Identification of the Maize PP2C Gene Family and Functional Studies on the Role of ZmPP2C15 in Drought Tolerance
Abstract
:1. Introduction
2. Results
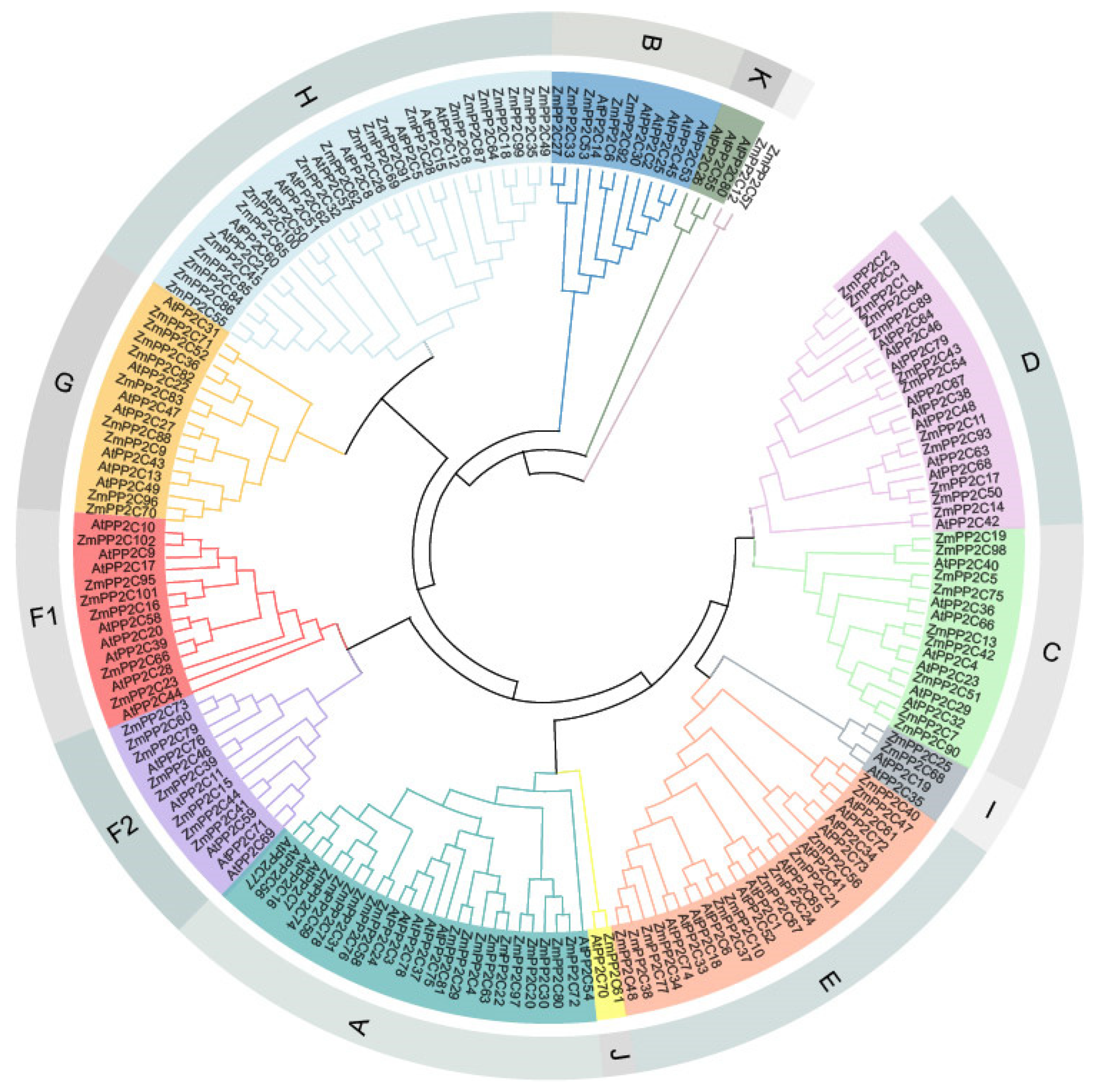
2.1. Identification and Evolutionary Analysis of the Maize PP2C Gene Family
2.2. Distribution of Maize PP2C Family Members on Chromosomes and Analysis of Gene Structure and Protein-Conserved Motifs
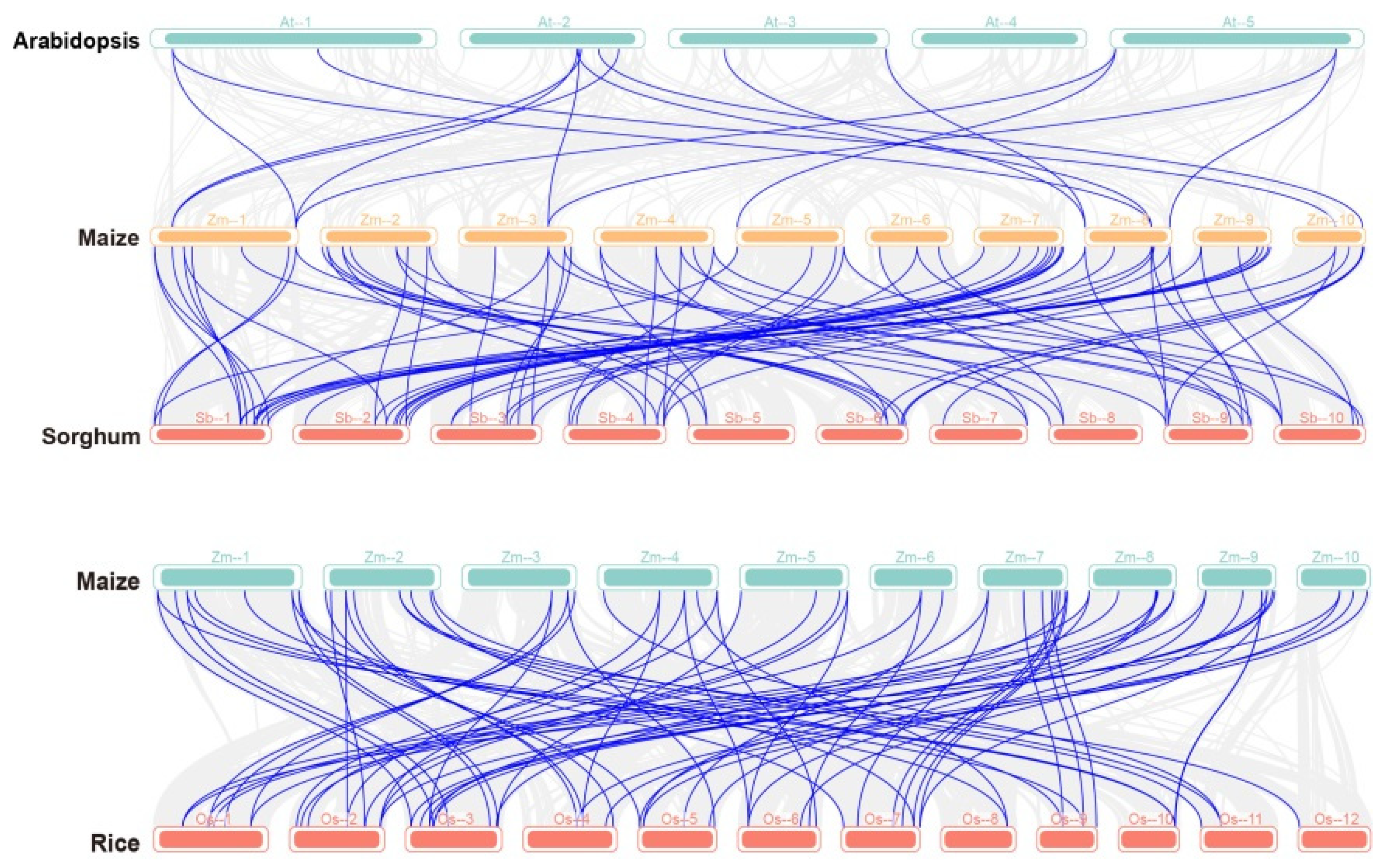
2.3. Collinearity Analysis between PP2C Gene Families in Sorghum, Rice, Arabidopsis, and Maize
2.4. Analysis of Candidate Genes for ZmPP2Cs under Drought and Rewatering Conditions
2.5. Expression Pattern Analysis of ZmPP2Cs in Response to Drought, ABA, and NaCl Treatments
2.6. Phenotypic Analysis of ZmPP2C15 Overexpression in A. thaliana under Drought Stress and Determination of Physiological Indexes
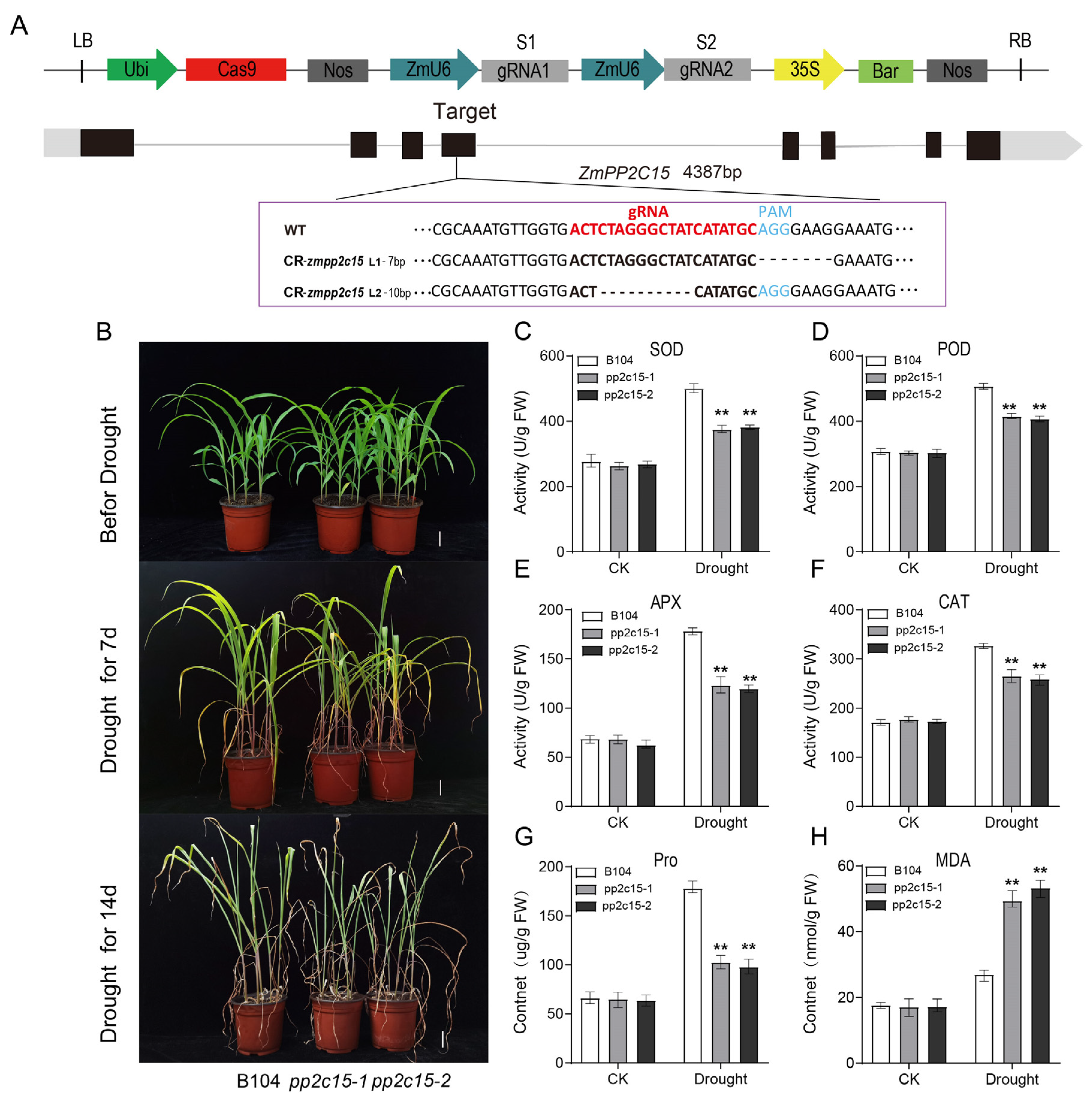
2.7. Analysis of Drought Tolerance in zmpp2c15 Maize Mutant Plants under Drought Stress
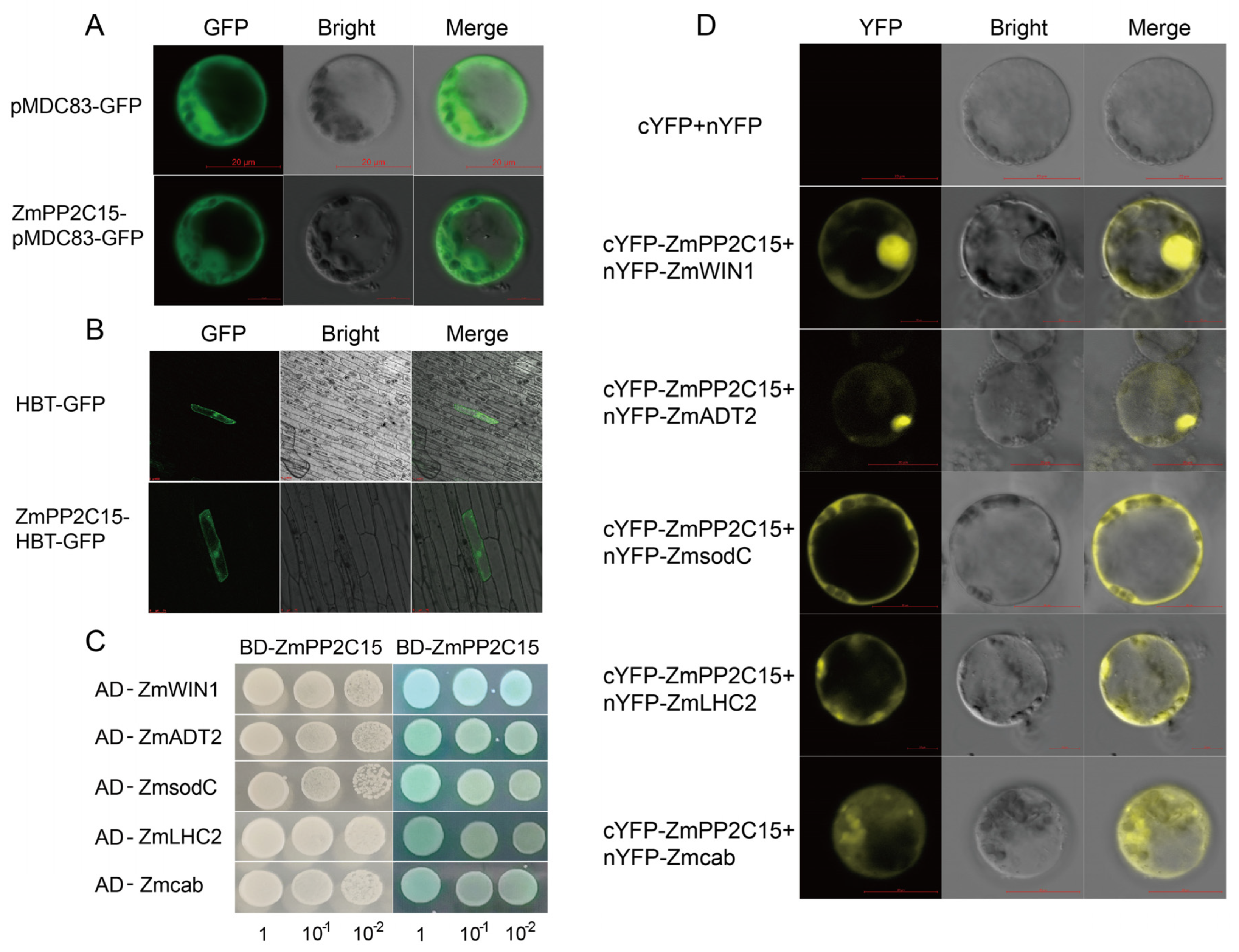
2.8. Analysis of the Subcellular Localization and Protein Interactions of ZmPP2C15
3. Discussion
4. Material Methods
4.1. Identification of the Maize PP2C Gene Family
4.2. Bioinformatics Analysis of the ZmPP2C Gene Family
4.3. Expression Profiling and Co-Expression Network Map Analysis of ZmPP2Cs
4.4. Plant Growing Conditions and Treatments
4.5. RNA Extraction, cDNA Synthesis, and qRT-PCR Analysis
4.6. Construction of Arabidopsis Overexpression Vectors, Obtaining Positive Plants, and Verifying Drought Tolerance
4.7. Construction of Maize Mutant Vectors, Obtaining Mutant Plants, and Verifying Drought Tolerance
4.8. Subcellular Localization of ZmPP2C15
