Phytochemical Elucidation and Effect of Maesa indica (Roxb.) Sweet on Alleviation of Potassium Dichromate-Induced Pulmonary Damage in Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Metabolic Profiling Using T-TOF LC/MS/MS
2.1.1. Flavonoids
Flavonol Derivatives
Flavone Derivatives
Flavanone Derivatives
Flavanol Derivatives
Phenolic Acids
Alkaloids
2.2. In-Vivo Study
2.2.1. Acute Toxicity
2.2.2. Investigation of the Lung Protection Activity of ME
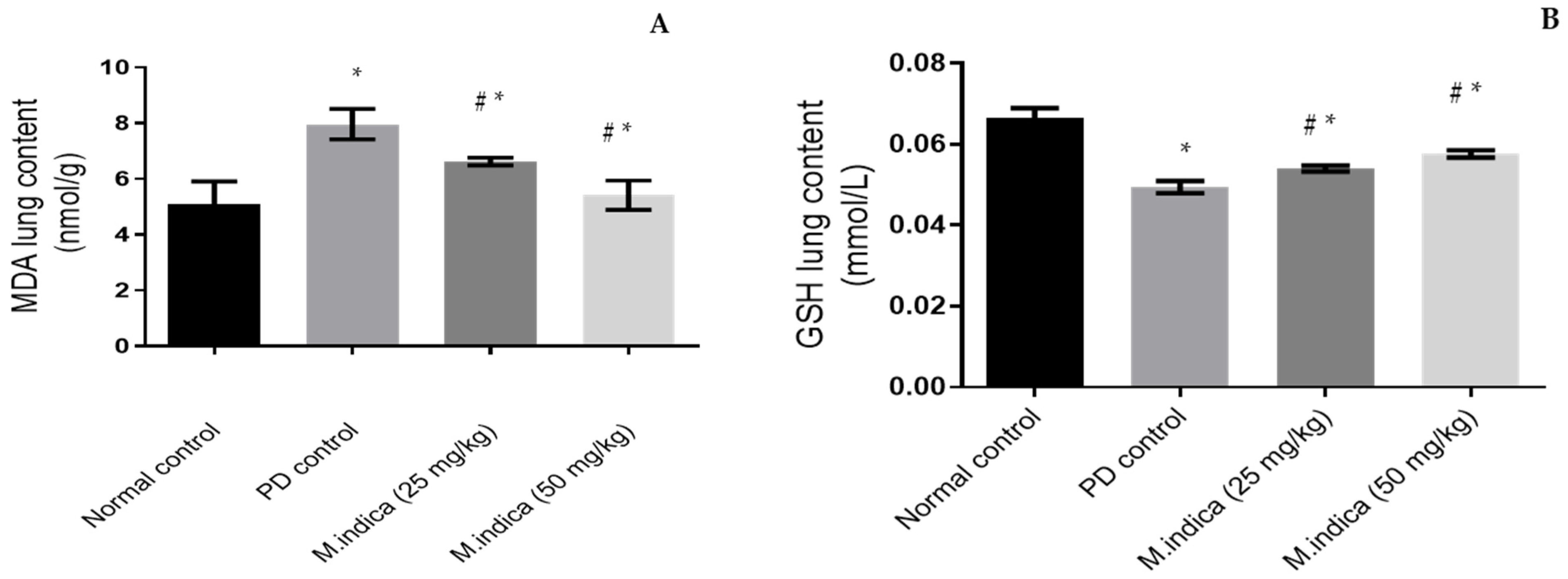
Effect of ME on GSH and MDA Lung Contents
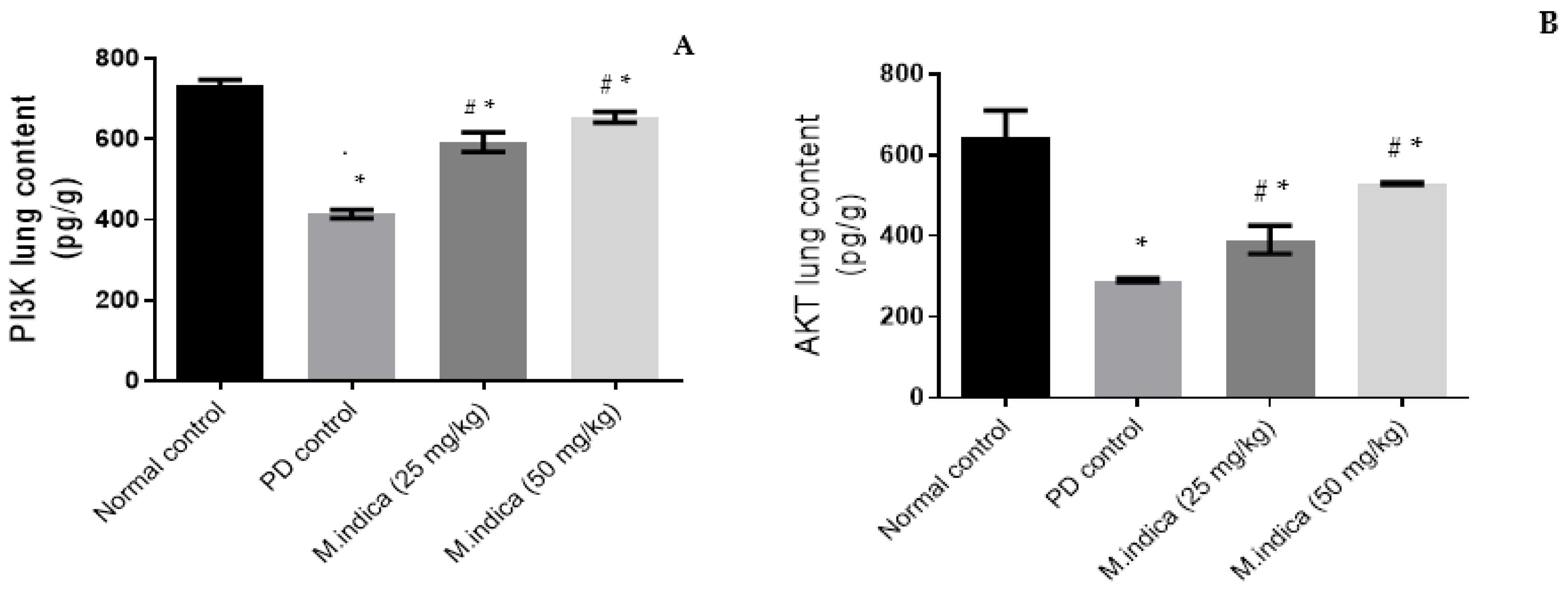
Effect of ME on AKt and PI3K Lung Contents
Histopathological Findings
3. Materials and Methods
3.1. Plant Materials
3.2. Extraction of Plant Materials
3.3. Metabolic Profiling Using T-TOF LC/MS/MS
3.3.1. Chemicals
3.3.2. Instrument
3.3.3. Chromatographic Conditions
3.3.4. Sample Preparation
3.4. The In-Vivo Study
3.4.1. Acute Toxicity Study
3.4.2. Investigation of Pulmonary Protection Activity
Animals
Drugs, Chemicals and Kits
Experimental Design of Pulmonary Damage
Biochemical Analysis
Estimation of MDA and GSH
Estimation of AKt and PI3K
Histological Examination
3.4.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bajpe, S.N.; Marulasiddaswamy, K.M.; Ramu, R.; Badiger, A.S.; Rudrappa, M.K.; Kini, R.K. Assessing DNA Barcodes Species Discriminating Ability and Phylogenetic Relation within Embelia Species. J. Appl. Biol. Biotechnol. 2022, 10, 2–7. [Google Scholar] [CrossRef]
- Shanmugam, S.; Baby, J.P.; Chandran, R.; Thankarajan, S.; Thangaraj, P. Maesa Indica: A Nutritional Wild Berry Rich in Polyphenols with Special Attention to Radical Scavenging and Inhibition of Key Enzymes, α-Amylase and α-Glucosidase. J. Food Sci. Technol. 2016, 53, 2957–2965. [Google Scholar] [CrossRef]
- Devarajan, N.; Ramalingam, S.; Subramaniam, S.M. Gas Chromatography Mass Spectroscopy Chromatogram and Antimicrobial Activity of Leaf Extracts of Blepharis maderaspatensis and Maesa indica. J. Herbs Spices Med. Plants 2015, 21, 267–282. [Google Scholar] [CrossRef]
- Jassim, S.A.A.; Naji, M.A. Novel Antiviral Agents: A Medicinal Plant Perspective. J. Appl. Microbiol. 2003, 95, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, F.A.M.; El-Hawary, S.S.; Abd El-Kader, E.M.; Alshehri, S.A.; Rabeh, M.A.; El-Mosallamy, A.E.M.K.; El Raey, M.A.; El Gedaily, R.A. Phytochemical Profiling and Antiviral Activity of Green Sustainable Nanoparticles Derived from Maesa indica (Roxb.) Sweet against Human Coronavirus 229E. Plants 2023, 12, 2813. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.A.; Salama, A.M.; El-Toumy, S.A.; Salama, A.A.A.; Tadros, S.H.; Gedaily, R.A.E. Novel Neuroprotective Potential of Bunchosia armeniaca (Cav.) DC against Lipopolysaccharide Induced Alzheimer’s Disease in Mice. Plants 2022, 11, 1792. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Lian, J.; Lin, J.; Zakaria, N.; Yahaya, B.H. Acute Lung Injury: Disease Modelling and the Therapeutic Potential of Stem Cells. In Cell Biology and Translational Medicine, Volume 10: Stem Cells in Tissue Regeneration; Springer: Berlin/Heidelberg, Germany, 2020; pp. 149–166. [Google Scholar]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef]
