RITA® Temporary Immersion System (TIS) for Biomass Growth Improvement and Ex Situ Conservation of Viola ucriana Erben & Raimondo
Abstract
1. Introduction
2. Results
2.1. In Vitro Seed Germination and Plantlets Cultivation
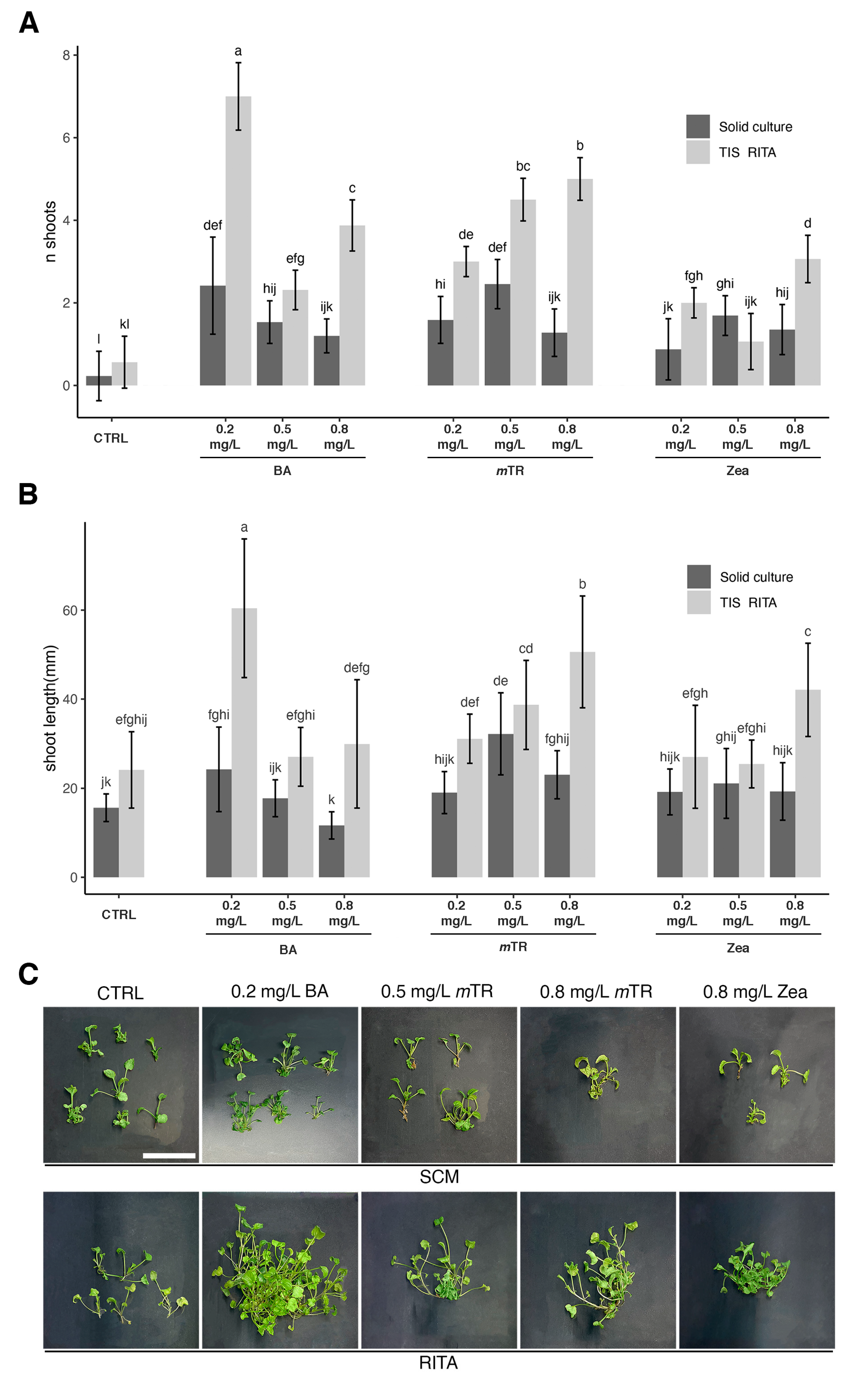
2.2. Effect of Culture System and PGRs on Shoot Multiplication and Shoot Length
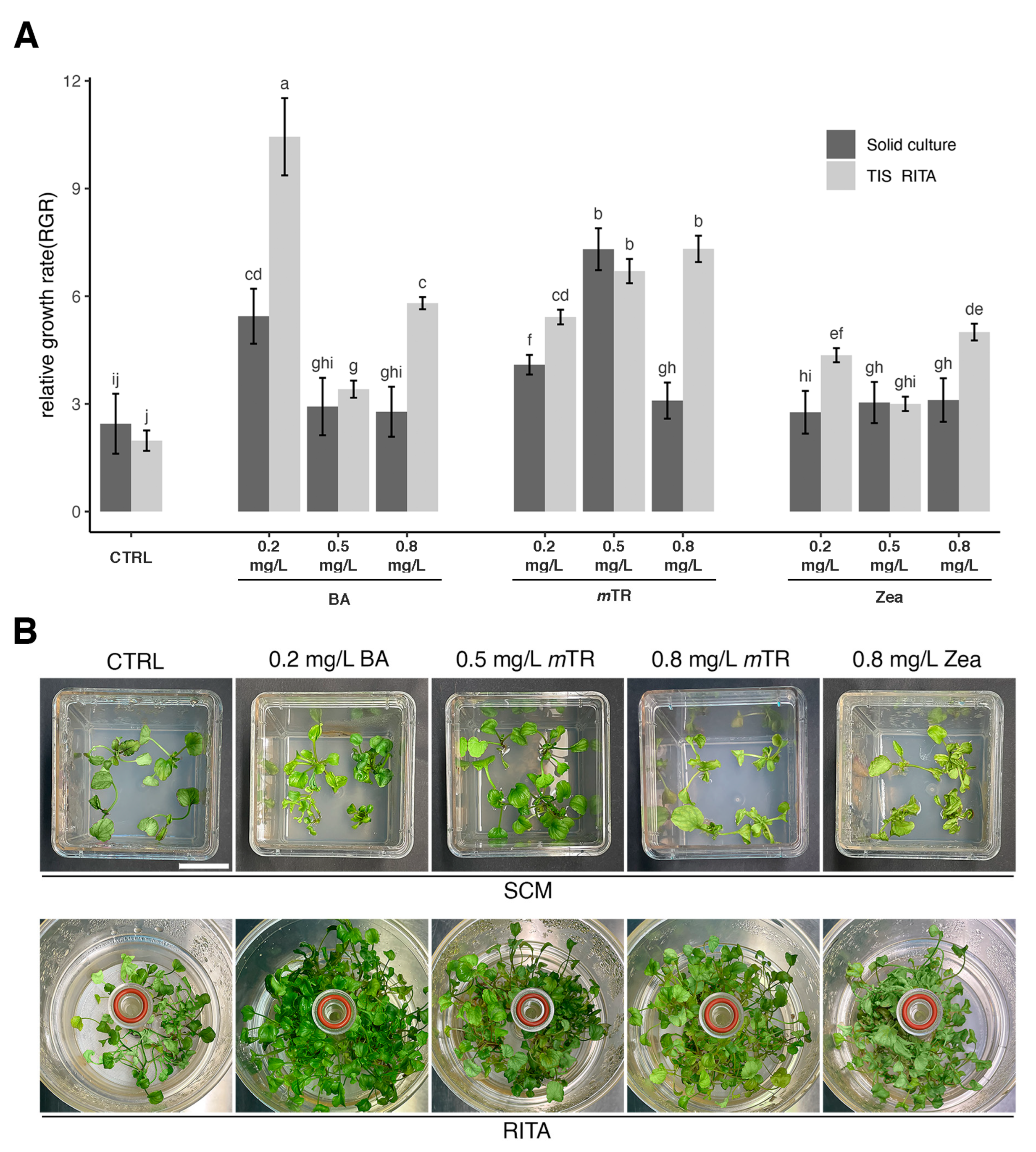
2.3. Effect of Culture Systems and PGRs on RGR Index
2.4. Rooting Phase in SCM, Rooting Plugs, and Acclimatization
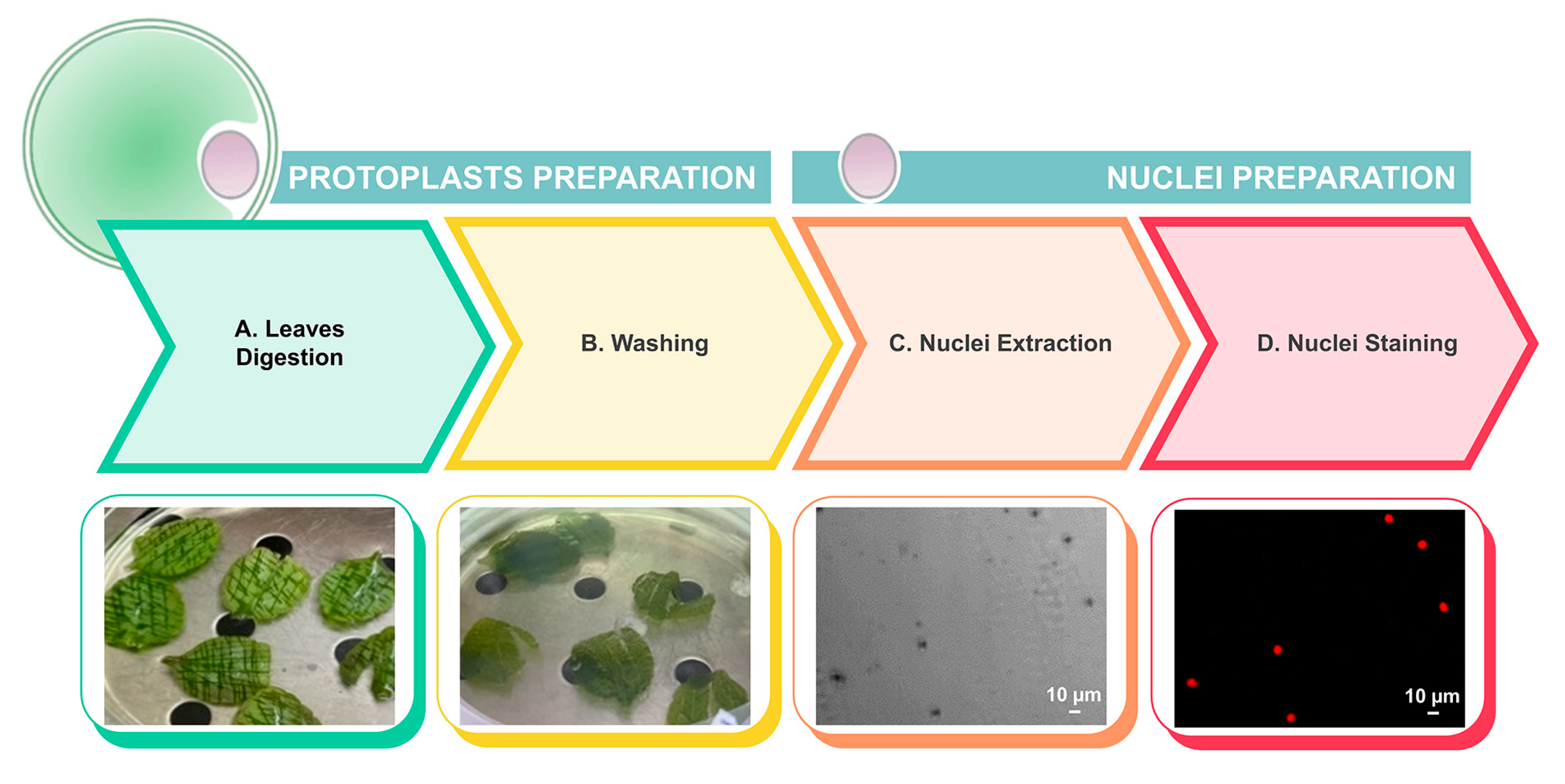
2.5. Ploidy Level Determination
3. Discussion
4. Materials and Methods
4.1. Seed Collection, Sterilization, and Pretreatment
4.2. Shoot Multiplication from Seedlings on Agar Medium
4.3. In Vitro Shoot Multiplication on RITA® TIS and SCM
4.4. Rooting
4.5. Statistics and Data Analysis
4.6. Plant Acclimatization
4.7. DNA Ploidy Level Determination After Protoplast Preparation and Nuclei Isolation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scoppola, A.; Lattanzi, E. Viola Section Melanium (Violaceae) in Italy. New Data on Morphology of Viola tricolor-Group. Webbia 2012, 67, 47–64. [Google Scholar] [CrossRef]
- Colombo, P.; Spadaro, V.; Raimondo, F.M. Morpho-Anatomical Analysis of Viola tineorum and V. ucriana (Violaceae) Endemic to the Mountains around Palermo (NW-Sicily). Bocconea 2007, 21, 233–247. [Google Scholar]
- Hühn, P.; Dillenberger, M.S.; Krause, S.; Kadereit, J.W. Polyploid Hybrid Speciation in the Calcarata Species Complex of Viola Section Melanium (Violaceae): Relating Hybrid Species to Parent Species Distribution and Ecology. Bot. J. Linn. Soc. 2023, 201, 309–328. [Google Scholar] [CrossRef]
- Krause, S.; Kadereit, J.W. Identity of the Calcarata Species Complex in Viola Sect. Melanium (Violaceae). Willdenowia 2020, 50, 195. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia; Edagricole: Bologna, Italy, 2017; Volume 2. [Google Scholar]
- Erben, M.; Raimondo, F.M. Viola tineorum e Viola ucriana Nuove Specie Dei Monti Del Palermitano (Sicilia). G. Bot. Ital. 1995, 129, 79–92. [Google Scholar] [CrossRef]
- Gianguzzi, L.; La Mantia, A. Viola ucriana; IUCN Red List of Threatened Species: Cambridge, UK, 2006. [Google Scholar]
