Xylem Hydraulics of Two Temperate Tree Species with Contrasting Growth Rates
Abstract
1. Introduction
2. Results
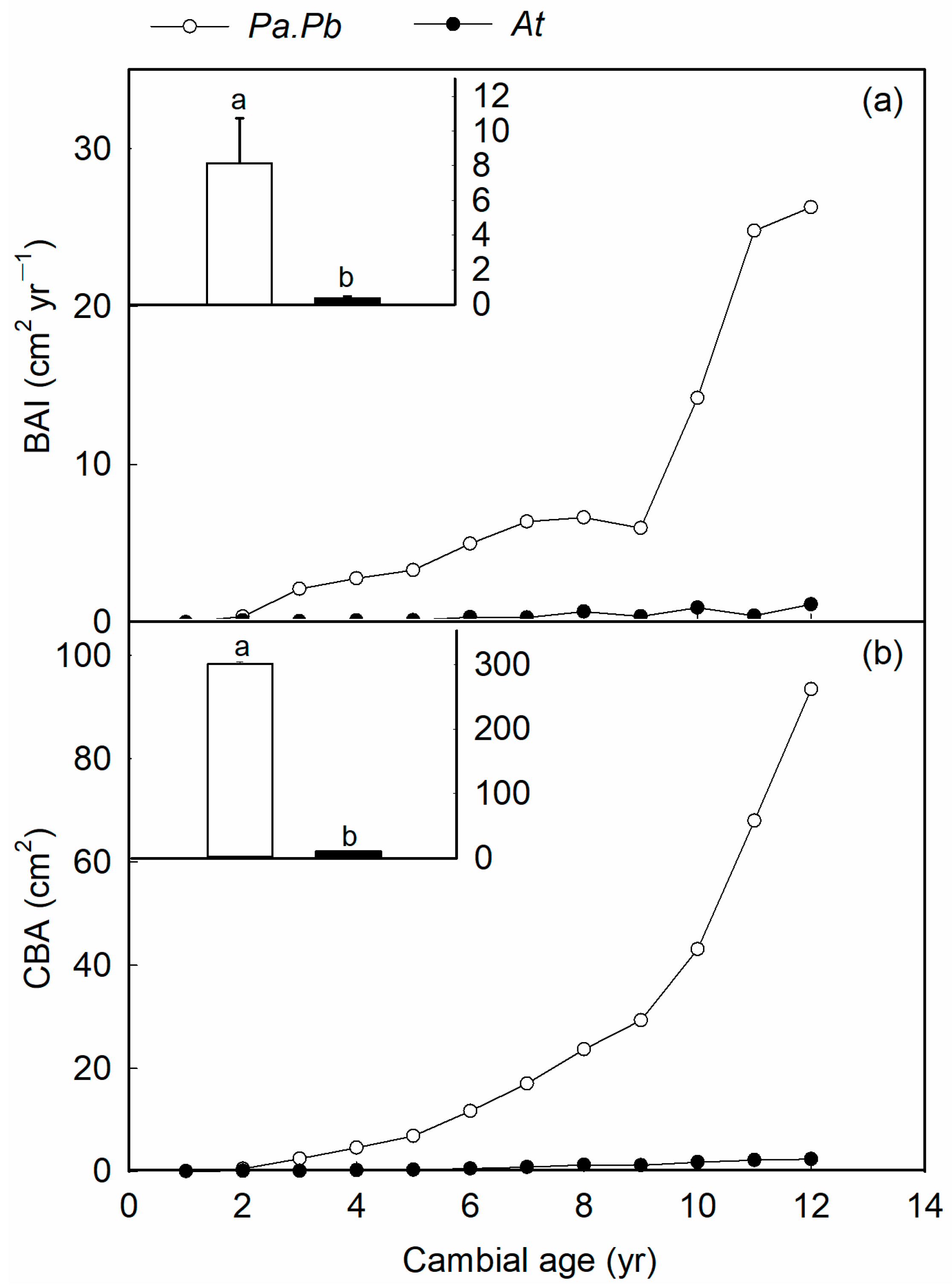
2.1. Radial Growth Rate
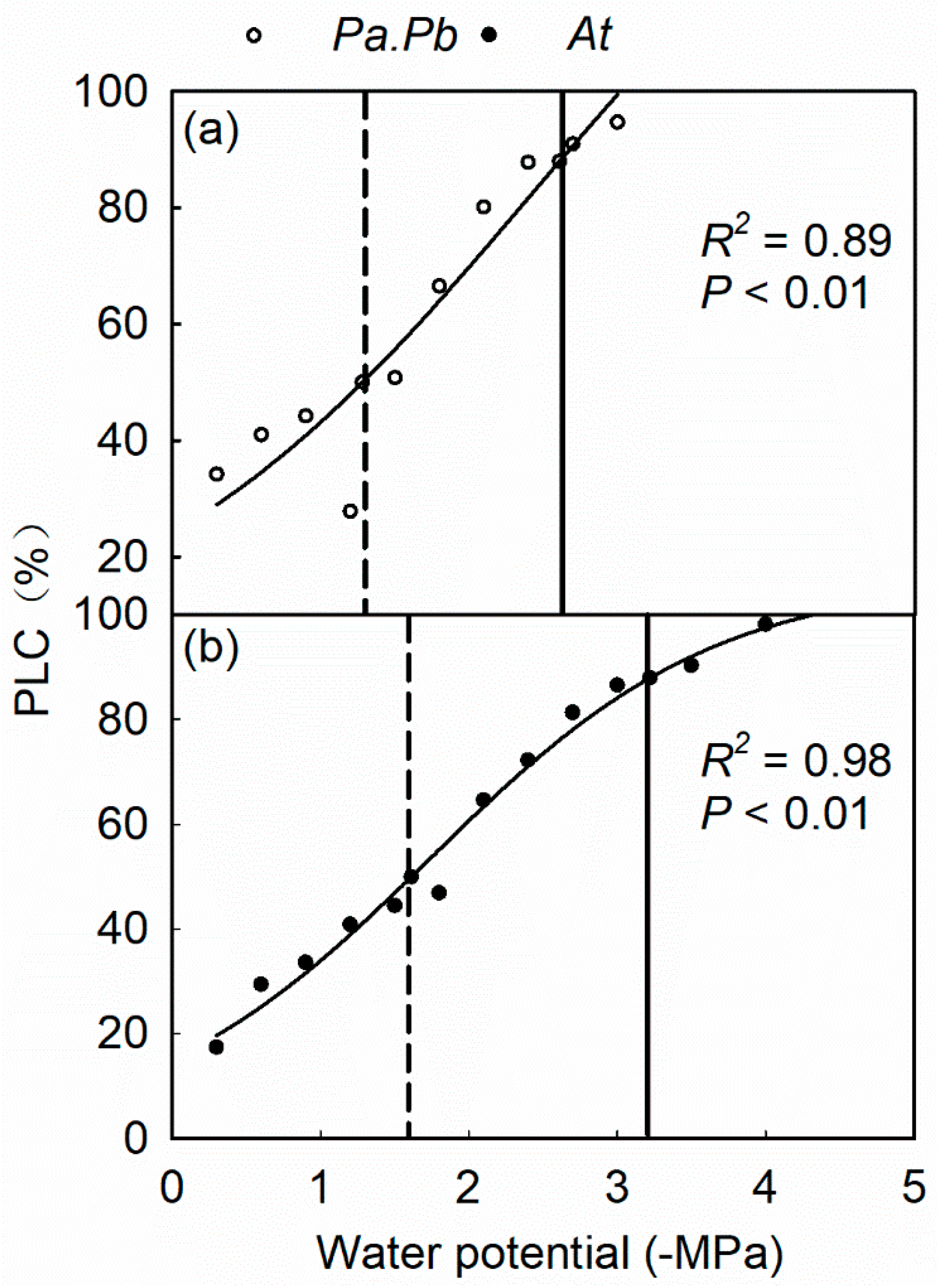
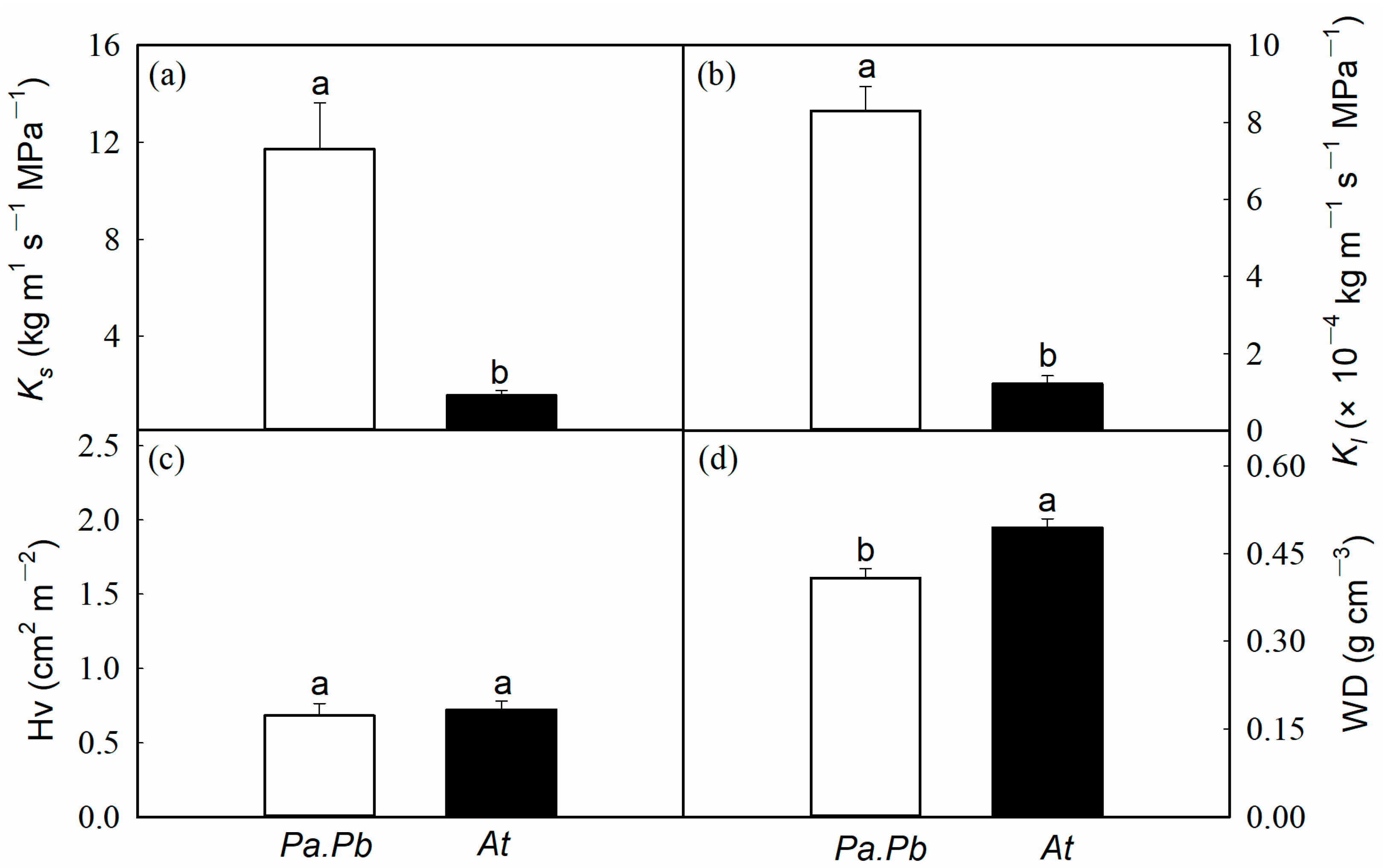
2.2. Efficiency and Safety of Water Transport in Xylem
2.3. Characteristics of Xylem Structure
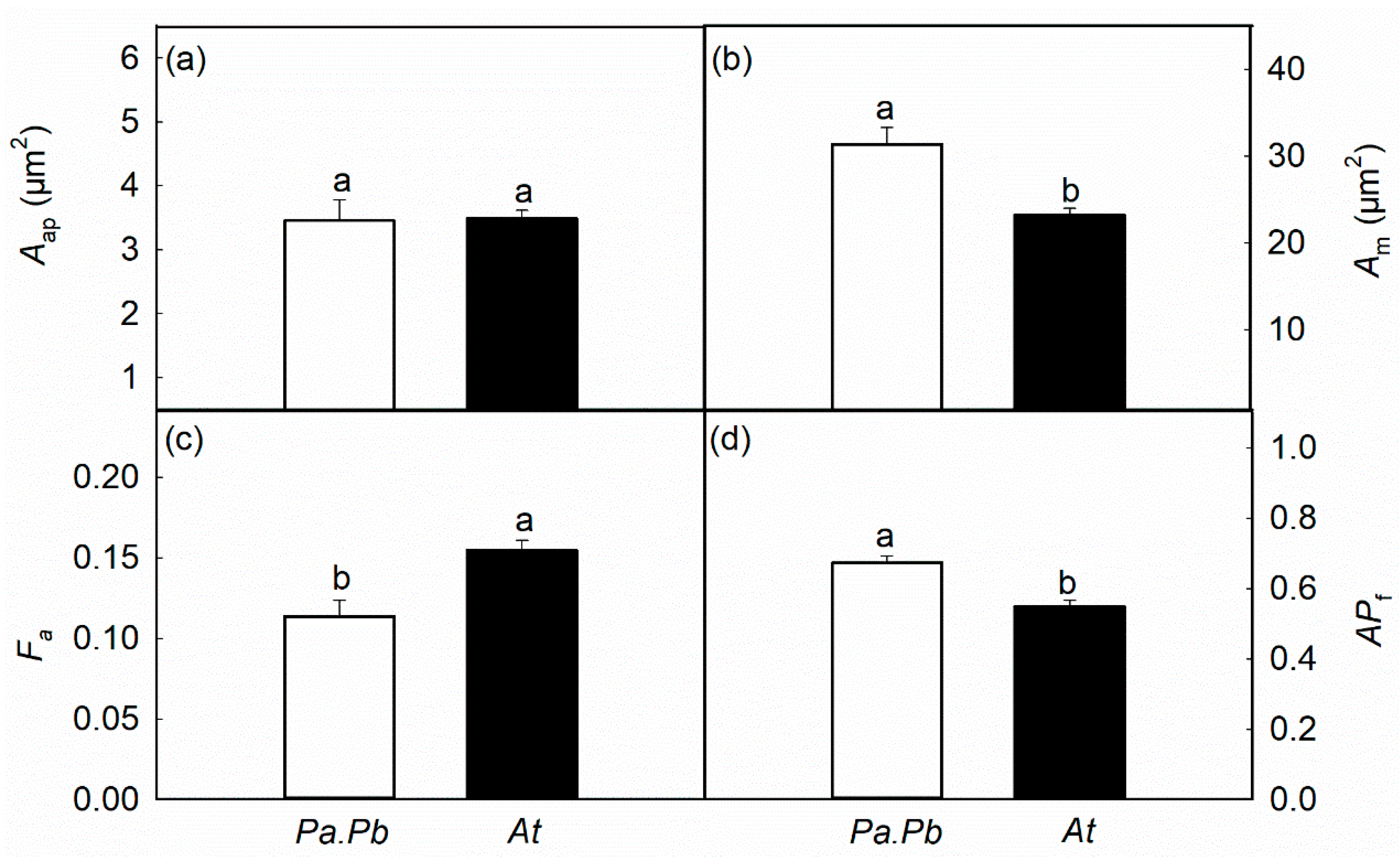
2.4. Leaf Functional Traits
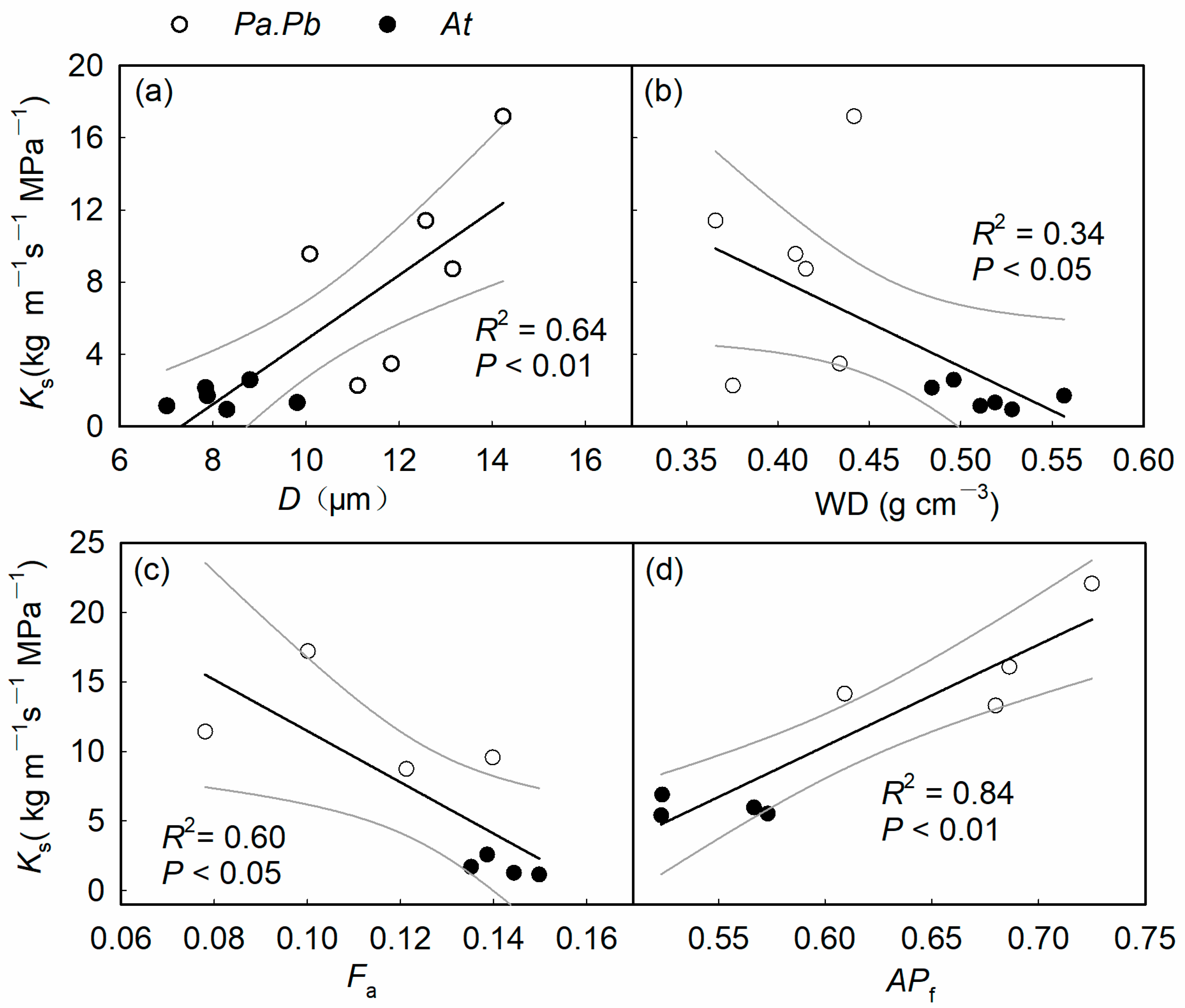
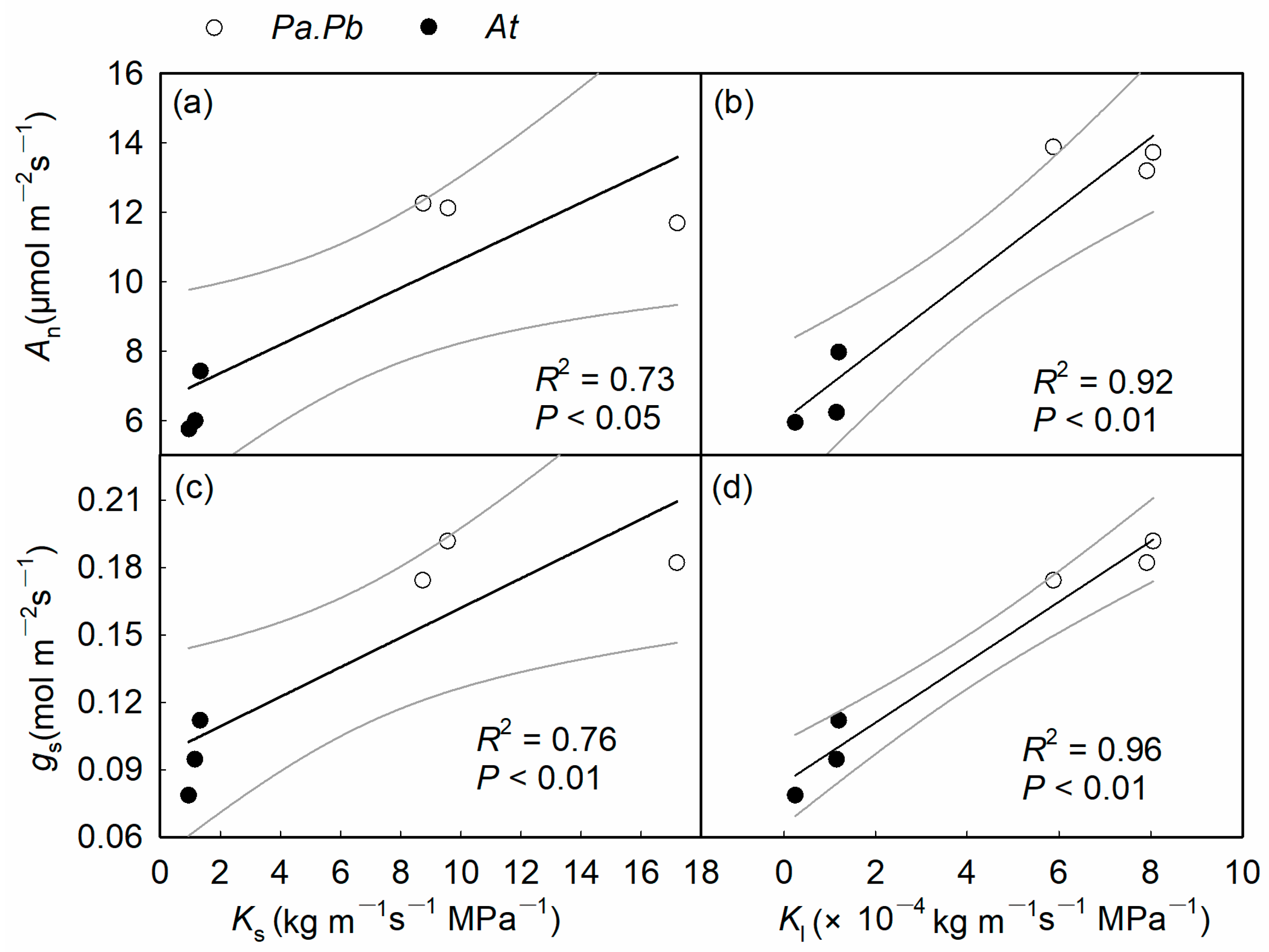
2.5. Relationship Between Functional Traits
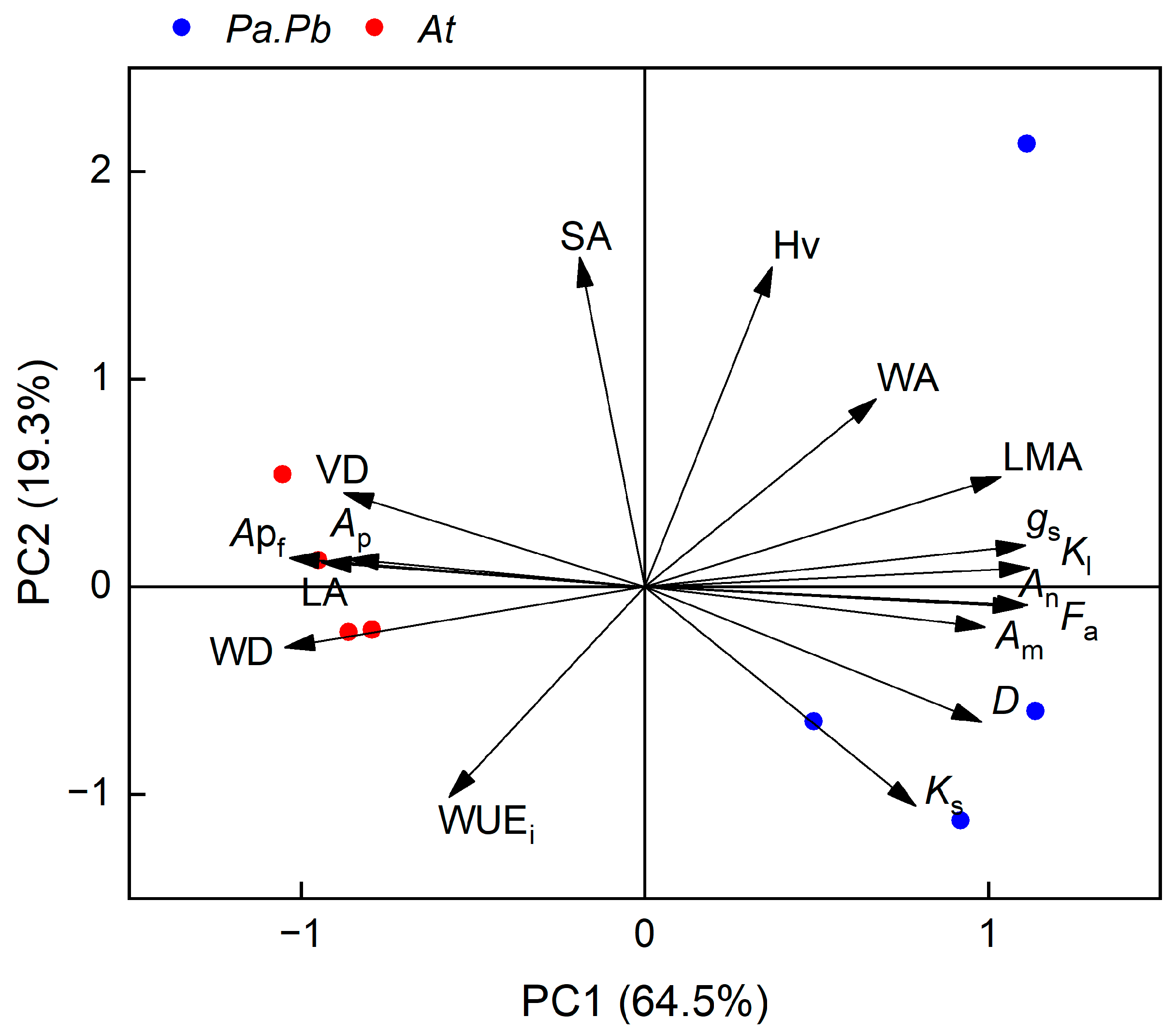
2.6. Results of the Principal Component Analysis
3. Discussion
3.1. Coordination Between Xylem Water Transport and Carbon Assimilation
3.2. The Trade-Off Between Hydraulic Efficiency and Safety
3.3. The Fast Versus Slow Growth Strategies
4. Materials and Methods
4.1. Study Site and Plant Materials
4.2. Tree-Ring Width and Basal Area Increment
4.3. Stem Hydraulic Traits
4.4. Stem Vulnerability Curves and Hydraulic Safety Margin
4.5. Xylem Anatomical Traits
4.6. Pit Anatomical Traits
4.7. Leaf Photosynthetic Gas Exchange and Leaf Anatomy
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| LA | LMA | WD | SA | WA | Kl | Ks | Hv | An | gs | Tr | WUEi | Aap | Am | Fa | Apf | D | VD | P50 | P88 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LA | ||||||||||||||||||||
| LMA | −0.78 ** | |||||||||||||||||||
| WD | 0.61 * | −0.75 ** | ||||||||||||||||||
| SA | 0.12 | 0.12 | 0.003 | |||||||||||||||||
| WA | −0.38 | 0.64 * | −0.63 * | 0.58 * | ||||||||||||||||
| Kl | −0.64 * | 0.85 ** | −0.83 ** | 0.02 | 0.70 ** | |||||||||||||||
| Ks | −0.45 | 0.50 | −0.57 * | −0.62* | 0.19 | 0.64 * | ||||||||||||||
| Hv | −0.39 | 0.59 * | −0.45 | 0.78** | 0.79 ** | 0.56 * | −0.20 | |||||||||||||
| An | −0.70 ** | 0.81 ** | −0.76 ** | 0.04 | 0.74 ** | 0.85 ** | 0.59 * | 0.45 | ||||||||||||
| gs | −0.80 ** | 0.82 ** | −0.86 ** | −0.21 | 0.56 * | 0.92 ** | 0.77 ** | 0.27 | 0.90 ** | |||||||||||
| Tr | −0.33 | 0.46 | −0.54 | 0.16 | 0.67 * | 0.58 * | 0.20 | 0.38 | 0.72 ** | 0.67 * | ||||||||||
| WUEi | 0.53 | −0.46 | 0.59 * | 0.39 | −0.09 | −0.58 * | −0.63 * | 0.05 | −0.28 | −0.65 * | −0.17 | |||||||||
