Abstract
Agricultural pests present a significant challenge to humanity, often managed through synthetic chemicals that, when misused, can cause irreversible harm to both the environment and human health. This study focuses on endemic plants from the Yucatán Peninsula in Mexico, particularly from the state of Campeche, to identify their historical uses and propose an updated list of species with pesticide potential in the region. We systematically reviewed specimens from the Center for Sustainable Development and Wildlife Management (CEDESU) herbarium and local databases. Of the 3084 specimens collected, 2524 (81.84%) were from Campeche. The collection encompasses 106 botanical families, 459 genera, and 747 species. The study identified 201 plant species from 48 taxonomic families that are endemic to the Yucatán Peninsula Biotic Province (YPBP), of which 123 species are exclusive to the Mexican Yucatán Peninsula (MYP), representing 61.19% of the endemic species. Campeche contains 134 species (66.66%), distributed across 96 genera and 43 families. Notably, 46.26% of the species (62 species) belong to the Mexican region, with 8 species (12.90%) exclusive to Campeche. The research revealed that 27.90% of the families and 19.79% of the genera present in the state have been the subject of previous scientific studies regarding their use as pesticides. The most extensively studied families were Euphorbiaceae and Fabaceae. However, there is a notable lack of research on endemic plants from the Yucatán Peninsula, underscoring the need for increased attention to these species. The identified genera and families contain chemical compounds with activity against significant pests, demonstrating substantial potential for the development of natural pesticides.
1. Introduction
Agricultural pests are a significant global issue because of their role in spreading diseases and causing substantial losses on important crops. They also degrade the quality of affected products, making them challenging to market [1]. According to Shashidhar et al. [2], a pest is any harmful organism that inflicts economic damage on crops and impacts non-target organisms within agroecosystems. Pests disrupt global food production, affecting crops during growth and sometimes even during post-harvest storage [3]. Synthetic chemicals. Synthetic chemicals have long been used to control pests in important crops. However, studies have found that some synthetic chemicals products like abamectin, cypermethrin, endosulfan, and imidacloprid can cause irreversible damage [4]. As demonstrated by Zhang et al. [5] in their study using mouse models, certain products can adversely affect the reproductive health of the subjects examined. Similarly, Kalefetoglu [6] highlights that abamectin is a harmful pesticide with a range of cytotoxic and genotoxic effects on non-target organisms.
The widespread and indiscriminate use of these pesticides not only promotes insect resistance but also results in environmental contamination, posing significant risks to human health and ecological balance [7]. To address these issues, it is crucial to develop pest control strategies that adopt an ecological approach. This includes exploring botanical alternatives for pest management, such as plant extracts with solvents, insecticidal plant powders, and essential oils. These natural options can serve as repellents, anti-feedants, insecticides, fungicides, herbicides, and nematicides [8].
The ethnobotanical use of natural products offers a viable and sustainable alternative for insect pest control due to the effectiveness of the secondary chemical compounds they contain. These compounds, such as monoterpenoids, sesquiterpenoids, phenylpropanoids, and alkaloids, are responsible for their insecticidal properties [9,10,11]. Plants have evolved over millions of years to develop defense mechanisms against insect attacks, including repellency and insecticidal actions [12].
Many plant species produce secondary metabolites with biological activity, which are extracted from roots, seeds, leaves, or fruits [13,14]. These extracts have led to the development of valuable products with insecticidal potential in adult and immature stages [15]. Additionally, these natural products help reduce the development of resistance to pests compared to synthetic insecticides [16]. Furthermore, many of these products adhere to international standards for environmentally friendly production [17].
Worldwide, there are 250,000 flowering plant species (Magnoliophyta), with about 22,351 species native to Mexico [18,19,20,21,22]. However, Mexico’s floral diversity is not yet documented, and a reliable total estimate remains elusive. According to Villaseñor [23], reports indicate that Mexico may have the highest level of endemism in the Americas and is recognized as a ‘megadiverse’ country, ranking among the top five in terms of floristic richness [24]. A recent study identifies 53 orders, 247 families, 2685 genera, and 21,841 species of flowering plants in Mexico, with 11,001 species being endemic [22].
Mexico consists of 32 states and is divided into eight regions, with the Southeast Region being one of them. This region includes the Yucatan Peninsula (MYP), covering the states of Campeche, Quintana Roo, and Yucatan, with a total area of 166,445.49 km2. Despite its size, the Yucatan Peninsula remains one of Mexico’s least explored and studied regions. Known as the Yucatan Peninsula Biotic Province (YPBP), it extends into the northern parts of Belize and Guatemala. Approximately 2327 plant species used for ethnobotanical purposes are found in the Yucatan Peninsula, distributed across 956 genera and 161 families. Among the families with the highest species richness are Fabaceae, Poaceae, Asteraceae, Orchidaceae, and Euphorbiaceae. According to Fernández et al. [25], the region’s total flora includes 99 endemic species, accounting for 4.27% [26]. While the Peninsula does not have high species diversity, it is of significant interest from a floristic and biogeographical perspective. This interest arises from the combination of elements from Central America, the Caribbean Sea Basin, and southern Mexico, mixed with endemic species, creating a unique flora [27,28]. The Yucatan Scientific Research Centre (CICY) from Mexico states that around 30% of the vascular plants in the MYP (648 species) have known medicinal uses [29]. According to the study by Méndez et al. [30], a total of 565 species across 370 genera and 107 families have been documented. However, most studies have focused on developing new drugs rather than exploring the potential of these plants for pest control in important crops.
To date, most research on plant-derived products has concentrated on their medicinal uses for antibacterial [31,32,33], antifungal [34,35,36], amebicidal [37], anti-inflammatory [38], sedative [39,40], spasmolytic [41], anti-arthritis [42], and antioxidant properties [43]. However, there is a lack of information regarding the efficacy of these plants in controlling arthropods, microorganisms, and weeds that are considered pests.
This review aims to assess the current status of endemic plants in the Yucatan Peninsula, with a particular focus on those in the state of Campeche that have been the least studied. It seeks to identify endemic plants utilized in agriculture and propose an updated list of species with potential pesticidal properties. The review will include species from the scientific collection of the herbarium at the Center for Studies in Sustainable Development and Wildlife Utilization (CEDESU) at the Autonomous University of Campeche (UACAM) and specialized literature. By addressing this knowledge gap, the review aspires to provide new insights into the pesticidal potential of endemic plants in the region. We hope that by highlighting the potential of these plants in southeastern Mexico, we can foster connections with various national and international institutions, thereby enabling the development of academic and research projects focused on discovering natural chemical alternatives to pesticides, reducing reliance on synthetic agrochemicals.
