Multi Characteristic Analysis of Vascular Cambium Cells in Populus euphratica Reveals Its Anti-Aging Strategy
Abstract
1. Introduction
2. Results
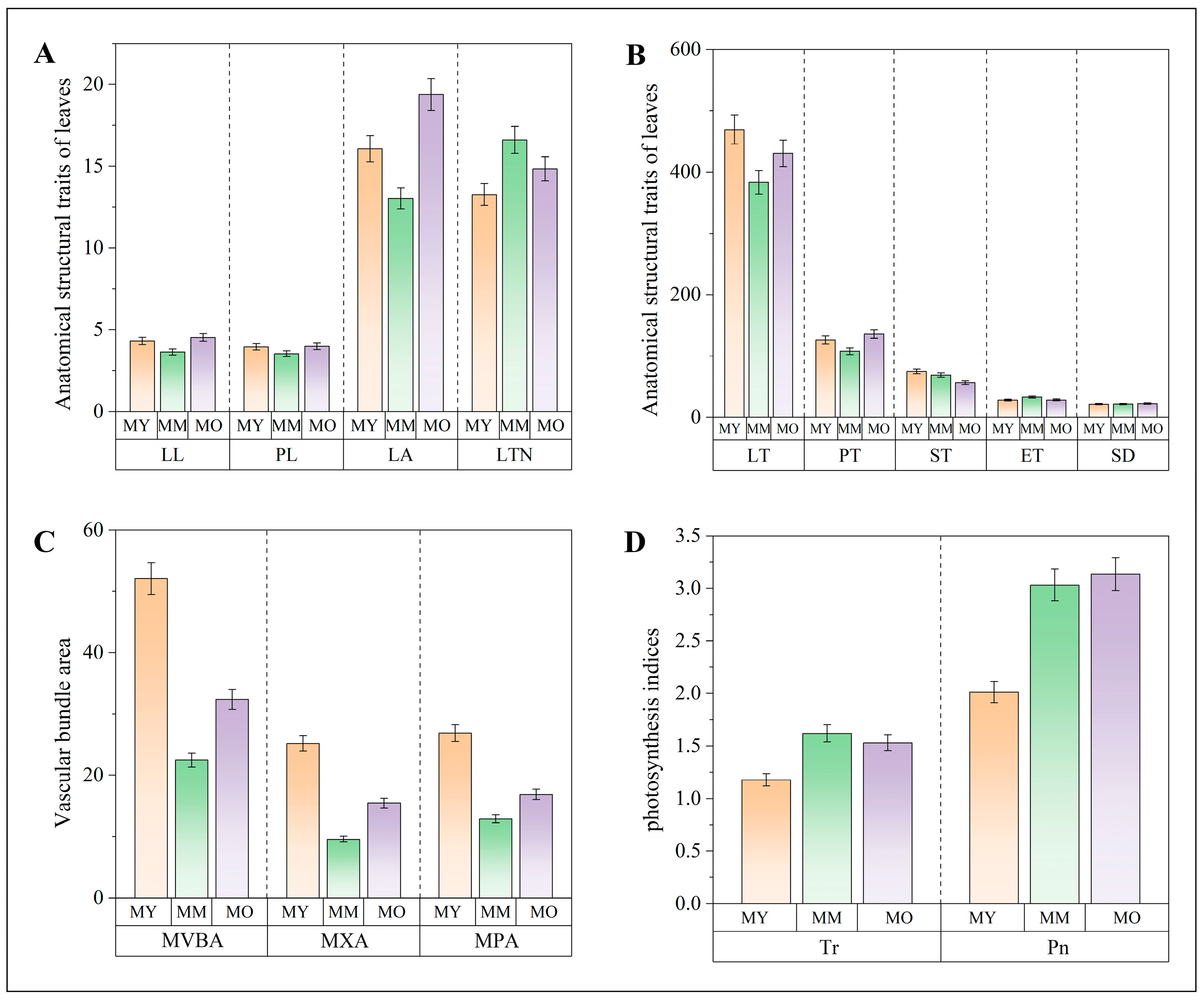
2.1. Evaluation of the Growth of Poplar at Different Ages
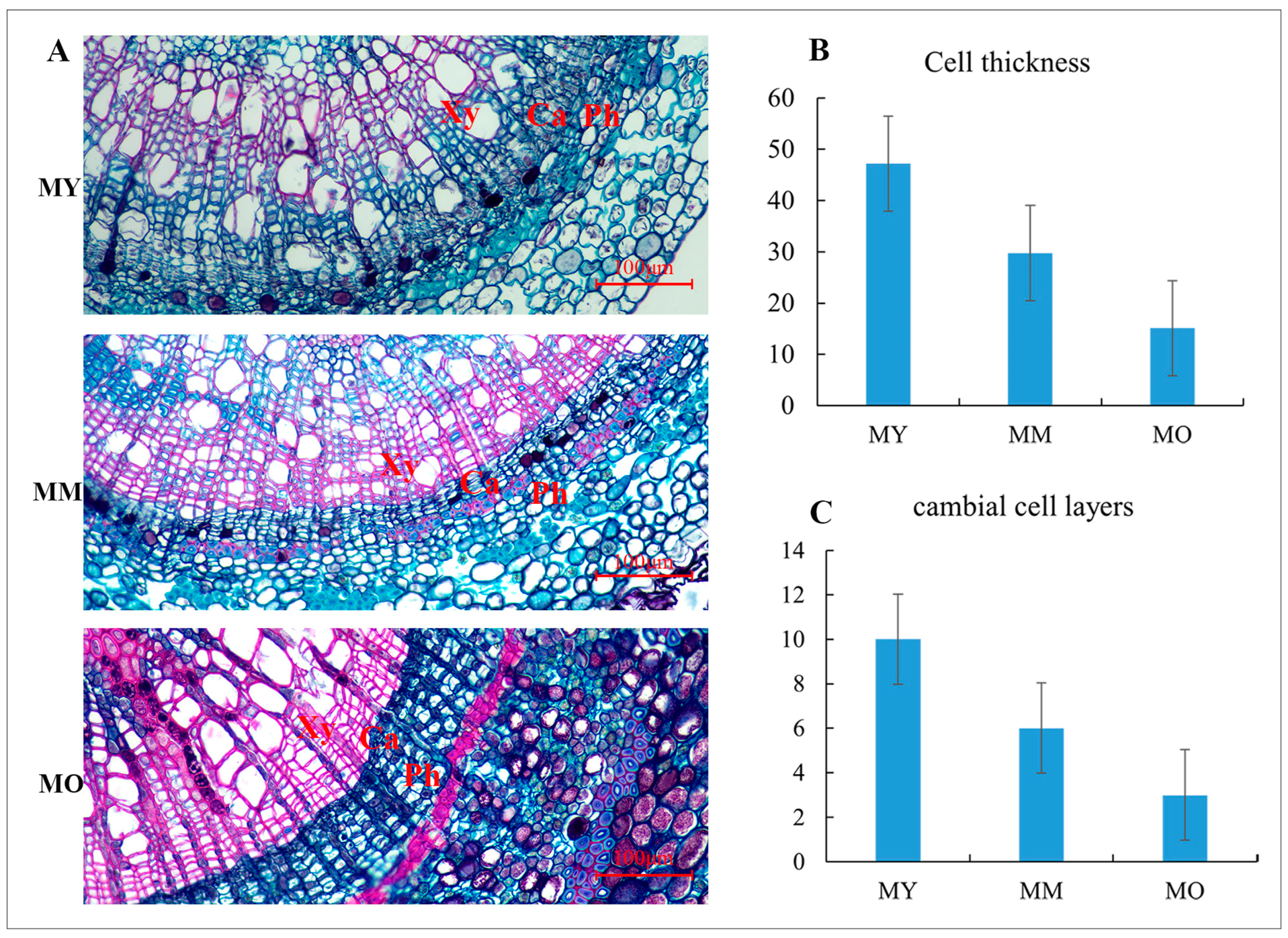
2.2. Anatomical Changes in Cambial Cells at Different Ages
2.3. Transcriptome Analysis of Different Tree Ages Vascular Cambium of P. euphratica
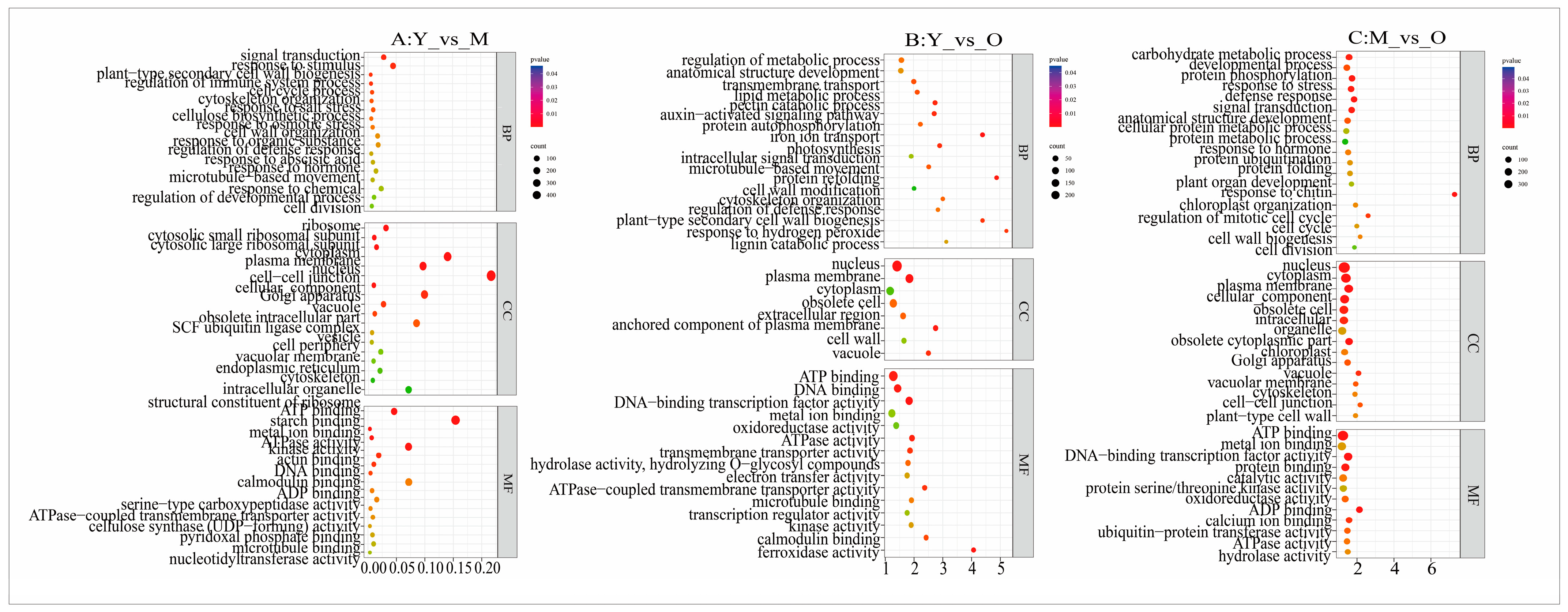
2.4. Functional Annotation
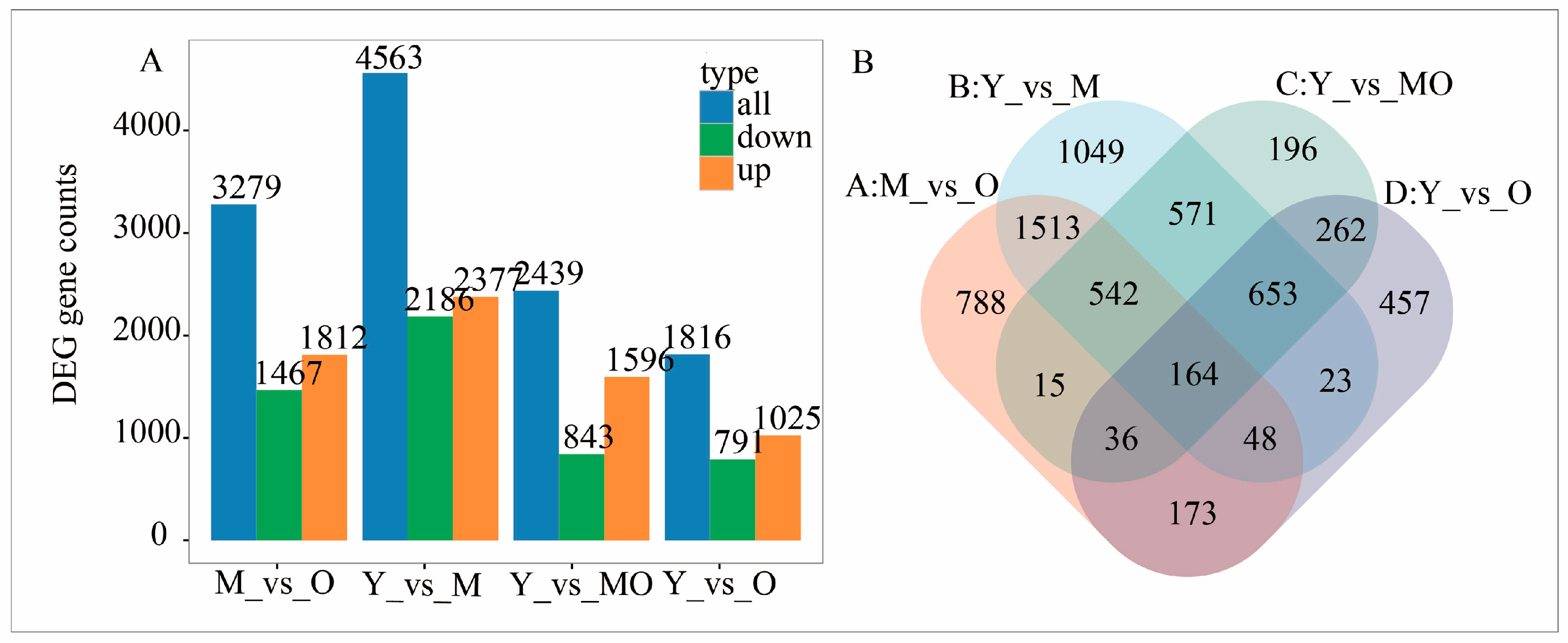
2.5. Differentially Expressed Genes in P. euphratica at Different Ages
2.6. Expression Patterns of DEGs Related to Cell Division and Differentiation
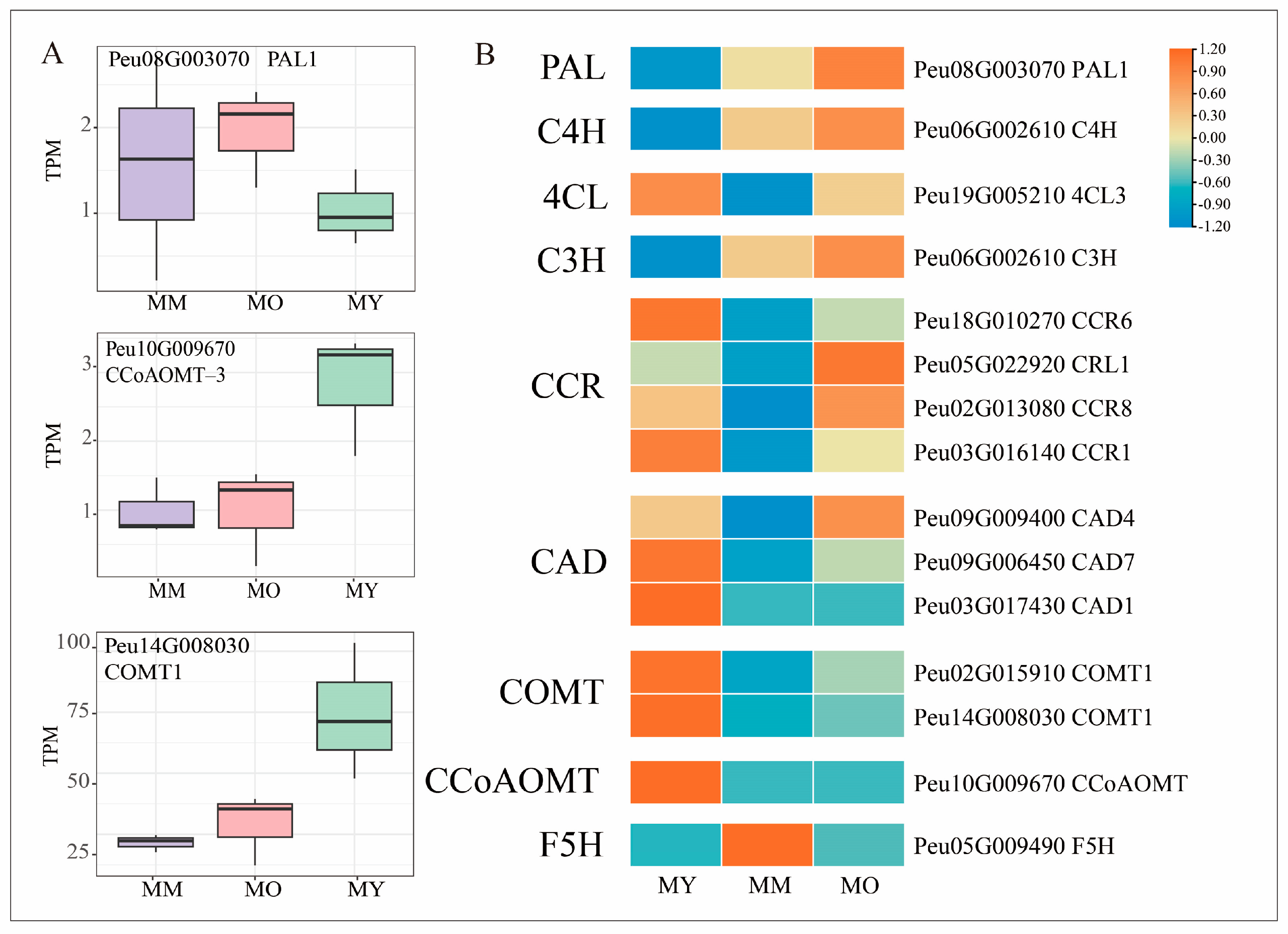
2.7. Expression Patterns of DEGs Related to Lignin_Biosynthesis
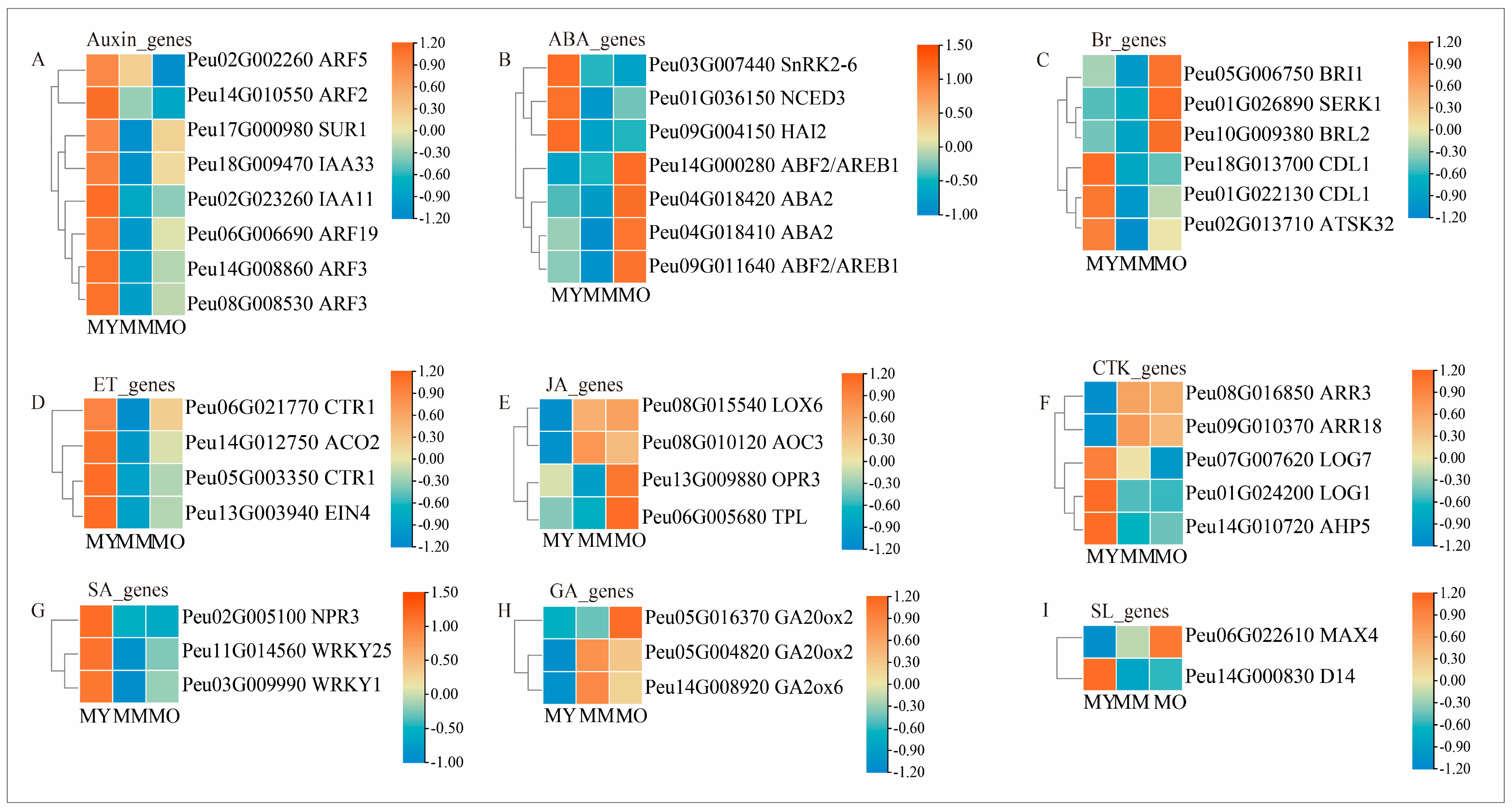
2.8. Expression Patterns of DEGs Related to Phytohormone
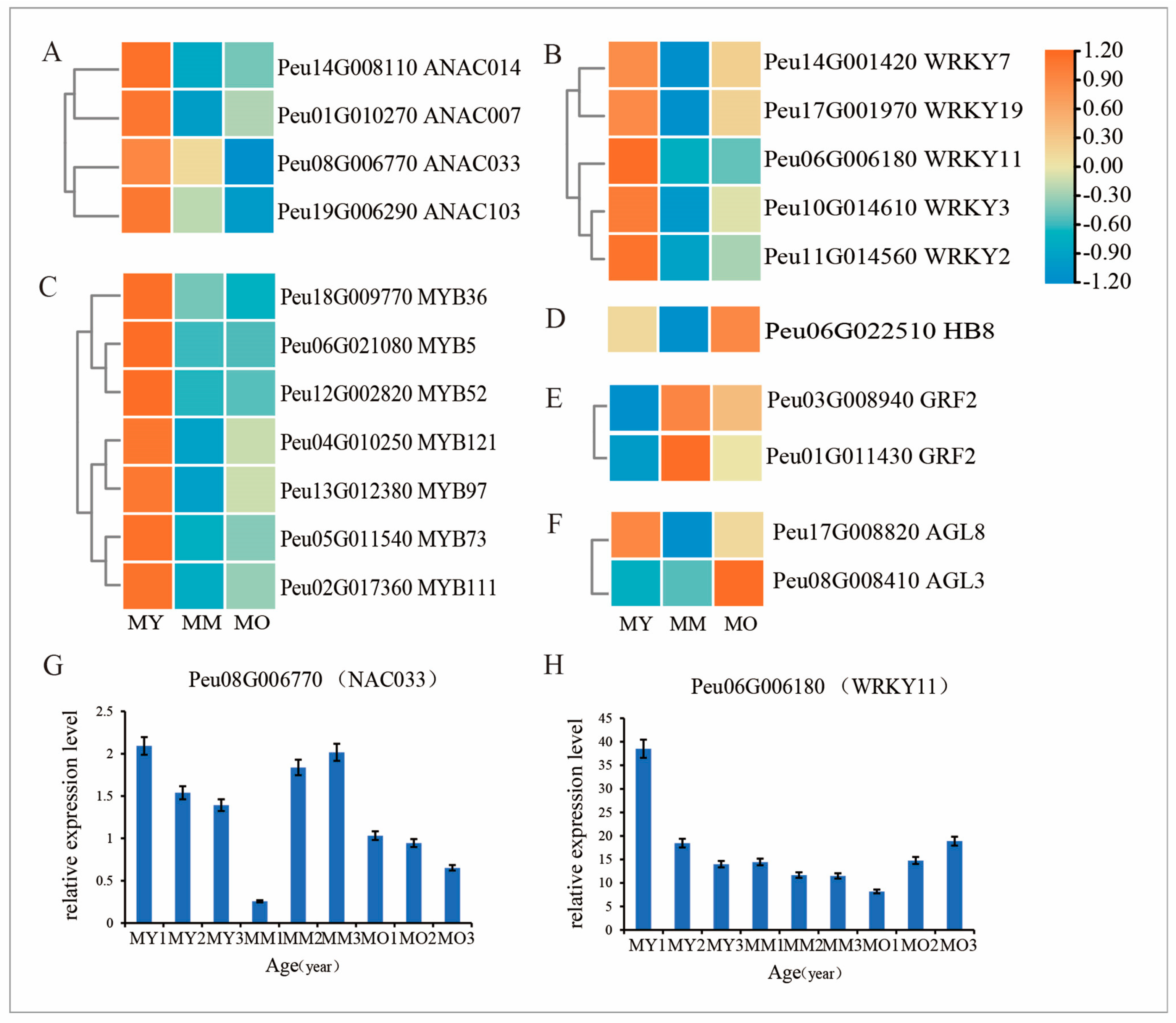
2.9. Expression Patterns of TF-Related DEGs
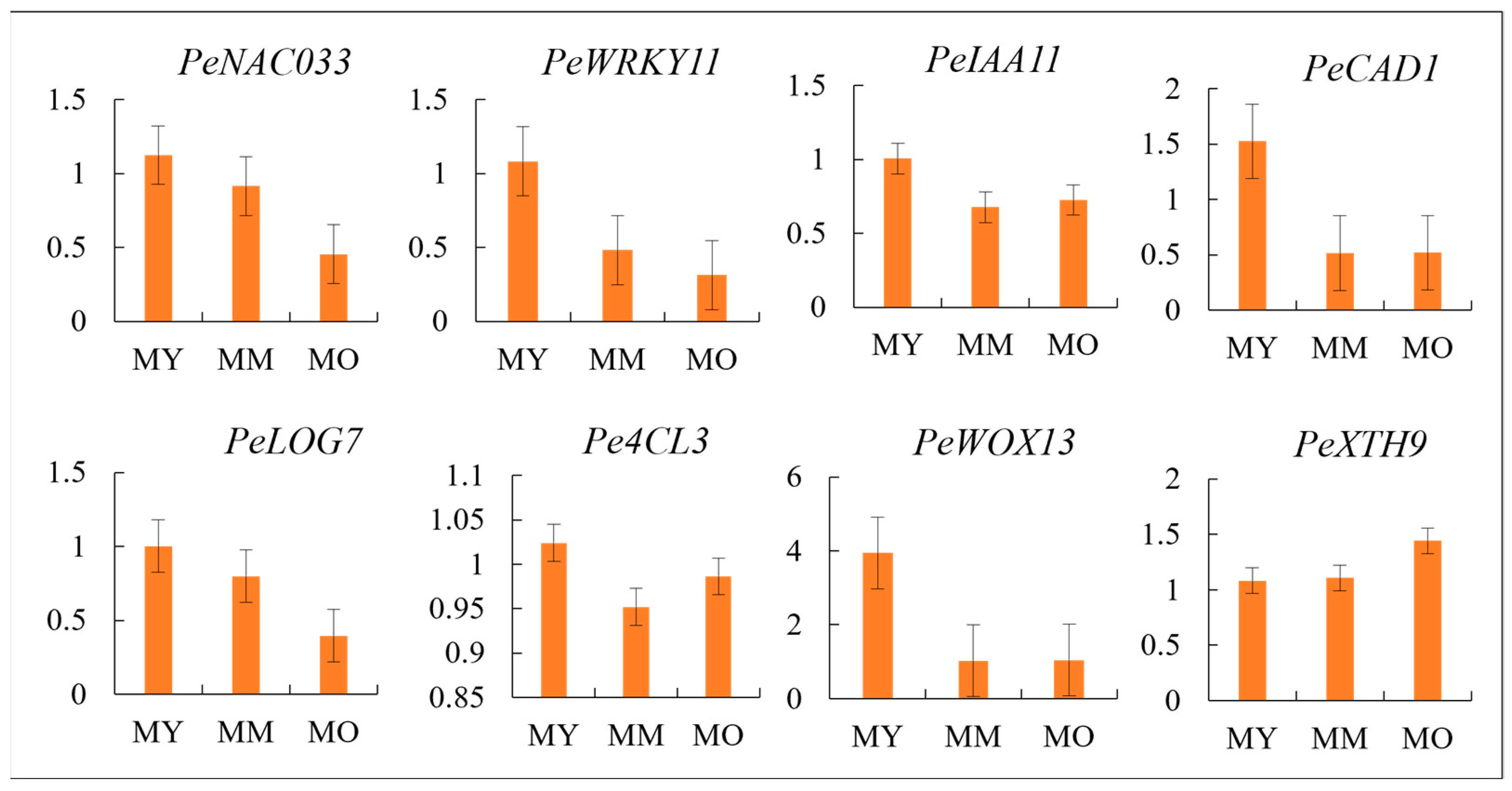
2.10. Validation of Candidate Differential Genes in Vascular Cambium
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. General Situation
5.2. Biological Material
5.3. Anatomical Observations of the Vascular Cambium
5.4. Morphological and Structural Analysis
5.5. Illumina RNA-Seq Library Construction and Sequencing
5.6. Bioinformatic Analysis of Illumina Data
5.7. Differential Expression Analysis
5.8. RT-qPCR Validation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhai, J.; Han, X.; Li, Z. Evaluation of Drought Resistance of Different Development Stages of Populus pruinosa Based on the Leaf Anatomical Structure. J. Tarim Univ. 2019, 31, 12–21. [Google Scholar]
- Munné-Bosch, S. Limits to Tree Growth and Longevity. Trends Plant Sci. 2018, 23, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ocaña, A.; Carmen García-López, M.; Jiménez-Ruiz, J.; Saniger, L.; Macías, D.; Navarro, F.; Oya, R.; Belaj, A.; de la Rosa, R.; Corpas, F.J.; et al. Identification of a gene involved in the juvenile-to-adult transition (JAT) in cultivated olive trees. Tree Genet. Genomes 2010, 6, 891–903. [Google Scholar] [CrossRef]