4.9. Validation of Protein Interactions Using Yeast Two-Hybrid (Y2H) and BiFC
4.10. Measurement of Physiological Indicators
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McMillen, M.S.; Mahama, A.A.; Sibiya, J.; Lübberstedt, T.; Suza, W.P. Improving drought tolerance in maize: Tools and techniques. Front. Genet. 2022, 13, 1001001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.; Xu, L.; Wang, A.; Huang, L.; Du, H.; Qiu, L.; Oelmüller, R. Drought stress responses in maize are diminished by Piriformospora indica. Plant Signal. Behav. 2017, 13, e1414121. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Zheng, Y.; Chachar, Z.; Zhang, X.; Zhou, G.; Zong, N.; Leng, P.; Zhao, J. Dissection of Maize Drought Tolerance at the Flowering Stage Using Genome-Wide Association Studies. Genes 2022, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Edmeades, G.O. Progress in Achieving and Delivering Drought Tolerance in Maize-An Update; ISAAA: Ithaca, NY, USA, 2013. [Google Scholar]
- Smith, R.D.; Walker, J.C. Plant protein phosphatases. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Koga, R.; Bohnert, H.J.; Fukuhara, T. Tissue- and environmental response-specific expression of 10 PP2C transcripts in Mesembryanthemum crystallinum. Mol. Genet. Genom. 1999, 261, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Awotunde, O.S.; Sugajska, E.; Zolnierowicz, S.; Muszyńska, G. Characterisation of two protein phosphatase 2A holoenzymes from maize seedlings. Biochim. Biophys. Acta. 2000, 1480, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Meskiene, I.; Bögre, L.; Glaser, W.; Balog, J.; Brandstötter, M.; Zwerger, K.; Ammerer, G.; Hirt, H. MP2C, a