0.35 | 2.84 ± 0.40 | 2.79 ± 0.44 | 2.89 ± 0.25 | 2.92 ± 0.49 | 3.24 ± 0.31 |
| T2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| T3 | 0.00 | 0.00 | 2.68 ± 0.14 | 2.74 ± 0.13 | 1.92 ± 1.38 | 1.07 ±1.35 | 1.29 ± 1.66 | 0.36 ± 0.94 | 0.00 |
| T4 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatment | Greenhouse Stem Diameter (cm) for R. mucronata | ||||||||
| Sep | Oct | Nov | Dec | Jan | Feb | Mar | Apr | May | |
| T1 | 10.6 ± 0.69 | 10.6 ± 0.69 | 10.6 ± 0.69 | 10.7 ± 0.67 | 10.94 ±1.17 | 10.6 ± 0.80 | 11.1 ± 1.27 | 11.14 ± 1.01 | 11.08 ± 1.13 |
| T2 | 10.7 ± 0.72 | 10.7 ± 0.72 | 10.7 ± 0.72 | 10.37 ± 0.97 | 10.64 ± 1.11 | 10.35 ± 0.88 | 9.92 ± 1.21 | 9.87 ± 1.20 | 9.87 ± 1.27 |
| T3 | 11.84 ± 0.77 | 11.84 ± 0.77 | 11.84 ± 0.77 | 11.14 ± 0.87 | 11.52 ± 0.75 | 11.21 ± 0.74 | 10.3 ± 0.62 | 10.11 ± 0.80 | 10.14 ± 1.09 |
| T4 | 12.01 ± 2.62 | 12.01 ±2.62 | 12.01 ± 2.62 | 11 ± 1.49 | 11.36 1.16 | 11.36 ± 1.16 | 9.69 ± 1.29 | 9.69 ± 1.29 | 9.69 ± 1.29 |
| Net House Stem Diameter (cm) for R. mucronata | |||||||||
| T1 | 9.89 ± 0.89 | 9.89 ± 0.89 | 9.89 ± 0.89 | 10.21 ± 1.04 | 10.94 ± 1.14 | 10.27 ± 1.65 | 10.04 ± 1.58 | 9.38 ± 2.24 | 8.93 ± 1.54 |
| T2 | 7.22 ± 1.00 | 7.22 ± 1.00 | 7.22 ± 1.00 | 7.22 ± 1.00 | 7.22 ± 1.00 | 7.22 ± 1.00 | 7.22 ± 1.00 | 7.22 ± 1.00 | 7.22 ± 1.00 |
| T3 | 9.06 ± 1.18 | 9.06 ± 1.18 | 9.54 ± 1.72 | 9.06 ± 1.18 | 9.06 ± 1.18 | 9.06 ± 1.18 | 9.06 ± 1.18 | 9.06 ± 1.18 | 9.06 ± 1.18 |
| T4 | 9.2 ± 1.11 | 9.2 ± 1.11 | 9.2 ± 1.11 | 9.2 ± 1.11 | 9.2 ± 1.11 | 9.2 ± 1.11 | 9.2 ± 1.11 | 9.2 ± 1.11 | 9.2 ± 1.11 |
| Treatment | Na | Ca | Mg | K | Treatment | Na | Ca | Mg | K | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. marina | GH | Leaves | T1 | 38.861 | 7.8463 | 0.576 | 0.711 | R. mucronata | GH | Leaves | T1 | 63.551 | 15.445 | 0.958 | 0.975 |
| T2 | 47.952 | 3.462 | 0.185 | 0.448 | T2 | 96.653 | 256.32 | 2.160 | 1.145 | ||||||
| T3 | 39.569 | 4.492 | 0.335 | 0.461 | T3 | 27.586 | 145.95 | 0.729 | 0.586 | ||||||
| T4 | 62.856 | 6.238 | 0.1543 | 0.4856 | T4 | 80.315 | 358.82 | 2.396 | 3.948 | ||||||
| Roots | T1 | 39.368 | 3.8944 | 1.398 | 0.617 | Roots | T1 | 38.833 | 172.25 | 0.936 | 0.954 | ||||
| T2 | 58.849 | 3.850 | 0.226 | 0.419 | T2 | 46.794 | 157.06 | 0.805 | 0.779 | ||||||
| T3 | 53.229 | 3.704 | 0.188 | 0.435 | T3 | 42.223 | 135.16 | 0.729 | 0.778 | ||||||
| T4 | 73.259 | 2.360 | 0.107 | 0.124 | T4 | 51.682 | 36.945 | 0.819 | 0.105 | ||||||
