Influence of pH on the Morphology and Cell Volume of Microscopic Algae, Widely Distributed in Terrestrial Ecosystems
Abstract
:1. Introduction
- Physiological criteria: growth reactions [62,63,64,65,66,67], photosynthesis intensity [68], carbohydrate and protein content [16], pigment composition and assimilation [69], chemical composition [17], chlorophyll “a” and “b” fluorescence [70], transmembrane electrochemical gradient [71], fatty acid production [72], cell division, and biological volume (cell volume) [58].
2. Results
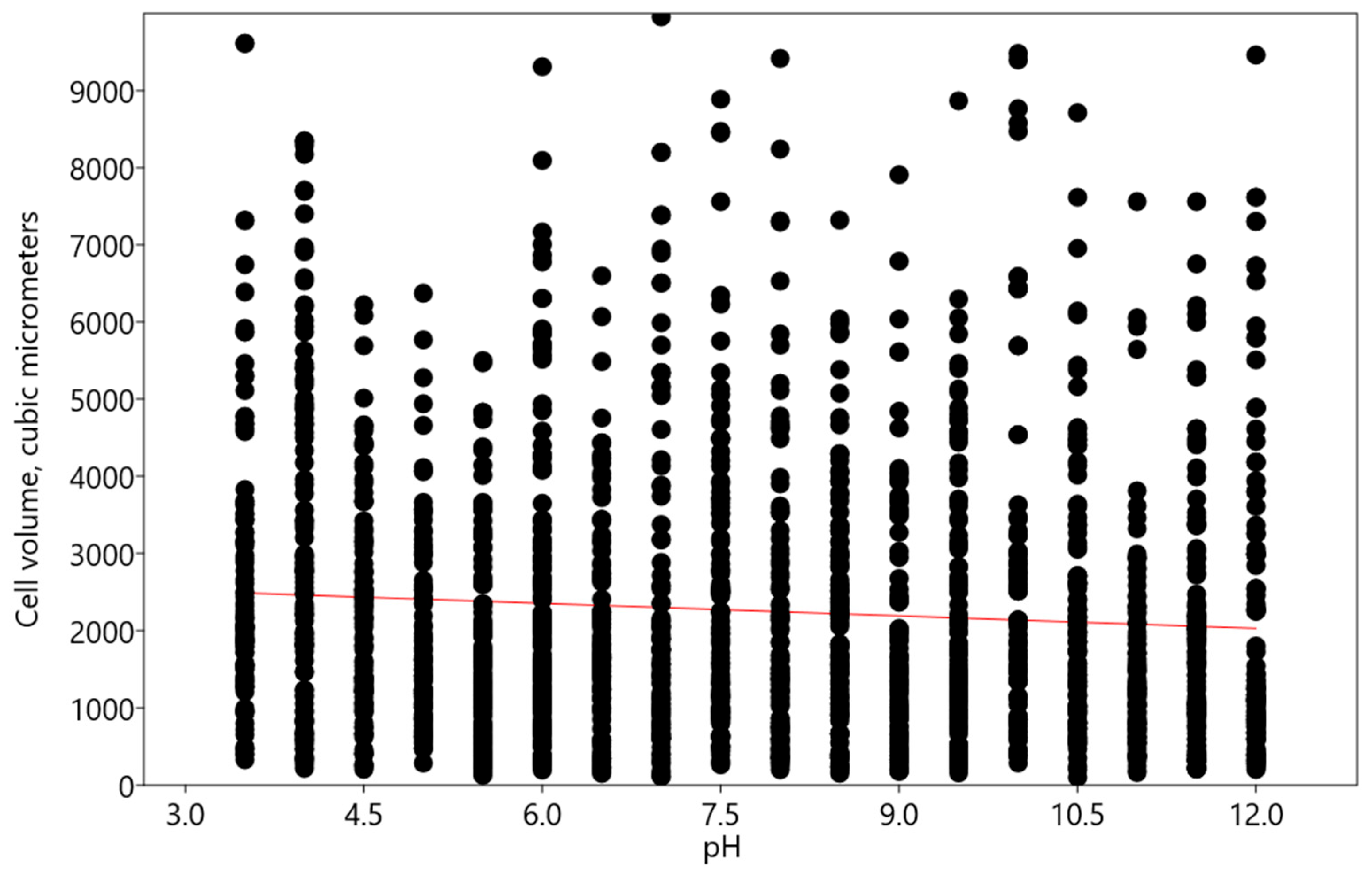
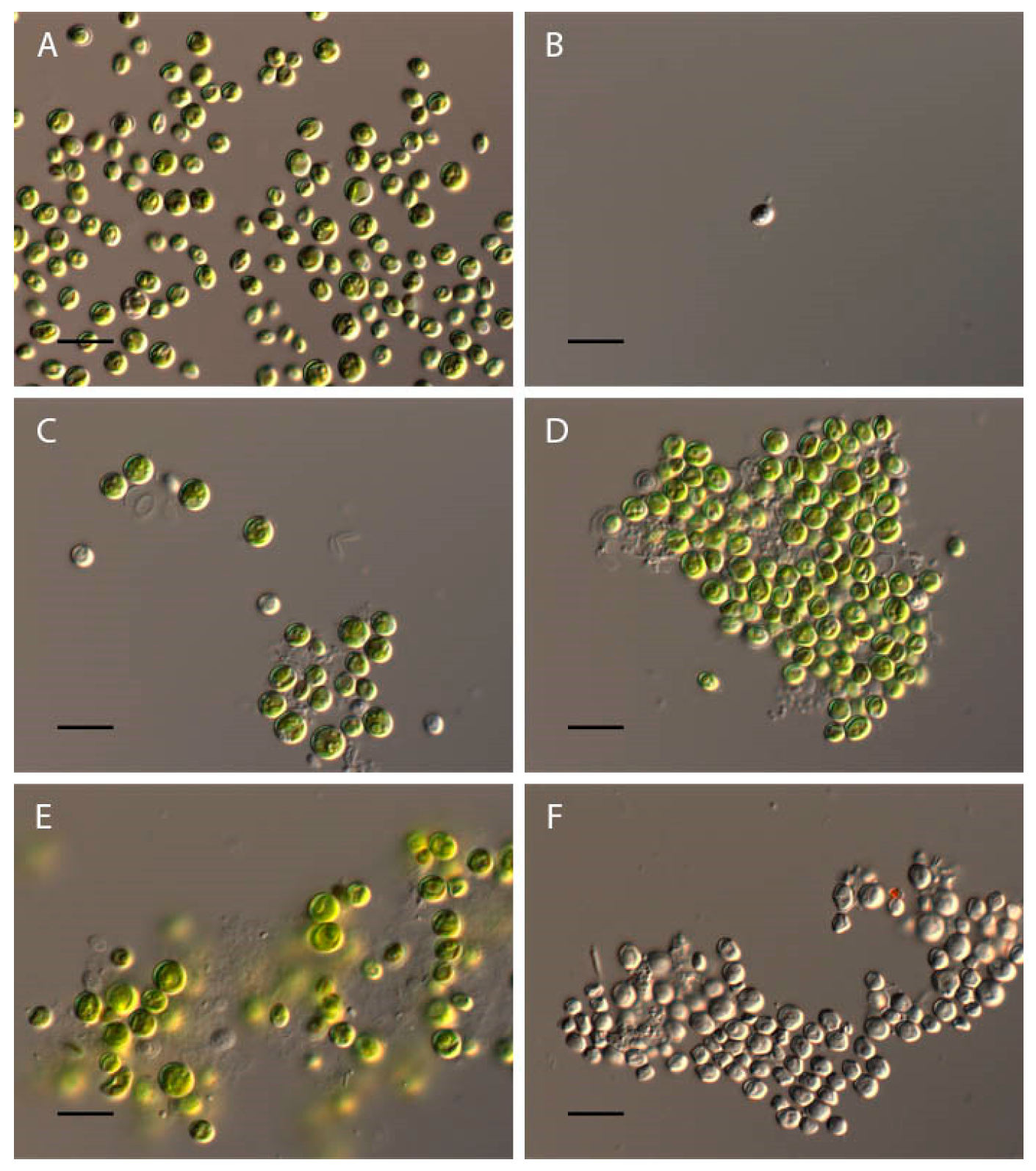
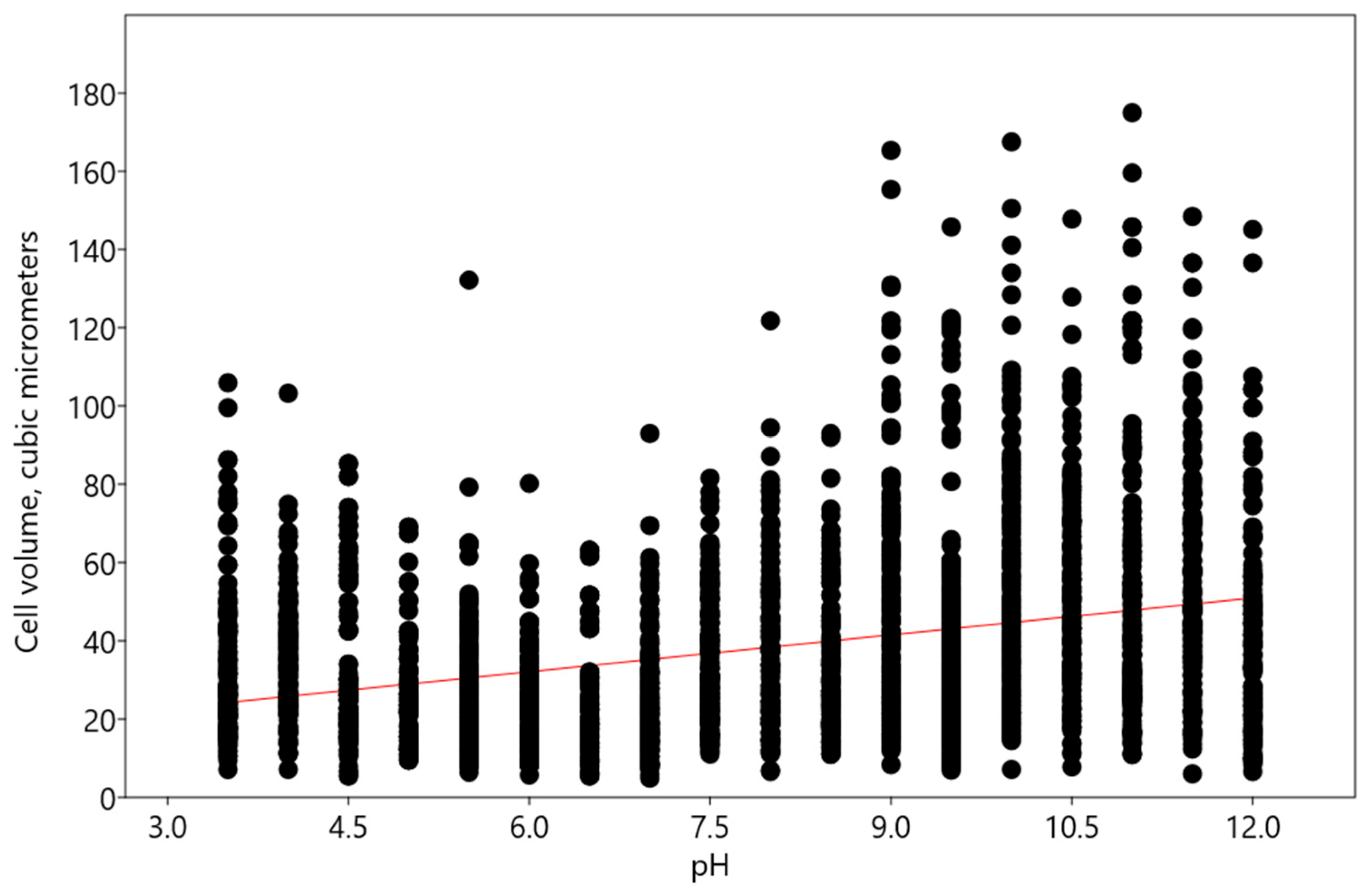
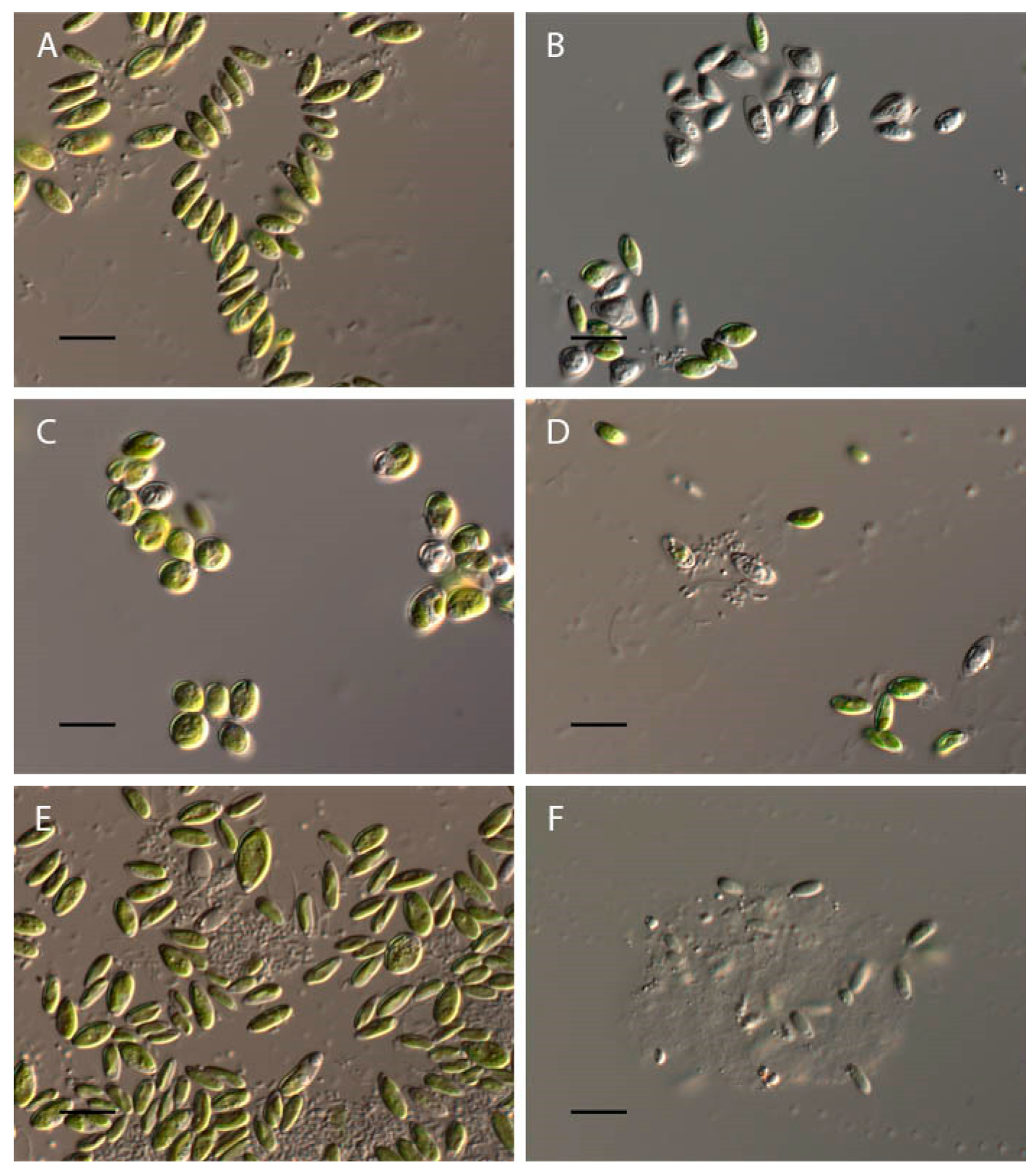
