Growth Allocation Shifts in the Invasive Hydrilla verticillata Under Interspecific Competition with Native Submerged Macrophytes
Abstract
1. Introduction
2. Results
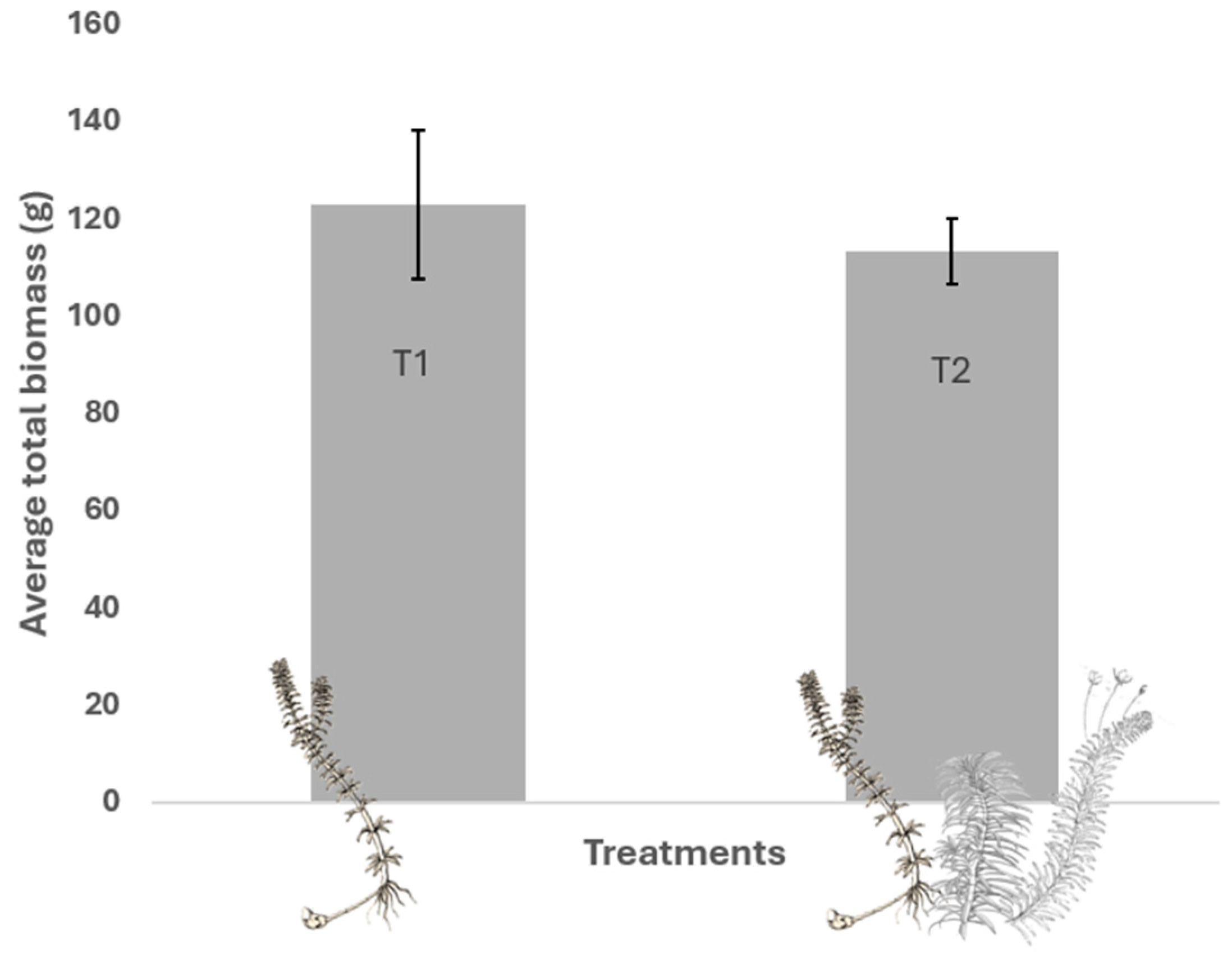
2.1. Total Biomass of Submerged Aquatic Macrophytes (SAMs)
2.2. Subterranean Biomass of Submerged Aquatic Macrophytes
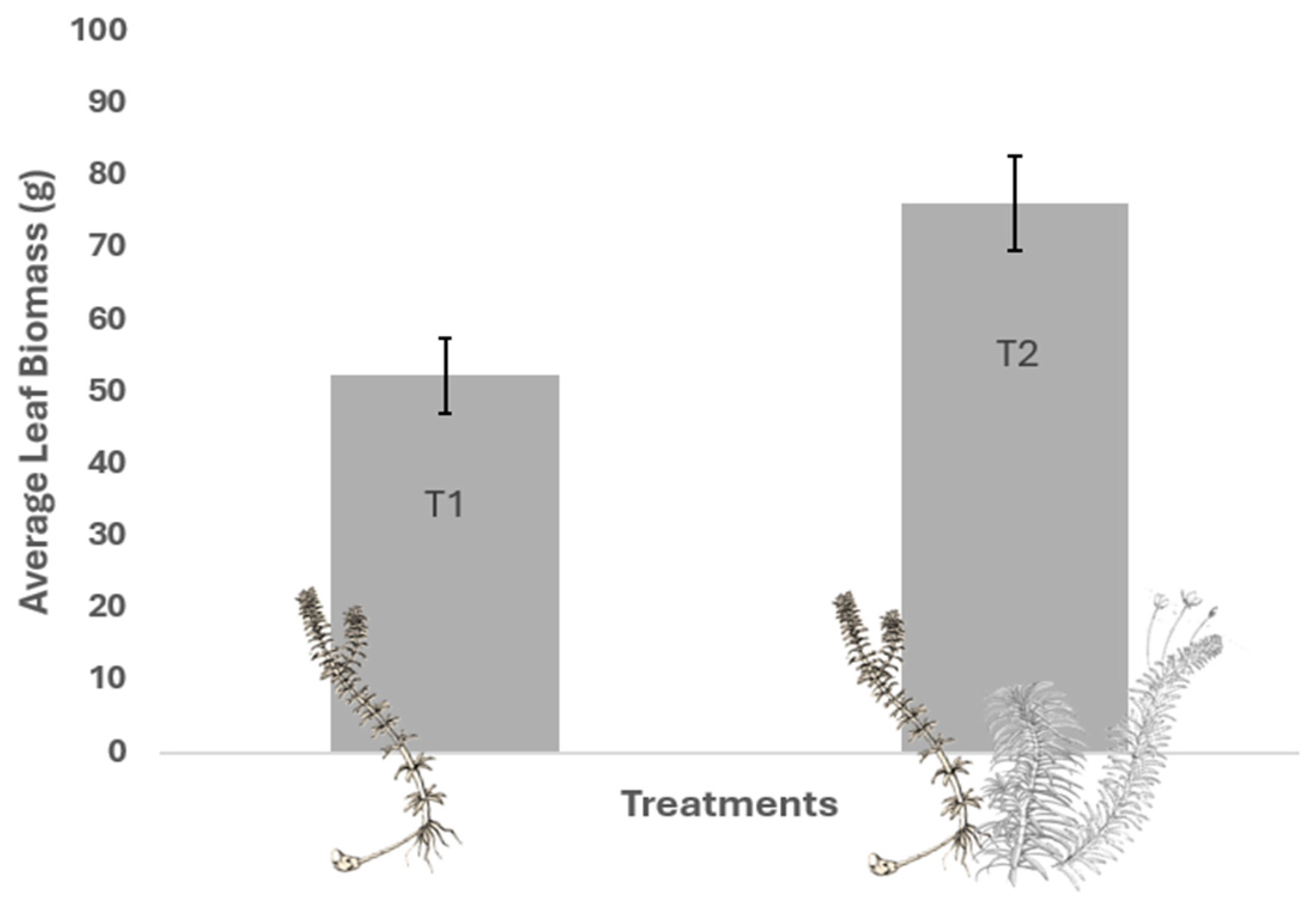
2.3. Total Foliar Biomass of Macrophytes
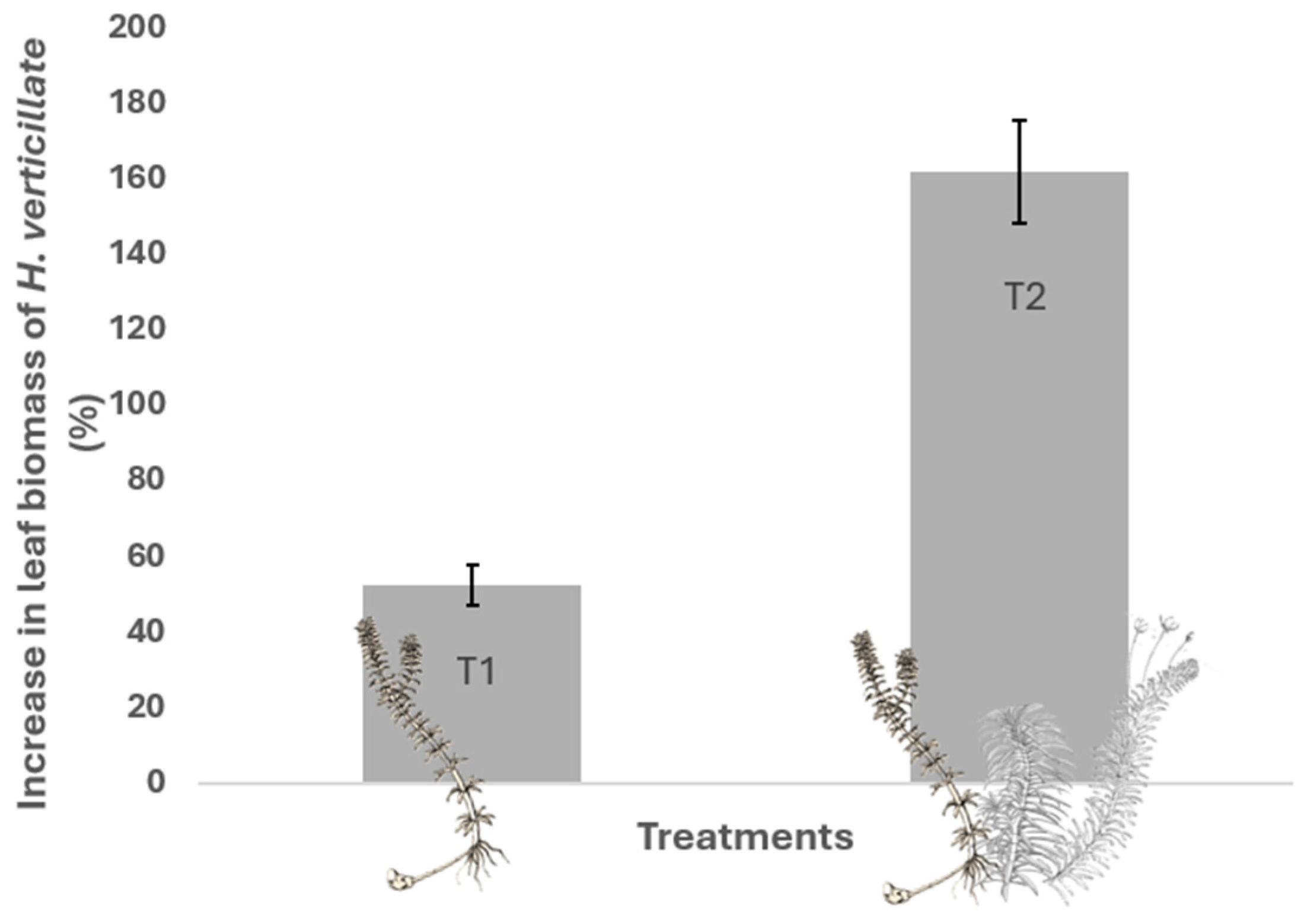
2.4. Foliar Biomass Increment of H. verticillata
3. Discussion
4. Materials and Methods
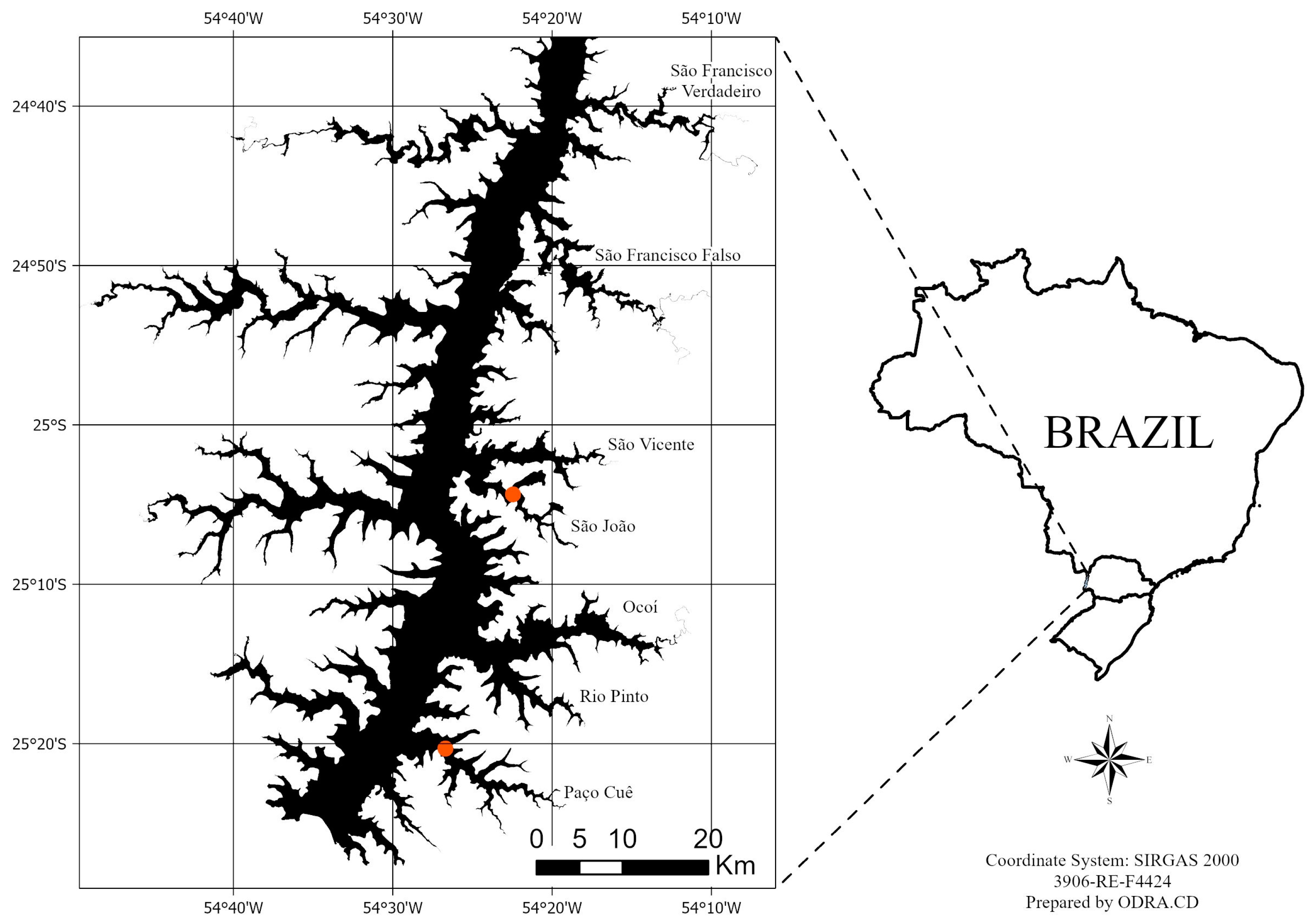
4.1. Collection Area of SAMs
4.2. Plant Processing and Mesocosm Setup
4.3. Data Collection
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trindade, C.R.T.; Landeiro, V.L.; Schneck, F. Macrophyte Functional Groups Elucidate the Relative Role of Environmental and Spatial Factors on Species Richness and Assemblage Structure. Hydrobiologia 2018, 823, 217–230. [Google Scholar] [CrossRef]
- Paz Cardozo, A.L.; Quirino, B.A.; Yofukuji, K.Y.; Ferreira Aleixo, M.H.; Fugi, R. Habitat Complexity and Individual Variation in Diet and Morphology of a Fish Species Associated with Macrophytes. Ecol. Freshw. Fish 2020, 30, 184–196. [Google Scholar] [CrossRef]
- Sousa, W.T.Z. Hydrilla verticillata (Hydrocharitaceae), a Recent Invader Threatening Brazil’s Freshwater Environments: A Review of the Extent of the Problem. Hydrobiologia 2011, 669, 1–20. [Google Scholar] [CrossRef]
- Thomaz, S.M. Ecosystem Services Provided by Freshwater Macrophytes. Hydrobiologia 2023, 850, 2757–2777. [Google Scholar] [CrossRef]
- Akhurst, D.J.; Jones, G.B.; Clark, M.; Reichelt-Brushett, A. Effects of Fish and Macrophytes on Phytoplankton and Zooplankton Community Structure in a Subtropical Freshwater Reservoir. Limnologica 2017, 62, 5–18. [Google Scholar] [CrossRef]
- Moi, D.A.; Evangelista, H.B.A.; Mormul, R.P.; Evangelista, L.R.; Thomaz, S.M. Ecosystem Multifunctionality and Stability Are Enhanced by Macrophyte Richness in Mesocosms. Aquat. Sci. 2021, 83, 53. [Google Scholar] [CrossRef]