| NH | Leaves | T1 | 72.312 | 36.0 | 0.153 | 0.388 | NH | Leaves | T1 | 70.852 | 48.776 | 0.765 | 0.761 | ||
| T2 | 58.893 | 71.56 | 0.2785 | 0.631 | T2 | 27.324 | 150.78 | 1.204 | 0.771 | ||||||
| T3 | 73.760 | 63.943 | 0.226 | 0.887 | T3 | 49.677 | 304.33 | 3.884 | 1.044 | ||||||
| T4 | 68.763 | 162.7 | 0.632 | 1.089 | T4 | 83.492 | 97.839 | 0.532 | 0.918 | ||||||
| Roots | T1 | 68.194 | 60.774 | 0.855 | 0.632 | Roots | T1 | 58.584 | 203.27 | 1.117 | 0.828 | ||||
| T2 | 52.881 | 91.846 | 0.367 | 0.864 | T2 | 68.913 | 96.072 | 0.522 | 0.801 | ||||||
| T3 | 74.214 | 105.43 | 0.322 | 1.051 | T3 | 65.849 | 29.47 | 0.793 | 0.655 | ||||||
| T4 | 67.575 | 153.91 | 0.877 | 1.121 | T4 | 70.103 | 41.858 | 0.783 | 0.558 |
| Months | Rainfall (mm) | Min. Air Temp (°C) | Max. Air Temp (°C) | Min. Relative Humidity (%) | Max. Relative Humidity (%) | Solar (MJ/m2) |
|---|---|---|---|---|---|---|
| Sept | 0.00 | 25.92 | 31.70 | 72.46 | 85.16 | 26.403 |
| Oct | 0.06 | 22.74 | 28.23 | 76.12 | 84.43 | 22.962 |
| Nov | 0.07 | 20.07 | 25.28 | 75.34 | 86.45 | 19.947 |
| Dec | 0.16 | 14.04 | 18.09 | 70.44 | 81.56 | 13.668 |
| Jan | 0.25 | 10.88 | 15.06 | 68.19 | 81.79 | 10.658 |
| Feb | 0.43 | 12.50 | 17.27 | 72.73 | 83.31 | 12.546 |
| Mar | 0.00 | 13.45 | 18.51 | 64.97 | 78.81 | 13.875 |
| Apr | 0.01 | 22.04 | 29.33 | 54.03 | 69.31 | 22.576 |
| May | 0.00 | 22.98 | 29.42 | 55.43 | 73.55 | 24.572 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimera, F.; Sobhi, B.; Omara, M.; Sewilam, H. Impact of Salinity Gradients on Seed Germination, Establishment, and Growth of Two Dominant Mangrove Species Along the Red Sea Coastline. Plants 2024, 13, 3471. https://doi.org/10.3390/plants13243471
Kimera F, Sobhi B, Omara M, Sewilam H. Impact of Salinity Gradients on Seed Germination, Establishment, and Growth of Two Dominant Mangrove Species Along the Red Sea Coastline. Plants. 2024; 13(24):3471. https://doi.org/10.3390/plants13243471
Chicago/Turabian StyleKimera, Fahad, Basma Sobhi, Mostafa Omara, and Hani Sewilam. 2024. "Impact of Salinity Gradients on Seed Germination, Establishment, and Growth of Two Dominant Mangrove Species Along the Red Sea Coastline" Plants 13, no. 24: 3471. https://doi.org/10.3390/plants13243471
APA StyleKimera, F., Sobhi, B., Omara, M., & Sewilam, H. (2024). Impact of Salinity Gradients on Seed Germination, Establishment, and Growth of Two Dominant Mangrove Species Along the Red Sea Coastline. Plants, 13(24), 3471. https://doi.org/10.3390/plants13243471