- Hudson, C.; Freeman, J.; Jones, R.; Potts, B.; Wong, M.; Weller, J.; Hecht, V.; Poethig, R.; Vaillancourt, R. Genetic Control of Heterochrony in Eucalyptus globulus. G3 Genes Genomes Genet. 2014, 4, 1235–1245. [Google Scholar] [CrossRef][Green Version]
- Wu, Z.; Jiang, Z.; Li, Z.; Jiao, P.; Zhai, J.; Liu, S.; Han, X.; Zhang, S.; Sun, J.; Gai, Z.; et al. Multi-omics analysis reveals spatiotemporal regulation and function of heteromorphic leaves in Populus. Plant Physiol. 2023, 192, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.D.; Zheng, W.J.; Chen, G.C.; Zhang, C.L.; Chen, J.; Wang, X.C. Differences in ultrastructure and photosynthetic characteristics between lanceolate and broadly ovate leaves of Populus euphratica. Acta Bot. Boreali-Occident. Sin. 2005, 25, 14–21. [Google Scholar]
- Hang, W.J.; Li, Z.J.; Yang, Z.P.; Liang, J.Y.; Bai, G.Z. Structural traits of Populus euphratica alien leaves and their relationship with breast height diameter. J. Ecol. 2010, 29, 2347–2352. [Google Scholar]
- Niinemets, Ü.; Kull, O. Effects of light availability and tree size on the architecture of assimilative surface in the canopy of Picea abies: Variation in shoot structure. Tree Physiol. 1995, 15, 791–798. [Google Scholar] [CrossRef][Green Version]
- Kang, J.-M.; Kojima, K.; Ide, Y.; Sasaki, S. Plantlet regeneration from leaf protoplasts ofPopulus euphratica. J. For. Res. 1996, 1, 99–102. [Google Scholar] [CrossRef]
- Meinzer, F.; Lachenbruch, B.; Dawson, T. Size- and Age-Related Changes in Tree Structure and Function; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Zang, D. Study on the Effects of Drought Stress on Photosynthesis and Chlorophyll Fluorescence of Apples and Leaf Aging Characteristics. Master’s Thesis, Northwest A&F University, Xianyang, China, 2011. [Google Scholar]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood Formation in Trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef]
