The Agronomic Potential of the Invasive Brown Seaweed Rugulopteryx okamurae: Optimisation of Alginate, Mannitol, and Phlorotannin Extraction
Abstract
:1. Introduction
2. Results and Discussion
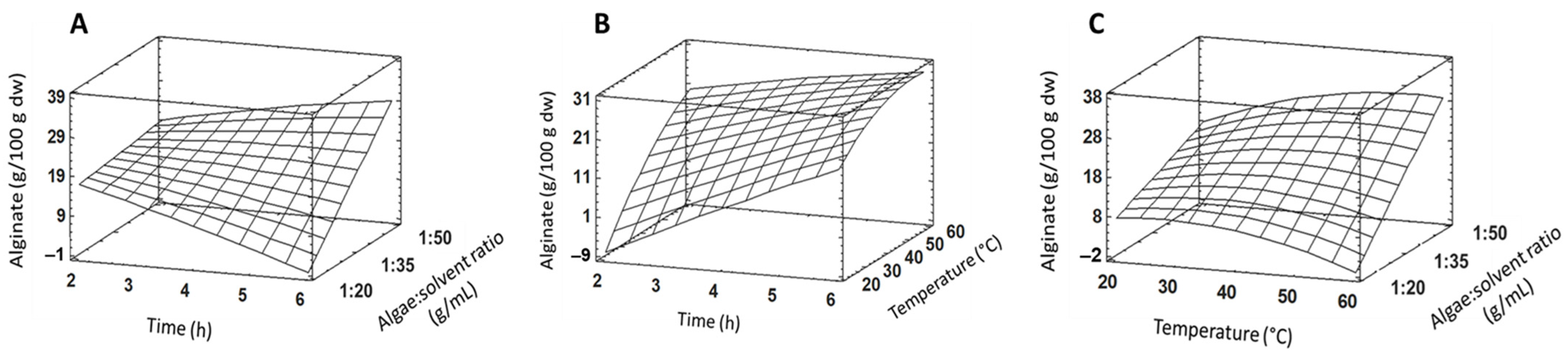
2.1. Extraction and Physicochemical Alginate Characterization
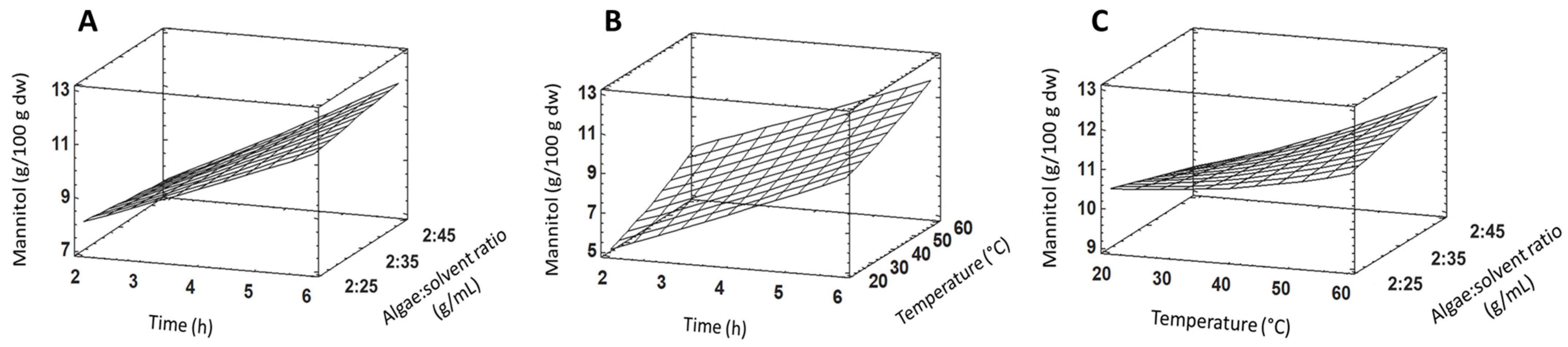
2.2. Mannitol Extraction
2.3. Phlorotannins Extraction
3. Materials and Methods
3.1. Solvents and Reagents
3.2. Samples
3.3. Alginate Extraction
3.4. Physicochemical Characterization of the Alginate Extracted from R. okamurae
3.5. Mannitol Extraction
3.6. Phlorotannins Extraction
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barcellos, L.; Pham, C.K.; Menezes, G.; Bettencourt, R.; Rocha, N.; Carvalho, M.; Felgueiras, H.P. A concise review on the potential applications of Rugulopteryx okamurae macroalgae. Mar. Drugs 2023, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Remya, R.R.; Samrot, A.V.; Kumar, S.S.; Mohanavel, V.; Karthick, A.; Chinnaiyan, V.K.; Umapathy, D.; Muhibbullah, M. Bioactive potential of brown algae. Adsorp. Sci. Technol. 2022, 2022, 9104835. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jeon, Y.J. Edible brown seaweeds: A review. J. Food Bioact. 2018, 2, 37–50. [Google Scholar] [CrossRef]
- Ismail, M.M.; El Zokm, G.M.; Miranda-López, J.M. Nutritional, bioactive compounds content, and antioxidant activity of brown seaweeds from the Red Sea. Front. Nutr. 2023, 10, 1210934. [Google Scholar] [CrossRef]
- Ummat, V.; Sivagnanam, S.P.; Rameshkumar, S.; Pednekar, M.; Fitzpatrick, S.; Rai, D.K.; Padamati, R.B.; O’Donnell, C.; Tiwari, B.K. Sequential extraction of fucoidan, laminarin, mannitol, alginate and protein from brown macroalgae Ascophyllum nodosum and Fucus vesiculosus. Int. J. Biol. Macromol. 2024, 256, 128195. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Bogolitsyn, K.; Parshina, A.; Mamatmyrodov, K.; Polomarchuk, D.; Popov, N. Recent advances in biochemistry of marine phaeophyta: Chemical analysis, structural studies and applications. In Studies in Natural Products Chemistry; Atta-ur-Rahman, F.R.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 435–486. [Google Scholar] [CrossRef]
- Omoarelojie, L.O.; Van Staden, J. Perspectives on the potentials of phlorotannins in enhancing phytoremediation performance. J. Plant Growth Regul. 2023, 42, 2972–2992. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Malde, M.K.; Eilertsen, K.E.; Elvevoll, E.O. Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H.; Kim, S.K. Marine algae as a potential pharmaceutical source for anti-allergic therapeutics. Process Biochem. 2012, 46, 386–394. [Google Scholar] [CrossRef]