- Gianguzzi, L.; Caldarella, O.; Di Pietro, R. A Phytosociological Analysis of the Brachypodium rupestre (Host) Roem. & Schult. Communities of Sicily. Plant Sociol. 2018, 55, 65–88. [Google Scholar] [CrossRef]
- Kilgore, S.; Havens, K.; Kramer, A.; Lythgoe, A.; MacKechnie, L.; De Vitis, M. Seed Collection, Storage, and Germination Practices May Affect Viola Reintroduction Outcomes. Nativ. Plants J. 2022, 23, 40–55. [Google Scholar] [CrossRef]
- Magrini, S.; Zucconi, L. Seed Germination of the Endangered Viola kitaibeliana and Other Italian Annual Pansies (Viola Section Melanium, Violaceae). Flora Mediterr. 2020, 30, 424–430. [Google Scholar] [CrossRef]
- Rani, K.; Groach, R.; Sharma, J.; Singh, N. In Vitro Direct Multiplication of Viola canescens Wall. Ex Roxb.: An Important Medicinal Plant. Ann. Phytomedicine Int. J. 2021, 10, 200–207. [Google Scholar] [CrossRef]
- Kendon, J.P.; Novotna, A.; Ramsay, M.M.; Porter, A.; Sarasan, V. Large Scale Propagation and in Vitro Weaning for the Restoration of Viola palustris to Support Assisted Colonisation of a Threatened Butterfly. Eurobiotech. J. 2021, 5, 170–179. [Google Scholar] [CrossRef]
- Businge, E.; Trifonova, A.; Schneider, C.; Rödel, P.; Egertsdotter, U. Evaluation of a New Temporary Immersion Bioreactor System for Micropropagation of Cultivars of Eucalyptus, Birch and Fir. Forests 2017, 8, 196. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary Immersion Systems in Plant Biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Li, J.-T.; Deng, D.-M.; Peng, G.-T.; Deng, J.-C.; Zhang, J.; Liao, B. Successful Micropropagation of the Cadmium Hyperaccumulator Viola baoshanensis (Violaceae). Int. J. Phytoremediation 2010, 12, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Naveed, I.; Muhammad, S.; Naqvi, S.; Mahmood, T. Standardization of Tissue Culture Conditions and Estimation of Free Scavenging Activity in Viola odorata L. Pak. J. Bot. 2013, 45, 197–202. [Google Scholar]
- Chalageri, G.; Babu, U.V. In Vitro Plant Regeneration via Petiole Callus of Viola patrinii and Genetic Fidelity Assessment Using RAPD Markers. Turk. J. Bot. 2012, 36, 358–368. [Google Scholar] [CrossRef]