| Aap | 0.26 | −0.05 | 0.20 | 0.36 | 0.20 | 0.06 | −0.36 | 0.29 | 0.16 | 0.03 | 0.50 | 0.14 | ||||||||
| Am | −0.45 | 0.67* | −0.77 ** | 0.03 | 0.65 * | 0.81 ** | 0.51 | 0.44 | 0.81 ** | 0.84 ** | 0.74 ** | −0.53 | 0.08 | |||||||
| Fa | −0.15 | 0.86 ** | −0.65 * | −0.12 | 0.50 | 0.70 ** | 0.65 * | 0.26 | 0.80 ** | 0.86 ** | 0.43 | −0.56 * | −0.14 | 0.70 ** | ||||||
| Apf | 0.57 * | −0.71 ** | 0.74 ** | 0.18 | −0.47 | −0.69 ** | −0.68 ** | −0.23 | −0.64 * | −0.76 ** | −0.36 | 0.61 * | 0.52 | −0.77 ** | −0.70 ** | |||||
| D | −0.73 ** | 0.68 * | −0.72 ** | −0.32 | 0.52 | 0.78 ** | 0.80 ** | 0.14 | 0.88 ** | 0.88 ** | 0.58 | −0.39 | −0.04 | 0.73 ** | 0.88 ** | −0.72 ** | ||||
| VD | 0.64 * | −0.59 * | 0.74 ** | 0.19 | −0.58 * | −0.72 ** | −0.65 * | −0.21 | −0.82 ** | −0.77 ** | −0.55 | 0.21 | 0.05 | −0.60 * | −0.76 ** | 0.59 * | −0.93 ** | |||
| P50 | 0.27 | −0.22 | −0.05 | 0.39 | 0.16 | −0.09 | −0.20 | 0.11 | −0.16 | −0.13 | 0.01 | −0.06 | 0.52 | 0.06 | −0.22 | 0.30 | −0.34 | 0.35 | ||
| P88 | 0.89 ** | −0.74 * | 0.64 | 0.35 | −0.25 | −0.81 ** | −0.79 * | −0.20 | −0.65 | −0.86 ** | 0.08 | 0.64 | 0.57 | −0.64 | −0.84 ** | 0.81 ** | −0.81 ** | 0.63 | 0.42 |

References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Hartmann, H.; Bastos, A.; Das, A.J.; Esquivel-Muelbert, A.; Hammond, W.M.; Martínez-Vilalta, J.; McDowell, N.G.; Powers, J.S.; Pugh, T.A.M.; Ruthrof, K.X.; et al. Climate change risks to global forest health: Emergence of unexpected events of elevated tree mortality worldwide. Annu. Rev. Plant Biol. 2022, 73, 673–702. [Google Scholar] [CrossRef] [PubMed]
- Cabon, A.; DeRose, R.J.; Shaw, J.D.; Anderegg, W.R.L. Declining tree growth resilience mediates subsequent forest mortality in the US Mountain West. Glob. Chang. Biol. 2023, 29, 4826–4841. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Vicente-Serrano, S.M.; Sánchez-Salguero, R.; Gutiérrez, E.; de Luis, M.; Sangüesa-Barreda, G.; Novak, K.; Rozas, V.; Tíscar, P.A.; et al. Forest resilience to drought varies across biomes. Glob. Chang. Biol. 2018, 24, 2143–2158. [Google Scholar] [CrossRef]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Benisiewicz, B.; Pawełczyk, S.; Niccoli, F.; Kabala, J.P.; Battipaglia, G. Drought impact on eco-physiological responses and growth performance of healthy and declining Pinus sylvestris L. trees growing in a dry area of southern Poland. Forests 2024, 15, 741. [Google Scholar] [CrossRef]