- Karuppusamy, S.; Rajauria, G.; Fitzpatrick, S.; Lyons, H.; McMahon, H.; Curtin, J.; Tiwari, B.K.; O’Donnell, C. Biological properties and health-promoting functions of laminarin: A comprehensive review of preclinical and clinical studies. Mar. Drugs 2022, 20, 772. [Google Scholar] [CrossRef]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, properties and applications of alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Yang, X.; Yao, L.; Hu, Z.; Li, H.; Xu, X.; Lu, J. Potential food and nutraceutical applications of alginate: A review. Mar. Drugs 2022, 20, 564. [Google Scholar] [CrossRef] [PubMed]
- Groisillier, A.; Shao, Z.; Michel, G.; Goulitquer, S.; Bonin, P.; Krahulec, S.; Nidetzky, B.; Duan, D.; Boyen, C.; Tonon, T. Mannitol metabolism in brown algae involves a new phosphatase family. J. Exp. Bot. 2014, 65, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Battacharyya, D.; Babgohari, M.Z.; Rathar, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Dayal, P.; Kumar, V.; Kumari, M. The plant bio-stimulant properties of seaweed extracts: Potential to mitigate climate change for sustainable agriculture. Food Sci. Rep. 2023, 4, 70–75. [Google Scholar]
- Qiqin, L.; Huaguang, Z.; Minxiu, S.; Qian, L.; Haijun, F.; Haimin, C.; Rui, Y. Improvement of soil structure and bacterial composition by long-term application of seaweed fertilizer. J. Soil Sci. Plant Nutr. 2023, 23, 5122–5132. [Google Scholar] [CrossRef]
- Santana, I.; Felix, M.; Bengoechea, C. Feasibility of invasive brown seaweed Rugulopteryx okamurae as source of alginate: Characterization of products and evaluation of derived gels. Polymers 2024, 16, 702. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. A green approach for alginate extraction from Sargassum muticum brown seaweed using ultrasound-assisted technique. Int. J. Biol. Macromol. 2019, 124, 451–459. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitate brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Fertah, M. Isolation and characterization of alginate from seaweed. In Seaweed Polysaccharides; Venkatesan, J., Anil, S., Kim, S.K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 11–26. [Google Scholar] [CrossRef]
- Cebrián-Lloret, V.; Cartan-Moya, S.; Martínez-Sanz, M.; Gómez-Cortés, P.; Calvo, M.V.; López-Rubio, A.; Martínez-Abad, A. Characterization of the invasive macroalgae Rugulopteryx okamurae for potential biomass valorisation. Food Chem. 2024, 440, 138241. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.G.S. Extraction and physicochemical characterization of Sargassum vulgare alginate from Brazil. Carbohydr. Res. 2007, 342, 2067–2074. [Google Scholar] [CrossRef]