- Salama, A.; Fayed, H.M.; Elgohary, R. L-Carnitine Alleviated Acute Lung Injuries Induced by Potassium Dichromate in Rats: Involvement of Nrf2/HO-1 Signaling Pathway. Heliyon 2021, 7, e07207. [Google Scholar] [CrossRef]
- Coetzee, J.J.; Bansal, N.; Chirwa, E.M.N. Chromium in Environment, Its Toxic Effect from Chromite-Mining and Ferrochrome Industries, and Its Possible Bioremediation. Expo. Health 2020, 12, 51–62. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in Metal-Induced Oxidative Stress and Human Disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, H.; Shimizu, Y.; Iwamae, S.; Hisada, T.; Ishizuka, T.; Iizuka, K.; Dobashi, K.; Mori, M. Evidence of Oxidative Stress in Asthma and COPD: Potential Inhibitory Effect of Theophylline. Respir. Med. 2000, 94, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, L.; Wu, X.; Li, R.; Wen, J.; Sha, J.; Wen, X. The PI3K/AKT Pathway in the Pathogenesis of Prostate Cancer. Front. Biosci. 2016, 21, 1084–1091. [Google Scholar]
- Meng, L.; Li, L.; Lu, S.; Li, K.; Su, Z.; Wang, Y.; Fan, X.; Li, X.; Zhao, G. The Protective Effect of Dexmedetomidine on LPS-Induced Acute Lung Injury through the HMGB1-Mediated TLR4/NF-κB and PI3K/Akt/MTOR Pathways. Mol. Immunol. 2018, 94, 7–17. [Google Scholar] [CrossRef] [PubMed]
- El-Gazar, A.A.; Emad, A.M.; Ragab, G.M.; Rasheed, D.M. Mentha pulegium L. (Pennyroyal, Lamiaceae) Extracts Impose Abortion or Fetal-Mediated Toxicity in Pregnant Rats; Evidenced by the Modulation of Pregnancy Hormones, MiR-520, MiR-146a, TIMP-1 and MMP-9 Protein Expressions, Inflammatory State, Certain Relate. Toxins 2022, 14, 347. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Samiotaki, M.; Panayotou, G.; Oreopoulou, V. Characterization of Flavonoid Subgroups and Hydroxy Substitution by HPLC-MS/MS. Molecules 2007, 12, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol Profiles of Vitis Vinifera Red Grapes and Their Single-Cultivar Wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Carle, R.; Berardini, N.; Mihalev, K.; Mollov, P. Flavonol Glycosides from Distilled Petals of Rosa Damascena Mill. Z. Naturforsch. Sect. C J. Biosci. 2005, 60, 379–384. [Google Scholar] [CrossRef]
- Hvattum, E. Determination of Phenolic Compounds in Rose Hip (Rosa canina) Using Liquid Chromatography Coupled to Electrospray Ionisation Tandem Mass Spectrometry and Diode-Array Detection. Rapid Commun. Mass Spectrom. 2002, 16, 655–662. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass Spectrometry in the Structural Analysis of Flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Abd Elkarim, A.S.; Abdelwahed, N.A.M.; Omer, E.A.; Elgamal, A.M.; ELsayed, W.M. Chenopodium murale Juice Shows Anti-Fungal Efficacy in Experimental Oral Candidiasis in Immunosuppressed Rats in Relation to Its Chemical Profile. Molecules 2023, 28, 4304. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Wen, J.-H.; Li, P.; Gao, W.; Yang, H. Target Profiling of Flavonol Glycosides in the Extract of Ginkgo biloba Leaf and Their Pharmacokinetics in Rat Plasma by Ultra-High-Performance Liquid Chromatography with Tandem Mass Spectrometry. J. Sep. Sci. 2022, 45, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Guijarro-Díez, M.; Nozal, L.; Marina, M.L.; Crego, A.L. Metabolomic Fingerprinting of Saffron by LC/MS: Novel Authenticity Markers. Anal. Bioanal. Chem. 2015, 407, 7197–7213. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Fernández, M.A.; Cerezo, A.B.; Canete-Rodriguez, A.M.; Troncoso, A.M.; García-Parrilla, M.C. Composition of Nonanthocyanin Polyphenols in Alcoholic-Fermented Strawberry Products Using LC–MS (QTRAP), High-Resolution MS (UHPLC-Orbitrap-MS), LC-DAD, and Antioxidant Activity. J. Agric. Food Chem. 2015, 63, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Khan, R.A.; Abdel-Hafez, A.A.; Abdel-Aziz, M.; Ahmed, E.; Enany, S.; Mahgoub, S.; Al-Rugaie, O.; Alsharidah, M.; Aly, M.S.A.; et al. Phytochemical Profiling, in Vitro and in Silico Anti-Microbial and Anti-Cancer Activity Evaluations and Staph GyraseB and h-TOP-IIβ Receptor-Docking Studies of Major Constituents of Zygophyllum coccineum L. Aqueous-Ethanolic Extract and Its Subsequent F. Molecules 2021, 26, 577. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, P. Combination of HPLC-Q-TOF-MS/MS, Network Pharmacology, and Molecular Docking to Reveal the Mechanism of Apple Pollen in the Treatment of Type 2 Diabetes Mellitus. Evid. Based Complement. Altern. Med. 2022, 2022, 3221196. [Google Scholar] [CrossRef]
- Attallah, N.G.M.; Negm, W.A.; Elekhnawy, E.; Elmongy, E.I.; Altwaijry, N.; El-Haroun, H.; El-Masry, T.A.; El-Sherbeni, S.A. Elucidation of Phytochemical Content of Cupressus macrocarpa Leaves: In Vitro and in Vivo Antibacterial Effect against Methicillin-Resistant Staphylococcus aureus Clinical Isolates. Antibiotics 2021, 10, 890. [Google Scholar] [CrossRef]
- Gholamalipour Alamdari, E.; Taleghani, A. New Bioactive Compounds Characterized by Liquid Chromatography–Mass Spectrometry and Gas Chromatography–Mass Spectrometry in Hydro-Methanol and Petroleum Ether Extracts of Prosopis Farcta (Banks & Sol.) JF Macbr Weed. J. Mass Spectrom. 2022, 57, e4884. [Google Scholar]
- Śliwka-Kaszyńska, M.; Anusiewicz, I.; Skurski, P. The Mechanism of a Retro-Diels–Alder Fragmentation of Luteolin: Theoretical Studies Supported by Electrospray Ionization Tandem Mass Spectrometry Results. Molecules 2022, 27, 1032. [Google Scholar] [CrossRef]
- Zhou, L.; Li, J.; Yan, C. Simultaneous Determination of Three Flavonoids and One Coumarin by LC–MS/MS: Application to a Comparative Pharmacokinetic Study in Normal and Arthritic Rats after Oral Administration of Daphne Genkwa Extract. Biomed. Chromatogr. 2018, 32, e4233. [Google Scholar] [CrossRef]
- Agus, S.; Achmadi, S.S.; Mubarik, N.R. Antibacterial Activity of Naringenin-Rich Fraction of Pigeon Pea Leaves toward Salmonella thypi. Asian Pac. J. Trop. Biomed. 2017, 7, 725–728. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Kerhoas, L.; Einhorn, J.; Ducrot, P.-H. Application of ESI/MS, CID/MS and Tandem MS/MS to the Fragmentation Study of Eriodictyol 7-O-Glucosyl-(1→2)-Glucoside and Luteolin 7-O-Glucosyl-(1→2)-Glucoside. Int. J. Mass Spectrom. 2005, 247, 93–100. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Mullen, W.; Burns, J.; Lean, M.E.J.; Brighenti, F.; Crozier, A. HPLC-MSn Analysis of Phenolic Compounds and Purine Alkaloids in Green and Black Tea. J. Agric. Food Chem. 2004, 52, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Saibabu, V.; Fatima, Z.; Khan, L.A.; Hameed, S. Therapeutic Potential of Dietary Phenolic Acids. Adv. Pharmacol. Pharm. Sci. 2015, 2015, 823539. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, H.; Li, Z.; Jiang, K.; Lee, M.-R. HPLC/QTOF-MS/MS Application to Investigate Phenolic Constituents from Ficus pandurata H. Aerial Roots. Biomed. Chromatogr. 2015, 29, 860–868. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Lozano-Castellón, J.; Mardones, C.; Pérez, A.J.; Saéz, V.; Riquelme, S.; von Baer, D.; Vallverdú-Queralt, A. Phenolic Profile of Grape Canes: Novel Compounds Identified by Lc-Esi-Ltq-Orbitrap-Ms. Molecules 2019, 24, 3763. [Google Scholar]