plant protein phosphatase 2C, functions as a negative regulator of mitogen-activated protein kinase pathways in yeast and plants. Proc. Natl. Acad. Sci. USA 1998, 95, 1938–1943. [Google Scholar] [CrossRef]
- Dong, L.; Ermolova, N.V.; Chollet, R. Partial purification and biochemical characterization of a heteromeric protein phosphatase 2A holoenzyme from maize (Zea mays L.) leaves that dephosphorylates C4 phosophoenolpyruvate carboxylase. Planta 2001, 213, 379–389. [Google Scholar] [CrossRef]
- Xiang, Y.; Sun, X.; Gao, S.; Qin, F.; Dai, M. Deletion of an Endoplasmic Reticulum Stress Response Element in a ZmPP2C-A Gene Facilitates Drought Tolerance of Maize Seedlings. Mol. Plant 2016, 10, 456–469. [Google Scholar] [CrossRef]
- Sugimoto, H.; Kondo, S.; Tanaka, T.; Imamura, C.; Muramoto, N.; Hattori, E.; Ogawa, K.; Mitsukawa, N.; Ohto, C. Overexpression of a novel Arabidopsis PP2C isoform, AtPP2CF1, enhances plant biomass production by increasing inflorescence stem growth. J. Exp. Bot. 2014, 65, 5385–5400. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Ahmad, N.; Wang, Y.; Ge, H.; Wang, Y.; Liu, W.; Li, X.; Wang, N.; Wang, F.; et al. CePP2C19 confers tolerance to drought by regulating the ABA sensitivity in Cyperus esculentus. BMC Plant Biol. 2023, 23, 1–15. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.B.; Wei, N.; Liu, Z.H.; Li, Y.; Zheng, Y.; Li, X.B. A type-2C protein phosphatase (GhDRP1) participates in cotton (Gossypium hirsutum) response to drought stress. Plant Mol. Biol. 2021, 107, 499–517. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Li, W.; Peng, Y.; Cao, Y.; Qu, J.; Sun, F.; Yang, Q.; Lu, Y.; Zhang, X.; Zheng, L.; et al. ZmPP2C26 Alternative Splicing Variants Negatively Regulate Drought Tolerance in Maize. Front. Plant Sci. 2022, 13, 851531. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ding, J.; Huang, W.; Yu, H.; Wu, S.; Li, W.; Mao, X.; Chen, W.; Xing, J.; Li, C.; et al. OsPP65 Negatively Regulates Osmotic and Salt Stress Responses Through Regulating Phytohormone and Raffinose Family Oligosaccharide Metabolic Pathways in Rice. Rice 2022, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Budak, H.; Akpinar, B.A. Plant miRNAs: Biogenesis, organization and origins. Funct. Integr. Genom. 2015, 15, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Epel, E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res. Rev. 2021, 73, 101507. [Google Scholar] [CrossRef] [PubMed]
- Chuong, N.N.; Hoang, X.L.T.; Nghia, D.H.T.; Dai, T.N.T.; Le Thi, V.-A.; Thao, N.P. Protein Phosphatase Type 2C Functions in Phytohormone-Dependent Pathways and in Plant Responses to Abiotic Stresses. Curr. Protein Pept. Sci. 2021, 22, 430–440. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, Z.; Wei, L.; Qiu, X.; Wang, G.; Liu, Z.; Fu, J.; Cao, L.; Wang, T. Overexpression of ZmPP2C55 positively enhances tolerance to drought stress in transgenic maize plants. Plant Sci. 2021, 314, 111127. [Google Scholar] [CrossRef]
- Krzywińska, E.; Kulik, A.; Bucholc, M.; Fernandez, M.A.; Rodriguez, P.L.; Dobrowolska, G. Protein phosphatase type 2C PP2CA together with ABI1 inhibits SnRK2.4 activity and regulates plant responses to salinity. Plant Signal. Behav. 2016, 11, e1253647. [Google Scholar] [CrossRef]