- Ayarza, J.; Coello, Y.; Nakamatsu, J. SEM-EDS study of ionically cross-linked alginate and alginic acid bead formation. Int. J. Polym. Anal. Charact. 2016, 22, 1–10. [Google Scholar] [CrossRef]
- Kaidi, S.; Bentiss, F.; Jama, C.; Khaya, K.; Belattmania, Z.; Reani, A.; Sabour, B. Isolation and structural characterization of alginates from the kelp species Laminaria ochroleuca and Saccorhiza polyschides from the Atlantic coast of Morocco. Coloids Interfaces 2022, 6, 51. [Google Scholar] [CrossRef]
- Grasdalen, H.; Bjorn, L.; Smidsrod, O. A p.m.r. study of the composition and sequence of uronate residues in alginates. Carbohydr. Res. 1979, 68, 23–31. [Google Scholar] [CrossRef]
- Ferreira-Anta, T.; Flórez-Fernández, N.; Torres, M.D.; Mazón, J.; Domínguez, H. Microwave-assisted hydrothermal processing of Rugulopteryx okamurae. Mar. Drugs 2023, 21, 319. [Google Scholar] [CrossRef]
- Song, B.; Liang, H.; Sun, R.; Peng, P.; Jiang, Y.; She, D. Hydrogel synthesis based on lignin/sodium alginate and application in agriculture. Int. J. Biol. Macromol. 2020, 144, 219–230. [Google Scholar] [CrossRef]
- Martínez-Cano, B.; Mendoza-Meneses, C.J.; García-Trejo, J.F.; Macías-Bobadilla, G.; Aguirre-Becerra, H.; Soto-Zarazúa, G.M.; Feregrino-Pérez, A.A. Review and perspectives of the use of alginate as a polymer matrix for microorganisms applied in agro-industry. Molecules 2022, 27, 4248. [Google Scholar] [CrossRef]
- Bogaert, K.A.; Delva, S.; De Clerck, O. Concise review of the genus Dictyota J.V. Lamouroux. J. Appl. Phycol. 2020, 32, 1521–1543. [Google Scholar] [CrossRef]
- Agabo-García, C.; Romero-García, L.I.; Álvarez-Gallego, C.J.; Blandino, A. Valorisation of the invasive alga Rugulopteryx okamurae through the production of monomeric sugars. Appl. Microbiol. Biotechnol. 2023, 107, 1971–1982. [Google Scholar] [CrossRef] [PubMed]
- Romero-Vargas, A.; Fdez-Güelfo, L.A.; Blandino, A.; Díaz, M.J.; Díaz, A.B. Rugulopteryx okamurae: Effect of hydrothermal acid pretreatment on the saccharification process. Bioresour. Technol. 2023, 388, 129721. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, P.; Subedi, R. Influence of mannitol priming on maize seeds under induced water stress. J. Agric. Crops 2020, 6, 27–31. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Iqbal, M.; Bharwana, S.A.; Siddiqi, Z.; Farid, M.; Ali, Q.; Saeed, R.; Rizwan, M. Mannitol alleviates chromium toxicity in wheat plants in relation to growth, yield, stimulation of anti-oxidative enzymes, oxidative stress and Cr uptake in sand and soil media. Ecotoxicol. Environ. Saf. 2015, 122, 1–8. [Google Scholar] [CrossRef]