- Wittemann, M.; Mujawamariya, M.; Ntirugulirwa, B.; Uwizeye, F.K.; Zibera, E.; Manzi, O.J.L.; Nsabimana, D.; Wallin, G.; Uddling, J. Plasticity and implications of water-use traits in contrasting tropical tree species under climate change. Physiol. Plant. 2024, 176, e14326. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.X.; Zhang, S.B.; Hao, G.Y.; Ferry Slik, J.W.; Cao, K.F. Hydraulic conductivity traits predict growth rates and adult stature of 40 Asian tropical tree species better than wood density. J. Ecol. 2012, 100, 732–741. [Google Scholar] [CrossRef]
- Hoeber, S.; Leuschner, C.; Köhler, L.; Arias-Aguilar, D.; Schuldt, B. The importance of hydraulic conductivity and wood density to growth performance in eight tree species from a tropical semi-dry climate. For. Ecol. Manag. 2014, 330, 126–136. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Hacke, U.G. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol. 2006, 26, 689–701. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Feild, T.S. Stem hydraulic supply is linked to leaf photosynthetic capacity: Evidence from New Caledonian and Tasmanian rainforests. Plant Cell Environ. 2000, 23, 1381–1388. [Google Scholar] [CrossRef]
- Feild, T.S.; Balun, L. Xylem hydraulic and photosynthetic function of Gnetum (Gnetales) species from Papua New Guinea. New Phytol. 2007, 177, 665–675. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M.; Gutiérrez, M.V. Hydraulic and photosynthetic co-ordination in seasonally dry tropical forest trees. Plant Cell Environ. 2002, 25, 1435–1444. [Google Scholar] [CrossRef]
- Guan, X.Y.; Wen, Y.; Zhang, Y.; Chen, Z.; Cao, K.F.; Martinez-Vilalta, J. Stem hydraulic conductivity and embolism resistance of Quercus species are associated with their climatic niche. Tree Physiol. 2023, 43, 234–247. [Google Scholar] [CrossRef]