- Luo, T.; Yang, T.; Wang, L.; Wang, R.; Wang, Y.; Yang, J.; Tong, Z.; Chen, F.; Wei, S.; Hei, P. Three-Stage Carbon Release Model during Macrophyte Decomposition. Ecol. Indic. 2023, 147, 109956. [Google Scholar] [CrossRef]
- Benítez-Mora, A.; Camargo, J.A. Ecological Responses of Aquatic Macrophytes and Benthic Macroinvertebrates to Dams in the Henares River Basin (Central Spain). Hydrobiologia 2014, 728, 167–178. [Google Scholar] [CrossRef]
- Han, X.; Feng, L.; Hu, C.; Chen, X. Wetland Changes of China’s Largest Freshwater Lake and Their Linkage with the Three Gorges Dam. Remote Sens. Environ. 2018, 204, 799–811. [Google Scholar] [CrossRef]
- Salgado, J.; Vélez, M.I.; González-Arango, C.; O’Dea, A. Protected Land Enhances the Survival of Native Aquatic Macrophytes and Limits Invasive Species Spread in the Panama Canal. Aquat. Conserv. 2023, 33, 737–750. [Google Scholar] [CrossRef]
- Pulzatto, M.M.; Cunha, E.R.; Dainez-Filho, M.S.; Thomaz, S.M. Association between the Success of an Invasive Macrophyte, Environmental Variables and Abundance of a Competing Native Macrophyte. Front. Plant Sci. 2019, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.A.; O’Hare, M.T.; McDonald, C.; Searle, K.R.; Daunt, F.; Stillman, R.A. Herbivore Regulation of Plant Abundance in Aquatic Ecosystems. Biol. Rev. 2016, 92, 1128–1141. [Google Scholar] [CrossRef]
- Gentilin-Avanci, C.; Pinha, G.D.; Petsch, D.K.; Mormul, R.P.; Thomaz, S.M. The Invasive Macrophyte Hydrilla verticillata Causes Taxonomic and Functional Homogenization of Associated Chironomidae Community. Limnology 2020, 22, 129–138. [Google Scholar] [CrossRef]
- Kennedy, T.A.; Naeem, S.; Howe, K.M.; Knops, J.M.H.; Tilman, D.; Reich, P. Biodiversity as a Barrier to Ecological Invasion. Nature 2002, 417, 636–638. [Google Scholar] [CrossRef]