- Żabicki, P.; Sliwinska, E.; Mitka, J.; Sutkowska, A.; Tuleja, M.; Migdałek, G.; Żabicka, J.; Słomka, A.; Kwiatkowska, M.; Kuta, E. Does Somaclonal Variation Play Advantageous Role in Conservation Practice of Endangered Species?: Comprehensive Genetic Studies of in Vitro Propagated Plantlets of Viola stagnina Kit. (Violaceae). Plant Cell Tissue Organ Cult. (PCTOC) 2019, 136, 339–352. [Google Scholar] [CrossRef]
- Slazak, B.; Sliwinska, E.; Saługa, M.; Ronikier, M.; Bujak, J.; Słomka, A.; Göransson, U.; Kuta, E. Micropropagation of Viola uliginosa (Violaceae) for Endangered Species Conservation and for Somaclonal Variation-Enhanced Cyclotide Biosynthesis. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 120, 179–190. [Google Scholar] [CrossRef]
- Jha, S.R.; Naz, R.; Asif, A.; Okla, M.K.; Soufan, W.; Al-Ghamdi, A.A.; Ahmad, A. Development of an In Vitro Propagation Protocol and a Sequence Characterized Amplified Region (SCAR) Marker of Viola serpens Wall. Ex Ging. Plants 2020, 9, 246. [Google Scholar] [CrossRef]
- Sychta, K.; Słomka, A.; Sliwinska, E.; Migdałek, G.; Kuta, E. From Cells Highly Tolerant to Zn and Pb to Fully Fertile Plants—Selection of Tolerant Lines with in Vitro Culture. Plant Physiol. Biochem. 2020, 146, 231–237. [Google Scholar] [CrossRef]
- Soni, M.; Kaur, R. Rapid in Vitro Propagation, Conservation and Analysis of Genetic Stability of Viola pilosa. Physiol. Mol. Biol. Plants 2014, 20, 95–101. [Google Scholar] [CrossRef][Green Version]
- Hai Son, N.; Thi Xuan Thao, L.; Thi Huong, T.; Trong Tuan, T. Effects Of Plant Growth Regulators On The Shoot Multiplication And Root Formation Of Violar tricolor L. Tạp chí Khoa học Công nghệ và Thực phẩm 2019, 19, 3–10. [Google Scholar]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal Variations and Their Applications in Horticultural Crops Improvement. 3 Biotech 2016, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Mallón, R.; Rodríguez-Oubiña, J.; González, M.L. In Vitro Propagation of the Endangered Plant Centaurea ultreiae: Assessment of Genetic Stability by Cytological Studies, Flow Cytometry and RAPD Analysis. Plant Cell Tissue Organ Cult. (PCTOC) 2010, 101, 31–39. [Google Scholar] [CrossRef]