- Zhang, C.; Khan, A.; Duan, C.Y.; Cao, Y.; Wu, D.D.; Hao, G.Y. Xylem hydraulics strongly influence the niche differentiation of tree species along the slope of a river valley in a water-limited area. Plant Cell Environ. 2022, 46, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Levionnois, S.; Jansen, S.; Wandji, R.T.; Beauchêne, J.; Ziegler, C.; Coste, S.; Stahl, C.; Delzon, S.; Authier, L.; Heuret, P. Linking drought-induced xylem embolism resistance to wood anatomical traits in Neotropical trees. New Phytol. 2020, 229, 1453–1466. [Google Scholar] [CrossRef]
- Wheeler, J.K.; Sperry, J.S.; Hacke, U.G.; Hoang, N. Inter-vessel pitting and cavitation in woody Rosaceae and other vesselled plants: A basis for a safety versus efficiency trade-off in xylem transport. Plant Cell Environ. 2005, 28, 800–812. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S. Functional and ecological xylem anatomy. Perspect. Plant Ecol. 2001, 4, 97–115. [Google Scholar] [CrossRef]
- Kaack, L.; Weber, M.; Isasa, E.; Karimi, Z.; Li, S.; Pereira, L.; Trabi, C.L.; Zhang, Y.; Schenk, H.J.; Schuldt, B.; et al. Pore constrictions in intervessel pit membranes provide a mechanistic explanation for xylem embolism resistance in angiosperms. New Phytol. 2021, 230, 1829–1843. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Choat, B.; Pletsers, A. Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am. J. Bot. 2009, 96, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lens, F.; Espino, S.; Karimi, Z.; Klepsch, M.; Schenk, H.J.; Schmitt, M.; Schuldt, B.; Jansen, S. Intervessel pit membrane thickness as a key determinant of embolism resistance in angiosperm xylem. IAWA J. 2016, 37, 152–171. [Google Scholar] [CrossRef]
- Levionnois, S.; Kaack, L.; Heuret, P.; Abel, N.; Ziegler, C.; Coste, S.; Stahl, C.; Jansen, S. Pit characters determine drought-induced embolism resistance of leaf xylem across 18 Neotropical tree species. Plant Physiol. 2022, 190, 371–386. [Google Scholar] [CrossRef]
- Liu, H.; Ye, Q.; Gleason, S.M.; He, P.; Yin, D. Weak tradeoff between xylem hydraulic efficiency and safety: Climatic seasonality matters. New Phytol. 2020, 229, 1440–1452. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, R.; Song, L.; Yan, T.; Na, E. Comparison of C:N:P stoichiometry in the plant-litter-soil system between poplar and elm plantations in the Horqin Sandy Land, China. Front. Plant Sci. 2021, 12, 655517. [Google Scholar] [CrossRef]