- Jiang, M.; Fu, S.; Chen, K.; Li, Q.; Jiang, W. Pharmacokinetic Analysis of Rosmarinic Acid and Its Analog in Rat Plasma Using Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. Sci. 2022, 60, 511–517. [Google Scholar] [CrossRef]
- Kurek, J. Alkaloids: Their Importance in Nature and Human Life; BoD—Books on Demand: Norderstedt, Germany, 2019. [Google Scholar]
- Kowmudi, G.; Nagappan, K.; Anoop, K.; Sailaja, M.; Narenderan, S.T. A Validated LC-MS/MS Method for the Quantification of Trigonelline in Marketed Dietary Supplements. Curr. Bioact. Compd. 2020, 16, 687–695. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Phenolics and Flavonoids Contents of Medicinal Plants, as Natural Ingredients for Many Therapeutic Purposes-A Review. IOSR J. Pharm 2020, 10, 42–81. [Google Scholar]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, Flavonoid-Rich Foods, and Cardiovascular Risk: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef]
- Salem, H.F.; Moubarak, G.A.; Ali, A.A.; Salama, A.A.A.; Salama, A.H. Budesonide-Loaded Bilosomes as a Targeted Delivery Therapeutic Approach against Acute Lung Injury in Rats. J. Pharm. Sci. 2023, 112, 760–770. [Google Scholar] [CrossRef]
- Liu, S.-C.; Lin, J.-T.; Wang, C.-K.; Chen, H.-Y.; Yang, D.-J. Antioxidant Properties of Various Solvent Extracts from Lychee (Litchi chinenesis Sonn.) Flowers. Food Chem. 2009, 114, 577–581. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila Aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Elgohary, R.; Amin, M.M.; Abd Elwahab, S. Immunomodulatory Effect of Protocatechuic Acid on Cyclophosphamide Induced Brain Injury in Rat: Modulation of Inflammosomes NLRP3 and SIRT1. Eur. J. Pharmacol. 2022, 932, 175217. [Google Scholar] [CrossRef] [PubMed]
- Afifi, N.A.; Ibrahim, M.A.; Galal, M.K. Hepatoprotective Influence of Quercetin and Ellagic Acid on Thioacetamide-Induced Hepatotoxicity in Rats. Can. J. Physiol. Pharmacol. 2018, 96, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Seeram, N.P.; Lee, R.; Feng, L.; Heber, D. Isolation and Identification of Strawberry Phenolics with Antioxidant and Human Cancer Cell Antiproliferative Properties. J. Agric. Food Chem. 2008, 56, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Sen, S.; Chanu, N.R.; Singh, A.B.; Lyngkhoi, C.; Kharlyngdoh, S.; Kalita, P. An Ethnomedicinal Survey of Antidiabetic Plants and Preliminary Evaluation of Antioxidant, Hypoglycemic Activity Maesa indica Leaves, a Folk Antidiabetic Plant of Manipur, India. Curr. Tradit. Med. 2021, 7, 286–294. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Hosseini, P.; Mehrabani, M.; Siahpoosh, A.; Goudarzi, M.; Khalili, H.; Malayeri, A. Attenuation of Bleomycin-Induced Pulmonary Fibrosis in Wistar Rats by Combination Treatment of Two Natural Phenolic Compounds: Quercetin and Gallic Acid. Nutr. Cancer 2021, 73, 2039–2049. [Google Scholar] [CrossRef]