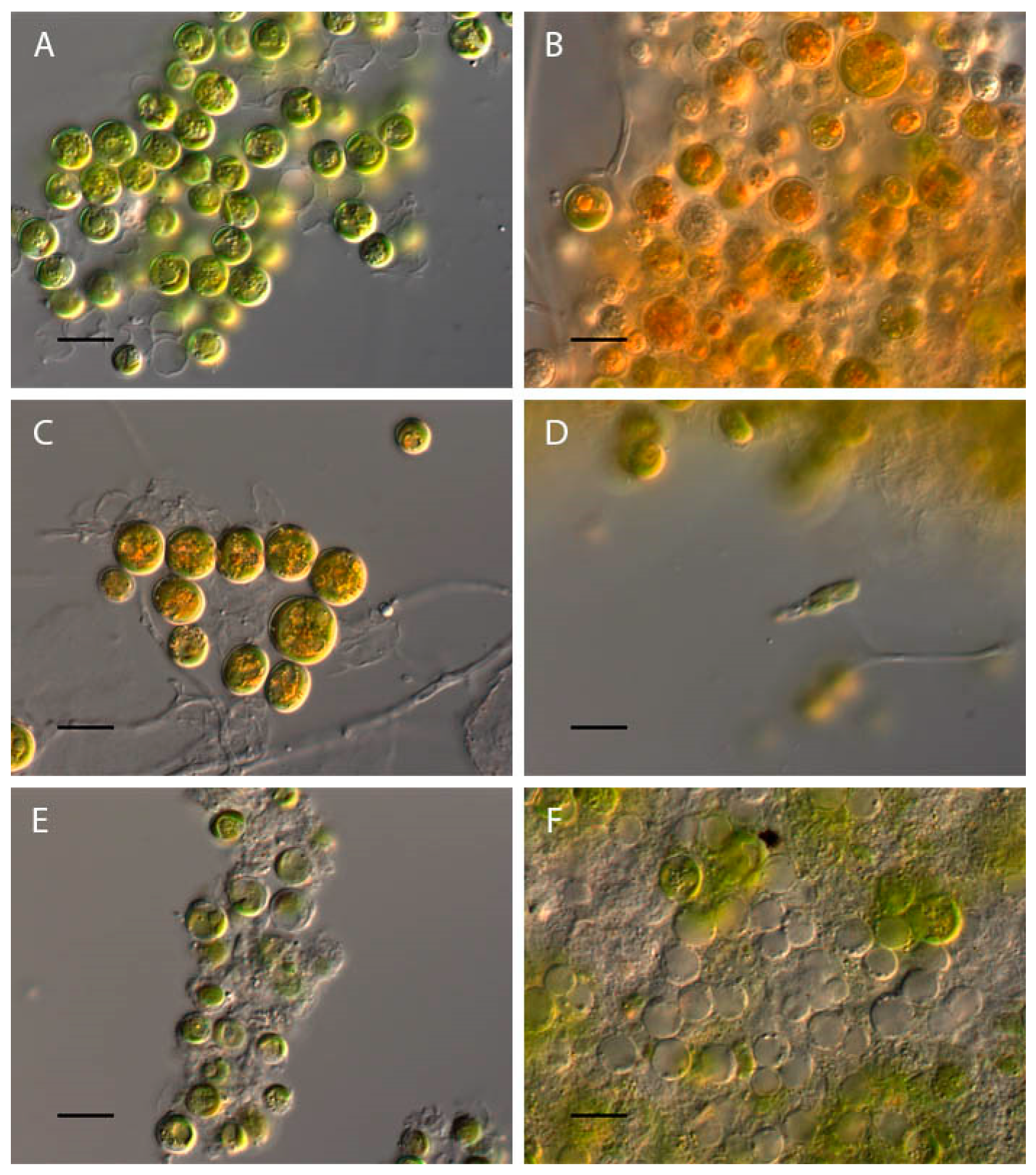
2.1. Bracteacoccus minor
2.2. Chlorococcum infusionum
2.3. Chlorella vulgaris
2.4. Pseudococcomyxa simplex
2.5. Vischeria magna
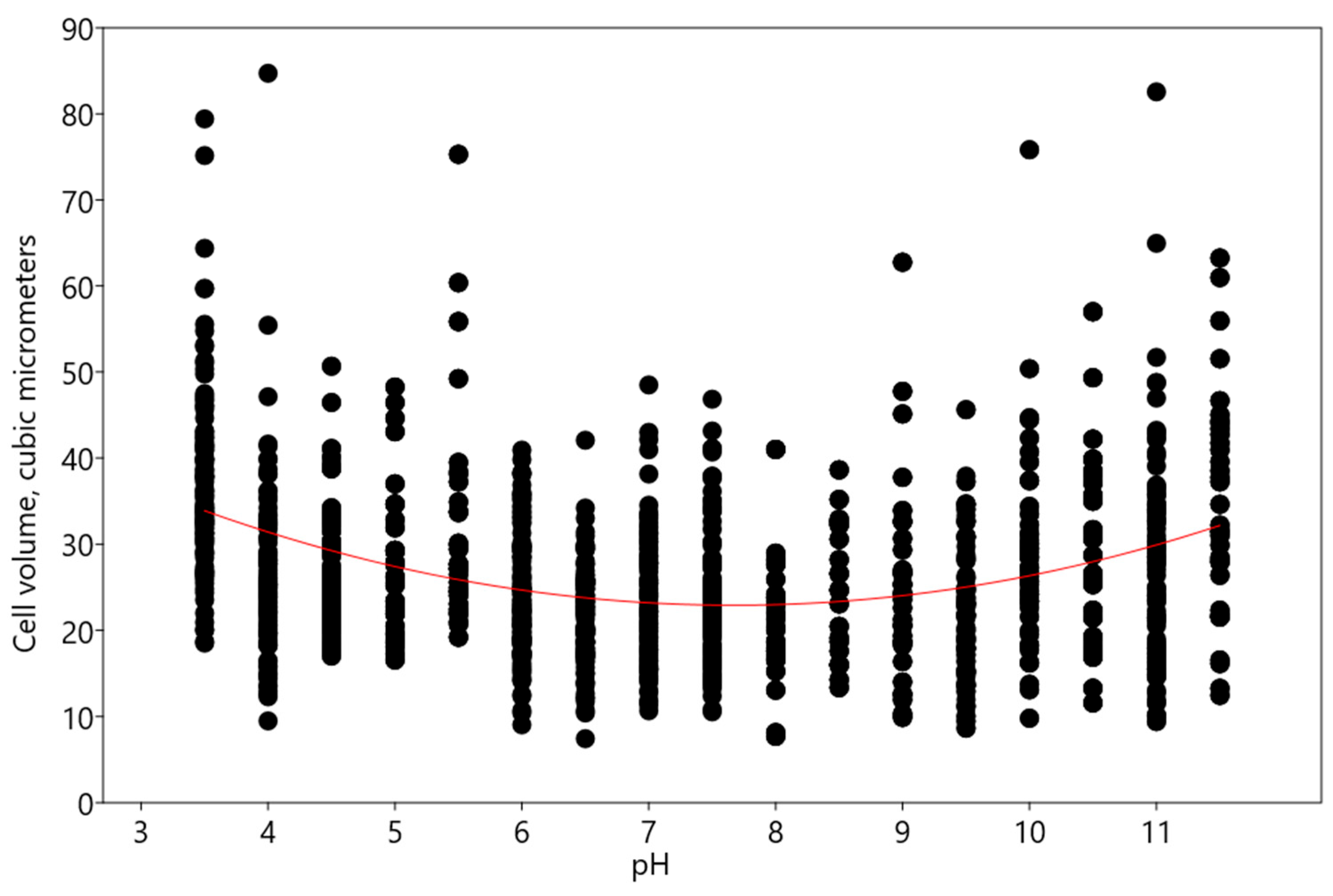
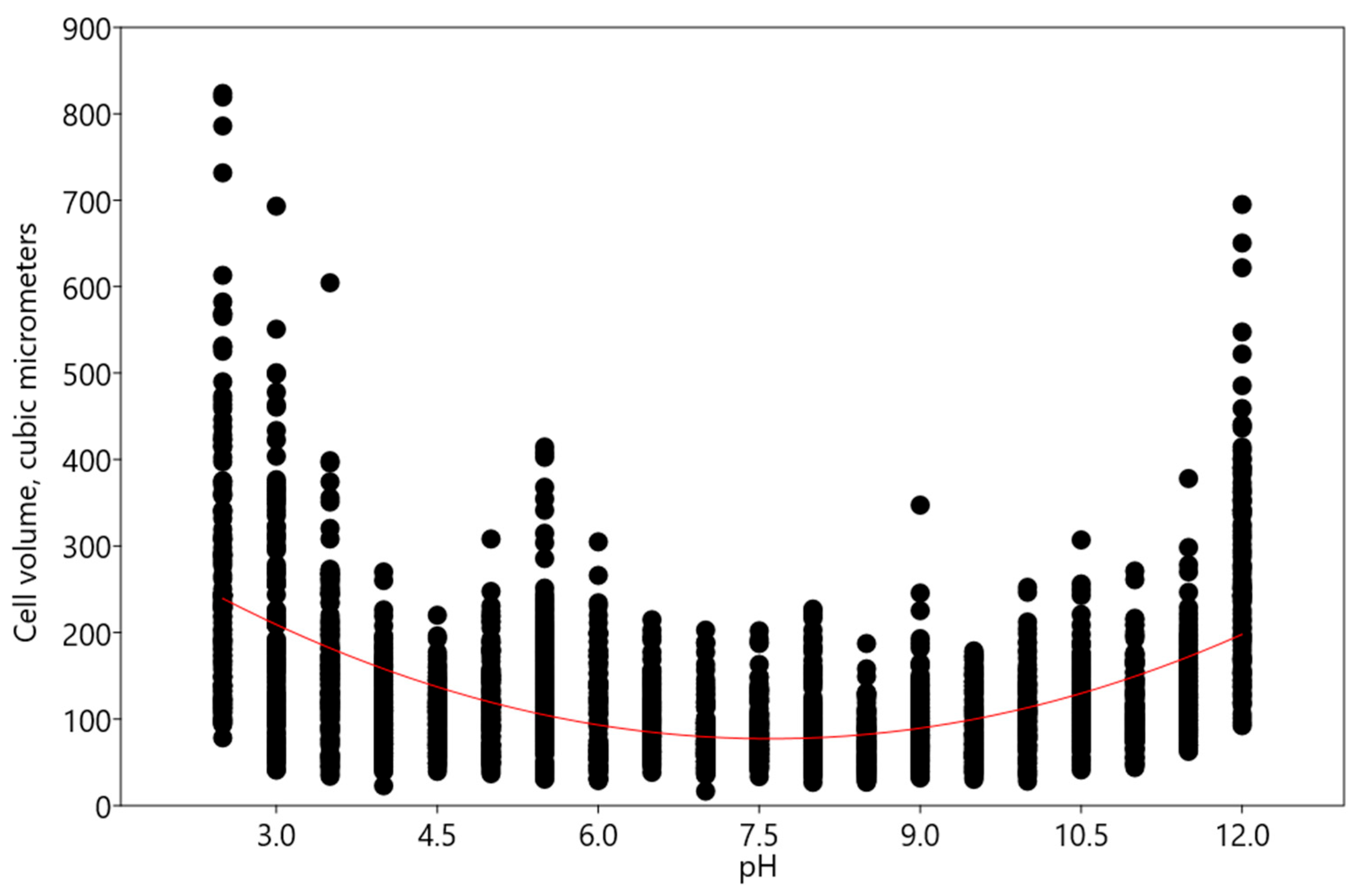
3. Discussion
- Minor changes according to the linear regression model—this type of reaction was characteristic of Bracteacoccus minor and Chlorococcum infusionum. It is necessary to note that desmid Euastrum binale also maintains a constant cell size when the pH changes [26]. It is possible that, for protection from extreme pH conditions, some species, such as Bracteacoccus minor, increased the oil content in their cells.