- Nhut, D.T.; Hai, N.T.; Thu, P.T.M.; Thi, N.N.; Hien, T.T.D.; Tuan, T.T.; Nam, N.B.; Huy, N.P.; Chien, H.X.; Jain, S.M. Protocol for Inducing Flower Color Somaclonal Variation in Torenia (Torenia fournieri Lind.). In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants. Methods in Molecular Biology; Lambardi, M., Ozudogru, E.A., Jain, S.M., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 994, pp. 455–462. [Google Scholar]
- Abreu, I.S.; Carvalho, C.R.; Clarindo, W.R. Massal Induction of Carica papaya L. ‘Golden’ Somatic Embryos and Somaclone Screening by Flow Cytometry and Cytogenetic Analysis. Cytologia 2014, 79, 475–484. [Google Scholar] [CrossRef]
- Kar, B.; Kuanar, A.; Singh, S.; Mohanty, S.; Joshi, R.K.; Subudhi, E.; Nayak, S. In Vitro Induction, Screening and Detection of High Essential Oil Yielding Somaclones in Turmeric (Curcuma longa L.). Plant Growth Regul. 2014, 72, 59–66. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Iglesias-Andreu, L.G.; Avilés-Viñas, S.A.; Gómez-Uc, E.; Canto-Flick, A.; Santana-Buzzy, N. Somaclonal Variation in Habanero Pepper (Capsicum chinense Jacq.) as Assessed ISSR Molecular Markers. HortScience 2014, 49, 481–485. [Google Scholar] [CrossRef]
- Thiem, B.; Śliwińska, E. Flow Cytometric Analysis of Nuclear DNA Content in Cloudberry (Rubus chamaemorus L.) in Vitro Cultures. Plant Sci. 2003, 164, 129–134. [Google Scholar] [CrossRef]
- Trajković, M.; Antonić, D.; Cingel, A.; Ghalawenji, N.; Subotić, A.; Jevremović, S. Advancement in Protocol for in Vitro Seed Germination, Plant Regeneration and Cryopreservation of Viola cornuta. 3 Biotech 2019, 9, 17. [Google Scholar] [CrossRef]
- Kunakhonnuruk, B.; Inthima, P.; Kongbangkerd, A. In Vitro Propagation of Rheophytic Orchid, Epipactis flava Seidenf.—A Comparison of Semi-Solid, Continuous Immersion and Temporary Immersion Systems. Biology 2019, 8, 72. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Wu, K.-L.; Zhang, J.-X.; Deng, R.-F.; Duan, J.; Teixeira da Silva, J.A.; Huang, W.-C.; Zeng, S.-J. Embryo Development in Association with Asymbiotic Seed Germination in Vitro of Paphiopedilum armeniacum S. C. Chen et F. Y. Liu. Sci. Rep. 2015, 5, 16356. [Google Scholar] [CrossRef]