- Habiba, U.; Ali, S.; Rizwan, M.; Ibrahim, M.; Hussain, A.; Shahid, M.R.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Alleviative role of exogenously applied mannitol in maize cultivars differing in chromium stress tolerance. Environ. Sci. Pollut. Res. 2019, 26, 5111–5121. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A source of bioactive phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, J.; He, S.; Cui, W.; Gao, F. The new products from brown seaweeds: Fucoxanthin and phlorotannins. In Sustainable Global Resources of Seaweeds Volume 2: Food, Pharmaceutical and Health Applications; Rao, A.R., Ravishankar, G.A., Eds.; Springer: Cham, Switzerland, 2022; pp. 181–202. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Kulkarni, M.G.; Stirk, W.A.; Van Staden, J. Eckol—A new plant growth stimulant from the brown seaweed Ecklonia maxima. J. Appl. Phycol. 2015, 27, 581–587. [Google Scholar] [CrossRef]
- Gomez, C.G.; Lambrecht, M.V.P.; Lozano, J.E.; Rinaudo, M.; Villar, M.A. Influence of the extraction–purification conditions on final properties of alginates obtained from brown algae (Macrocystis pyrifera). Int. J. Biol. Macromol. 2009, 44, 365–371. [Google Scholar] [CrossRef]
- Cameron, M.C.; Ross, A.G.; Percival, E.G.V. Methods for the routine estimation of mannitol, alginic acid, and combined fucose in seaweeds. J. Soc. Chem. Ind. 1948, 67, 161–164. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.; Jouini, M.; Amor, H.B.H.; Mzoughi, Z.; Dridi, M.; Said, R.B.; Bouraoui, A. Phytochemical analysis and evaluation of the antioxidant, anti-inflammatory, and antinociceptive potential of phlorotannin–rich fractions from three Mediterranean brown seaweeds. Mar. Biotechnol. 2018, 20, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Ank, G.; Da Gama, B.A.P.; Pereira, R.C. Latitudinal variation in phlorotannin contents from Southwestern Atlantic brown seaweeds. PeerJ 2019, 7, e7379. [Google Scholar] [CrossRef]
- Belattmania, Z.; Kaidi, S.; El-Atouani, S.; Katif, C.; Bentiss, F.; Jama, C.; Reani, A.; Sabour, B.; Vasconcelos, V. Isolation and FTIR-ATR and 1H NMR characterization of alginates from the main alginophyte species of the Atlantic coast of Morocco. Molecules 2020, 25, 4335. [Google Scholar] [CrossRef]
- Connan, S.; Goulard, F.; Stiger, V.; Deslandes, E.; Gall, E.A. Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot. Mar. 2004, 47, 410–416. [Google Scholar] [CrossRef]
- Creis, E.; Delage, L.; Charton, S.; Goulitquer, S.; Leblanc, C.; Potin, P.; Gall, E.A. Constitutive or inducible protective mechanisms against UVB radiation in the brown alga Fucus vesiculosus? A study of gene expression and phlorotannin content responses. PLoS ONE 2015, 10, e0128003. [Google Scholar] [CrossRef]