- Xu, H.; Cao, D.; Feng, J.; Wu, H.; Lin, J.; Wang, Y. Transcriptional regulation of vascular cambium activity during the transition from juvenile to mature stages in Cunninghamia lanceolata. J. Plant Physiol. 2016, 200, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, J.; Jin, B.; Zhao, J.; Xu, H.; Lu, Z.; Li, W.; Li, X.; Li, L.; Liang, E.; et al. Multifeature analyses of vascular cambial cells reveal longevity mechanisms in old Ginkgo biloba trees. Proc. Natl. Acad. Sci. USA 2020, 117, 2201–2210. [Google Scholar] [CrossRef]
- Li, X.; Liang, E.; Gričar, J.; Prislan, P.; Rossi, S.; Čufar, K. Age dependence of xylogenesis and its climatic sensitivity in Smith fir on the south-eastern Tibetan Plateau. Tree Physiol. 2012, 33, 48–56. [Google Scholar] [CrossRef]
- Hardtke, C.S.; Berleth, T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998, 17, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto-Kitano, M.; Kusumoto, T.; Tarkowski, P.; Kinoshita-Tsujimura, K.; Václavíková, K.; Miyawaki, K.; Kakimoto, T. Cytokinins are central regulators of cambial activity. Proc. Natl. Acad. Sci. USA 2008, 105, 20027–20031. [Google Scholar] [CrossRef]
- Sehr, E.M.; Agusti, J.; Lehner, R.; Farmer, E.E.; Schwarz, M.; Greb, T. Analysis of secondary growth in the Arabidopsis shoot reveals a positive role of jasmonate signalling in cambium formation. Plant J. Cell Mol. Biol. 2010, 63, 811–822. [Google Scholar] [CrossRef]
- Wang, C.; Liu, N.; Geng, Z.; Ji, M.; Wang, S.; Zhuang, Y.; Wang, D.; He, G.; Zhao, S.; Zhou, G.; et al. Integrated transcriptome and proteome analysis reveals brassinosteroid-mediated regulation of cambium initiation and patterning in woody stem. Hortic. Res. 2022, 9, uhab048. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Kondo, Y.; Fukuda, H. TDIF Peptide Signaling Regulates Vascular Stem Cell Proliferation via the WOX4 Homeobox Gene in Arabidopsis. Plant Cell 2010, 22, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Baima, S.; Possenti, M.; Matteucci, A.; Wisman, E.; Altamura, M.M.; Ruberti, I.; Morelli, G. The arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol. 2001, 126, 643–655. [Google Scholar] [CrossRef]