- Leyva-Ovalle, O.R.; Bello-Bello, J.J.; Murguía-González, J.; Núñez-Pastrana, R.; Ramírez-Mosqueda, M.A. Micropropagation of Guarianthe skinneri (Bateman) Dressler et W. E. Higging in Temporary Immersion Systems. 3 Biotech 2020, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-D.; Kwon, S.-H.; Murthy, H.N.; Yun, S.-W.; Pyo, S.-S.; Park, S.-Y. Temporary Immersion Bioreactor System as an Efficient Method for Mass Production of In Vitro Plants in Horticulture and Medicinal Plants. Agronomy 2022, 12, 346. [Google Scholar] [CrossRef]
- Benelli, C.; De Carlo, A. In Vitro Multiplication and Growth Improvement of Olea europaea L. Cv Canino with Temporary Immersion System (PlantformTM). 3 Biotech 2018, 8, 317. [Google Scholar] [CrossRef]
- Kikowska, M.; Danek, K.; Gornowicz-Porowska, J.; Thiem, B. Application of Temporary Immersion System RITA® for Efficient Biomass Multiplication and Production of Artificial Seeds for Ex Situ Conservation of Linnaea borealis L. Plant Cell Tissue Organ Cult. (PCTOC) 2022, 151, 673–680. [Google Scholar] [CrossRef]
- Georgiev, V.; Ivanov, I.; Berkov, S.; Pavlov, A. Temporary Immersion Systems for Amaryllidaceae Alkaloids Biosynthesis by Pancratium maritimum L. Shoot Culture. J. Plant Biochem. Biotechnol. 2014, 23, 389–398. [Google Scholar] [CrossRef]
- Anglana, C.; Capaci, P.; Barozzi, F.; Migoni, D.; Rojas, M.; Stigliano, E.; Di Sansebastiano, G.P.D.; Papadia, P. Dittrichia viscosa Selection Strategy Based on Stress Produces Stable Clonal Lines for Phytoremediation Applications. Plants 2023, 12, 2499. [Google Scholar] [CrossRef]
- Anglana, C.; Barozzi, F.; Capaci, P.; Migoni, D.; Rojas, M.; Fanizzi, F.P.; Di Sansebastiano, G.-P. Characterization of Three Species of Aquatic Mosses in Axenic Culture for Biomonitoring and Biotechnological Applications. Aquat. Bot. 2024, 193, 103762. [Google Scholar] [CrossRef]
- Michoux, F.; Ahmad, N.; McCarthy, J.; Nixon, P.J. Contained and High-level Production of Recombinant Protein in Plant Chloroplasts Using a Temporary Immersion Bioreactor. Plant Biotechnol. J. 2011, 9, 575–584. [Google Scholar] [CrossRef]