2. Results
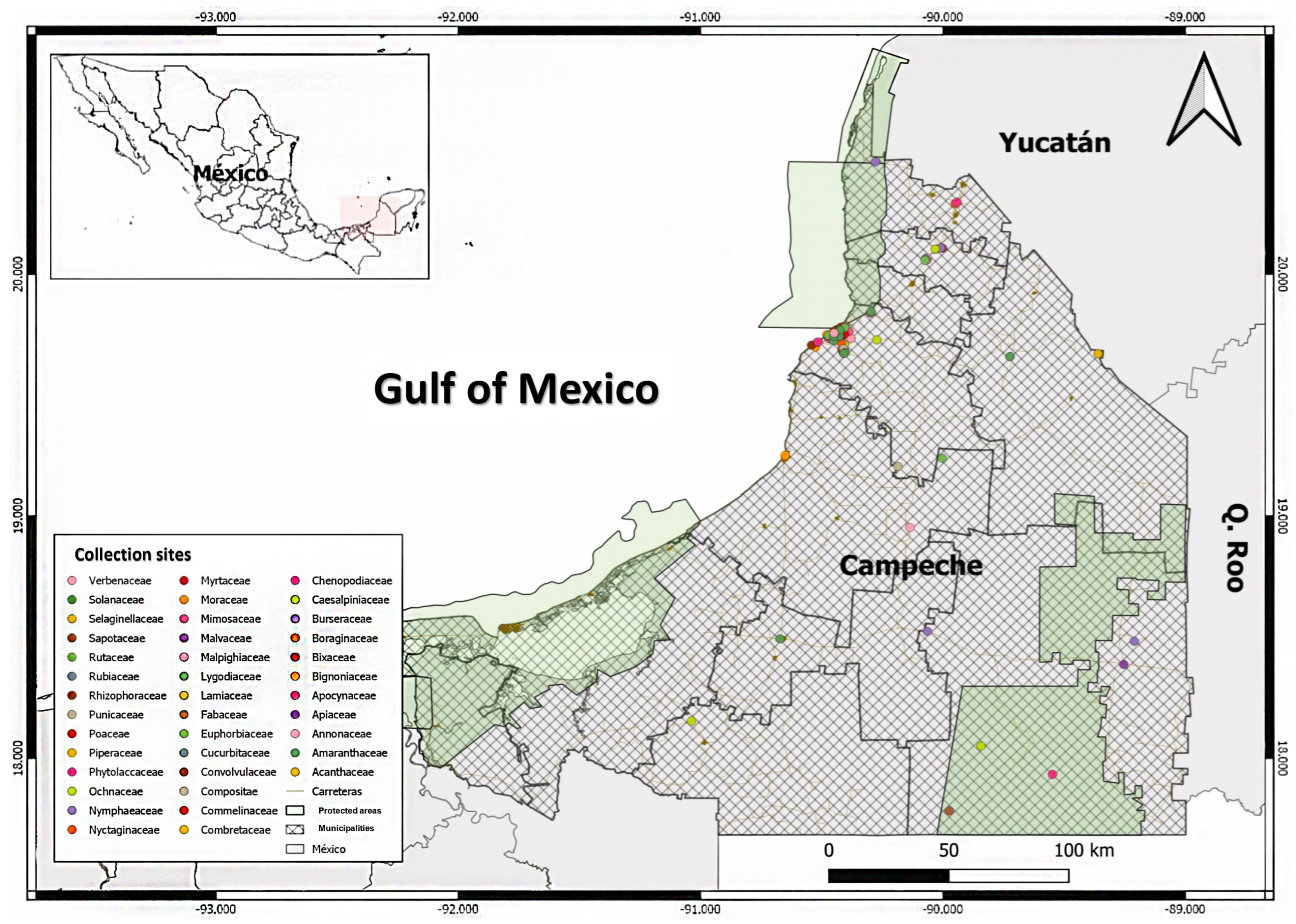
The review and organization of the material in the CEDESU-UACAM Herbarium revealed 3084 plant specimens collected from the states of Campeche, Chiapas, Oaxaca, and Veracruz. Among these specimens, 81.84% (2524) are from Campeche (Figure 1). The Campeche collection includes specimens from 106 botanical families, spread across 459 genera and 747 species. Notably, 83 species among these have yet to be identified by researchers.
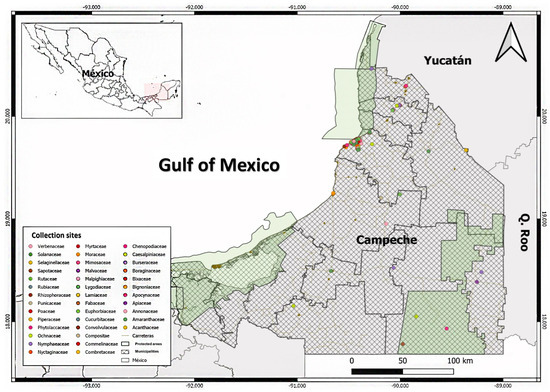
Figure 1.
Distribution of botanical families among the specimens in the Herbarium of the Scientific Collection at CEDESU-UACAM, highlighting the proportion of species collected from the state of Campeche.
Based on a review of the local literature, scientific databases, herbarium collections, and National Commission for the Knowledge and Use of Biodiversity of Mexico CONABIO’s website, we found that endemic species from each state in the Yucatan Peninsula (MYP)—which includes Campeche, Quintana Roo, and Yucatan—also exhibit endemism with other areas within the Yucatan Peninsula Biotic Province (YPBP). The YPBP extends to Guatemala and Belize as well. Within the YPBP, we identified 48 taxonomic families and 201 plant species from 135 endemic genera, with 123 species being exclusive to the MYP. This represents 61.19% of the species endemic to the Mexican region (Table 1). The compilation of a floristic list of YPBP endemic species found in Campeche revealed that 66.66% (134 species) of these species are present in the state. The distribution of these species spans across 96 genera and 43 botanical families. Among these, 46.26% (62 species) are exclusive to the Mexican region (MYP), and 12.90% (8 species) are exclusive to Campeche.

Table 1.
Updated list of endemic plants in the Yucatan Peninsula Biotic Province (YPBP), along with their distribution in the Mexican states comprising the Yucatan Peninsula (MYP).
In the review of species occurring in the state of Campeche, it was found that 27.90% (n = 12) of the taxonomic families are reported in at least one study on pest control. Among the genera present in the state (n = 96), 19.79% (n = 19) have been investigated in previous studies. For species (n = 134), 17.16% (n = 23) have been mentioned in some form of study (Table 2).

Table 2.
Number of botanical genera and species present in the state of Campeche, belonging to the Yucatan Peninsula Biotic Province (YPBP) that have been studied for pests’ control.
Among the endemic species of previous studies, the most represented families were Euphorbiaceae and Fabaceae. The Euphorbiaceae family includes the following species: Acalypha gaumeri, Cnidoscolus souzae, Croton arboreus, Croton chichenensis, and Jatropha gaumeri. The Fabaceae family is represented by Gliricidia maculata, Havardia albicans, Lonchocarpus castilloi, Lonchocarpus xuul, Lonchocarpus yucatanensis, Platymiscium yucatanum, and Senegalia gaumeri (Table 3).

Table 3.
Updated list of endemic plants of the Biotic Province of the Yucatan Peninsula (YPBP), with previous reports on chemical characterization and use for pest control.
3. Discussion
The search for new biological resources that can be used, to ensure safety and satisfaction for humans, is crucial today. In this context, the wild flora of the Yucatan Peninsula, combined with the ethnobotanical knowledge of Mayan culture regarding its medicinal and gastronomic uses, represents a valuable resource for bio-prospecting.
