Anatomical Mechanisms of Leaf Blade Morphogenesis in Sasaella kogasensis ‘Aureostriatus’
Abstract
1. Introduction
2. Results
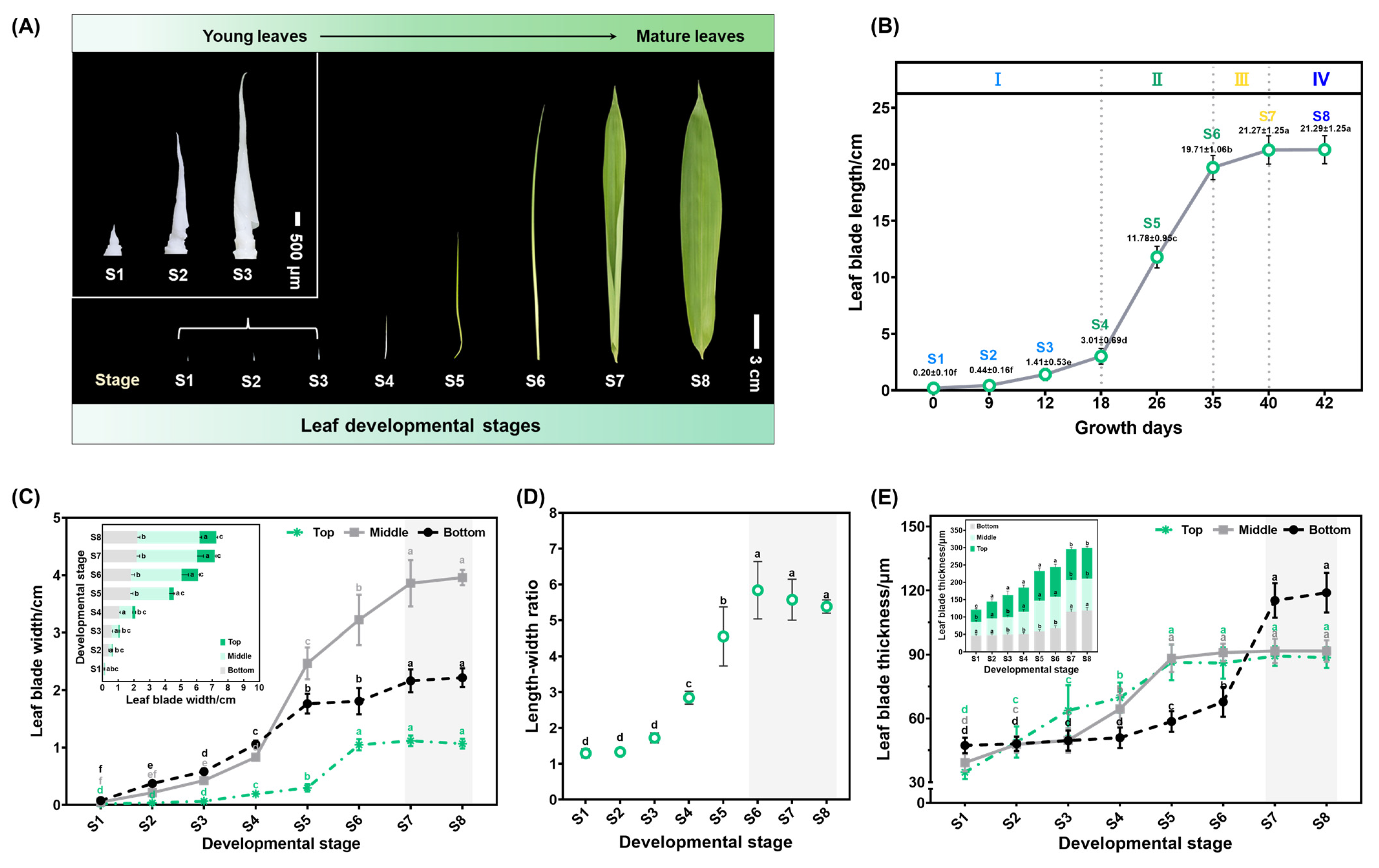
2.1. Morphological Characteristics of Leaf Blade Development of S. kogasensis ‘Aureostriatus’
2.2. Three-Dimensional Morphological Characterization of S. kogasensis ‘Aureostriatus’ Leaf Blades
2.3. Anatomical Characteristics of Leaves of S. kogasensis ‘Aureostriatus’ during Rapid Elongation
2.3.1. Division and Differentiation of Epidermal Cells during Rapid Elongation
2.3.2. Division, Expansion, and Degradation of Mesophyll Cells during Rapid Elongation
2.4. Anatomical Characteristics of the Medial–Lateral and Adaxial–Abaxial Axes during Development of S. kogasensis ‘Aureostriatus’
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. A Survey of Morphological Indicators of Leaf Developmental Processes in S. kogasensis ‘Aureostriatus’
4.3. Investigation of the Growth Pattern of Representative Leaf Blades of S. kogasensis ‘Aureostriatus’
4.4. Cytological Observations of Leaf Epidermal and Mesophyll Cells during the Period of Rapid Elongation
4.5. Observation of the Microstructure of the Leaf Epidermis during Rapid Elongation
4.6. Data Analysis and Graphics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsukaya, H. Organ shape and size: A lesson from studies of leaf morphogenesis. Curr. Opin. Plant Biol. 2003, 6, 57–62. [Google Scholar] [CrossRef]
- Neelima, S. Leaf development in angiosperms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 419–446. [Google Scholar] [CrossRef]
- Didier, R.; Therese, M.; Cris, K. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 2000, 12, 507–518. [Google Scholar] [CrossRef]
- John, L.B.; Yuval, E.; Stuart, F.B. Establishment of polarity in angiosperm lateral organs. Trends Genet. 2002, 18, 134–141. [Google Scholar] [CrossRef]
- Micol, J.L.; Hake, S. The development of plant leaves. Plant Physiol. 2003, 131, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Tomotsugu, K.; Nobutaka, M.; Motoaki, S.; Kazuo, S.; Masaru, O.-T. Tcp transcription factors regulate the activities of asymmetric leaves1 and mir164, as well as the auxin response, during differentiation of leaves in Arabidopsis. Plant Cell 2010, 22, 3574–3588. [Google Scholar] [CrossRef]
- Sarvepalli, K.; Nath, U. Cin-tcp transcription factors: Transiting cell proliferation in plants. IUBMB Life 2018, 70, 718–731. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Y.; Teotia, S.; Zhang, Z.; Tang, G. The making of leaves: How small rna networks modulate leaf development. Front. Plant Sci. 2018, 9, 824. [Google Scholar] [CrossRef]