- Rigoulot, S.B.; Petzold, H.E.; Williams, S.P.; Brunner, A.M.; Beers, E.P. Populus trichocarpa clade A PP2C protein phosphatases: Their stress-induced expression patterns, interactions in core abscisic acid signaling, and potential for regulation of growth and development. Plant Mol. Biol. 2019, 100, 303–317. [Google Scholar] [CrossRef]
- Miao, J.; Li, X.; Li, X.; Tan, W.; You, A.; Wu, S.; Tao, Y.; Chen, C.; Wang, J.; Zhang, D.; et al. OsPP2C09, a negative regulatory factor in abscisic acid signalling, plays an essential role in balancing plant growth and drought tolerance in rice. New Phytol. 2020, 227, 1417–1433. [Google Scholar] [CrossRef]
- Yu, X.; Han, J.; Li, L.; Zhang, Q.; Yang, G.; He, G. Wheat PP2C-a10 regulates seed germination and drought tolerance in transgenic Arabidopsis. Plant Cell Rep. 2020, 39, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, H.; Wang, Y.; Qiu, X.; Gao, G.; Zhu, A.; Chen, P.; Wang, X.; Chen, K.; Chen, J.; et al. Genome-Wide Identification and Expression Analysis of BnPP2C Gene Family in Response to Multiple Stresses in Ramie (Boehmeria nivea L.). Int. J. Mol. Sci. 2023, 24, 15282. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.; Corpas, F.J.; Borsani, O.; Barroso, J.B.; Monza, J. Water stress induces a differential and spatially distributed nitro-oxidative stress response in roots and leaves of Lotus japonicus. Plant Sci. 2013, 201–202, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into Drought Stress Signaling in Plants and the Molecular Genetic Basis of Cotton Drought Tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, L.; Xiao, B.; Li, D.; Xing, X.; Kong, X.; Li, D. Enhanced tolerance to low temperature in tobacco by over-expression of a new maize protein phosphatase 2C, ZmPP2C2. J. Plant Physiol. 