- Fang, L.D.; Ning, Q.R.; Guo, J.J.; Gong, X.W.; Zhu, J.J.; Hao, G.Y. Hydraulic limitation underlies the dieback of Populus pseudo-simonii trees in water-limited areas of northern China. For. Ecol. Manag. 2021, 483, 118764. [Google Scholar] [CrossRef]
- Mo, Z.H. Study on Response Characteristics of Part of Maple Species from Other Places on the Drought Stress. Master’s Thesis, Shandong Agricultural University, Taian, China, 2009. [Google Scholar]
- Gleason, S.M.; Westoby, M.; Jansen, S.; Choat, B.; Hacke, U.G.; Pratt, R.B.; Bhaskar, R.; Brodribb, T.J.; Bucci, S.J.; Cao, K.F.; et al. Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world’s woody plant species. New Phytol. 2015, 209, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Cornelissen, H. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Zhu, S.D.; Song, J.J.; Li, R.H.; Ye, Q. Plant hydraulics and photosynthesis of 34 woody species from different successional stages of subtropical forests. Plant Cell Environ. 2012, 36, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.E.; Ehleringer, J.R. Gender-specific physiology, carbon isotope discrimination, and habitat distribution in boxelder, Acer negundo. Ecology 1993, 74, 798–815. [Google Scholar] [CrossRef]
- Choat, B.; Cobb, A.R.; Jansen, S. Structure and function of bordered pits: New discoveries and impacts on whole-plant hydraulic function. New Phytol. 2007, 177, 608–626. [Google Scholar] [CrossRef] [PubMed]
- Hacke, U.G.; Spicer, R.; Schreiber, S.G.; Plavcová, L. An ecophysiological and developmental perspective on variation in vessel diameter. Plant Cell Environ. 2016, 40, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Preston, K.A.; Cornwell, W.K.; DeNoyer, J.L. Wood density and vessel traits as distinct correlates of ecological strategy in 51 California coast range angiosperms. New Phytol. 2006, 170, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.J.; Kitajima, K.; Kraft, N.J.B.; Reich, P.B.; Wright, I.J.; Bunker, D.E.; Condit, R.; Dalling, J.W.; Davies, S.J.; Díaz, S.; et al. Functional traits and the growth–mortality trade-off in tropical trees. Ecology 2010, 91, 3664–3674. [Google Scholar] [CrossRef]
- PlavcovÁ, L.; Hacke, U.G.; Sperry, J.S. Linking irradiance-induced changes in pit membrane ultrastructure with xylem vulnerability to cavitation. Plant Cell Environ. 2010, 34, 501–513. [Google Scholar] [CrossRef]