- Petruzzella, A.; Manschot, J.; van Leeuwen, C.H.A.; Grutters, B.M.C.; Bakker, E.S. Mechanisms of Invasion Resistance of Aquatic Plant Communities. Front. Plant Sci. 2018, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Liu, Z.; Zhang, Q.; Tian, X.; Ju, R.; Li, B.; van Kleunen, M.; Chase, J.M.; Wu, J. Genotype Diversity Enhances Invasion Resistance of Native Plants via Soil Biotic Feedbacks. Ecol. Lett. 2024, 27, e14384. [Google Scholar] [CrossRef] [PubMed]
- Lososová, Z.; Bello, F.; Chytrý, M.; Kühn, I.; Pyšek, P.; Sádlo, J.; Winter, M.; Zelený, D. Alien Plants Invade More Phylogenetically Clustered Community Types and Cause Even Stronger Clustering. Glob. Ecol. Biogeogr. 2015, 24, 786–794. [Google Scholar] [CrossRef]
- Qian, H.; Qian, S.; Sandel, B. Phylogenetic Structure of Alien and Native Species in Regional Plant Assemblages across China: Testing Niche Conservatism Hypothesis versus Niche Convergence Hypothesis. Glob. Ecol. Biogeogr. 2022, 31, 1864–1876. [Google Scholar] [CrossRef]
- Qian, H. Intercontinental Comparison of Phylogenetic Relatedness in Introduced Plants at the Transition from Naturalization to Invasion: A Case Study on the Floras of South Africa and China. Plant Divers. 2023, 45, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Divíšek, J.; Chytrý, M.; Beckage, B.; Gotelli, N.J.; Lososová, Z.; Pyšek, P.; Richardson, D.M.; Molofsky, J. Similarity of Introduced Plant Species to Native Ones Facilitates Naturalization, but Differences Enhance Invasion Success. Nat. Commun. 2018, 9, 4631. [Google Scholar] [CrossRef] [PubMed]
- Dick, J.T.A.; Alexander, M.E.; Ricciardi, A.; Laverty, C.; Downey, P.O.; Xu, M.; Jeschke, J.M.; Saul, W.-C.; Hill, M.P.; Wasserman, R.; et al. Functional Responses Can Unify Invasion Ecology. Biol. Invasions 2017, 19, 1667–1672. [Google Scholar] [CrossRef]
- Hulme, P.E.; Bernard-Verdier, M. Comparing Traits of Native and Alien Plants: Can We Do Better? Funct. Ecol. 2017, 32, 117–125. [Google Scholar] [CrossRef]
- Vitti, S.; Pellegrini, E.; Casolo, V.; Trotta, G.; Boscutti, F. Contrasting Responses of Native and Alien Plant Species to Soil Properties Shed New Light on the Invasion of Dune Systems. J. Plant Ecol. 2020, 13, 667–675. [Google Scholar] [CrossRef]