- Ilegems, M.; Douet, V.; Meylan-Bettex, M.; Uyttewaal, M.; Brand, L.; Bowman, J.L.; Stieger, P.A. Interplay of auxin, KANADI and Class III HD-ZIP transcription factors in vascular tissue formation. Development 2010, 137, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Mitsuda, N.; Iwase, A.; Yamamoto, H.; Yoshida, M.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. NAC Transcription Factors, NST1 and NST3, Are Key Regulators of the Formation of Secondary Walls in Woody Tissues of Arabidopsis. Plant Cell 2007, 19, 270–280. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.L.; Zhong, R.; Ye, Z.H. MYB83 is a direct target of SND1 and acts redundantly with MYB46 in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell Physiol. 2009, 50, 1950–1964. [Google Scholar] [CrossRef]
- Zhou, J.; Lee, C.; Zhong, R.; Ye, Z.H. MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell 2009, 21, 248–266. [Google Scholar] [CrossRef]
- Rudolph, K.L. DNA-methylation aging at single-cell level. Nat. Aging 2021, 1, 1086–1087. [Google Scholar] [CrossRef]
- Watkinson, A. Plant senescence. Trends Ecol. Evol. 1992, 7, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Efroni, I.; Han, S.-K.; Kim, H.J.; Wu, M.-F.; Steiner, E.; Birnbaum, K.D.; Hong, J.C.; Eshed, Y.; Wagner, D. Regulation of Leaf Maturation by Chromatin-Mediated Modulation of Cytokinin Responses. Dev. Cell 2013, 24, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Fritz, A.K.; Paulsen, G.M.; Bai, G.; Pandravada, S.; Gill, B.S. Modeling and mapping QTL for senescence-related traits in winter wheat under high temperature. Mol. Breed. 2010, 26, 163–175. [Google Scholar] [CrossRef]
- Wang, B.S.; Wang, S.P. Research progress on the response of plant leaf traits to climate change. J. Plant Ecol. 2015, 39, 206–216. [Google Scholar]
- Wojciechowska, N.; Sobieszczuk-Nowicka, E.; Bagniewska-Zadworna, A. Plant organ senescence—Regulation by manifold pathways. Plant Biol. 2018, 20, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.W.; Li, C.W. Epigenetic regulation of plant leaf senescence. Acta Bot. Sin. 2014, 49, 729–737. [Google Scholar]