- Elisha, I.L.; Dzoyem, J.-P.; McGaw, L.J.; Botha, F.S.; Eloff, J.N. The Anti-Arthritic, Anti-Inflammatory, Antioxidant Activity and Relationships with Total Phenolics and Total Flavonoids of Nine South African Plants Used Traditionally to Treat Arthritis. BMC Complement. Altern. Med. 2016, 16, 307. [Google Scholar] [CrossRef]
- Itu, M.H.; Islam, M.; Repon, A.U.; Mozumder, M.O.F.; Aziz, A.I.; Barua, N.; Ahfter, F.; Khan, M.F.; Choudhury, K.A.A. Investigation of Phytochemicals and in Vitro Anti-Arthritic Activity of Methanol Extract of Maesa Indica (Roxb.) Leaves. J. Pharmacogn. Phytochem. 2019, 8, 319–323. [Google Scholar]
- Rashidi, R.; Rezaee, R.; Shakeri, A.; Hayes, A.W.; Karimi, G. A Review of the Protective Effects of Chlorogenic Acid against Different Chemicals. J. Food Biochem. 2022, 46, e14254. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, H.; Zhang, Y.; Zhang, Z. Protective Effects of Chlorogenic Acid on Cerebral Ischemia/Reperfusion Injury Rats by Regulating Oxidative Stress-Related Nrf2 Pathway. Drug Des. Devel. Ther. 2020, 14, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Takashima, K.; Matsushima, M.; Hashimoto, K.; Nose, H.; Sato, M.; Hashimoto, N.; Hasegawa, Y.; Kawabe, T. Protective Effects of Intratracheally Administered Quercetin on Lipopolysaccharide-Induced Acute Lung Injury. Respir. Res. 2014, 15, 150. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.-T.; Xiao, L.; Zhu, L.; Wang, Q.; Yan, T. Anti-Inflammatory Effects of Apigenin in Lipopolysaccharide-Induced Inflammatory in Acute Lung Injury by Suppressing COX-2 and NF-KB Pathway. Inflammation 2014, 37, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, N.S.; Jaafar, I.S. Natural Products as A Promising Therapy for SARS CoV-2; An Overview. Iraqi J. Pharm. Sci. 2021, 30, 29–40. [Google Scholar] [CrossRef]
- Yang, C.; Yang, W.; He, Z.; He, H.; Yang, X.; Lu, Y.; Li, H. Kaempferol Improves Lung Ischemia-Reperfusion Injury via Antiinflammation and Antioxidative Stress Regulated by SIRT1/HMGB1/NF-κB Axis. Front. Pharmacol. 2020, 10, 1635. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-H.; Yang, J.-J.; Yang, M.-L.; Li, Y.-C.; Kuan, Y.-H. Rutin Decreases Lipopolysaccharide-Induced Acute Lung Injury via Inhibition of Oxidative Stress and the MAPK–NF-κB Pathway. Free Radic. Biol. Med. 2014, 69, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Uzun, F.G.; Demir, F.; Kalender, S.; Bas, H.; Kalender, Y. Protective Effect of Catechin and Quercetin on Chlorpyrifos-Induced Lung Toxicity in Male Rats. Food Chem. Toxicol. 2010, 48, 1714–1720. [Google Scholar] [CrossRef]
- Zhao, M.; Li, C.; Shen, F.; Wang, M.; Jia, N.; Wang, C. Naringenin Ameliorates LPS-Induced Acute Lung Injury through Its Anti-Oxidative and Anti-Inflammatory Activity and by Inhibition of the PI3K/AKT Pathway. Exp. Ther. Med. 2017, 14, 2228–2234. [Google Scholar] [CrossRef]
- Sanbongi, C.; Takano, H.; Osakabe, N.; Sasa, N.; Natsume, M.; Yanagisawa, R.; Inoue, K.; Kato, Y.; Osawa, T.; Yoshikawa, T. Rosmarinic Acid Inhibits Lung Injury Induced by Diesel Exhaust Particles. Free Radic. Biol. Med. 2003, 34, 1060–1069. [Google Scholar] [CrossRef]