2010, 167, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Shi, W.S.; Liu, Y.; Gao, X.M.; Hu, B.; Sun, H.R.; Li, X.Y.; Yang, Y.; Li, X.F.; Liu, Z.B.; et al. MdPP2C24/37, Protein Phosphatase Type 2Cs from Apple, Interact with MdPYL2/12 to Negatively Regulate ABA Signaling in Transgenic Arabidopsis. Int. J. Mol. Sci. 2022, 23, 14375. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, Y.M.; Zhai, H.; Cai, H.; Ji, W.; Luo, X.; Li, j.; Bai, X. AtPP2CG1, a protein phosphatase 2C, positively regulates salt tolerance of Arabidopsis in abscisic acid-dependent manner. Biochem. Biophys. Res. Commun. 2012, 422, 710–715. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, L.; Cai, G.; Lv, C.; Yang, H.; Ren, X.; Hu, B.; Zhou, X.; Jiang, T.; Xiang, Y.; et al. Genome-Wide Identification and Characterization of the PP2C Family from Zea mays and Its Role in Long-Distance Signaling. Plants 2023, 12, 3153. [Google Scholar] [CrossRef]
- Xing, B.; Gu, C.; Zhang, T.; Zhang, Q.; Yu, Q.; Jiang, J.; Liu, G. Functional Study of BpPP2C1 Revealed Its Role in Salt Stress in Betula platyphylla. Front. Plant Sci. 2021, 11, 617635. [Google Scholar] [CrossRef]
- Lu, J.; Wang, L.; Zhang, Q.; Ma, C.; Su, X.; Cheng, H.; Guo, H. AmCBF1 Transcription Factor Regulates Plant Architecture by Repressing GhPP2C1 or GhPP2C2 in Gossypium hirsutum. Front. Plant Sci. 2022, 13, 914206. [Google Scholar] [CrossRef]