- Grime, J.P. Competitive Exclusion in Herbaceous Vegetation. Nature 1973, 242, 344–347. [Google Scholar] [CrossRef]
- Stohlgren, T.J.; Jarnevich, C.; Chong, G.W.; Evangelista, P.H. Scale and Plant Invasions: A Theory of Biotic Acceptance. Preslia 2006, 78, 405–426. [Google Scholar]
- Silveira, M.J.; Thomaz, S.M. Interspecific Associations between Hydrilla verticillata and Three Dominant Native Genera of Submerged Macrophytes Are Taxa Dependent. Aquat. Sci. 2019, 81, 1–8. [Google Scholar] [CrossRef]
- Callaway, R.M.; Waller, L.P.; Diaconu, A.; Pal, R.; Collins, A.B.; Mueller-Schaerer, H.; Maron, J.L. Escape from Competition: Neighbors Reduce Centaurea stoebe Performance at Home but Not Away. Ecology 2011, 92, 2208–2213. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yuan, G.; Cao, T.; Ni, L.; Zhang, M.; Wang, S. An Alternative Mechanism for Shade Adaptation: Implication of Allometric Responses of Three Submersed Macrophytes to Water Depth. Ecol. Res. 2012, 27, 1087–1094. [Google Scholar] [CrossRef]
- Bouma, T.J.; De Vries, M.B.; Low, E.; Peralta, G.; Tánczos, I.C.; van de Koppel, J.; Herman, P.M.J. Trade-off Related to Ecosystem Engineering: A Case Study on Stiffness of Emerging Macrophytes. Ecology 2005, 86, 2187–2199. [Google Scholar] [CrossRef]
- Gratani, L. Plant Phenotypic Plasticity in Response to Environmental Factors. Adv. Bot. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Pereto, S.C.A.S.; dos Santos Ribas, L.G.; Wojciechowski, J.; Ceschin, F.; Dittrich, J.; Valões Bezerra, L.A.; Padial, A.A. Trade-off in Leaf and Root Investment of an Abundant Aquatic Macrophyte in a Neotropical Floodplain. Fundam. Appl. Limnol. 2016, 188, 309–314. [Google Scholar] [CrossRef]