- Taylan, M.; Kaya, H.; Demir, M.; Evliyaoğlu, O.; Sen, H.S.; Fırat, U.; Keles, A.; Yilmaz, S.; Sezgi, C. The Protective Effects of Caffeic Acid Phenethyl Ester on Acetylsalicylic Acid-Induced Lung Injury in Rats. J. Investig. Surg. 2016, 29, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Abdalhameid, E.; El-Haleim, A.; Enas, A.; Abdelsalam, R.M.; Georgy, G.S.; Fawzy, H.M.; Kenawy, S.A. Cinnamic Acid Mitigates Methotrexate-Induced Lung Fibrosis in Rats: Comparative Study with Pirfenidone. Naunyn. Schmiedeberg’s Arch. Pharmacol. 2023, 397, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Du, L.; Zhao, L.; Shang, R.; Liu, D.; Jing, Q.; Liang, J.; Ren, Y. The Total Alkaloids of Aconitum Tanguticum Protect against Lipopolysaccharide-Induced Acute Lung Injury in Rats. J. Ethnopharmacol. 2014, 155, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Shang, J.-H.; Pu, S.-B.; Wang, H.-S.; Wang, B.; Liu, L.; Liu, Y.-P.; Hong-Mei, S.; Luo, X.-D. Effect of Total Alkaloids from Alstonia Scholaris on Airway Inflammation in Rats. J. Ethnopharmacol. 2016, 178, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Mieczan, T.; Wójcik, G. Importance of Redox Equilibrium in the Pathogenesis of Psoriasis—Impact of Antioxidant-Rich Diet. Nutrients 2020, 12, 1841. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M.; Mehta, M.; Paudel, K.R.; Satija, S.; Chellappan, D.K.; Dureja, H.; Gupta, G.; Tambuwala, M.M.; Negi, P.; et al. Plants Derived Therapeutic Strategies Targeting Chronic Respiratory Diseases: Chemical and Immunological Perspective. Chem. Biol. Interact. 2020, 325, 109125. [Google Scholar] [CrossRef] [PubMed]
- El Kady, W.M.; Salama, A.A.A.; Desoukey, S.Y.; Hagag, E.G.; El-Shenawy, S.M.; El-Shanawany, M.A. Comparative DNA Profiling, Botanical Identification and Biological Evaluation of Gazania longiscapa DC and Gazania rigens L. Bull. Fac. Pharm. Cairo Univ. 2015, 53, 129–145. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Salama, A.; Salama, R.A.A. Dunaliella Salina Attenuates Diabetic Neuropathy Induced by STZ in Rats: Involvement of Thioredoxin. Biomed Res. Int. 2020, 2020, 1295492. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic Method for Quantitative Determination of Nanogram Amounts of Total and Oxidized Glutathione: Applications to Mammalian Blood and Other Tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Basha, M.; Salama, A.; Noshi, S.H. Soluplus®based Solid Dispersion as Fast Disintegrating Tablets: A Combined Experimental Approach for Enhancing the Dissolution and Antiulcer Efficacy of Famotidine. Drug Dev. Ind. Pharm. 2020, 46, 253–263. [Google Scholar] [CrossRef]
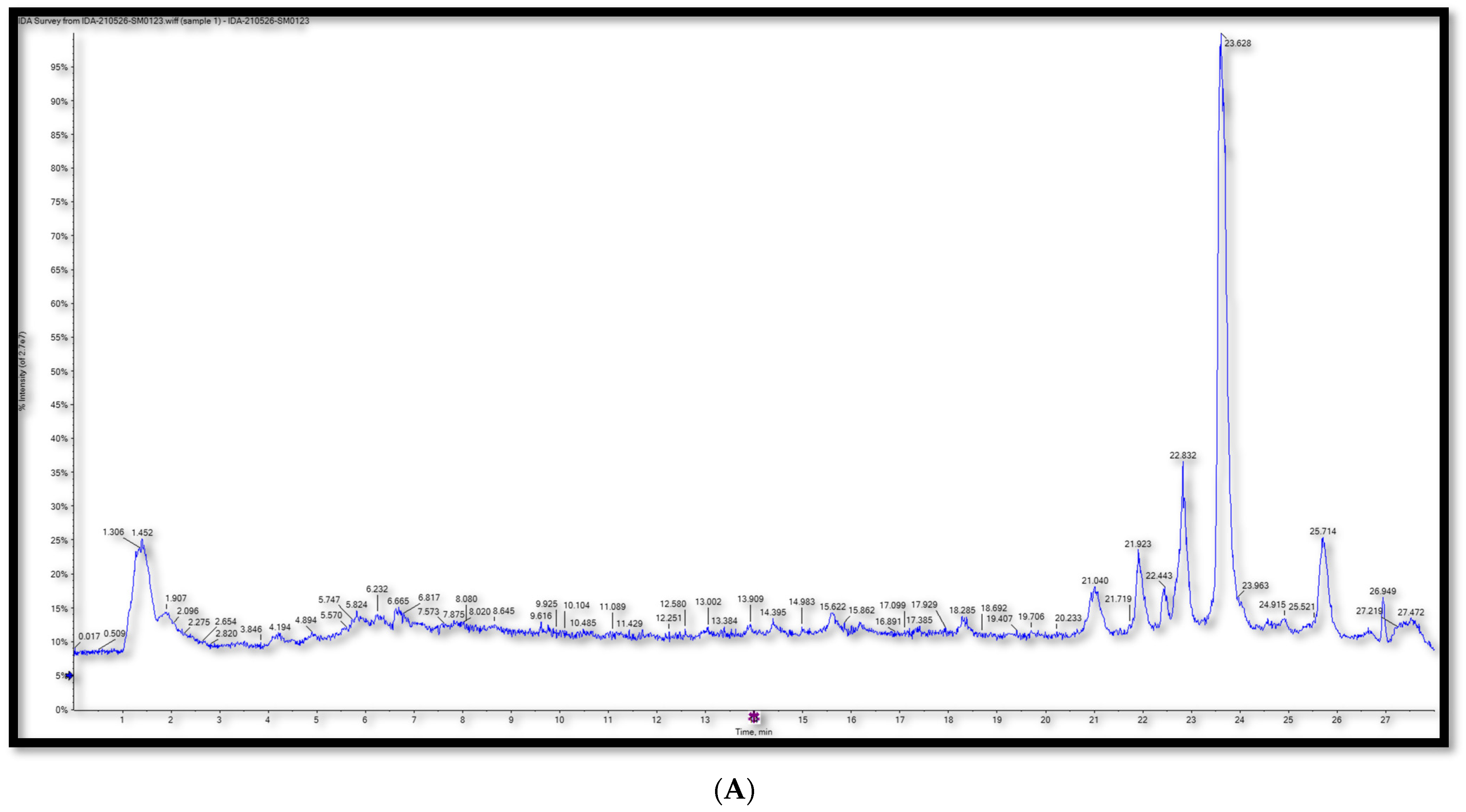


| No. | RT (min) | Mol. Ion m/z | Identified Compound | Molecular Formula | Error (ppm) | Fragment Ions | |
|---|---|---|---|---|---|---|---|
| [M − H]− | [M + H]+ | ||||||
| Flavonols | |||||||
| 3. | 1.22153 | 319.0688 | 317.0546 | Myricetin | C15H10O8 | 1.8 | 317.0546, 281.1009, 225.0102 |
| 7. | 1.33837 | 477.0106 | Quercetin-3-Glucuronide | C21H18O13 | −1.3 | 477.0106, 301.0204, 271.0723, 242.9609, 163.0403, 151.0251 | |
| 10. | 1.41537 | 433.0459 | Quercetin-3-D-xyloside | C20H18O11 | −0.8 | 433.0459, 301.0204 | |
| 29. | 4.7067 | 593.1576 | Kaempferol-7-neohesperidoside | C27H30O15 | −1.8 | 593.1576, 285.0364 | |
| 30. | 4.90885 | 609.1462 | 611.1603 | Rutin | C27H30O16 | −1 | 611.1603, 303.110, 143.03 |