- Manuela, D.; Xu, M. Patterning a leaf by establishing polarities. Front. Plant Sci. 2020, 11, 568730. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Zhou, Y.; Zhang, Y.; Qin, A.; Yu, X.; Zhao, Z.; Wu, R.; Guo, C.; Bawa, G.; et al. Identification of novel regulators required for early development of vein pattern in the cotyledons by single-cell rna-sequencing. Plant J. 2022, 110, 7–22. [Google Scholar] [CrossRef]
- Detlef, W.; Gerd, J. Stem cells that make stems. Nature 2002, 415, 751–754. [Google Scholar] [CrossRef]
- Braybrook, S.A.; Kuhlemeier, C. How a plant builds leaves. Plant Cell 2010, 22, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Veit, B. Determination of cell fate in apical meristems. Curr. Opin. Plant Biol. 2004, 7, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Canales, C.; Grigg, S.; Tsiantis, M. The formation and patterning of leaves: Recent advances. Planta 2005, 221, 752–756. [Google Scholar] [CrossRef]
- Sun, B.; Looi, L.-S.; Guo, S.; He, Z.; Gan, E.-S.; Huang, J.; Xu, Y.; Wee, W.-Y.; Ito, T. Timing mechanism dependent on cell division is invoked by polycomb eviction in plant stem cells. Science 2014, 343, 1248559. [Google Scholar] [CrossRef]
- Poethig, R. Leaf morphogenesis in flowering plants. Plant Cell 1997, 9, 1077–1087. [Google Scholar] [CrossRef]
- Andrew, H. Axioms and axes in leaf formation? Curr. Opin. Plant Biol. 1999, 2, 56–60. [Google Scholar] [CrossRef]
- Breuninger, H.; Lenhard, M. Control of tissue and organ growth in plants. Curr. Top. Dev. Biol. 2010, 91, 185–220. [Google Scholar] [CrossRef]
- Beemster, G.T.S.; Avramova, V.; Sprangers, K. Kinematic analysis of cell division and expansion: Quantifying the cellular basis of growth and sampling developmental zones in Zea mays leaves. J. Vis. Exp. 2016, 118, e54887. [Google Scholar] [CrossRef]
- Gonzalez, N.; Vanhaeren, H.; Inzé, D. Leaf size control: Complex coordination of cell division and expansion. Trends Plant Sci. 2012, 17, 332–340. [Google Scholar] [CrossRef]
- Isabelle, M.; François, G.; Jean-Louis, D. Generation of form and associated mass deposition during leaf development in grasses: A kinematic approach for non-steady growth. Ann. Bot. 1997, 80, 673–683. [Google Scholar] [CrossRef][Green Version]
- Nath, U.; Crawford, B.C.W.; Carpenter, R.; Coen, E. Genetic control of surface curvature. Science 2003, 299, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Andriankaja, M.; Dhondt, S.; De Bodt, S.; Vanhaeren, H.; Coppens, F.; De Milde, L.; Mühlenbock, P.; Skirycz, A.; Gonzalez, N.; Beemster, G.T.S.; et al. Exit from proliferation during leaf development in Arabidopsis thaliana: A not-so-gradual process. Dev. Cell 2012, 22, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Tomohiko, T.; Hirokazu, T.; Hirofumi, U. Two independent and polarized processes of cell elongation regulate leaf blade. Development 1996, 1222, 1589–1600. [Google Scholar] [CrossRef]