- Cook, C.D.K.; Lüönd, R. A Revision of the Genus Hydrilla (Hydrocharitaceae). Aquat. Bot. 1982, 13, 485–504. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Xiao, S.; Chen, R.; Luo, C.; Zhu, T.; Cao, T.; Ni, L.; Xie, P.; Su, H.; et al. Effects of Benthivorous Fish Disturbance on Chlorophyll a Contents in Water and the Growth of Two Submersed Macrophytes with Different Growth Forms under Two Light Regimes. Sci. Total Environ. 2020, 704, 135269. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.V.C.; Dainez-Filho, M.S.; Bertoncin, A.P.S.; Muniz, C.M.; Meurer, T.; Figueiredo, B.R.S.; Thomaz, S.M.; Fávaro, S.L.; Mormul, R.P. Native Snails Choose an Invasive Macrophyte over a Native Macrophyte as a Food Resource. Can. J. Zool. 2019, 97, 362–367. [Google Scholar] [CrossRef]
- Simberloff, D.; Rejmánek, M. Encyclopedia of Biological Invasions; University of California Press: Berkeley, CA, USA, 2011; p. 792. [Google Scholar]
- Bianchini, I.; Cunha-Santino, M.B.; Milan, J.A.M.; Rodrigues, C.J.; Dias, J.H.P. Growth of Hydrilla verticillata (L.f.) Royle under Controlled Conditions. Hydrobiologia 2010, 644, 301–312. [Google Scholar] [CrossRef]
- Mormul, R.P.; Ferreira, F.A.; Michelan, T.S.; Carvalho, P.; Silveira, M.; Thomaz, S.M. Aquatic Macrophytes in the Large, Sub-Tropical Itaipu Reservoir, Brazil. Rev. Biol. Trop. 2009, 58, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.D.K.; Urmi-König, K. A Revision of the Genus Egeria (Hydrocharitaceae). Aquat. Bot. 1984, 19, 73–96. [Google Scholar] [CrossRef]
- Gubiani, É.A.; Thomaz, S.M.; Bini, L.M.; Piana, P.A. Metapopulation Models Predict the Temporal Response of Two Macrophytes to Drought in a Subtropical Water Reservoir. Ecol. Eng. 2017, 100, 1–7. [Google Scholar] [CrossRef]
- Bini, L.M.; Thomaz, S.M. Prediction of Egeria Najas and Egeria Densa Occurrence in a Large Subtropical Reservoir (Itaipu Reservoir, Brazil-Paraguay). Aquat. Bot. 2005, 83, 227–238. [Google Scholar] [CrossRef]
- Florêncio, F.M.; Rosado, A.; Leal, R.P.; Vecchia, A.D. Unexpected Coexistence of a Native and an Invasive Macrophyte: A Functional versus Environmental Niche Perspective. Hydrobiologia 2024, 1–19. [Google Scholar] [CrossRef]