- Fanglan, L.; Weikai, B. Responses of the morphological and anatomical structure ofthe plant leaf to environmental change. Chin. Bull. Bot. 2005, 22, 118–127. [Google Scholar]
- Diao, J.; Zeng, X.; Zhong, F. A Comparative Study of Old Leaf Morphology and Structure of Different Ages of Aegiceras Corniculatum. J. Jiaying Univ. 2015, 33, 59–64. [Google Scholar]
- Zhao, P.; Feng, M.; Jiao, P.; Li, Z. Morphological and anatomical characteristics of leaves at different developmental stages of Populus euphratica and their relationship with breast diameter. Arid Zone Res. 2016, 33, 1071–1080. [Google Scholar]
- Zhao, L.; Liu, W.; Yang, X.; Gao, X.; Hang, Y. Drought resistant adaptation strategies of Populus euphratica alien leaves. Jiangsu Agric. Sci. 2021, 49, 130–135. [Google Scholar]
- Zang, Y.; Wang, D.-X.; Song, B.; Lv, D.; Tao, W. Leaf anatomical structure based drought resistance evaluation of 11 urban forest plants in Xining city. J. Northwest A F Univ. (Nat. Sci. Ed.) 2014, 42, 86–92+98. [Google Scholar]
- Zhang, P.; Mu, F.; Song, H.; Qu, Y.; Wang, K.; Fen, B. Anatomical Structure and Drought Resistance in Broomcorn Millet Leaf. Trans. Chin. Soc. Agric. Mach. 2013, 44, 119–126. [Google Scholar]
- Gonzalez-Paleo, L.; Ravetta, D.A. Relationship between photosynthetic rate, water use and leaf structure in desert annual and perennial forbs differing in their growth. Photosynthetica 2018, 56, 1177–1187. [Google Scholar] [CrossRef]
- Yu, W.; Gao, Y.; Pang, Y.; Wang, Z.; Fu, B. Response of leaf morphology and structure of Anemone shikokiana to heterogeneous habitats and altitude changes. Acta Ecol. Sin. 2019, 39, 4413–4420. [Google Scholar]
- Shi, K.; Yang, Q.; Guo, X. Comparison on Leaf Anatomical Structures Between Female and Male Plants of Eucommia ulmoides. J. Hebei Norm. Univ. Sci. Technol. 2017, 31, 24–27+80. [Google Scholar]
- Yang, M. Anatomical Structure of Vitex negundo L. var. heterophylla (Franch.) Rehd. Leaves and Its Ecology Adaptation. Hubei Agric. Sci. 2014, 53, 2356–2358+2361. [Google Scholar]