- Beemster, G.T.S.; Veylder, L.D.; Vercruysse, S.; West, G.; Rombaut, D.; Hummelen, P.V.; Galichet, A.; Gruissem, W.; Inzé, D.; Vuylsteke, M. Genome-wide analysis of gene expression profiles associated with cell cycle transitions in growing organs of Arabidopsis. Plant Physiol. 2005, 138, 734–743. [Google Scholar] [CrossRef]
- Kalve, S.; Fotschki, J.; Beeckman, T.; Vissenberg, K.; Beemster, G.T.S. Three-dimensional patterns of cell division and expansion throughout the development of Arabidopsis thaliana leaves. J. Exp. Bot. 2014, 65, 6385–6397. [Google Scholar] [CrossRef]
- Tang, H.B.; Wang, J.; Wang, L.; Shang, G.D.; Xu, Z.G.; Mai, Y.X.; Liu, Y.T.; Zhang, T.Q.; Wang, J.W. Anisotropic cell growth at the leaf base promotes age-related changes in leaf shape in Arabidopsis thaliana. Plant Cell 2023, 35, 1386–1407. [Google Scholar] [CrossRef]
- Schnyder, H.; Seo, S.; Rademacher, I.F.; Kühbauch, W. Spatial distribution of growth rates and of epidermal cell lengths in the elongation zone during leaf development in Lolium perenne L. Planta 1990, 181, 423–431. [Google Scholar] [CrossRef]
- Bertrand, M.; Matthieu, R.; François, T. The elongation rate at the base of a maize leaf shows an invariant pattern. J. Exp. Bot. 2001, 52, 1259–1268. [Google Scholar] [CrossRef]
- Lieve, B.; Fabio, F.; Elizabeth, V.V.; Hans, L. Epidermal cell division and cell elongation in two Aegilops species with contrasting leaf elongation rates. Funct. Plant Biol. 2003, 30, 425–432. [Google Scholar] [CrossRef]
- Fournier, C.; Durand, J.L.; Ljutovac, S.; Schäufele, R.; Gastal, F.; Andrieu, B. A functional–structural model of elongation of the grass leaf and its relationships with the phyllochron. New Phytol. 2005, 166, 881–894. [Google Scholar] [CrossRef]
- Zhu, Y.; Chang, L.; Tang, L.; Jiang, H.; Zhang, W.; Cao, W. Modelling leaf shape dynamics in rice. NJAS Wagening. J. Life Sci. 2009, 57, 73–81. [Google Scholar] [CrossRef]
- Sprangers, K.; Thys, S.; van Dusschoten, D.; Beemster, G.T.S. Gibberellin enhances the anisotropy of cell expansion in the growth zone of the maize leaf. Front. Plant Sci. 2020, 11, 1163. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ding, Y. Observations on megasporogenesis, microsporogenesis and development of the male and female gametophytes of Arundinaria simonii f. Heterophylla. Sci. Silvae Sin. 2013, 49, 168–175. [Google Scholar] [CrossRef]
- Wei, Q.; Jiao, C.; Guo, L.; Ding, Y.; Cao, J.; Feng, J.; Dong, X.; Mao, L.; Sun, H.; Yu, F.; et al. Exploring key cellular processes and candidate genes regulating the primary thickening growth of moso underground shoots. New Phytol. 2016, 214, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jiang, M.; Zhang, L.; Lin, S.; Ding, Y. Fruit morphological characteristics of thirteen bamboo species. J. Plant Resour. Environ. 2016, 25, 96–103. [Google Scholar] [CrossRef]
- Lin, S.; Fu, H.; Wan, Y.; Zhang, S.; Zhu, R.; Wang, F.; Ding, Y. Anther development and floral morphology characteristics of Bambusa oldhami ‘xia zao’ zsx. J. Nanjing For. Univ. 2019, 43, 7–13. [Google Scholar] [CrossRef]