A key aspect of bio-prospecting is the variability in bioactive compound content within plants of the same family or genus, which can depend on factors such as developmental and handling conditions. For example, promising results have been obtained from the evaluation of plants of the genus Cnidoscolus for antimicrobial activity against Escherichia coli (Enterobacteriaceae) and Pseudomonas aeruginosa (Pseudomonadaceae). This suggests that C. souzae, an endemic species of the Yucatan Peninsula, may possess compounds with similar bioactive properties. Some note that members of the same botanical family and species within the same genus may exhibit similar characteristics and potential due to their common evolutionary ancestry. They share a genetic pool that enables them to synthesize similar chemical compounds. By occupying analogous ecological niches, these species develop traits and compounds that enhance their survival in comparable environments. Different species can produce similar metabolites because they often conserve the biosynthetic pathways for metabolite production within their taxonomic groups [71,72,73,74].
Moreover, studies have shown that the Cactaceae family is renowned for its high levels of triterpenes and sterols, compounds that possess the potential to control insects and bacteria [47]. For example, Selenicereus grandiflorus, a species from this family, may contain terpenoids with biological efficacy. Assessments conducted in entomological cages have shown that aqueous extracts of Croton itzaeus (stem) are also effective against M. javanica. Plants contain terpenoids, which are compounds with an isoprene-based structure bound to oxygen [75]. Terpenoids affect insects by slowing food passage through the gut and reducing digestibility by inhibiting the secretion of digestive enzymes such as proteases. This inhibition leads to physical weakening and impaired growth and development. Saponins, with a chemical structure similar to insect molting hormones, act as inhibitors or antagonists, disrupting the molting and metamorphosis processes [76].
Despite the rich biodiversity of the Yucatan Peninsula, nobody has reported the use of several endemic species, such as the genus Justicia, in pest control. However, researchers have conducted studies on other species within the same genus, such as Justicia spicigera (Acanthaceae), and have discovered that they possess flavonoids and tannins, which are secondary compounds known for their insecticidal properties [77,78]. Jatropha curcas seeds contain the compound Jatropherol-I, which has insecticidal properties [79]. This finding suggests that Jatropha gaumeri may also possess similar compounds. Jatropha curcas seed extracts have shown efficacy against the maize weevil Sitophilus zeamais (Coleoptera: Curculionidae) in stored grains, without affecting the germination of treated seeds [80]. In addition, J. gossypifolia leaf extract has shown toxicity to Spodoptera litura (Lepidoptera: Noctuoidea) larvae (24-h LC50, 6.56 mgmL−1) [81].
Despite the existing knowledge, J. gaumeri remains an understudied species in insect control. However, studies have shown that species of the genus Justicia are a rich source of active biomolecules with diverse biological activities, including terpenes [82]. Species of the genus Acacia have shown insecticidal effects, as evidenced by aqueous, ethanolic, and acetonic extracts of the aerial parts of Acacia modesta, which affect adults of the mosquito Culex pipiens [83]. However, there has been no evaluation of the species Acacia gentlei in pest control.
In our study, we found a significant diversity of plant species from several botanical families that are effective in pest control. However, there are currently no reports of products derived from endemic species of Campeche and the Yucatan Peninsula (YMP) being used for pest insect management. This underscores an obvious need to continue the search for biomolecules with the potential to be integrated into ecological strategies within integrated pest management.
Several organisms produced natural products, which comprise organic molecules with complex chemical structures, and include both primary and secondary metabolites. Secondary metabolites, in particular, are small organic molecules that are not essential for the growth, development, or reproduction of the producing organism. Instead, they are synthesized at specific stages of the life cycle or under particular environmental conditions [84,85]. We can classify these compounds based on their composition, chemical structure, synthesis route, or solubility in different solvents. A simple classification based on chemical structure includes three major groups: phenolic compounds (such as coumarins, flavonoids, and tannins), nitrogen compounds (alkaloids), and carbon and hydrogen compounds (terpenoids) [86,87,88].
Plant-produced metabolites serve as defense mechanisms and exhibit a broad spectrum of activity, affecting insects at cellular, tissue, or general organism levels [86]. These metabolites disrupt cellular and physiological processes responsible for homeostasis, leading to insecticidal effects such as inhibition of feeding, alterations in development, reduced fecundity, deformations in successive generations, interference with vital enzyme activity, alterations in the nervous system, blockage of metabolic pathways, behavioral impairment, and reduced insect populations [85,89,90,91,92,93,94,95,96].
For instance, alkaloids are prevalent and active compounds, with over 12,000 variants described to date [86]. Studies have shown that alkaloids exhibit a wide range of biological activities by interfering with various physiological mechanisms in insects. When alkaloids enter an insect, they trigger the production of reactive oxygen species (ROS), such as oxygen ions, free radicals, and peroxides. This increase in ROS leads to oxidative stress, which changes the mitochondrial membrane potential and causes cell death. Alkaloids prompt the opening of cell membrane channels, elevating calcium ion levels within the cell and leading to apoptosis [85,97,98].
Alkaloids also affect the hormonal balance of insects, during metamorphosis. They can disrupt the function of the prothoracicotropic hormone (PTTH), which activates the prothoracic glands to synthesize ecdysone (the molting hormone) and juvenile hormone. Disruption in the production of these hormones can cause larvae to remain in the larval stage for an extended period or, sometimes, reach the pupal stage but emerge with malformations such as deformed wings and reduced fecundity [99,100]. Alkaloids also have potent effects on the nervous system of insects. They mimic acetylcholine, an essential neurotransmitter, by binding to acetylcholine receptors on the cell membrane. This interaction alters membrane permeability, leading to spasmodic contractions, convulsions, and death of the insect [101]. Alkaloids have neurotoxic effects that manifest as decreased locomotion, tremors in appendages and abdominal segments, and altered food intake. These effects can lead to reproductive disturbances, such as reduced fecundity in females, inhibited sexual maturity, and decreased hatching rates [102].
Plants also produce flavonoids, which are compounds derived from the shikimate and acetyl coenzyme A pathways. Flavonoids represent one of the most diverse chemical groups, with over 5000 compounds identified [75]. These compounds disrupt the detoxification system of insects by reducing the activity of glutathione-S-transferase and esterase enzymes and decreasing mitochondrial activity. This reduction affects ubiquinone oxidoreductase, an enzyme crucial for energy production, affecting feeding and movement processes [103]. Tannins are another class of complex phenolic compounds, classified into hydrolyzable and condensed tannins. Their primary effect on insects is to cause fatal midgut injury through oxidative stress induced by peroxides generated during tannin oxidation [104,105].
Researchers have attributed the biosynthesis of bicyclic aromatic compounds called coumarins to the shikimate pathway [106]. These compounds are further classified into four groups: hydroxycoumarins, furanocoumarins, pyranocoumarins, and glycosylated coumarins [107]. Coumarins conjugate with the enzymes transaminase and cytochrome P450, inhibiting the detoxification system of insects [108]. We can extract plant compounds using various methods, both conventional (such as maceration and boiling) and non-conventional (such as microwaving and ultrasound), among others. Essential oils derived from these plants exhibit a range of mechanisms of action. Their primary property is the ability to alter the lipid bilayer of cells. Some essential oils can have synergistic effects when combined with botanical insecticides, enhancing their efficacy up to sixfold. These oils may exhibit multiple effects, including direct toxicity, growth inhibition, repulsion, and alteration of insect behavior [109,110].