- An increase in acidic and alkaline pH ranges according to the polynomial regression model—such changes were observed in Pseudoccomixa simplex and Vischeria magna cells. Perhaps these algae could utilize the regulation of biovolume under stress conditions of low and high pH levels. In addition, Vischeria magna increases the synthesis of oil-containing metabolites.
- An increase in alkaline pH range according to the linear regression model was found in experiments with Chlorella vulgaris.
4. Materials and Methods
4.1. Strains Cultivation and Methodic of Experiments
4.2. Statistical Analysis
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kushner, D.J. Microbial Life in Extreme Conditions; Mir: Moscow, Russia, 1981; 519p. [Google Scholar]
- Odum, Y. Fundamentals of Ecology; Mir: Moscow, Russia, 1975; 740p. [Google Scholar]
- Coleman, J.R.; Colman, B. Effect of external pH on carbon accumulation and CO2 fixation in blue-green alga. In Proceedings of the Fifth International Congress on Photosynthesis, Halkidiki, Greece, 7–13 September 1980; Volume 123, pp. 89–90. [Google Scholar]
- Reddy, H.; Balasubramanian, A.; Shantaram, M.V. Influence of pH on growth (14 C)-carbon metabolism and nitrogen fixation by two blue-green algae. Phycos 1980, 19, 45–51. [Google Scholar]
- Rachlin, J.; Grosso, A. The effects of pH on the growth of Chlorella vulgaris and its interactions with cadmium toxicity. Arch. Environ. Contam. Toxicol. 1991, 20, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W. The effect of the pH on the division rate of the coccolithophonid Cricoshaera elongate. J. Phycol. 1966, 2, 33–42. [Google Scholar]
- McLean, R.J.; Trainor, F.R. Fasciculochloris, a new chlorosphaeracean alga from Connecticut soil. Phycologia 1965, 4, 145–148. [Google Scholar] [CrossRef]
- Hopkins, E.F.; Wann, F.B. Relation of hydrogen-ion concentration to growth of Chlorella and to the availability of iron. Bot. Gaz. 1926, 81, 353–376. [Google Scholar] [CrossRef]
- Maciasr, F.M. Effect of pH of the medium on the availability of chelated iron for Chlamydomonas mundane. J. Protozool. 1965, 12, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.W.; Park, K. Bicarbonate utilization by marine phytoplankton in photosynthesis. Physiol. Plan. 1962, 15, 43–45. [Google Scholar]
- Rao, K.V.N. Determination of optimum pH range for the growth of Oocystis marssonii Lemm. in the three media differing in nitrogen source. Indian J. Plant Physiol. 1963, 6, 2–10. [Google Scholar]
- Spencer, C.P. Theoretical aspects of the control of pH in natural sea water and synthetic culture media for marine algae. Bot. Mar. 1966, 9, 3–4. [Google Scholar] [CrossRef]
- Ikemori, M.; Noshida, K. Inorganic carbon sourse and the inhibitory effect of diamox on the photosynthesis of marine algae, Ulva pertusa. Annu. Rep. Noto Mar. Lab. 1967, 7, 63–81. [Google Scholar]
- Thomas, E.A.; Tregunna, E.B. Bicarbonate ion assimilation in photosynthesis by Sargassum muticum. Can. J. Bot. 1968, 46, 256–261. [Google Scholar] [CrossRef]
- Raven, J. The mechanism of photosynthetic use of bicarbonate by Hydrodictyon africanum. J. Exp. Bot. 1968, 46, 82–85. [Google Scholar]
- Khaili, Z.; Asker, M.S.; El-Sayed, S.; Kobbia, I.A. Responces of Dunaliella bardawil and Chlorella ellipsoidea to pH stress. Acta Bot. Hung. 2010, 52, 123–135. [Google Scholar] [CrossRef]
- Jabir, T.F.; Noor Abbood, H.A.; Salman, F.S.; Hafit, A.Y. Influence of pH, pesticide and radiation interactions on the chemical composition of Chlorella vulgaris algae. IOP Conf. Ser. Earth Environ. Sci. 2021, 722, 012046. [Google Scholar] [CrossRef]
- Gollerbach, M.M.; Shtina, E.A. Soil Algae; Nauka: Leningrad, Russia, 1969; p. 228. (In Russian) [Google Scholar]
- Schneider, S.C.; Kahlert, M.; Kelly, M.G. Interactions between pH and nutrients on benthic algae in streams and consequences for ecological status assessment and species richness patterns. Sci. Total Environ. 2013, 444, 73–84. [Google Scholar] [CrossRef]
- Thomas, B.T.; Rajan, R.; Saranyamol, S.T.; Bhagya, M.V. Influence of pH on the diversity of soil algae. J. Glob. Biosci. 2017, 6, 5088–5094. [Google Scholar]
- Novakovskaya, I.V.; Dubrovskiy, Y.A.; Patova, E.N.; Novakovskiy, A.B.; Sterlyagova, I.N. Influence of ecological factors on soil algae in different types of mountain tundra and sparse forests in the Northern Urals. Phycologia 2020, 59, 320–329. [Google Scholar] [CrossRef]
- Zhang, H.; Zong, R.; He, H.; Liu, K.; Yan, M.; Miao, Y.; Ma, B.; Huang, X. Biogeographic distribution patterns of algal community in different urban lakes in China: Insights into the dynamics and co-existence. J. Environ. Sci. 2021, 100, 216–227. [Google Scholar] [CrossRef]