- Sousa, W.T.Z.; Thomaz, S.M.; Murphy, K.J.; Silveira, M.J.; Mormul, R.P. Environmental Predictors of the Occurrence of Exotic Hydrilla verticillata (L.f.) Royle and Native Egeria Najas Planch. In a Sub-Tropical River Floodplain: The Upper River Paraná, Brazil. Hydrobiologia 2009, 632, 65–78. [Google Scholar] [CrossRef]
- Fasoli, J.V.B.; Mormul, R.P.; Cunha, E.R.; Thomaz, S.M. Plasticity Responses of an Invasive Macrophyte Species to Inorganic Carbon Availability and to the Interaction with a Native Species. Hydrobiologia 2018, 817, 227–237. [Google Scholar] [CrossRef]
- Liu, X.; Hou, Z.; Li, F.; Xie, Y.; Li, Y.; Yu, X. Competition between Potamogeton Malaianus and Hydrilla verticillata in Response to Different Water Level Conditions. Hydrobiologia 2023, 850, 3031–3041. [Google Scholar] [CrossRef]
- Funk, J.L.; Vitousek, P.M. Resource-Use Efficiency and Plant Invasion in Low-Resource Systems. Nature 2007, 446, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.; Nelson, C.R.; Atwater, D.Z. Response of Bluebunch Wheatgrass to Invasion: Differences in Competitive Ability among Invader-Experienced and Invader-Naïve Populations. Funct. Ecol. 2018, 32, 1857–1866. [Google Scholar] [CrossRef]
- Thomaz, S.M. Fatores Ecológicos Associados à Colonização E Ao Desenvolvimento de Macrófitas Aquáticas E Desafios de Manejo. Planta Daninha 2002, 20, 21–33. [Google Scholar] [CrossRef]
- Thiébaut, G. Impact of Mechanical Removal on the Regeneration and Colonization Abilities of the Alien Aquatic Macrophyte Egeria densa. Life 2023, 13, 2004. [Google Scholar] [CrossRef] [PubMed]
- Netherland, M.D.; Getsinger, K.D. Scaling Studies for Submersed Aquatic Plant Management Research. J. Aquat. Plant Manag. 2018, 56, 10–16. [Google Scholar]
- Ricklefs, R.E. A Economia da Natureza; Guanabara Koogan: Rio De Janeiro, Brazil, 2010; p. 14. [Google Scholar]
- Simepar. Meteorological Data: Itaipu Binational Station. Paraná Meteorological and Environmental Monitoring System. Available online: https://www.simepar.br (accessed on 20 October 2022).