Endemic botanical species from Campeche and the Yucatan Peninsula, belonging to taxonomic families known for pest control, have considerable potential in this area. However, the future use of these species for agricultural pest management is uncertain. Most current research focuses on identifying and characterizing chemical compounds for pharmaceutical applications, rather than exploring their potential in pest control. This underutilization contrasts with the progress made in other regions, where endemic flora has been effectively harnessed for developing plant-based biopesticides.
India and China lead globally in this field, leveraging their biodiversity and traditional use of botanical extracts with insecticidal properties. The United States follows in third place, emphasizing the technological production and commercialization of biopesticides [111]. Examples of endemic species with insecticide properties include Azadirachta indica (Meliaceae) from India, valued for its natural insecticidal extracts [112]; Sophora flavescens (Fabaceae) from China, effective against insects and fungi [113]; and Maclura pomifera (Moraceae), an endemic U.S. species recognized for its insect-repellent properties [114].
In comparison, species endemic to the Yucatan Peninsula, such as C. souzae and J. gaumeri, remain largely unexplored despite the region’s rich ethnobotanical tradition. This highlights the need for increased research investment to identify and characterize the secondary metabolites of these plants and integrate them into ecological pest management strategies. The experiences of India and China could serve as valuable models for advancing the development and adoption of biopesticides from local botanical resources.
4. Materials and Methods
A comprehensive review of relevant research articles was conducted by querying two major academic databases, SCOPUS and Web of Science (WOS), over the past ten years. The objective was to identify studies that assessed the insecticidal activity and chemical composition of endemic plants.
Additionally, a review was carried out on the botanical material stored in the Herbarium and Scientific Collection of CEDESU-UACAM, along with the local literature by Carnevali et al. [115] and Valencia-Gutiérrez et al. [116]. The search terms used included ‘insecticidal activity’, ‘pesticide’, ‘endemic’, ‘chemical composition’, and ‘Mexico’, and these terms were applied to the abstract, title, and keyword fields in both English and Spanish.
The database of the National Commission for the Knowledge and Use of Biodiversity of Mexico (CONABIO, https://www.biodiversidad.gob.mx, accessed on 1 January 2024) was also consulted. Duplicates were removed from the citation list, and the abstracts and full texts of each manuscript were reviewed. This process resulted in an updated list of endemic plants from the Yucatan Peninsula (MYP) and the Yucatan Peninsula Biotic Province (YPBP), including those specific to the state of Campeche. The database searches and the literature review were finalized on 15 July 2024.
5. Conclusions
The literature review shows that there is a significant limitation in research on the endemic plants of the Yucatan Peninsula. This research gap emphasizes the urgent need to shift focus towards these unique species. The genera and taxonomic families found in this region have shown the potential to harbor chemical compounds that could act on important pests, showing that they could provide valuable opportunities for the development of new pest control strategies. Despite their potential, there is a scarcity of existing sources addressing extraction technologies, chemical analysis, and pest control related to these species. This highlights a significant knowledge gap and emphasizes the need for more in-depth studies in these areas. Further research into the extraction of bioactive compounds, their chemical characterization, and their efficacy in pest control could not only expand our understanding of the endemic plants of the Yucatan Peninsula but also contribute to the development of more sustainable and effective pest management methods.
Author Contributions
Conceptualization, N.A.-H., C.G.-E. and D.H.-G.; methodology, N.A.-H., C.G.-E. and D.H.-G.; software, B.H.Z.-N., F.D.-N. and B.Q.-G.; validation, B.H.Z.-N., F.D.-N. and B.Q.-G.; investigation, N.A.-H., C.G.-E., D.H.-G., B.H.Z.-N., F.D.-N. and B.Q.-G.; resources, E.L.-A., N.L.-V., S.O.-S., I.V.L.-S. and L.D.C.-P.; data curation, E.L.-A., N.L.-V., S.O.-S., I.V.L.-S. and L.D.C.-P.; writing—original draft preparation, N.A.-H., B.H.Z.-N., F.D.-N., B.Q.-G., C.G.-E., D.H.-G., E.L.-A., N.L.-V., S.O.-S., I.V.L.-S. and L.D.C.-P.; writing—review and editing, N.A.-H., B.H.Z.-N., F.D.-N., B.Q.-G., C.G.-E., D.H.-G., E.L.-A., N.L.-V., S.O.-S., I.V.L.-S. and L.D.C.-P.; visualization, N.A.-H., C.G.-E. and D.H.-G.; supervision, B.H.Z.-N., F.D.-N. and B.Q.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All the associated data are available in the manuscript.
Acknowledgments
The first author extends their gratitude to the National Council for Humanities, Science and Technology (CONAHCYT–Mexico) for the scholarship offered. Additionally, the author thanks to CIIDIR Oaxaca of the Instituto Politécnico Nacional (IPN) of Mexico, and the Universidad Autonoma de Campeche (UACAM-CEDESU), for their invaluable information and support in this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dimetry, N.Z. Different Plant Families as Bioresource for Pesticides. In Advances in Plant Biopesticides; Singh, D., Ed.; Springer: London, UK, 2014; pp. 1–20. [Google Scholar]
- Cheeti, S.; Kumar, G.S.; Priyanka, J.S.; Firdous, G.; Ranjeeva, P.R. Pest detection and classification using YOLO and CNN. Ann. Rom. Soc. Cell Biol. 2021, 25, 15295–15300. [Google Scholar]
- Kulkarni, J.; Kapse, N.; Kulkarni, O.K. Plant-based pesticides for control of Helicoverpa armigera on Cucumis. Asian Agric. Hist. 2009, 13, 327–332. [Google Scholar]
- Cerna, C.; Aguirre, C.L.; Flores, M.; Guervara, L.; Landeros, J.; Ochoa, Y. Susceptibility to Bactericera cockerelli (Sulc) (Hemiptera: Triozidae) to insecticides in the state of Nuevo Leon, Mexico. Resist. Pest Manag. Newsl. 2010, 19, 14–17. [Google Scholar]
- Zhang, Y.; Kong, C.; Chi, H.; Li, J.; Xing, J.; Wang, F.; Zhai, Q. Effect of a beta-cypermethrin and emamectin benzoate pesticide mixture on reproductive toxicity in male mice in a greenhouse environment. Toxicol. Mech. Methods 2020, 30, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kalefetoglu Macar, T. Investigation of cytotoxicity and genotoxicity of abamectin pesticide in Allium cepa L. Environ. Sci. Pollut. Res. 2021, 28, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Cunill, J.M. Current status of pesticide effects on environment, human health and its eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, A.; Rather, M.A.; Jain, V.; Rasool, S.; Nazir, R.; Malik, N.A.; Majid, S.A. Plant-based natural products as potential eco-friendly and safer biopesticides: A comprehensive overview of their advantages over conventional pesticides, limitations, and regulatory aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Sowmya-Sujatha, S. Insight of botanical-based biopesticides against economically important pests. Int. J. Pharm. Life Sci. 2012, 3, 2138–2148. [Google Scholar]
- Yactayo, C.J.P.; Tang, H.V.; Mendoza, J.; Christensen, S.A.; Block, A.K. Plant defense chemicals against insect pests. Agronomy 2020, 10, 1156. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant secondary metabolites: An opportunity for circular economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef]
- Lagunes, A. Uso de Extractos y Polvos Vegetales y Polvos Minerales Para el Combate de Plagas del Maíz y del Fríjol en la Agricultura de Subsistencia; Colegio de Postgraduados, USAID, CONACYT, BORUCONSA: Texcoco, México, 1994; 35p. [Google Scholar]
- Upasani, S.M.; Kotkar, H.M.; Mendki, P.S.; Maheshwari, V.L. Partial characterization and insecticidal properties of Ricinus communis L. foliage flavonoids. Pest Manag. Sci. 2003, 59, 1349–1354. [Google Scholar] [CrossRef]
- Simmonds, M.S. Flavonoid–insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef]
- Calvo-Irabien, L.M. Native Mexican aromatic flora and essential oils: Current research status, gaps in knowledge, and agro-industrial potential. Ind. Crop. Prod. 2018, 111, 807–822. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- USDA. Programa Nacional Orgánico: Reglamento Final; United States Department of Agriculture: Washington, DC, USA, 2009. Available online: http://somexpro.org/wpcontent/uploads/2009/02/nop09.pdf (accessed on 2 September 2024).