- Hajek, P.; Leuschner, C.; Hertel, D.; Delzon, S.; Schuldt, B. Trade-offs between xylem hydraulic properties, wood anatomy and yield in Populus. Tree Physiol. 2014, 34, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Poorter, L.; Horsting, A.; Delzon, S.; Sterck, F.; Geitmann, A. Pit and tracheid anatomy explain hydraulic safety but not hydraulic efficiency of 28 conifer species. J. Exp. Bot. 2022, 73, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Hacke, U.G.; Jansen, S. Embolism resistance of three boreal conifer species varies with pit structure. New Phytol. 2009, 182, 675–686. [Google Scholar] [CrossRef]
- Lens, F.; Sperry, J.S.; Christman, M.A.; Choat, B.; Rabaey, D.; Jansen, S. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytol. 2010, 190, 709–723. [Google Scholar] [CrossRef]
- Zhang, W.W.; Song, J.; Wang, M.; Liu, Y.Y.; Li, N.; Zhang, Y.J.; Holbrook, N.M.; Hao, G.Y. Divergences in hydraulic architecture form an important basis for niche differentiation between diploid and polyploid Betula species in NE China. Tree Physiol. 2017, 37, 604–616. [Google Scholar] [CrossRef]
- Moreno-Gutiérrez, C.; Dawson, T.E.; Nicolás, E.; Querejeta, J.I. Isotopes reveal contrasting water use strategies among coexisting plant species in a Mediterranean ecosystem. New Phytol. 2012, 196, 489–496. [Google Scholar] [CrossRef]
- Song, Y.; Sterck, F.; Sass-Klaassen, U.; Li, C.; Poorter, L. Growth resilience of conifer species decreases with early, long-lasting and intense droughts but cannot be explained by hydraulic traits. J. Ecol. 2022, 110, 2088–2104. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; López, B.C.; Loepfe, L.; Lloret, F. Stand- and tree-level determinants of the drought response of Scots pine radial growth. Oecologia 2011, 168, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; He, J.; Smith, N.G.; Yang, H.; Liu, J.; Chen, L.; Tao, J.; Luo, W. Tree growth rate-mediated trade-off between drought resistance and recovery in the Northern Hemisphere. Proc. R. Soc. B Biol. Sci. 2024, 291, 20241427. [Google Scholar] [CrossRef]