- Hu, C.-D.; Chinenov, Y.; Kerppola, T.K. Visualization of Interactions among bZIP and Rel Family Proteins in Living Cells Using Bimolecular Fluorescence Complementation. Mol. Cell 2002, 9, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.E.; Kim, Y.; Huh, W.-K.; Park, H.-O. Bimolecular Fluorescence Complementation (BiFC) Analysis: Advances and Recent Applications for Genome-Wide Interaction Studies. J. Mol. Biol. 2015, 427, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Guo, X.; Zhao, D.; Zhang, Q.; Jiang, Y.; Wang, Y.; Peng, X.; Wei, Y.; Zhai, Z.; Zhao, W.; et al. Cloning and expression profiling of the PacSnRK2 and PacPP2C gene families during fruit development, ABA treatment, and dehydration stress in sweet cherry. Plant Physiol. Biochem. 2017, 119, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, C.; Li, L.; Gao, L.; Hu, G.; Zhang, Y.; Reynolds, M.P.; Zhang, X.; Jia, J.; Mao, X.; et al. DIW1 encoding a clade I PP2C phosphatase negatively regulates drought tolerance by dephosphorylating TaSnRK1.1 in wheat. J. Integr. Plant Biol. 2023, 65, 1918–1936. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shi, Y.; Wang, Y.; Liu, F.; Li, Z.; Qi, J.; Wang, Y.; Zhang, J.; Yang, S.; Wang, Y.; et al. The clade F PP2C phosphatase ZmPP84 negatively regulates drought tolerance by repressing stomatal closure in maize. New Phytol. 2022, 237, 1728–1744. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, w202–w208. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Cao, L.; Ma, C.; Ye, F.; Pang, Y.; Wang, G.; Fahim, A.M.; Lu, X. Genome-wide identification of NF-Y gene family in maize (Zea mays L.) and the positive role of ZmNF-YC12 in drought resistance and recovery ability. Front. Plant Sci. 2023, 17, 1159955. [Google Scholar] [CrossRef]