- Mittermeier, R.A. Primate diversity and the tropical forest. In Biodiversity; Wilson, E.O., Ed.; National Academy Press: Washington, DC, USA, 1988; pp. 145–154. [Google Scholar]
- Akeroyd, J.; Synge, H. Higher plant diversity. In Global Biodiversity. Status of the Earth’s Living Resources; Groombridge, B., Ed.; Chapman & Hall: London, UK, 1992; pp. 64–87. [Google Scholar]
- Mittermeier, R.A.; Goettsch, M.C. La importancia de la diversidad biológica de México. In México Ante los Retos de la Biodiversidad; Sarukhán, J., Dirzo, R., Eds.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Tlalpan, Ciudad de Mexico, Mexico, 1992; pp. 63–73. [Google Scholar]
- Villaseñor, J.L. Diversidad y distribución de las magnoliophyta de México. Interciencia 2003, 28, 160–167. [Google Scholar]
- Villaseñor, J.L.; Ortiz, E. Biodiversidad de las plantas con flores (División Magnoliophyta) en México. Rev. Mex. Biodivers. 2014, 85, 134–142. [Google Scholar] [CrossRef]
- Villaseñor, J.L. Los géneros de plantas vasculares en México. Bol. Soc. Bot. Mex. 2004, 75, 105–135. [Google Scholar]
- Sánchez-K, J.G. Riqueza de especies, clasificación y listado de las gramíneas (Poaceae) de México. Acta Bot. Mex. 2019, 126, e1379. [Google Scholar]
- Fernández, G.C.; Tapia, J.L.; Duno, R.; Ramírez, I.M.; Can, L.; Hernández, S.; Castillo, A. La flora de la Península de Yucatán Mexicana: 250 años de conocimiento florístico. Biodiversitas 2012, 101, 6–10. [Google Scholar]
- Duno-de Stefano, R.; Ramírez Morillo, I.M.; Tapia-Muñoz, J.L.; Hernández-Aguilar, S.; Can, L.; Cetzal-Ix, W.; Méndez-Jiménez, N.; Zamora-Crescencio, P.; Gutiérrez-Báez, C.; Carnevali-Fernández-Concha, G. Aspectos generales de la flora vascular de la Península de Yucatán, México. Bot. Sci. 2018, 96, 515–532. [Google Scholar] [CrossRef]
- Estrada-Loera, E. Phytogeographic relationships of the Yucatan Peninsula. J. Biogeogr. 1991, 18, 687–697. [Google Scholar] [CrossRef]
- Rzedowski, J. 1ra. Edición digital; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Tlalpan, Ciudad de Mexico, Mexico, 2006. [Google Scholar]
- Magaña, A.M.; Gama Campillo, L.M.; Mariaca Méndez, R. El uso de las plantas medicinales en las comunidades Maya-Chontales de Nacajuca, Tabasco, México. Polibotánica 2010, 29, 213–262. [Google Scholar]
- Méndez, M.; Durán, S.; Campos, S.; Dorantes, A. Flora medicinal. In Biodiversidad y Desarrollo Humano en Yucatán; Durán, S., Méndez, M., Eds.; Centro de Investigación Científica de Yucatán, Programa de Pequeñas Donaciones del Fondo para el Medio Ambiente Mundial, Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Secretaría de Desarrollo Urbano y Medio Ambiente de Yucatán: Mérida, Yucatán, México, 2010; pp. 349–352. [Google Scholar]
- Lang, G.; Buchbauer, G. A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. Flavour Fragr. J. 2012, 27, 13–39. [Google Scholar] [CrossRef]
- Guerra-Boone, L.; Alvarez-Roma, R.; Salazar-Aranda, R.; Torres-Cirio, A.; Rivas-Galindo, V.M.; Waksman de Torres, N.; Gonzalez-Gonzalez, G.; Perez-Lopez, L.A. Chemical composition, antimicrobial, and antioxidant activities of the essential oils from Magnolia grandiflora, Chrysanctinia mexicana, and Schinus molle found in northeast Mexico. Nat. Prod. Commun. 2013, 8, 135–138. [Google Scholar] [PubMed]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Rubio-Rosas, E.; Lozoya-Gloria, E.; Mosso González, C.; Ramón-Canul, L.G.; Cruz-Durán, R. Essential oil composition and biological/pharmacological properties of Salmea scandens (L.) DC. Food Control 2015, 57, 177–184. [Google Scholar] [CrossRef]
- Saad, N.Y.; Muller, C.D.; Lobstein, A. Major bioactivities and mechanism of action of essential oils and their components. Flavour Fragr. J. 2013, 28, 269–279. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- López-Meneses, A.K.; Plascencia-Jatomea, M.; Lizardi-Mendoza, J.; Rosas-Burgos, E.C.; Luque-Alcaraz, A.G.; Cortez-Rocha, M.O. Antifungal and antimycotoxigenic activity of essential oils from Eucalyptus globulus, Thymus capitatus, and Schinus molle. Food Sci. Technol. 2015, 35, 664–671. [Google Scholar] [CrossRef]
- Monzote, L.; Alarcón, O.; Setzer, W.N. Antiprotozoal activity of essential oils. Agric. Conspec. Sci. 2012, 77, 167–175. [Google Scholar]
- Carrera-Martínez, C.A.; Rosas-López, R.; Rodríguez-Monroy, M.A.; Canales-Martínez, M.M.; Román-Guerrero, A.; Jiménez-Alvarado, R. Chemical composition and in vivo anti-inflammatory activity of Bursera morelensis Ramírez essential oil. J. Essent. Oil Bear. Plants 2014, 17, 758–768. [Google Scholar]
- Anaya-Eugenio, G.D.; Rivero-Cruz, I.; Rivera-Chávez, J.; Mata, R. Hypoglycemic properties of some preparations and compounds from Artemisia ludoviciana Nutt. J. Ethnopharmacol. 2014, 155, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Lenardao, E.J.; Savegnago, L.; Jacob, R.G.; Victoria, F.N. Antinociceptive effect of essential oils and their constituents: An update review. J. Braz. Chem. Soc. 2016, 27, 435–474. [Google Scholar] [CrossRef]
- Zavala-Mendoza, D.; Grasa, L.; Zavala-Sánchez, M.; Pérez-Gutiérrez, S.; Murillo, M. Antispasmodic effects and action mechanism of essential oil of Chrysactinia mexicana A. Gray on rabbit ileum. Molecules 2016, 21, 783. [Google Scholar] [CrossRef] [PubMed]
- Flores-San Martin, D.; Perea-Flores, M.D.J.; Morales-López, J.; Centeno-Álvarez, M.M.; Pérez-Ishiwara, G.; Pérez-Hernández, N.; Pérez-Hernández, E. Effect of Heterotheca inuloides essential oil on rat cytoskeleton articular chondrocytes. Nat. Prod. Res. 2013, 6419, 37–41. [Google Scholar]