- Guo, J.J.; Gong, X.W.; Fang, L.D.; Jiang, D.M.; Ala, M.; Bucci, S.J.; Scholz, F.G.; Goldstein, G.; Hao, G.Y. Switching of dominant positions between two sand-fixing shrub species during the dune revegetation process is underlain by their contrasting xylem hydraulics and water-use strategies. Land Degrad. Dev. 2020, 31, 1195–1205. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, C.; Zhou, Z.; Li, Z. Co-ordinated performance of leaf hydraulics and economics in 10 Chinese temperate tree species. Funct. Plant Biol. 2016, 43, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.S.; Goldstein, G.; Meinzer, F.C.; Fisher, J.B.; Machado, K.; Woodruff, D.; Jones, T. Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 2004, 140, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Fichot, R.; Chamaillard, S.; Depardieu, C.; Le Thiec, D.; Cochard, H.; Barigah, T.S.; Brignolas, F. Hydraulic efficiency and coordination with xylem resistance to cavitation, leaf function, and growth performance among eight unrelated Populus deltoides × Populus nigra hybrids. J. Exp. Bot. 2011, 62, 2093–2106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.Q.; He, X.Y.; Chen, W.; Chen, Y.H.; Zhang, Y.; Ning, Z.H. Quantitative analysis of urban forest structure: A case study on Shenyang arboretum. Chin. J. Appl. Ecol. 2003, 14, 2090–2094. [Google Scholar] [CrossRef]
- Chang, Y.F. Study on Renewal Evaluation of Natural Quality of Representative Agricultural Land in Liaoning Province. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2022. [Google Scholar]
- Xu, S.; Xu, W.; Chen, W.; He, X.; Huang, Y.; Wen, H. Leaf phenological characters of main tree species in urban forest of Shenyang. PLoS ONE 2014, 9, e99277. [Google Scholar] [CrossRef]
- Wang, A.Y.; Cui, H.X.; Gong, X.W.; Guo, J.J.; Wu, N.; Hao, G.Y. Contrast in vulnerability to freezing-induced xylem embolism contributes to divergence in spring phenology between diffuse- and ring-porous temperate trees. For. Ecosyst. 2022, 9, 100070. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 51–67. [Google Scholar]
- Witkowski, E.T.F.; Lamont, B.B. Leaf specific mass confounds leaf density and thickness. Oecologia 1991, 88, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L. Spatial and temporal variation in plant hydraulic traits and their relevance for climate change impacts on vegetation. New Phytol. 2014, 205, 1008–1014. [Google Scholar] [CrossRef]