- Cuong, D.X.; Boi, V.N.; Van, T.T.T.; Hau, L.N. Effect of storage time on phlorotannin content and antioxidant activity of six Sargassum species from Nhatrang Bay, Vietnam. J. Appl. Phycol. 2016, 28, 567–572. [Google Scholar] [CrossRef]
- Deyab, M.A.; El-Katony, T.M.; El-Adl, M.F.; Ward, F.M. Temporal variation in chemical composition of Dictyota dichotoma (Hudson) J.V. Lamouroux (Dictyotales, Phaeophyceae) from Red Sea coast, Egypt. J. Coast. Life Med. 2017, 5, 149–155. [Google Scholar] [CrossRef]
- Erpel, F.; Mariotti-Celis, M.S.; Parada, J.; Pedreschi, F.; Pérez-Correa, J.R. Pressurized hot liquid extraction with 15% v/v glycerol-water as an effective environment-friendly process to obtain Durvillaea incurvata and Lessonia spicata phlorotannin extracts with antioxidant and antihyperglycemic potential. Antioxidants 2021, 10, 1105. [Google Scholar] [CrossRef]
- Hachemi-Benmalek, N.; Nouani, A.; Benchabane, A. Valorization of brown algae (Cystoseira caespitosa) from local region in Algeria for sodium alginate extraction and their application in the immobilization of microbial pectinases. Alger. J. Environ. Sci. Technol. 2019, 5, 1155–1162. [Google Scholar]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Khaya, K.; Raja, A.; Katif, C.; Bentiss, F.; Jama, C.; Reani, A.; Sabour, B.; Belattmania, Z. Chemical composition and antioxidant properties of Treptacantha baccata (Fucales, Ochrophyta) from the Atlantic coast of Morocco. Int. J. Algae 2022, 24, 159–174. [Google Scholar] [CrossRef]
- Kuda, T.; Taniguchi, E.; Nishizawa, M.; Araki, Y. Fate of water-soluble polysaccharides in dried Chorda filum a brown alga during water washing. J. Food Compos. Anal. 2002, 15, 3–9. [Google Scholar] [CrossRef]
- Lemesheva, V.; Islamova, R.; Stepchenkova, E.; Shenfeld, A.; Birkemeyer, C.; Tarakhovskaya, E. Antibacterial, antifungal and algicidal activity of phlorotannins, as principal biologically active components of ten species of brown algae. Plants 2023, 12, 821. [Google Scholar] [CrossRef]
- López-Hortas, L.; Flórez-Fernández, N.; Mazón, J.; Domínguez, H.; Torres, M.D. Relevance of drying treatment on the extraction of high valuable compounds from invasive brown seaweed Rugulopteryx okamurae. Algal Res. 2023, 69, 102917. [Google Scholar] [CrossRef]
- Manns, D.; Deutschle, A.L.; Saake, B.; Meyer, A.S. Methodology for quantitative determination of the carbohydrate composition of brown seaweeds Laminariaceae. R. Soc. Chem. Adv. 2014, 4, 25736. [Google Scholar] [CrossRef]
- Miller, I.J. Alginate composition of some New-Zealand brown seaweeds. Phytochemistry 1996, 41, 1315–1317. [Google Scholar] [CrossRef]