- Rodríguez-García, I.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; González-Aguilar, G.A.; Moctezuma, E.; Gutiérrez-Pacheco, M.; Tapia-Rodríguez, M.R.; Ortega-Ramírez, L.A.; Zavala, A.-J.F. Oregano (Lippia graveolens) essential oil added within pectin edible coatings prevents fungal decay and increases the antioxidant capacity of treated tomatoes. J. Sci. Food Agric. 2016, 96, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal-Alejo, J.; Tun-Suárez, J.M.; Moguel-Catzín, S.; Marbán-Mendoza, N.; Medina-Baizabal, L.; Simá-Polanco, P.; Peraza-Sánchez, S.R.; Gamboa-Angulo, M.M. In vitro sensitivity of Meloidogyne incognita to extracts from native Yucatecan plants. Nematropica 2006, 36, 89–97. [Google Scholar]
- Gamboa-Angulo, M.M.; Cristóbal-Alejo, J.; Medina-Baizabal, I.L.; Chi-Romero, F.; Méndez-González, R.; Sima-Polanco, P.; May-Pat, F. Antifungal properties of selected plants from the Yucatan Peninsula, Mexico. World J. Microbiol. Biotechnol. 2008, 24, 1955–1959. [Google Scholar] [CrossRef]
- Vargas-Díaz, A.A.; Gamboa-Angulo, M.; Medina-Baizabal, I.L.; Pérez-Brito, D.; Cristóbal-Alejo, J.; Ruiz-Sánchez, E. Evaluation of native Yucatecan plant extracts against Alternaria chrysanthemi and antifungal activity spectrum of Acalypha gaumeri. Rev. Mex. Fitopatol. 2014, 32, 1–11. [Google Scholar]
- Salazar, J.R.; Loza-Mejía, M.A.; Soto-Cabrera, D. Chemistry, biological activities and in silico bioprospection of sterols and triterpenes from Mexican columnar Cactaceae. Molecules 2020, 25, 1649. [Google Scholar] [CrossRef]
- Villafaña-Simá, J.R.; Cáceres-Farfán, M.; Méndez-González, M.; Dorantes, A.; Borges-Argáez, R. Actividad antimicrobiana de la corteza de Diospyros bumelioides Standl. In V Reunión Nacional de Investigación en Productos Naturales; Guadalajara: Jalisco, México, 2008; p. 1. [Google Scholar]
- Vera-Ku, M.; Mena-Reynoso, M.; Alpuche-Aguilar, D.; Gamboa-León, R.; Rosado-Vallado, M.E. Leishmanicidal, cytotoxic and antifungal activity of medicinal plants used against cutaneous diseases in Mayan traditional medicine. Int. J. Indig. Med. Plants 2015, 48, 1793. [Google Scholar]
- García-Sosa, K.; Aldana-Pérez, R.; Moo, R.V.E.; Simá-Polanco, P.; Peña-Rodríguez, L.M. Dinimbidiol ether, a novel bioactive dimeric diterpene from the root extract of Cnidoscolus souzae. Nat. Prod. Commun. 2017, 12, 1391–1392. [Google Scholar] [CrossRef]
- Zapata-Estrella, H.E.; Sánchez-Pardenilla, A.D.; García-Sosa, K.; Escalante-Erosa, F.; de Campos-Buzzi, F.; Meira-Quintão, N.L.; Cechinel-Filho, V.; Peña-Rodríguez, L.M. Bioactive metabolites from Cnidoscolus souzae and Acmella pilosa. Nat. Prod. Commun. 2014, 9, 1319–1321. [Google Scholar] [CrossRef]
- Aguilar-Guadarrama, A.B.; Rios, M.Y. Three new sesquiterpenes from Croton arboreous. J. Nat. Prod. 2004, 67, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Merlin-Cervantes, M.A.; Contreras-Marín, F.; Balam-Uc, E.; Simá-Polanco, P.; Medina-Baizabal, I.L.; Gamboa-Ángulo, M. Evaluación antifúngica de la raíz de Croton chichenesis colectada en dos poblaciones de Yucatán. Rev. Latinoam. Quim. 2012, 39, 1–4. [Google Scholar]
- Can-Aké, R.; Erosa-Rejón, G.; May-Pat, F.; Peña-Rodríguez, L.M.; Peraza-Sánchez, S.R. Bioactive terpenoids from roots and leaves of Jatropha gaumeri. Rev. Soc. Quím. Mex. 2004, 48, 11–14. [Google Scholar]
- Murad, K.U.F.; Nukmal, N.; Setyaningrum, E. The effect of storage time on the raw material of insecticide candidate from Gliricidia maculata leaves on toxicities stability to control mealybug. IOP Conf. Ser. Earth Environ. Sci. 2021, 739, 012075. [Google Scholar] [CrossRef]
- Rosado-Aguilar, J.A.; Arjona-Cambranes, K.; Torres-Acosta, J.F.J.; Rodríguez-Vivas, R.I.; Bolio-González, M.E.; Ortega-Pacheco, A.; Alzina-López, A.; Gutiérrez-Ruiz, E.J.; Gutiérrez-Blanco, E.A.; Aguilar-Caballero, A.J. Plant products and secondary metabolites with acaricide activity against ticks. Vet. Parasitol. 2017, 238, 66–76. [Google Scholar] [CrossRef]
- Hernández-Orduño, G.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Aguilar-Caballero, A.J.; Reyes-Ramirez, R.R.; Hoste, H.; Calderón-Quintana, J.A. In vitro anthelmintic effect of Acacia gaumeri, Havardia albicans, and quebracho tannin extracts on a Mexican strain of Haemonchus contortus L3 larvae. Trop. Subtrop. Agroecosyst. 2008, 8, 191–197. [Google Scholar]
- Reyes-Chilpa, R.; Viveros-Rodríguez, N.; Gómez-Garibay, F. Antitermitic activity of Lonchocarpus castilloi flavonoids and heartwood extracts. J. Chem. Ecol. 1995, 21, 455–463. [Google Scholar] [CrossRef]
- Gómez-Garibay, F.; Reyes-Chilpa, R.; Quijano, L.; Calderón-Pardo, J.S.; Ríos-Castillo, T. Methoxy furan auranols with fungistatic activity from Lonchocarpus castilloi. Phytochemistry 1990, 29, 459–463. [Google Scholar] [CrossRef]
- Borges-Argáez, R.; Balnbury, L.; Flowers, A.; Giménez-Turba, A.; Ruiz, G.; Waterman, P.G.; Peña-Rodríguez, L.M. Cytotoxic and antiprotozoal activity of flavonoids from Lonchocarpus spp. Phytomedicine 2007, 14, 530–533. [Google Scholar] [CrossRef]
- Borges-Argáez, R.; Peña-Rodríguez, L.M.; Waterman, P.G. Flavonoids from two Lonchocarpus species of the Yucatan Peninsula. Phytochemistry 2002, 60, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Yam-Puc, A.; Peña-Rodríguez, L.M. Isocordoin derivatives from the root extract of Lonchocarpus xuul. J. Mex. Chem. Soc. 2009, 53, 12–14. [Google Scholar] [CrossRef]
- Castañeda-Ramírez, G.S.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Borges-Argáez, R.; Cáceres-Farfán, M.; Mancilla-Montelongo, G.; Mathieu, C. Bio-guided fractionation to identify Senegalia gaumeri leaf extract compounds with anthelmintic activity against Haemonchus contortus eggs and larvae. Vet. Parasitol. 2019, 270, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Estrada, A.E.; Ruiz-Sánchez, E.; Gamboa-Ángulo, M. Activity of Eugenia winzerlingii Standl. extracts on Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae). Rev. Prot. Veg. 2015, 30, 38. [Google Scholar]