- Lavoie, M.; Le Faucheur, S.; Boullemant, A.; Fortin, C.; Campbell, P.G.C. The influence of pH on algal cell membrane permeability and its implications for the uptake of lipophilic metal complexes. J. Phycol. 2012, 48, 293–302. [Google Scholar] [CrossRef]
- Lacroux, J.; Trably, E.; Bernet, N.; Steyer, J.P.; van Lis, R. Mixotrophic growth of microalgae on volatile fatty acids is determined by their undissociated form. Algal Res. 2020, 47, 101870. [Google Scholar] [CrossRef]
- Caron, D.A.; Lie, A.A.Y.; Buckowski, T.; Turneer, J.; Frabotta, K. The Effect of pH and Salinity on the Toxicity and Growth of the Golden Alga, Prymnesium parvum. Protist 2023, 174, 125927. [Google Scholar] [CrossRef]
- Černá, K.; Neustupa, J. The pH-related morphological variations of two acidophilic species of Desmidiales (Viridiplantae) isolated from a lowland peat bog, Czech Republic. Aquat. Ecol. 2010, 44, 409–419. [Google Scholar] [CrossRef]
- Comeau, S.; Edmunds, P.J.; Lantz, C.A.; Carpenter, R.C. Water flow modulates the response of coral reef communities to ocean acidification. Sci. Rep. 2019, 4, 6681. [Google Scholar] [CrossRef] [PubMed]
- Chowdury, K.H.; Nahar, N.; Deb, U.K. The growth factors involved in microalgae cultivation for biofuel production: A Review. Comput. Water Energy Environ. Eng. 2020, 9, 185–215. [Google Scholar] [CrossRef]
- Malavasi, V.; Soru, S.; Cao, G. Extremophile Microalgae: The potential for biotechnological application. J. Phycol. 2020, 56, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, K.; Davarpanah, A. Design and construction of a micro-photo bioreactor in order to dairy wastewater treatment by micro-algae: Parametric study. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 611–624. [Google Scholar] [CrossRef]
- Han, W.; Jin, W.; Li, Z.; Wei, Y.; He, Z.; Chen, C.; Qin, C.; Chen, Y.; Tu, R.; Zhou, X. Cultivation of Microalgae for Lipid Production Using Municipal Wastewater. Process Saf. Environ. Prot. 2021, 155, 155–165. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Ahuja, V.; Chandel, N.; Gurav, R.; Bhatia, R.K.; Govarthanan, M.; Tyagi, V.K.; Kumar, V.; Pugazendhi, A.; Banu, R.; et al. Advances in algal biomass pretreatment and its valorisation into biochemical and bioenergy by the microbial processes. Bioresour. Technol. 2022, 358, 127437. [Google Scholar]
- Karimi, Z.; Blersch, D.M.; Davis, V.A. Design and analysis of a flow way photobioreactor for substrate assessment in attached cultivation of filamentous green algae. Algal Res. 2022, 66, 102801. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Park, J.; Ralph, P.J.; Craiggs, R.J. Improved microalgal productivity and nutrient removal through operating wastewater high rate algal ponds in series. Algal Res. 2020, 47, 101850. [Google Scholar] [CrossRef]
- Yadav, G.; Mathimani, T.; Sekar, M.; Sindhu, R.; Pugazhendhi, A. Strategic evaluation of limiting factors affecting algal growth—An approach to waste mitigation and carbon dioxide sequestration. Sci. Total Environ. 2021, 796, 991–149049. [Google Scholar] [CrossRef] [PubMed]
- Shtina, E.A.; Gollerbach, M.M. Ecology of the Soil Algae; M. Nauka: Moscow, Russia, 1976; 143p. (In Russian) [Google Scholar]
- Andreeva, V.M. Soil and Aerophilic Green Algae (Chlorophyta: Tetrasporales, Chlorococcales, Chlorosarcinales); Nauka: Saint Petersburg, Russia, 1998; 351p. [Google Scholar]
- Kostikov, I.; Romanenko, P.; Demchenko, P.; Darienko, T.M.; Mikhayljuk, T.I.; Rybchinskiy, O.V.; Solonenko, A.M. Soil Algae of Ukraine; Phytosotsiologichniy Center: Kiev, Ukraine, 2001; p. 300. [Google Scholar]
- Büdel, B.; Darienko, T.; Deutschewitz, K.; Dojani, S.; Friedl, T.; Mohr, K.I.; Salisch, M.; Reisser, W.; Weber, B. Southern African biological soil crusts are ubiquitous and highly diverse in drylands, being restricted by rainfall frequency. Microb. Ecol. 2009, 57, 229–247. [Google Scholar] [CrossRef]
- Škaloud, P. Species composition and diversity of aero-terrestrial algae and cyanobacteria of the Boreč Hill ventaroles. Fottea 2009, 9, 65–80. [Google Scholar] [CrossRef]
- Ettl, H.; Gärtner, G. Syllabus der Boden-, Luft- und Flechtenalgen; Gustav Fischer Verlag: Stuttgart, Germany, 1995; 721p. [Google Scholar]
- Lukešová, A.; Hoffmann, L. Soil algae flora from acid rain impacted forest areas of the Krušne hory Mts. 1. Algal communities. Vegatatio 1996, 125, 123–136. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. World-Wide Electronic Publication, National University of Ireland, Galway. 22 November 2018. AlgaeBase. Available online: https://www.algaebase.org (accessed on 12 November 2023).