- Nair, D.; Vanuopadath, M.; Balasubramanian, A.; Iyer, A.; Ganesh, S.; Anil, A.N.; Vikraman, V.; Pillai, P.; Bose, C.; Nair, B.G.; et al. Phlorotannins from Padina tetrastromatica: Structural characterization and functional studies. J. Appl. Phycol. 2019, 31, 3131–3141. [Google Scholar] [CrossRef]
- Olsson, J.; Toth, G.B.; Albers, E. Biochemical composition of red, green and brown seaweeds on the Swedish west coast. J. Appl. Phycol. 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- Percival, E.; Young, M. Carbohydrates of the brown seaweeds: Part III. Desmarestia aculeata. Carbohydr. Res. 1974, 32, 195–201. [Google Scholar] [CrossRef]
- Pérez, R. Ces algues qui nous entourent. In Conception Actuelle, Rôle Dans la Biosphère, Utilisation, Culture; Editions Quae: Versailles, France, 1997. [Google Scholar]
- Rashedy, S.H.; Abd El Hafez, M.A.E.; Dar, M.A.; Cotas, J.; Pereira, L. Evaluation and characterization of alginate extracted from brown seaweed collected in the Red Sea. Appl. Sci. 2021, 11, 6290. [Google Scholar] [CrossRef]
- Shibata, T.; Kawaguchi, S.; Hama, Y.; Inagaki, M.; Yamaguchi, K.; Nakamura, T. Local and chemical distribution of phlorotannins in brown algae. J. Appl. Phycol. 2004, 16, 291–296. [Google Scholar] [CrossRef]
- Trica, B.; Delattre, C.; Gros, F.; Ursu, A.V.; Dobre, T.; Djelveh, G.; Michaud, P.; Oancea, F. Extraction and characterization of alginate from an edible brown seaweed (Cystoseira barbata) harvested in the Romanian Black Sea. Mar. Drugs 2019, 17, 405. [Google Scholar] [CrossRef]
- Zhang, R.; Yuen, A.K.L.; Nys, R.; Masters, A.F.; Maschmeyer, T. Step by step extraction of bio-actives from the brown seaweeds, Carpophyllum flexuosum, Carpophyllum plumosum, Ecklonia radiata and Undaria pinnatifida. Algal Res. 2020, 52, 102092. [Google Scholar] [CrossRef]
- Zubia, M.; Payri, C.; Deslandes, E. Alginate, mannitol, phenolic compounds and biological activities of two range-extending brown algae, Sargassum mangarevense and Turbinaria ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia). J. Appl. Phycol. 2008, 20, 1033–1043. [Google Scholar] [CrossRef]
- García Gómez, J.C.; Sempere Valverde, J.; Ostalé Valriberas, E.; Martínez, M.; Olaya Ponzone, L.; Roi González, A.; Espinosa Torre, F.; Sánchez Moyano, J.E.; Megina Martínez, C.; Parada, J.A. Rugulopteryx okamurae (EY Dawson) IK Hwang, WJ Lee & HS Kim (Dictyotales, Ochrophyta), alga exótica “explosiva” en el estrecho de Gibraltar. Observaciones preliminares de su distribución e impacto. Almoraima Rev. Estud. Campogibraltareños 2018, 49, 97–113. [Google Scholar]