- Wei, Q.; Guo, L.; Jiao, C.; Fei, Z.; Chen, M.; Cao, J.; Ding, Y.; Yuan, Q. Characterization of the developmental dynamics of the elongation of a bamboo internode during the fast growth stage. Tree Physiol. 2019, 39, 1201–1214. [Google Scholar] [CrossRef]
- Chen, M.; Guo, L.; Ramakrishnan, M.; Fei, Z.; Vinod, K.K.; Ding, Y.; Jiao, C.; Gao, Z.; Zha, R.; Wang, C.; et al. Rapid growth of moso bamboo (Phyllostachys edulis): Cellular roadmaps, transcriptome dynamics, and environmental factors. Plant Cell 2022, 34, 3577–3610. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, C.; Yao, W.; Zhang, L.; Ding, Y.; Yang, Z.; Lin, S. Comparative phylogenomic analyses and co-expression gene network reveal insights in flowering time and aborted meiosis in woody bamboo, Bambusa oldhamii ‘xia zao’ zsx. Front. Plant Sci. 2022, 13, 3240. [Google Scholar] [CrossRef]
- Wang, G.; Yu, F.; Wu, H.; Hu, S.; Wu, S.; Pei, N.; Shi, J.; Lambers, H. Roots originating from different shoot parts are functionally different in running bamboo, Phyllostachys glauca. Funct. Ecol. 2023, 37, 1082–1094. [Google Scholar] [CrossRef]
- Yao, W.; Li, C.; Lin, S.; Wang, J.; Fan, T.; Zhao, W. The structures of floral organs and reproductive characteristics of an ornamental bamboo species, Pleioblastus pygmaeus. Hortic. Plant J. 2023, 9, 589–601. [Google Scholar] [CrossRef]
- Ding, Y.; Lin, S.; Wei, Q.; Yao, W.; Que, F.; Li, L. Advances in developmental biology of bamboos. J. Nanjing For. Univ. 2022, 46, 23–40. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, Q. Studies on the comparative anatomy of bamboo leaves and its significance for bamboo systematic taxonomy. J. Nanjing For. Univ. 1994, 18, 1–6. [Google Scholar]
- Yue, Y.; Cao, H.; Tang, F. Advance in bamboo chemical in gredients and its utilizations. J. Anhui Agric. Univ. 2007, 34, 328–333. [Google Scholar] [CrossRef]
- Choi, D.; Cho, K.-A.; Na, M.-S.; Choi, H.-S.; Kim, Y.-O.; Lim, D.-H.; Cho, S.J.; Cho, H. Effect of bamboo oil on antioxidative activity and nitrite scavenging activity. J. Ind. Eng. Chem. 2008, 14, 765–770. [Google Scholar] [CrossRef]
- Nirmala, C.; Bisht, M.S.; Bajwa, H.K.; Santosh, O. Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci. Technol. 2018, 77, 91–99. [Google Scholar] [CrossRef]
- Su, J.; Lin, S.; Shi, W.; Wang, X.; Zheng, X.; Wan, Y.; Ding, Y. Anatomical observation and three- dimensional construction of leaf blades from six bamboos. J. Nanjing For. Univ. 2020, 44, 47–53. [Google Scholar] [CrossRef]