- Du, H.W.; Luo, Y.M.; Lv, S.S.; Sun, J.F.; Wang, Y.K.; Hu, X.L.; Tian, Z.Q. Analysis on ZmCDPK7 enhancing the thermotolerance of maize protoplasts. J. Henan Agric. Univ. 2021, 55, 621–630. [Google Scholar]
- Hollender, C.A.; Liu, Z. Bimolecular fluorescence complementation (BiFC) assay for protein-protein interaction in onion cells using the helios gene gun. J. Vis. Exp. 2010, 40, 1963. [Google Scholar]
- Sun, Q.; Hu, J.J. Research Technology of Plant Physiology; Northwest A&F University Press: Xianyang, China, 2006. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.; Cao, L.; Ye, F.; Ma, C.; Liang, X.; Song, Y.; Lu, X. Identification of the Maize PP2C Gene Family and Functional Studies on the Role of ZmPP2C15 in Drought Tolerance. Plants 2024, 13, 340. https://doi.org/10.3390/plants13030340
Pang Y, Cao L, Ye F, Ma C, Liang X, Song Y, Lu X. Identification of the Maize PP2C Gene Family and Functional Studies on the Role of ZmPP2C15 in Drought Tolerance. Plants. 2024; 13(3):340. https://doi.org/10.3390/plants13030340
Chicago/Turabian StylePang, Yunyun, Liru Cao, Feiyu Ye, Chenchen Ma, Xiaohan Liang, Yinghui Song, and Xiaomin Lu. 2024. "Identification of the Maize PP2C Gene Family and Functional Studies on the Role of ZmPP2C15 in Drought Tolerance" Plants 13, no. 3: 340. https://doi.org/10.3390/plants13030340
APA StylePang, Y., Cao, L., Ye, F., Ma, C., Liang, X., Song, Y., & Lu, X. (2024). Identification of the Maize PP2C Gene Family and Functional Studies on the Role of ZmPP2C15 in Drought Tolerance. Plants, 13(3), 340. https://doi.org/10.3390/plants13030340