| 34. | 5.39382 | 315.1088 | 3′-methoxy-4′,5,7-trihydroxyflavonol | C16H12O7 | 1 | 315.1088, 300.236 | |
| 36. | 6.16062 | 463.0924 | Quercetin-4′-glucoside | C21H20O12 | −5.9 | 463.0924, 301.0264 | |
| 37. | 6.22428 | 447.0976 | Quercitrin (Quercetin-3-O-rhamnoside) | C21H20O11 | −7.9 | 477.0996, 429.2153, 401.1227, 301.2084 | |
| 41. | 6.62018 | 447.0948 | Kaempferol-3-O-glucoside | C21H20O11 | −1.7 | 447.0948, 285.0409, 255.025 | |
| 42. | 6.73458 | 477.0996 | Isorhamnetin-3-O-glucoside | C22H22O12 | 7.2 | 477.0996, 315.0204, 301.110 | |
| 43. | 7.07877 | 301.0503 | 303.046 | Quercetin | C15H10O7 | 7.3 | 303.046, 285.1349, 153.2648, 137.0592 |
| 44. | 7.09293 | 465.1005 | Hyperoside (Quercetin 3-galactoside) | C21H20O12 | 2.3 | 465.1005, 303.0500 | |
| 46. | 7.7529 | 301.0998 | 3 5 7-trihydroxy-4′-methoxyflavone (Diosmetin) | C16H12O6 | 4.3 | 301.0998, 283.0971, 255.0724 | |
| Flavones | |||||||
| 12. | 1.44153 | 417.0578 | Kaempferol-3-O-alpha-L-arabinoside | C20H18O10 | −0.8 | 417.0578, 285.0406, 284.0327 | |
| 35. | 5.74847 | 445.0771 | Baicalein-7-O-glucuronide | C21H18O11 | 0.4 | 445.0771, 269.045, 175.0244 | |
| 38. | 6.23795 | 591.1369 | Acacetin-7-O-rutinoside (Linarin) | C28H32O14 | −0.8 | 591.1369, 445.0101, 283.0318 | |
| 39. | 6.3527 | 285.0396 | Luteolin | C15H10O6 | 2.5 | 285.0396, 163.6529, 151.005 | |
| 40. | 6.61593 | 415.1646 | Puerarin | C21H20O9 | −6.7 | 415.1646, 295.0405, 253.1208 | |
| 47. | 7.8312 | 431.0983 | apigenin-7-O-glucoside | C21H20O10 | 0.8 | 431.0983, 269.0423, 268.0355 | |
| 51. | 8.86035 | 269.0807 | Formononetin | C16H12O4 | 1.8 | 269.0807, 237.0812, 213.0736 | |
| 52. | 10.0624 | 269.0419 | Apigenin | C15H10O5 | 3.2 | 269.0419, 117.0327 | |
| 54. | 12.3865 | 433.114 | Apigenin 8-C-glucoside (vitexin) | C21H20O10 | −0.8 | 433.114, 415.1061, 313.0744 | |
| Flavanones | |||||||
| 1. | 1.182367 | 271.0123 | Naringenin | C15H12O5 | 0.1 | 271.0123, 151.005, 119.051, 107.014 | |
| 45. | 7.487216 | 301.1187 | Hesperetin | C16H14O6 | 1.9 | 301.1187, 283.1092, 161.0413 | |
| 53. | 11.38955 | 289.1188 | 3′ 4′ 5 7-tetrahydroxyflavanone (fustin) | C15H12O6 | 2.1 | 271.0123, 151.005, 119.051, 107.014 | |
| Flavanols | |||||||
| 34. | 5.53515 | 289.011 | catechin | C15H14O5 | 2.1 | 289.011, 245.098 | |
| Phenolic acids | |||||||
| 19. | 1.49335 | 353.0888 | Chlorogenic acid | C16H18O9 | 0.8 | 353.0888, 191.0546, 173.0496, 135.100 | |
| 24. | 2.353867 | 149.0598 | Trans-Cinnamic acid | C9H8O2 | −0.3 | 149.0598, 131.0492, 105.0540 | |
| 26. | 3.325267 | 137.0248 | P-hydroxybenzoic acid | C7H6O3 | −0.9 | 137.0239, 93.0341 | |
| 31. | 4.95135 | 153.0183 | 3,4-Dihydroxybenzoic acid (Protocatechuic acid) | C7H6O4 | 3.7 | 153.0183, 109.0297, 108.0218 | |
| 48. | 7.857533 | 359.0174 | Rosmarinic acid | C18H16O8 | 0.4 | 359.0174, 315.0241, 161.0540, 135.0709 | |
| 49. | 8.054216 | 411.1733 | γ-Tocotrienol (vitamin E) | C28H42O2 | 1.2 | 411.1733, 409.8878, 242.12191, 100.4870 | |
| 50. | 8.084617 | 179.1066 | Daphnetin | C9H6O4 | 0.1 | 179.1066, 135.03, 77.0404 | |
| 55. | 14.40788 | 179.0549 | Caffeic acid | C9H8O4 | 4.5 | 179.0549, 163.0349, 138.0005, 135.0644, 109.0569 | |
| Amino acids | |||||||
| 2. | 1.205733 | 162.0765 | Carnitine | C7H15NO3 | 0.5 | 162.0765, 85.0287, 72.421 | |