- Gao, Z.; Guo, L.; Chen, M.; Yu, F.; Wei, Q. Characterization of the development dynamics within the linear growth bamboo leaf. Physiol. Plant. 2021, 172, 1518–1534. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, Z.; Xiang, Y.; Guo, L.; Zhang, C.; Que, F.; Yu, F.; Wei, Q. Characterization of anatomical features, developmental roadmaps, and key genes of bamboo leaf epidermis. Physiol. Plant. 2022, 174, e13822. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, Z.; Wang, L.; Wang, J.; Wang, S.; Fei, B.; Chen, C.; Shi, C.; Liu, X.; Zhang, H.; et al. Chromosome-level reference genome and alternative splicing atlas of moso bamboo (Phyllostachys edulis). GigaScience 2018, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hu, Y.; Li, J.; Yu, Z.; Guo, Q. Genome-wide identification and expression analysis of the plant u-box protein gene family in Phyllostachys edulis. Front. Genet. 2021, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Lieven, D.V.; Tom, B.; Gerrit, T.S.B.; Luc, K.; Franky, T.; Isabelle, L.; Van Der Schueren, E.; Sara, M.; Mirande, N.; Dirk, I. Functional analysis of cyclin-dependent kinase iinhibitors of Arabidopsis. Plant Cell 2001, 13, 1653–1667. [Google Scholar] [CrossRef]
- Chen, A.; Zhao, W.; Ruan, Y.; Guo, C.; Zhang, W.; Shi, J.; Yang, G.; Yu, F. Pattern of emergence and degradation of Phyllostachys edulis ‘pachyloen’ shoot and the changes of nutrient composition during degradation. Sci. Silvae Sin. 2019, 55, 32–40. [Google Scholar] [CrossRef]
- Liu, P.; Dong, W.; Zheng, J.; Pu, C.; Zhang, M. Anatiomical strucyure of Qiongzhuea tumidinoda leaves. J. Northwest For. Univ. 2018, 33, 110–115. [Google Scholar] [CrossRef]
- Long, C.; Liu, T.; Yu, F.; Yang, Q.; Shi, J.; Yang, G. Comparative anatomy of leaves between Phyllostachys edulis ‘pachyloen’ and Phyllostachys edulis. J. Anhui Agric. Univ. 2014, 42, 39–44. [Google Scholar] [CrossRef]
- Zheng, J.; Dong, W.; Liu, P.; Yin, Z.; Wu, Y. Anatomical structure of Neosinocalamus affinis leaves. For. Inventory Plan. 2019, 44, 148–152. [Google Scholar] [CrossRef]
- Muller, B.; Bourdais, G.; Reidy, B.; Bencivenni, C.; Massonneau, A.S.; Condamine, P.; Rolland, G.L.; Conéjéro, G.V.; Rogowsky, P.; Tardieu, F.O. Association of specific expansins with growth in maize leaves is maintained under environmental, genetic, and developmental sources of variation. Plant Physiol. 2007, 143, 278–290. [Google Scholar] [CrossRef]
- Macadam, J.W.; Nelson, C.J. Specific leaf weight in zones of cell division, elongation and maturation in tall fescue leaf blades. Ann. Bot. 1987, 59, 369–376. [Google Scholar] [CrossRef]
- Nelissen, H.; Rymen, B.; Coppens, F.; Dhondt, S.; Fiorani, F.; Beemster, G.T.S. Kinematic analysis of cell division in leaves of mono- and dicotyledonous species: A basis for understanding growth and developing refined molecular sampling strategies. Methods Mol. Biol. 2013, 959, 247–264. [Google Scholar] [CrossRef]