| Block | Ti (h) | Te (°C) | Ra (g/mL) | Algyield (g/100 g dw) | Ti (h) | Te (°C) | Ra (g/mL) | Man Yield (g/100 g dw) | Ti (h) | Te (°C) | Ra (g/mL) | Et (%) | Phl Yield (g/100 g dw) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 60 | 1:20 | 7.8 | 6 | 20 | 2:45 | 8.3 | 2 | 20 | 1:25 | 50 | 0.208 |
| 1 | 2 | 60 | 1:50 | 19.5 | 6 | 40 | 2:25 | 11.0 | 3 | 20 | 1:40 | 30 | 0.223 |
| 1 | 6 | 60 | 1:35 | 14.1 | 2 | 40 | 2:35 | 7.1 | 1 | 60 | 1:40 | 50 | 0.331 |
| 1 | 6 | 20 | 1:50 | 17.0 | 2 | 60 | 2:45 | 8.2 | 3 | 60 | 1:25 | 10 | 0.188 |
| 1 | 2 | 20 | 1:20 | 8.0 | 6 | 60 | 2:35 | 11.0 | 1 | 20 | 1:10 | 10 | 0.100 |
| 1 | 6 | 40 | 1:20 | 6.7 | 4 | 40 | 2:45 | 8.7 | 1 | 40 | 1:25 | 30 | 0.181 |
| 1 | 4 | 40 | 1:50 | 18.2 | 4 | 60 | 2:25 | 8.8 | 2 | 40 | 1:40 | 10 | 0.222 |
| 1 | 2 | 40 | 1:35 | 12.1 | 2 | 20 | 2:25 | 7.0 | 2 | 60 | 1:10 | 30 | 0.106 |
| 1 | 4 | 20 | 1:35 | 7.9 | 4 | 20 | 2:35 | 7.5 | 3 | 40 | 1:10 | 50 | 0.117 |
| 2 | 4 | 60 | 1:20 | 8.6 | 6 | 20 | 2:45 | 11.1 | 2 | 20 | 1:25 | 50 | 0.185 |
| 2 | 2 | 60 | 1:50 | 20.5 | 6 | 40 | 2:25 | 10.5 | 3 | 20 | 1:40 | 30 | 0.192 |
| 2 | 6 | 60 | 1:35 | 13.7 | 2 | 40 | 2:35 | 6.4 | 1 | 60 | 1:40 | 50 | 0.319 |
| 2 | 6 | 20 | 1:50 | 19.1 | 2 | 60 | 2:45 | 7.0 | 3 | 60 | 1:25 | 10 | 0.193 |
| 2 | 2 | 20 | 1:20 | 8.5 | 6 | 60 | 2:35 | 12.3 | 1 | 20 | 1:10 | 10 | 0.111 |
| 2 | 6 | 40 | 1:20 | 6.6 | 4 | 40 | 2:45 | 8.0 | 1 | 40 | 1:25 | 30 | 0.186 |
| 2 | 4 | 40 | 1:50 | 20.0 | 4 | 60 | 2:25 | 10.5 | 2 | 40 | 1:40 | 10 | 0.220 |
| 2 | 2 | 40 | 1:35 | 13.5 | 2 | 20 | 2:25 | 6.9 | 2 | 60 | 1:10 | 30 | 0.100 |
| 2 | 4 | 20 | 1:35 | 6.1 | 4 | 20 | 2:35 | 8.5 | 3 | 40 | 1:10 | 50 | 0.133 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Alginate | |||||
| A: Time | 10.584 | 1 | 10.584 | 14.36 | 0.0053 |
| B: Temperature | 57.037 | 1 | 57.037 | 77.41 | 0.0000 |
| C: Algae/solvent ratio | 37.453 | 1 | 37.453 | 50.83 | 0.0001 |
| AA | 0.762 | 1 | 0.762 | 1.03 | 0.3390 |
| AB | 18.347 | 1 | 18.347 | 24.90 | 0.0011 |
| AC | 34.850 | 1 | 34.850 | 47.30 | 0.0001 |
| BB | 23.363 | 1 | 23.363 | 31.71 | 0.0005 |
| BC | 18.875 | 1 | 18.875 | 25.62 | 0.0010 |
| Blocks | 1.561 | 1 | 1.561 | 2.12 | 0.1837 |
| Total error | 5.894 | 8 | 0.737 | ||
| Total (corr.) | 474.363 | 17 | |||
| R2 = 98.78%; R2 adjusted for degrees of freedom (df) = 97.36%; Standard error of estimate = 0.8584; Mean absolute error = 0.4617. | |||||
| Mannitol | |||||
| A: Time | 9.362 | 1 | 9.362 | 10.22 | 0.0127 |
| B: Temperature | 1.700 | 1 | 1.700 | 1.86 | 0.2103 |
| C: Algae/solvent ratio | 0.301 | 1 | 0.301 | 0.33 | 0.5824 |
| AA | 0.006 | 1 | 0.006 | 0.01 | 0.9384 |
| AB | 0.004 | 1 | 0.004 | 0.00 | 0.9462 |