- Meinzer, F.C.; McCulloh, K.A.; Lachenbruch, B.; Woodruff, D.R.; Johnson, D.M. The blind men and the elephant: The impact of context and scale in evaluating conflicts between plant hydraulic safety and efficiency. Oecologia 2010, 164, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.H.; Hao, G.Y. Divergence between ring- and diffuse-porous wood types in broadleaf trees of Changbai Mountains results in substantial differences in hydraulic traits. Chin. J. Appl. Ecol. 2018, 29, 352–360. [Google Scholar] [CrossRef]



| Tree Species | Ψmd (MPa) | P50 (MPa) | P88 (MPa) | HSM50 (MPa) | HSM88 (MPa) |
|---|---|---|---|---|---|
| Populus alba L. × P. berolinensis Dippel | −1.72 ± 0.21 b | −1.26 ± 0.12 a | −1.61 ± 0.17 a | −0.98 ± 0.14 b | −1.26 ± 0.12 b |
| Acer truncatum | −1.02 ± 0.18 a | −2.50 ± 0.23 a | −3.12 ± 0.12 b | 0.31 ± 0.27 a | 1.82 ± 0.14 a |
| Tree Species | LS (cm2) | LMA (g m−2) | LT (μm) | PT (μm) | ST (μm) | PT/ST |
|---|---|---|---|---|---|---|
| Populus alba × P. berolinensis | 10.60 ± 0.8 b | 108.58 ± 8.9 a | 172.20 ± 2.7 a | 80.72 ± 1.4 a | 53.29 ± 1.2 a | 1.53 ± 0.02 a |
| Acer truncatum | 29.20 ± 2.0 a | 48.23 ± 4.7 b | 121.24 ± 3.0 b | 36.92 ± 1.6 b | 44.70 ± 1.0 b | 0.84 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.-Y.; Lu, Y.-J.; Cui, H.-X.; Liu, S.-S.; Li, S.-Q.; Hao, G.-Y. Xylem Hydraulics of Two Temperate Tree Species with Contrasting Growth Rates. Plants 2024, 13, 3575. https://doi.org/10.3390/plants13243575
Wang A-Y, Lu Y-J, Cui H-X, Liu S-S, Li S-Q, Hao G-Y. Xylem Hydraulics of Two Temperate Tree Species with Contrasting Growth Rates. Plants. 2024; 13(24):3575. https://doi.org/10.3390/plants13243575
Chicago/Turabian StyleWang, Ai-Ying, Yi-Jun Lu, Han-Xiao Cui, Shen-Si Liu, Si-Qi Li, and Guang-You Hao. 2024. "Xylem Hydraulics of Two Temperate Tree Species with Contrasting Growth Rates" Plants 13, no. 24: 3575. https://doi.org/10.3390/plants13243575
APA StyleWang, A.-Y., Lu, Y.-J., Cui, H.-X., Liu, S.-S., Li, S.-Q., & Hao, G.-Y. (2024). Xylem Hydraulics of Two Temperate Tree Species with Contrasting Growth Rates. Plants, 13(24), 3575. https://doi.org/10.3390/plants13243575