| 5. | 1.286033 | 146.0468 | L-Glutamic acid | C5H9NO4 | −0.9 | 146.0468, 128.0347, 102.0588, 100.0358, 91.0552 | |
| 6. | 1.293733 | 104.1054 | N,N-Dimethylglycine | C4H9NO2 | 0.7 | 104.1054, 60.0829, 59.0735, 58.0663 | |
| 8. | 1.344233 | 156.0427 | Histidine | C6H9N3O2 | −0.9 | 156.0427, 110.0036, 93.0434, 83.0586, 68.9831 | |
| 13. | 1.4579 | 132.0641 | trans-4-Hydroxy-L-proline | C5H9NO3 | 3.3 | 132.0641, 86.0656, 68.0452, 57.0568 | |
| 14. | 1.4670 | 133.0591 | L-Asparagine | C4H8N2O3 | 2.9 | 133.0591, 116.0347, 87.0537, 74.0284, 70.0329 | |
| 15. | 1.47007 | 116.0712 | L-Proline | C5H9NO2 | 1.3 | 116.0712, 70.0657 | |
| 16. | 1.4797 | 146.0919 | L-β-Homoleucine | C7H15NO2 | 0.7 | 146.0919, 87.1103, 86.0969, 69.0715 | |
| 17. | 1.47985 | 116.0707 | Norvaline | C5H11NO2 | 1.2 | 116.0707, 59.0301 | |
| 20. | 1.598517 | 130.0858 | Hydroxy proline | C5H9NO3 | 4.8 | 130.0858, 113.04095 | |
| 21. | 1.660383 | 175.1184 | L-Arginine | C6H14N4O2 | −0.1 | 175.1184, 158.0913, 116.068, 70.0659 | |
| 22. | 1.903867 | 130.0483 | L-5-Oxoproline | C5H7NO3 | 7.5 | 130.0483, 84.0805, 56.048 | |
| 23. | 2.049333 | 134.0461 | Adenine | C5H5N5 | 2.5 | 134.0461, 107.0416 | |
| 25. | 2.374333 | 166.0866 | L-Phenylalanine | C9H11NO2 | −2 | 166.0866, 120.0792, 103.0544, 91.054, 79.0511 | |
| 27. | 3.403083 | 203.0845 | L-tryptophan | C11H12N2O2 | −0.3 | 188.0687, 170.0324, 159.0884, 142.0646, 132.0801 | |
| 28. | 4.472367 | 144.0445 | L-β-Homoisoleucine | C7H15NO2 | 0.7 | 144.0445, 87.1103, 86.0969, 69.0715 | |
| 56 | 15.9923 | 385.1516 | S-Adenosyl-L-homocysteine | C14H20N6O5S | 0.3 | 385.1516, 275.1985, 133.0981 | |
| Alkaloids | |||||||
| 11. | 1.43257 | 138.052 | Trigonelline | C7H7NO2 | 2.3 | 138.052, 110.0599, 94.0651, 92.0489 | |
| 32. | 5.00988 | 195.086 | Caffeine | C8H10N4O2 | 3.2 | 195.086, 163.034, 138.005, 95.0816 | |
| Carbohydrates | |||||||
| 4. | 1.26053 | 195.0521 | Gluconate | C6H12O7 | 1.7 | 195.0521, 159.8957, 116.933, 99.9293, 76.9712 | |
| 9. | 1.35473 | 179.0557 | D-tagatose | C6H12O6 | 0.6 | 179.0557, 89.0245, 71.0138, 59.0136, 43.0184 | |
| 18. | 1.48225 | 341.1109 | Galactinol dihydrate | C12H22O11 | −2.3 | 341.1073, 295.093, 179.061, 161.0456, 143.0326 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelgawad, F.A.M.; El-Hawary, S.S.; El-Kader, E.M.A.; Alshehri, S.A.; Rabeh, M.A.; El-Mosallamy, A.E.M.K.; Salama, A.; El Gedaily, R.A. Phytochemical Elucidation and Effect of Maesa indica (Roxb.) Sweet on Alleviation of Potassium Dichromate-Induced Pulmonary Damage in Rats. Plants 2024, 13, 338. https://doi.org/10.3390/plants13030338
Abdelgawad FAM, El-Hawary SS, El-Kader EMA, Alshehri SA, Rabeh MA, El-Mosallamy AEMK, Salama A, El Gedaily RA. Phytochemical Elucidation and Effect of Maesa indica (Roxb.) Sweet on Alleviation of Potassium Dichromate-Induced Pulmonary Damage in Rats. Plants. 2024; 13(3):338. https://doi.org/10.3390/plants13030338
Chicago/Turabian StyleAbdelgawad, Fatma Alzahra M., Seham S. El-Hawary, Essam M. Abd El-Kader, Saad Ali Alshehri, Mohamed Abdelaaty Rabeh, Aliaa E. M. K. El-Mosallamy, Abeer Salama, and Rania A. El Gedaily. 2024. "Phytochemical Elucidation and Effect of Maesa indica (Roxb.) Sweet on Alleviation of Potassium Dichromate-Induced Pulmonary Damage in Rats" Plants 13, no. 3: 338. https://doi.org/10.3390/plants13030338