- Brodribb, T.J.; Jordan, G.J. Water supply and demand remain balanced during leaf acclimation of Nothofagus cunninghamii trees. New Phytol. 2011, 192, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Carinsmurphy, M.R.; Jordan, G.J.; Brodribb, T.J. Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant Cell Environ. 2012, 35, 1407–1418. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Chen, Y.; Wang, X.; Pen, S.; Chen, L.; Wang, R.; Ma, L.; Li, Z.; Li, M. Study on leaf physiological characteristics of ancient Camellia oleifera Abel. Jiangsu Agric. Sci. 2019, 47, 188–191. [Google Scholar]
- Su, P.; Zhang, L.; Du, M.; Bi, Y.; Zhao, A.; Liu, X. Photosynthetic character and water use efficiency of different leaf shapes of Populus euphratica and their response to CO2 enrichment. Acta Phytoecol. Sin. 2003, 27, 34–40. [Google Scholar]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 2007, 152, 1–12. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, J.; Zhao, J.; Xu, H.; Wang, L.; Jin, B. Cytological and miRNA expression changes during the vascular cambial transition from the dormant stage to the active stage in Ginkgo biloba L. Trees 2016, 30, 2177–2188. [Google Scholar] [CrossRef]
- Deslauriers, A.; Giovannelli, A.; Rossi, S.; Castro, G.; Fragnelli, G.; Traversi, L. Intra-annual cambial activity and carbon availability in stem of poplar. Tree Physiol. 2009, 29, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Etchells, J.P.; Mishra, L.S.; Kumar, M.; Campbell, L.; Turner, S.R. Wood Formation in Trees Is Increased by Manipulating PXY-Regulated Cell Division. Curr. Biol. CB 2015, 25, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Song, D.; Sun, J.; Wang, X.; Li, L. PtrHB7, a class III HD-Zip gene, plays a critical role in regulation of vascular cambium differentiation in Populus. Mol. Plant 2013, 6, 1331–1343. [Google Scholar] [CrossRef]