- Kawaletz, H.; Mölder, I.; Annighöfer, P.; Terwei, A.; Zerbe, S.; Ammer, C. Back to the Roots: How Do Seedlings of Native Tree Species React to the Competition by Exotic Species? Ann. For. Sci. 2014, 71, 337–347. [Google Scholar] [CrossRef]
- Yannelli, F.A.; MacLaren, C.; Kollmann, J. Moving Away from Limiting Similarity during Restoration: Timing of Arrival and Native Biomass Are Better Proxies of Invasion Suppression in Grassland Communities. Front. Ecol. Evol. 2020, 8, 238. [Google Scholar] [CrossRef]
- Yannelli, F. Applying Competition Theory to Ensure Ecological Restoration and Prevent Plant Invasions. Biodiversity 2021, 22, 82–86. [Google Scholar] [CrossRef]
- Sakata, Y.; Craig, T.P. An Exotic Herbivore Reinforces Competition between Exotic and Native Plants. J. Ecol. 2021, 109, 2740–2753. [Google Scholar] [CrossRef]
- Trotta, G.; Boscutti, F.; Jamoneau, A.; Decocq, G.; Chiarucci, A. There Is Room for Everyone: Invasion Credit Cannot Be Inferred from the Species–Area Relationship in Fragmented Forests. Appl. Veg. Sci. 2023, 26, 12745. [Google Scholar] [CrossRef]
- Kortz, A.; Hejda, M.; Pergl, J.; Kutlvašr, J.; Petřík, P.; Sádlo, J.; Vítková, M.; Vojík, M.; Pyšek, P. Impacts of Native and Alien Plant Dominants at Different Spatial Scales. NeoBiota 2024, 92, 29–43. [Google Scholar] [CrossRef]
- Gioria, M.; Carta, A.; Balogianni, V.; Fornara, D.; Pyšek, P.; Osborne, B.A. Changes in the Functional and Phylogenetic Diversity of Above- and Below-Ground Plant Communities Invaded by Two Alien Herbs. NeoBiota 2023, 88, 75–101. [Google Scholar] [CrossRef]
- Weiner, J. Allocation, Plasticity and Allometry in Plants. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- Mooney, H.A. The Carbon Balance of Plants. Annu. Rev. Ecol. Syst. 1972, 3, 315–346. [Google Scholar] [CrossRef]
- Lerdau, M.T.; Monson, R.K.; Ehleringer, J.R. The Carbon Balance of Plants: Economics, Optimization, and Trait Spectra in a Historical Perspective. Oecologia 2023, 203, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Gruntman, M.; Segev, U. Effect of Residence Time on Trait Evolution in Invasive Plants: Review and Meta-Analysis. NeoBiota 2024, 91, 99–124. [Google Scholar] [CrossRef]
- Umaña, M.N.; Cao, M.; Lin, L.; Swenson, N.G.; Zhang, C. Trade-Offs in Above- and Below-Ground Biomass Allocation Influencing Seedling Growth in a Tropical Forest. J. Ecol. 2020, 109, 1184–1193. [Google Scholar] [CrossRef]
- Maberly, S.C.; Gontero, B. Trade-Offs and Synergies in the Structural and Functional Characteristics of Leaves Photosynthesizing in Aquatic Environments. Adv. Photosynth. Respir. 2018, 307–343. [Google Scholar] [CrossRef]
- Aschehoug, E.T.; Brooker, R.; Atwater, D.Z.; Maron, J.L.; Callaway, R.M. The Mechanisms and Consequences of Interspecific Competition among Plants. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 263–281. [Google Scholar] [CrossRef]
- Tan, X.; Guo, X.; Guo, W.; Liu, S.; Du, N. Invasive Rhus Typhina Invests More in Height Growth and Traits Associated with Light Acquisition than Do Native and Non-Invasive Alien Shrub Species. Trees 2018, 32, 1103–1112. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Li, G.; Mou, C. Disentangling Interspecific and Intraspecific Variability in Height–Diameter Allometry of Dominant Tree Species in the Rocky Mountains across Broad Spatial Scales. For. Int. J. For. Res. 2023, 97, 363–375. [Google Scholar] [CrossRef]
- de Castro, W.A.C.; Almeida, R.V.; Leite, M.B.; Marrs, R.H.; Matos, D.S. Invasion Strategies of the White Ginger Lily Hedychium coronarium J. König (Zingiberaceae) under Different Competitive and Environmental Conditions. Environ. Exp. Bot. 2016, 127, 55–62. [Google Scholar] [CrossRef]