- Muñoz-Alcayaga, C.; Soto, J.; Román-Figueroa, C.; Paneque, M. Ex Situ Conservation of Atriplex taltalensis I.M. Johnst. via In Vitro Culturing of Its Axillary Shoots. Diversity 2022, 15, 13. [Google Scholar] [CrossRef]
- Ivanova, M.; van Staden, J. Effect of Ammonium Ions and Cytokinins on Hyperhydricity and Multiplication Rate of in Vitro Regenerated Shoots of Aloe polyphylla. Plant Cell Tissue Organ Cult. 2008, 92, 227–231. [Google Scholar] [CrossRef]
- Ochatt, S.J.; Patat-Ochatt, E.M.; Moessner, A. Ploidy Level Determination within the Context of in Vitro Breeding. Plant Cell Tissue Organ Cult. (PCTOC) 2011, 104, 329–341. [Google Scholar] [CrossRef]
- Loureiro, J.; Rodriguez, E.; Dolezel, J.; Santos, C. Two New Nuclear Isolation Buffers for Plant DNA Flow Cytometry: A Test with 37 Species. Ann. Bot. 2007, 100, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Cires, E.; Cuesta, C.; Fernández Casado, M.Á.; Nava, H.S.; Vázquez, V.M.; Fernández Prieto, J.A. Isolation of Plant Nuclei Suitable for Flow Cytometry from Species with Extremely Mucilaginous Compounds: An Example in the Genus viola L. (Violaceae). An. Del Jardín Botánico De Madr. 2011, 68, 139–154. [Google Scholar] [CrossRef]
- Doležel, J.; Greilhuber, J.; Suda, J. Estimation of Nuclear DNA Content in Plants Using Flow Cytometry. Nat. Protoc. 2007, 2, 2233–2244. [Google Scholar] [CrossRef]
- Li, X.-M.; Jenke, H.; Strauss, S.; Bazakos, C.; Mosca, G.; Lymbouridou, R.; Kierzkowski, D.; Neumann, U.; Naik, P.; Huijser, P.; et al. Cell-Cycle-Linked Growth Reprogramming Encodes Developmental Time into Leaf Morphogenesis. Curr. Biol. 2024, 34, 541–556.e15. [Google Scholar] [CrossRef]
- Andriankaja, M.; Dhondt, S.; De Bodt, S.; Vanhaeren, H.; Coppens, F.; De Milde, L.; Mühlenbock, P.; Skirycz, A.; Gonzalez, N.; Beemster, G.T.S.; et al. Exit from Proliferation during Leaf Development in Arabidopsis thaliana: A Not-So-Gradual Process. Dev. Cell 2012, 22, 64–78. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gatti, E.; Sgarbi, E.; Ozudogru, E.A.; Lambardi, M. The Effect of Plantform TM Bioreactor on Micropropagation of Quercus robur in Comparison to a Conventional in Vitro Culture System on Gelled Medium, and Assessment of the Microenvironment Influence on Leaf Structure. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2017, 151, 1129–1136. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio Team: Boston, MA, USA, 2020. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Di Sansebastiano, G.P.; Barozzi, F. Transient Secretory Enzyme Expression in Leaf Protoplasts to Characterize SNARE Functional Classes in Conventional and Unconventional Secretion. In Methods in Molecular Biology (Clifton, N.J.); Humana Press: New York, NY, USA, 2017; Volume 1662, pp. 209–221. [Google Scholar]
- Schmuths, H.; Meister, A.; Horres, R.; Bachmann, K. Genome Size Variation among Accessions of Arabidopsis thaliana. Ann. Bot. 2004, 93, 317–321. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capaci, P.; Barozzi, F.; Forciniti, S.; Anglana, C.; Iuele, H.; Accogli, R.A.; Carra, A.; Lenucci, M.S.; del Mercato, L.L.; Di Sansebastiano, G.P. RITA® Temporary Immersion System (TIS) for Biomass Growth Improvement and Ex Situ Conservation of Viola ucriana Erben & Raimondo. Plants 2024, 13, 3530. https://doi.org/10.3390/plants13243530
Capaci P, Barozzi F, Forciniti S, Anglana C, Iuele H, Accogli RA, Carra A, Lenucci MS, del Mercato LL, Di Sansebastiano GP. RITA® Temporary Immersion System (TIS) for Biomass Growth Improvement and Ex Situ Conservation of Viola ucriana Erben & Raimondo. Plants. 2024; 13(24):3530. https://doi.org/10.3390/plants13243530
Chicago/Turabian StyleCapaci, Piergiorgio, Fabrizio Barozzi, Stefania Forciniti, Chiara Anglana, Helena Iuele, Rita Annunziata Accogli, Angela Carra, Marcello Salvatore Lenucci, Loretta L. del Mercato, and Gian Pietro Di Sansebastiano. 2024. "RITA® Temporary Immersion System (TIS) for Biomass Growth Improvement and Ex Situ Conservation of Viola ucriana Erben & Raimondo" Plants 13, no. 24: 3530. https://doi.org/10.3390/plants13243530
APA StyleCapaci, P., Barozzi, F., Forciniti, S., Anglana, C., Iuele, H., Accogli, R. A., Carra, A., Lenucci, M. S., del Mercato, L. L., & Di Sansebastiano, G. P. (2024). RITA® Temporary Immersion System (TIS) for Biomass Growth Improvement and Ex Situ Conservation of Viola ucriana Erben & Raimondo. Plants, 13(24), 3530. https://doi.org/10.3390/plants13243530