- Das Gupta, M.; Nath, U. Divergence in patterns of leaf growth polarity is associated with the expression divergence of mir396. Plant Cell 2015, 27, 2785–2799. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, P.M.; Bonetta, D.; Tsukaya, H.; Dengler, R.E.; Dengler, N.G. Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev. Biol. 1999, 215, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.H.; Nelson, C.J. Elongation of the grass leaf and its relationship to the phyllochron. Crop Sci. 1995, 35, 4–10. [Google Scholar] [CrossRef]
- MacAdam, J.W.; Volenec, J.J.; Nelson, C.J. Effects of nitrogen on mesophyll cell division and epidermal cell elongation in tall fescue leaf blades. Plant Physiol. 1989, 89, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Brandis, D. Remarks on the structure of bamboo leaves. Trans. Linn. Soc. Lond. Ser. Bot. 1907, 7, 69–92. [Google Scholar] [CrossRef]
- Yang, S.M.; Jiang, Z.H.; Ren, H.Q. Present status and advances in anatomical characteristics of bamboo. World Bamboo Ratt. 2006, 3, 1–6+12. [Google Scholar] [CrossRef]
- Vega, A.S.; Castro, M.A.; Guerreiro, C. Ontogeny of fusoid cells in Guadua species (Poaceae, Bambusoideae, Bambuseae): Evidence for transdifferentiation and possible functions. Flora-Morphol. Distrib. Funct. Ecol. Plants 2016, 222, 13–19. [Google Scholar] [CrossRef]
- Metcalfe, C.R. Some thoughts on the structure of bamboo leaves. Bot. Mag. 1956, 69, 391–400. [Google Scholar] [CrossRef]
- Leandro, T.D.; Rodrigues, T.M.; Clark, L.G.; Scatena, V.L. Fusoid cells in the grass family Poaceae (Poales): A developmental study reveals homologies and suggests new insights into their functional role in young leaves. Ann. Bot. 2018, 122, 833–848. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Fu, H.J.; Su, J.J.; Wan, Y.W.; Zhang, C.X.; Ding, Y.L.; Lin, S.Y. Desilication of bamboo leaves for paraffin slices preparation and their cytological study on eleven bamboo species. J. Nanjing For. Univ. 2019, 43, 74–80. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Lv, Z.; Zhang, H.; Yue, J.; Zhang, X.; Li, L.; Huang, F.; Lin, S. Anatomical Mechanisms of Leaf Blade Morphogenesis in Sasaella kogasensis ‘Aureostriatus’. Plants 2024, 13, 332. https://doi.org/10.3390/plants13030332
Zhao W, Lv Z, Zhang H, Yue J, Zhang X, Li L, Huang F, Lin S. Anatomical Mechanisms of Leaf Blade Morphogenesis in Sasaella kogasensis ‘Aureostriatus’. Plants. 2024; 13(3):332. https://doi.org/10.3390/plants13030332
Chicago/Turabian StyleZhao, Wanqi, Zhuo Lv, Hanjiao Zhang, Jiahui Yue, Xu Zhang, Long Li, Feiyi Huang, and Shuyan Lin. 2024. "Anatomical Mechanisms of Leaf Blade Morphogenesis in Sasaella kogasensis ‘Aureostriatus’" Plants 13, no. 3: 332. https://doi.org/10.3390/plants13030332
APA StyleZhao, W., Lv, Z., Zhang, H., Yue, J., Zhang, X., Li, L., Huang, F., & Lin, S. (2024). Anatomical Mechanisms of Leaf Blade Morphogenesis in Sasaella kogasensis ‘Aureostriatus’. Plants, 13(3), 332. https://doi.org/10.3390/plants13030332