- Qiu, Z.; Wan, L.; Chen, T.; Wan, Y.; He, X.; Lu, S.; Wang, Y.; Lin, J. The regulation of cambial activity in Chinese fir (Cunninghamia lanceolata) involves extensive transcriptome remodeling. New Phytol. 2013, 199, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Bonawitz, N.D.; Kim, J.I.; Tobimatsu, Y.; Ciesielski, P.N.; Anderson, N.A.; Ximenes, E.; Maeda, J.; Ralph, J.; Donohoe, B.S.; Ladisch, M.; et al. Disruption of Mediator rescues the stunted growth of a lignin-deficient Arabidopsis mutant. Nature 2014, 509, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Mohnen, D. A review of xylan and lignin biosynthesis: Foundation for studying Arabidopsis irregular xylem mutants with pleiotropic phenotypes. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 212–241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Tang, S.; Zhang, H.; He, M.; Liu, J.; Zhi, H.; Sui, Y.; Liu, X.; Jia, G.; Zhao, Z.; et al. DROOPY LEAF1 controls leaf architecture by orchestrating early brassinosteroid signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 21766–21774. [Google Scholar] [CrossRef]
- Balmant, K.M.; Noble, J.D.; Alves, F.C.; Dervinis, C.; Conde, D.; Schmidt, H.W.; Vazquez, A.I.; Barbazuk, W.B.; Campos, G.L.; Resende, M.F.R., Jr.; et al. Xylem systems genetics analysis reveals a key regulator of lignin biosynthesis in Populus deltoides. Genome Res. 2020, 30, 1131–1143. [Google Scholar] [CrossRef]
- Hu, Q.; Xiao, S.; Guan, Q.; Tu, L.; Sheng, F.; Du, X.; Zhang, X. The laccase gene GhLac1 modulates fiber initiation and elongation by coordinating jasmonic acid and flavonoid metabolism. Crop J. 2020, 8, 522–533. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Yu, J.; Wang, Z.; Sun, Y.; Li, S.; Lin, Y.J.; Chiang, V.L.; Li, W.; Wang, J.P. Transcriptional reprogramming of xylem cell wall biosynthesis in tension wood. Plant Physiol. 2021, 186, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Vaahtera, L.; Schulz, J.; Hamann, T. Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants 2019, 5, 924–932. [Google Scholar] [CrossRef]
- Qi, T.; Wang, J.; Huang, H.; Liu, B.; Gao, H.; Liu, Y.; Song, S.; Xie, D. Regulation of Jasmonate-Induced Leaf Senescence by Antagonism between bHLH Subgroup IIIe and IIId Factors in Arabidopsis. Plant Cell 2015, 27, 1634–1649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nieminen, K.; Serra, J.A.; Helariutta, Y. The formation of wood and its control. Curr Opin Plant Biol 2014, 17, 56–63. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Gampala, S.S.; Rock, C.D. Abscisic acid signaling in seeds and seedlings. Plant Cell 2002, 14 (Suppl. S1), S15–S45. [Google Scholar] [CrossRef]