- Moo-Koh, F.A.; Cristóbal-Alejo, J.; Reyes-Ramírez, A.; Tun-Suárez, J.M.; Sandoval-Luna, R.; Ramírez-Pool, J.A. Actividad in vitro del extracto acuoso del Bonellia flammea contra hongos fitopatógenos. Agrociencia 2014, 48, 833–845. [Google Scholar]
- Domínguez-Carmona, D.B.; Escalante-Erosa, F.; García-Sosa, K.; Ruiz-Pinell, G.; Gutiérrez-Yapu, D.; Chan-Bacab, M.J.; Moo-Puc, R.E.; Veitch, N.C.; Giménez-Turba, A.; Peña-Rodríguez, L.M. Metabolites from roots of Colubrina greggii var. yucatanensis and evaluation of their antiprotozoan, cytotoxic, and antiproliferative activities. J. Braz. Chem. Soc. 2011, 22, 1279–1285. [Google Scholar] [CrossRef]
- Melchor-Martínez, E.M.; Tamez-Fernández, J.F.; González-González, G.M.; Silva-Mares, D.A.; Waksman-Minsky, N.; Pérez-López, L.A.; Rivas-Galindo, V.M. Active flavonoids from Colubrina greggii var. greggii S. Watson against clinical isolates of Candida spp. Molecules 2021, 26, 5760. [Google Scholar] [CrossRef]
- Palacios, F.; Gladstone, S. Eficacia del farnesol y de un extracto de semilla de ayote como repelentes de Atta mexicana. Manejo Integr. Plagas Agroecol. 2003, 68, 89–91. [Google Scholar]
- Polanco-Hernández, G.M. Metabolitos Con Actividad Tripanocida Producidos Por Plantas Nativas de la Península de Yucatán. Tesis de Maestría, Posgrado en Ciencias Biológicas, Centro de Investigación Científica de Yucatán, A.C., Mérida, Yucatán, México, 2011; p. 86. [Google Scholar]
- Sánchez-Medina, A.; Stevenson, P.C.; Habtemariam, S.; Peña-Rodríguez, L.M.; Corcoran, O.; Mallet, A.I.; Veitch, N.C. Triterpenoid saponins from a cytotoxic root extract of Sideroxylon foetidissimum subsp. gaumeri. Phytochemistry 2009, 70, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Vleminckx, J.; Salazar, D.; Fortunel, C.; Mesones, I.; Dávila, N.; Lokvam, J.; Fine, P.V. Divergent secondary metabolites and habitat filtering both contribute to tree species coexistence in the Peruvian Amazon. Front. Plant Sci. 2018, 9, 836. [Google Scholar] [CrossRef] [PubMed]
- Beran, F.; Köllner, T.G.; Gershenzon, J.; Tholl, D. Chemical convergence between plants and insects: Biosynthetic origins and functions of common secondary metabolites. New Phytol. 2019, 223, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Qu, Q.; Yang, Z.; Wang, M.; Liu, G.; Xue, S. Variations and factors characterizing ecological niches of species in a stable grassland plant community. Ecol. Indic. 2021, 128, 107846. [Google Scholar] [CrossRef]
- Vázquez-Luna, A.; Pérez-Flores, L.; Díaz-Sobac, R. Biomoléculas con actividad insecticida: Una alternativa para mejorar la seguridad alimentaria. Cienc. Tecnol. Aliment. 2007, 5, 306–313. [Google Scholar] [CrossRef]
- Geyter, L.E.D.E. Toxicity and Mode of Action of Steroid and Terpenoid Secondary PLANT metabolites against Economically Important Pest Insects in Agriculture; Faculty of Bioscience Engineering, Ghent University: Ghent, Belgium, 2012. [Google Scholar]
- Dominguez, X.A.; Alcorn, J.B. Screening of medicinal plants used by Huastec Mayans of northeastern Mexico. J. Ethnopharmacol. 1985, 13, 139. [Google Scholar] [CrossRef]
- Euler, K.L.; Alam, M. Isolation of kaempferitrin from Justicia spicigera. J. Nat. Prod. 1982, 45, 220. [Google Scholar] [CrossRef]
- Jing-X, L.D.Q.; Olofsson, C.S.; Salehi, A.; Surve, V.V.; Caballero, J. CaV2.3 calcium channels control second-phase insulin release. J. Clin. Investig. 2005, 115, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Ohazurike, N.C.; Omuh, M.O.; Emeribe, E.O. The use of seed extracts of the physic nut (Jatropha curcas L.) in the control of maize weevil (Sitophilus zeamaise M.) in stored maize grains (Zea mays L.). Glob. J. Agric. Sci. 2003, 2, 86–88. [Google Scholar] [CrossRef]
- Phowichit, S.; Buatippawan, S.; Bullangpoti, V. Insecticidal activity of Jatropha gossypifolia L. (Euphorbiaceae) and Cleome viscosa L. (Capparidaceae) on Spodoptera litura (Lepidoptera: Noctuidae): Toxicity and carboxylesterase and glutathione-S-transferase activity studies. Commun. Agric. Appl. Biol. Sci. 2008, 73, 611–619. [Google Scholar] [PubMed]
- Mohammed, A.E.; Al-Keridis, L.A.; Rahman, I.; Alotaibi, M.O.; Suliman, R.S.; Alrajhi, A.M.; Elobeid, M.M.; Alothman, M.R.; Alhomaidi, E.A.; Korany, S.M. Silver nanoparticles formation by Jatropha integerrima and LC/MS-QTOF-based metabolite profiling. Nanomaterials 2021, 11, 2400. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.E.; Ibrahim, R.R.; Hussien, A.; Salem, K.; Gonaid, M.H.; Soliman, H.S.M. Phytochemical screening and insecticidal activity of different extracts of Acacia modesta Wall. on adult Culex pipiens mosquito. Egypt. J. Hosp. Med. 2018, 73, 8022–8030. [Google Scholar]
- Monfil, V.O.; Casas-Flores, S. Molecular mechanisms of biocontrol in Trichoderma spp. and their applications in agriculture. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 77–94. [Google Scholar]
- Teichert, I.; Nowrousian, M.; Pöggeler, S.; Kück, U. The filamentous fungus Sordaria macrospora as a genetic model to study fruiting body development. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2014; Volume 87, pp. 199–244. [Google Scholar]
- Chowański, S.; Adamski, Z.; Marciniak, P.; Rosiński, G.; Büyükgüzel, E.; Büyükgüzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F.; et al. A review of bioinsecticidal activity of Solanaceae alkaloids. Toxins 2016, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.R. Natural Products; Abel, E.W., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2003. [Google Scholar]