| AC | 0.053 | 1 | 0.053 | 0.06 | 0.8154 |
| BB | 0.053 | 1 | 0.053 | 0.06 | 0.8168 |
| BC | 0.092 | 1 | 0.092 | 0.10 | 0.7596 |
| Blocks | 0.720 | 1 | 0.720 | 0.79 | 0.4012 |
| Total error | 7.330 | 8 | 0.916 | ||
| Total (corr.) | 54.771 | 17 | |||
| R2 = 86.62%; R2 adjusted for degrees of freedom (df) = 71.56%; Standard error of estimate = 0.9572; Mean absolute error = 0.5778. | |||||
| Phlorotannin’s | |||||
| A: Time | 0.0040 | 1 | 0.0040 | 32.51 | 0.0005 |
| B: Temperature | 0.0001 | 1 | 0.0001 | 1.00 | 0.3462 |
| C: Algae/solvent ratio | 0.0187 | 1 | 0.0187 | 153.71 | 0.0000 |
| D: Ethanol proportion | 0.0028 | 1 | 0.0028 | 23.04 | 0.0014 |
| AA | 0.0005 | 1 | 0.0005 | 4.34 | 0.0707 |
| AB | 0.0003 | 1 | 0.0003 | 2.66 | 0.1415 |
| AC | 0.0008 | 1 | 0.0008 | 6.73 | 0.0319 |
| BB | 0.00009 | 1 | 0.00009 | 0.76 | 0.4100 |
| Blocks | 0.00008 | 1 | 0.00008 | 0.62 | 0.4522 |
| Total error | 0.00097 | 8 | 0.00012 | ||
| Total (corr.) | 0.0775 | 17 | |||
| R2 = 98.74%; R2 adjusted for degrees of freedom (df) = 97.33%; Standard error of estimate = 0.0110; Mean absolute error = 0.0062. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rincón-Cervera, M.A.; de Burgos-Navarro, I.; Chileh-Chelh, T.; Belarbi, E.-H.; Álvarez-Corral, M.; Carmona-Fernández, M.; Ezzaitouni, M.; Guil-Guerrero, J.L. The Agronomic Potential of the Invasive Brown Seaweed Rugulopteryx okamurae: Optimisation of Alginate, Mannitol, and Phlorotannin Extraction. Plants 2024, 13, 3539. https://doi.org/10.3390/plants13243539
Rincón-Cervera MA, de Burgos-Navarro I, Chileh-Chelh T, Belarbi E-H, Álvarez-Corral M, Carmona-Fernández M, Ezzaitouni M, Guil-Guerrero JL. The Agronomic Potential of the Invasive Brown Seaweed Rugulopteryx okamurae: Optimisation of Alginate, Mannitol, and Phlorotannin Extraction. Plants. 2024; 13(24):3539. https://doi.org/10.3390/plants13243539
Chicago/Turabian StyleRincón-Cervera, Miguel A., Irene de Burgos-Navarro, Tarik Chileh-Chelh, El-Hassan Belarbi, Miriam Álvarez-Corral, Minerva Carmona-Fernández, Mohamed Ezzaitouni, and José L. Guil-Guerrero. 2024. "The Agronomic Potential of the Invasive Brown Seaweed Rugulopteryx okamurae: Optimisation of Alginate, Mannitol, and Phlorotannin Extraction" Plants 13, no. 24: 3539. https://doi.org/10.3390/plants13243539
APA StyleRincón-Cervera, M. A., de Burgos-Navarro, I., Chileh-Chelh, T., Belarbi, E.-H., Álvarez-Corral, M., Carmona-Fernández, M., Ezzaitouni, M., & Guil-Guerrero, J. L. (2024). The Agronomic Potential of the Invasive Brown Seaweed Rugulopteryx okamurae: Optimisation of Alginate, Mannitol, and Phlorotannin Extraction. Plants, 13(24), 3539. https://doi.org/10.3390/plants13243539