- Li, W.F.; Ding, Q.; Chen, J.J.; Cui, K.M.; He, X.Q. Induction of PtoCDKB and PtoCYCB transcription by temperature during cambium reactivation in Populus tomentosa Carr. J. Exp. Bot. 2009, 60, 2621–2630. [Google Scholar] [CrossRef] [PubMed]
- Busov, V.B.; Johannes, E.; Whetten, R.W.; Sederoff, R.R.; Spiker, S.L.; Lanz-Garcia, C.; Goldfarb, B. An auxin-inducible gene from loblolly pine (Pinus taeda L.) is differentially expressed in mature and juvenile-phase shoots and encodes a putative transmembrane protein. Planta 2004, 218, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, H.X.; Dillon, S.K.; Southerton, S.G. Generation and analysis of expressed sequence tags from six developing xylem libraries in Pinus radiata D. Don. BMC Genom. 2009, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Gai, Z.; Sun, J.; Zhai, J.; Qiu, C.; Wu, Z.; Li, Z. Multi Characteristic Analysis of Vascular Cambium Cells in Populus euphratica Reveals Its Anti-Aging Strategy. Plants 2024, 13, 3549. https://doi.org/10.3390/plants13243549
Han X, Gai Z, Sun J, Zhai J, Qiu C, Wu Z, Li Z. Multi Characteristic Analysis of Vascular Cambium Cells in Populus euphratica Reveals Its Anti-Aging Strategy. Plants. 2024; 13(24):3549. https://doi.org/10.3390/plants13243549
Chicago/Turabian StyleHan, Xiaoli, Zhongshuai Gai, Jianhao Sun, Juntuan Zhai, Chen Qiu, Zhihua Wu, and Zhijun Li. 2024. "Multi Characteristic Analysis of Vascular Cambium Cells in Populus euphratica Reveals Its Anti-Aging Strategy" Plants 13, no. 24: 3549. https://doi.org/10.3390/plants13243549
APA StyleHan, X., Gai, Z., Sun, J., Zhai, J., Qiu, C., Wu, Z., & Li, Z. (2024). Multi Characteristic Analysis of Vascular Cambium Cells in Populus euphratica Reveals Its Anti-Aging Strategy. Plants, 13(24), 3549. https://doi.org/10.3390/plants13243549