- Kuete, V. Bioactivity of plant constituents against vancomycin-resistant enterococci. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Rai, M.K., Kon, K.V., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 23–30. [Google Scholar]
- Wink, M. Interference of alkaloids with neuroreceptors and ion channels. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 21, pp. 3–122. [Google Scholar]
- Adeyemi, M.M.H. The potential of secondary metabolites in plant material as deterrents against insect pests: A review. Afr. J. Pure Appl. Chem. 2010, 4, 243–246. [Google Scholar]
- López, M.D.; Pascual-Villalobos, M.J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crop. Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- Nenaah, G.E. Chemical composition, toxicity and growth inhibitory activities of essential oils of three Achillea species and their nano-emulsions against Tribolium castaneum (Herbst). Ind. Crop. Prod. 2014, 53, 252–260. [Google Scholar] [CrossRef]
- Maazoun, A.M.; Hlel, T.B.; Hamdi, S.H.; Belhadj, F.; Jemaa, J.M.B.; Marzouki, M.N. Screening for insecticidal potential and acetylcholinesterase activity inhibition of Urginea maritima bulbs extract for the control of Sitophilus oryzae (L.). J. Asia-Pac. Entomol. 2017, 20, 752–760. [Google Scholar] [CrossRef]
- Rosado-Aguilar, J.A.; Rodríguez-Vivas, R.I.; Borges-Argaez, R. Acaricidal activity of Havardia albicans and Caesalpinia gaumeri methanolic leaf extracts on Rhipicephalus microplus and its toxicity to laboratory animals. Exp. Appl. Acarol. 2017, 71, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Adamski, Z.; Marciniak, P.; Ziemnicki, K.; Büyükgüzel, E.; Erdem, M.; Büyükgüzel, K.; Ventrella, E.; Falabella, P.; Cristallo, M.; Salvia, R.; et al. Potato leaf extract and its component, α-solanine, exert similar impacts on development and oxidative stress in Galleria mellonella L. Arch. Insect Biochem. Physiol. 2014, 87, 26–39. [Google Scholar] [CrossRef]
- Macedo-Márquez, A. La producción de especies reactivas de oxígeno (EROS) en las mitocondrias de Saccharomyces cerevisiae. Rev. Esp. Cienc. Quím.-Biol. 2012, 15, 97–103. [Google Scholar]
- Ge, Y.; Liu, P.; Yang, R.; Zhang, L.; Chen, H.; Camara, I.; Liu, Y.; Shi, W. Insecticidal constituents and activity of alkaloids from Cynanchum mongolicum. J. Agric. Food Chem. 2015, 63, 17483–17492. [Google Scholar] [CrossRef]
- Orozco-Sánchez, F.; Rodríguez-Monroy, M. Cell suspension culture of Azadirachta indica for production of bioinsecticide. Rev. Mex. Ing. Quím. 2007, 6, 251–258. [Google Scholar]
- Celis, Á.; Mendoza, C.; Pachón, M.; Cardona, J.; Delgado, W.; Cuca, L.E. Extractos vegetales utilizados como biocontroladores con énfasis en la familia Piperaceae: Una revisión. Agron. Colomb. 2010, 26, 97–106. [Google Scholar]
- Abbassi, K.; Atay-Kadiri, Z.; Ghaout, S. Biological effects of alkaloids extracted from three plants of Moroccan arid areas on the desert locust. Physiol. Entomol. 2003, 28, 232–236. [Google Scholar] [CrossRef]
- Pavela, A.; Maggi, F.; Lannarelli, R.; Benelli, G. Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Trop. 2019, 193, 181–190. [Google Scholar] [CrossRef]
- Galati, G.; Sabzevari, O.; Wilson, J.X.; O’Brien, P.J. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicol. 2002, 177, 91–104. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, P.C. Tannins in plant-herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.G.; Rennó, M.N.; Figueroa-Villar, J.D. Coumarins as cholinesterase inhibitors: A review. Chem.-Biol. Interact. 2016, 254, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crop. Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Wen, Z.; Zeng, R.; Sen-Niu, G. Ecological significance of induction of broad-substrate cytochrome P450s by natural and synthetic inducers in Helicoverpa zea. Pest Biochem. Physiol. 2009, 94, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Tejero, C.M.; Guirao, P.; Pascual, V.M.J. El Uso de Aceites Esenciales Como Insecticidas y Repelentes de Pulgones; La Alberca: Murcia, España, 2017; 60p. [Google Scholar]
- Lugo, T.J.J.; Morales, F. Uso de los aceites esenciales en el control de plagas. Rev. Artrop. Salud. 2017, 7, 1–10. [Google Scholar]
- Smith, H.; Idris, O.; Maboeta, M. Global trends of green pesticide research from 1994 to 2019: A bibliometric analysis. J. Toxicol. 2021, 637516, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Adusei, S.; Azupio, S. Neem: A novel biocide for pest and disease control of plants. J. Chem. 2022, 778554, 1–12. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Huang, L.; Wang, J.; Huang, X. Sophora flavescens Ait.: Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2015, 172, 10–29. [Google Scholar] [CrossRef]
- Ferro, M. A cultural and entomological review of the Osage orange (Maclura pomifera (Raf.) Schneid.) (Moraceae) and the origin and early spread of “hedge apple” folklore. Southeastern Nat. 2014, 13, 1–34. [Google Scholar] [CrossRef]
- Carnevali Fernández-Concha, G.; Tapia-Muñoz, J.L.; Duno de Stefano, R.; Ramírez, I.M. Flora Ilustrada de la Península de Yucatán: Listado Florístico; Centro de Investigación Científica de Yucatán, A.C.: Mérida, Mexico, 2010. [Google Scholar]
- Valencia-Gutiérrez, M.; López-Méndez, M.; Góngora-Chin, R.E.; García-Ramírez, M. Plantas del Patrimonio Campechano y Sus Usos Tradicionales; Universidad Autónoma de Campeche-ISBN: Campeche, México, 2021; 114p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).