- John, D.M.; Witton, B.A.; Brook, A.J. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae; Cambridge University Press: Cambridge, UK, 2005; 702p. [Google Scholar]
- Johansen, J.R.; Rushfort, S.R.; Brotherson, J.D. The algal flora of Navajo National Monumant, Arizona, U.S.A. Nova Hedwig. 1983, 38, 501–553. [Google Scholar]
- Broady, P.A. Diversity, distribution and dispersal of Antarctic terrestrial algae. Biodivers. Conserv. 1996, 5, 1307–1335. [Google Scholar] [CrossRef]
- Lukešova, A. Soil algae in brown coal and lignite post-mining areas in Central Europe (Czech Republic and Germany). Restor. Ecol. 2001, 9, 341–350. [Google Scholar] [CrossRef]
- Flechtner, V.R.; Johansen, J.R.; Belnap, J. The biological soil crusts of the San Nicolas Island: Enigmatic algae from a geographically isolated ecosystems. West. N. Am. Nat. 2008, 68, 405–436. [Google Scholar] [CrossRef]
- Moskvich, N.P. The experience of using algae in the study of the sanitary state of the soil. Bot. J. 1973, 58, 412–416. [Google Scholar]
- Novakovskaya, I.V.; Patova, E.N. Soil Algae of Spruce Forests and Their Changes in Conditions of Aerotechnogenic Pollution; Komi Scientific Center: Syktyvkar, Russia, 2012; 128p. (In Russian) [Google Scholar]
- Novichkova-Ivanova, L.N. Changes of synusions of soil algae of Franz Josef Land. Bot. J. 1963, 47, 42. [Google Scholar]
- Shtina, E.A. Soil algae as pioneers of the overgrowth of technogenic substrates and indicators of the state of disturbed lands. J. Gen. Biol. 1985, 46, 435–443. [Google Scholar]
- Neganova, L.B.; Shilova, I.I.; Shtina, E.A. Algoflora of technogenic sands of oil and gas producing areas of the Middle Ob region and the impact of oil pollution on it. Ecology 1978, 3, 29–35. [Google Scholar]
- Kaštovska, K.; Elster, J.; Stibal, M.; Šantrůčkova, H. Microbial assemblages in soil microbial succession after glacial retreat in Svalbard (High Arctic). Microb. Ecol. 2005, 50, 396–407. [Google Scholar] [CrossRef]
- Gomez, S.R.; Johansen, J.R.; Lowe, R.L. Epilithic aerial algae of Great Smoky Mountains National Park. Biologia 2003, 58, 603–615. [Google Scholar]
- Johansen, J.R.; Lowe, R.; Gomez, S.R.; Kociolek, J.P.; Makosky, S.A. New algal records for the Great Smoky Mountains National Park, U.S.A., with an annotated checklist of all reported algal species for the park. Algol. Stud. 2004, 11, 17–44. [Google Scholar] [CrossRef]
- Shtina, E.L.; Bolyshev, N.I. Algae communities in soils of dry and desert steppes. Bot. J. 1963, 48, 670–680. [Google Scholar]
- Hargreaves, J.; Whitton, B. Effect of pH on growth of acid stream algae. Eur. J. Phycol. 1976, 11, 215–223. [Google Scholar] [CrossRef]
- Zuppini, A.; Gerotto, C.; Baldan, B. Programmed cell death and adaptation: Two different types of abiotic stress response in a unicellular chlorophyte. Plant Cell Physiol. 2010, 51, 884–895. [Google Scholar] [CrossRef]
- Bastien, C.; Côté, R. Effects du cuirve sur l’ultrastructure de Scenedesmus quadricauda et Chlorella vulgaris. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1989, 74, 57–71. [Google Scholar] [CrossRef]
- Agrawal, S.C. Effects of different factors on the akinete germination in Pithophora oedogonia (Mont.) Wittrock. J. Basic Microbiol. 1986, 26, 195–199. [Google Scholar] [CrossRef]
- Rothe, S.; Schumann, R.; Karsten, U. Effect of temperature and photon influence rate on growth rates of two epiphytic diatom species from Kongsfjorden. Ber. Polar Meeresforsch. 2004, 492, 136–146. [Google Scholar]
- Liu, C.G.; Jin, X.C.; Sun, L.; Zhong, Y.; Dai, S.G.; Zhuang, Y.Y. Influence of pH on growth and species composition of algae in freshwater ecosystems. J. Agro-Environ. Sci. 2005, 24, 294–298. [Google Scholar]
- Zhan, Y.-J.; Wang, X.-L.; Yang, R.-J.; Zhang, Y.-Y. Influence of Cd (II) on growth of 8 species of marine microalgae. Environ. Sci. 2006, 27, 720–726. [Google Scholar]
- Middelboe, A.L.; Hansen, P.J. Direct effects of pH and inorganic carbon on macrolalgal photosynthesis and growth. Mar. Biol. Res. 2007, 3, 134–144. [Google Scholar] [CrossRef]
- Mitrovic, S.M.; Hitchcock, J.N.; Davie, A.W.; Ryan, D.A. Growth responses of Cyclotella meneghiniana (Bacillariophyceae) to various temperatures. J. Plankton Res. 2010, 32, 1217–1221. [Google Scholar] [CrossRef]
- Gigova, L.; Ivanova, N.; Gacheva, G.; Andreeva, R.; Furnadzhieva, S. Response of Trachydiscus minutes (Xanthophyceae) to temperature and light. J. Phycol. 2012, 48, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Karandashova, I.V.; Elanskaya, I.V. Genetic control and mechanisms of cyanobacteria resistance to salt and hyperosmotic stress. Genetics 2005, 41, 1589–1600. [Google Scholar] [CrossRef]
- Backor, M.; Fahselt, D.; Davidson, R.D.; Wu, C.T. Effects of copper on wild and tolerant strains of the lichen photobiont Trebouxia erici (Chlorophyta) and possible tolerance mechanism. Arch. Environ. Contam. Toxicol. 2003, 45, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Piovár, J.; Stavrou, E.; Kimákova, T.; Kadukova, J.; Bačkor, M. Influence of long-term exposure to copper on the lichen photobiont Trebouxia erici and free-living algae Scenedesmus quadricauda. Plant Growth Regul. 2011, 63, 81–88. [Google Scholar] [CrossRef]
- Messerli, M.A.; Amaral-Zettler, L.A.; Zettler, E.; Jung, S.-K.; Smith, P.J.S.; Sogin, M.L. Life in acidic pH imposes as increased energetic cost for a eukaryotic acidophile. J. Exp. Biol. 2005, 208, 2569–2579. [Google Scholar] [CrossRef]
- Almutairi, A.W.; Toulibah, H.E. Effect of salinity and pH on fatty acid profile of the Green Algae Tetraselmis suecica. J. Pet. Environ. Biotechnol. 2017, 8, 3–8. [Google Scholar] [CrossRef]
- Li, K.W.; McLaughlin, F.; Lovejoy, C.; Carmack, E.C. Smallest algae thrive as the Arctic Ocean freshens. Science 2009, 326, 539. [Google Scholar] [CrossRef]
- Bratbak, G. Bacterial biovolume and biomass estimation. Appl. Environ. Microbiol. 1985, 49, 1488–1493. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Interrelated influence of iron, light and cell size on marine phytoplankton growth. Nature 1997, 390, 389–392. [Google Scholar] [CrossRef]