- Dostál, P. Reproductive Strategies of Native Plant Populations Altered by a Plant Invasion. Funct. Ecol. 2023, 37, 2500–2510. [Google Scholar] [CrossRef]
- Bora, L.S.; Padial, A.A. A Global Review on Invasive Traits of Macrophytes and Their Link to Invasion Success. Acta Limnol. Bras. 2023, 35, 20. [Google Scholar] [CrossRef]
- Fagúndez, J.; Sánchez, A. High Variability and Multiple Trade-Offs in Reproduction and Growth of the Invasive Grass Cortaderia selloana after Cutting. Weed Res. 2024, 64, 251–260. [Google Scholar] [CrossRef]
- Smith, K.E.; Zhou, M.; Flis, P.; Jones, D.; Bishopp, A.; Yant, L. The Evolution of the Duckweed Ionome Mirrors Losses in Structural Complexity. Ann. Bot. 2024, 133, 997–1006. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, H.; Lu, Q.; Ma, J.; Shen, Y.; Wang, G. Different Responses of Leaf and Root Economics Spectrum to Grazing Time at the Community Level in Desert Steppe, China. Sci. Total Environ. 2023, 909, 168547. [Google Scholar] [CrossRef] [PubMed]
- Douce, P.; Saiz, H.; Benot, M.; Mermillod-Blondin, F.; Simon, L.; Renault, D.; Vallier, F.; Oury, Y.; Fontaine, M.; Bittebiere, A. Functional Characteristics rather than Co-Occurrences Determine the Outcome of Interactions between Neighbouring Plants in Sub-Antarctic Ponds: Consequences for Macrophyte Community Biomass. Freshw. Biol. 2023, 68, 561–576. [Google Scholar] [CrossRef]
- Thomaz, S.M.; Carvalho, P.; Mormul, R.P.; Ferreira, F.A.; Silveira, M.J.; Michelan, T.S. Temporal Trends and Effects of Diversity on Occurrence of Exotic Macrophytes in a Large Reservoir. Acta Oecologica Int. J. Ecol. 2009, 35, 614–620. [Google Scholar] [CrossRef]
- Thomaz, S.M.; Bini, L.M. Ecologia E Manejo de Macrófitas Aquáticas; Eduem: Maringá, Brazil, 2003; pp. 19–38. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 24 April 2024).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Microsoft Corporation. Microsoft Excel. 2024. Available online: https://www.microsoft.com/en-us/microsoft-365/excel (accessed on 27 September 2024).


| Treatment | Total Biomass | Subterranean Biomass | Foliar Biomass | H. verticillata Biomass Increment | ||||
|---|---|---|---|---|---|---|---|---|
| (Tukey Model) | (Tukey Model) | (Tukey Model) | (Tukey Model) | |||||
| Estimate | z-Value | Estimate | z-Value | Estimate | z-Value | Estimate | z-Value | |
| T1 × T2 | −0.08 | −0.63 | −0.63 | −3.07 ** | 0.37 | 3.29 ** | 2100.2 | 9.782 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa, L.; Vieira, L.A.; Michelan, T.S.; Vale, A.H.; Chiba de Castro, W.A. Growth Allocation Shifts in the Invasive Hydrilla verticillata Under Interspecific Competition with Native Submerged Macrophytes. Plants 2024, 13, 3500. https://doi.org/10.3390/plants13243500
da Costa L, Vieira LA, Michelan TS, Vale AH, Chiba de Castro WA. Growth Allocation Shifts in the Invasive Hydrilla verticillata Under Interspecific Competition with Native Submerged Macrophytes. Plants. 2024; 13(24):3500. https://doi.org/10.3390/plants13243500
Chicago/Turabian Styleda Costa, Letícia, Luíz Alberto Vieira, Thaísa Sala Michelan, Alvaro Herrera Vale, and Wagner Antonio Chiba de Castro. 2024. "Growth Allocation Shifts in the Invasive Hydrilla verticillata Under Interspecific Competition with Native Submerged Macrophytes" Plants 13, no. 24: 3500. https://doi.org/10.3390/plants13243500
APA Styleda Costa, L., Vieira, L. A., Michelan, T. S., Vale, A. H., & Chiba de Castro, W. A. (2024). Growth Allocation Shifts in the Invasive Hydrilla verticillata Under Interspecific Competition with Native Submerged Macrophytes. Plants, 13(24), 3500. https://doi.org/10.3390/plants13243500