- Weisse, T.; Scheffel, U.; Stadler, P.; Foissner, W. Local adaptation among geographically distant clones of the cosmopolitan freshwater ciliate Meseres corlissi. II. Response to pH. Aquat. Microb. Ecol. 2007, 47, 289–297. [Google Scholar] [CrossRef]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Sun, J.; Liu, D. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef]
- Borics, G.; Lerf, V.; Enikő, T.; Stanković, I.; Pickó, L.; Béres, V.; Várbíró, G. Biovolume and surface area calculations for microalgae, using realistic 3D models. Sci. Total Environ. 2021, 773, 145538. [Google Scholar] [CrossRef] [PubMed]
- Finkel, Z.V. Light absorption and size scaling of the light limited metabolism in marine diatoms. Limnol. Oceanogr. 2001, 46, 86–94. [Google Scholar] [CrossRef]
- Raven, J.A.; Kubler, J.E. New light on the scaling of metabolic rate with the size of algae. J. Phycol. 2002, 38, 11–16. [Google Scholar] [CrossRef]
- Tang, E.P.Y. The allelometry of algal growth rates. J. Plankton Res. 1995, 17, 1325–1335. [Google Scholar] [CrossRef]
- Tang, E.P.Y.; Peters, R.H. The allelometry of algal respiration. J. Plankton Res. 1995, 17, 303–315. [Google Scholar] [CrossRef]
- Sarthou, G.; Timmermans, K.R.; Blain, S.; Treguer, P. Growth physiology and fate of diatoms in the ocean: A review. J. Sea Res. 2005, 53, 25–42. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatom: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; 747p. [Google Scholar]
- Irwin, A.J.; Finkel, Z.V.; Schofield, O.M.E.; Falkowski, P.G. Scaling-up from nutrient physiology to the size-structure of phytoplankton communities. J. Plankton Res. 2006, 28, 459–471. [Google Scholar] [CrossRef]
- Agusti, S. Allometric scaling of light absorption and scattering by phytoplankton cells. Can. J. Fish. Aquat. Sci. 1991, 48, 763–767. [Google Scholar] [CrossRef]
- Joint, I.; Pomroy, A. Allometric estimation of the productivity of phytoplankton assemblages. Mar. Ecol. Prog. Ser. 1988, 47, 161–168. [Google Scholar] [CrossRef]
- Connolly, J.A.; Oliver, M.J.; Beaulieu, J.M.; Knight, C.A.; Tomanek, L.; Moline, M.A. Correlated evolution of genome size and cella volume in diatoms (Bacillariophyceae). J. Phycol. 2008, 44, 124–131. [Google Scholar] [CrossRef]
- Hein, M.; Pedersen, M.F.; Sand-Jensen, K. Size-dependent nitrogen uptake in micro- and macroalgae. Mar. Ecol. Prog. Ser. 1995, 118, 247–253. [Google Scholar] [CrossRef]
- Vogel, S. Life’s Devices: The Physical World of Animals and Plants; Princeton University Press: Princeton, NJ, USA, 1988; 369p. [Google Scholar]
- Borics, G.; Várbíró, G.; Falucskai, J.; Végvári, Z.; T-Krasznai, E.; Görgényi, J.; B-Béres, V.; Lerf, V. A two-dimensional morphospace for cyanobacteria and microalgae: Morphological diversity, evolutionary relatedness, and size constraints. Freshw. Biol. 2023, 68, 115–126. [Google Scholar] [CrossRef]
- Polishuk, V.; Kostikov, I.Y.; Taran, N.Y.; Voitsitsky, V.M.; Budzanivska, I.; Khyzhnyak, S.; Trokhymets, V.M. The complex studying of Antarctic biota. Ukr. Antarct. J. 2009, 8, 293–301. [Google Scholar] [CrossRef]
- Neustupa, J.; Skaloud, P. Diversity of subaerial algae and cyanobacteria on tree bark in tropical mountain habitats. Biologia 2008, 63, 806–812. [Google Scholar] [CrossRef]
- Kessler, E.; Kramer, H. Physiologische untersuchungen an einer ungewöhnlich saureresistenten Chlorella. Arch. Microbiol. 1960, 37, 245–255. [Google Scholar]
- Gimmler, H.; Weis, U. Dunaliella acidophila–life at pH 1.0. In Dunaliella, Physiology, Biochemistry and Biotechnology; Avron, M., Amotz, A.B., Eds.; CRC Press: Boca Raton, FL, USA, 1992; pp. 99–133. [Google Scholar]
- Gross, W. Ecophysiology of algae living in highly acidic environments. Hydrobiologia 2000, 433, 31–37. [Google Scholar] [CrossRef]
- Bahr, M.; Díaz, I.; Dominguez, A.; González Sánchez, A.; Muñoz, R. Microalgal biotechnology as a platform for an integral biogas upgrading and nutrient removal from anaerobic effluents. Environ. Sci. Technol. 2014, 48, 573–581. [Google Scholar] [CrossRef] [PubMed]
- González-Sánchez, A.; Revah, S.; Deshusses, M.A. Alkaline biofiltration of H2S odors. Environ. Sci. Technol. 2008, 42, 7398–7404. [Google Scholar] [CrossRef]
- González-Sánchez, A.; Revah, S. The effect of chemical oxidation on the biological 396 sulfide oxidation by an alkaliphilic sulfoxidizing bacterial consortium. Enzym Microb. Technol. 2007, 40, 292–298. [Google Scholar] [CrossRef]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and cyanobacterial cultivation: The supply of nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Franco-Morgado, M.; Alcántara, C.; Noyola, A.; Muñoz, R.; González-Sánchez, A. A study of photosynthetic biogas upgrading based on a high rate algal pond under alkaline conditions: Influence of the illumination regime. Sci. Total Environ. 2017, 592, 419–425. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Mathew, A.K.; Pandey, A.; Sukumaran, R.K. Harvesting of microalgal biomass: Efficient method for flocculation through pH modulation. Bioresour. Technol. 2016, 213, 216–221. [Google Scholar] [CrossRef]
- Gondi, R.; Kavitha, S.; Yukesh Kannah, R.; Karthikeyan, O.P.; Kumar, G.; Vinay Kumar Tyagi, V.K.; Banu, J.R. Algal-based system for removal of emerging pollutants from waste water: A review. Bioresour. Technol. 2022, 344, 126245. [Google Scholar] [CrossRef] [PubMed]
- Oruganti, R.K.; Katam, K.; Show, P.L.; Gadhamshetty, V.; Upadhyayula, V.K.K.; Bhattacharyya, D. A Comprehensive Review on the Use of Algal-Bacterial Systems for Wastewater Treatment with Emphasis on Nutrient and Micropollutant Removal. Bioengineered 2022, 13, 10412–10453. [Google Scholar] [CrossRef]
- Olaveson, M.M.; Stokes, P.M. Responses of the acidophilic Euglena mutabilis (Euglenophyceae) to carbon enrichment at pH 3. J. Phycol. 1989, 25, 529–539. [Google Scholar] [CrossRef]
- Graham, J.M.; Arancibia-Avila, P.; Graham, L.E. Effects of pH and selected metals on growth of the filamentous green alga Mougeotia under acidic conditions. J. Limnol. Oceanogr. 1996, 41, 263–270. [Google Scholar] [CrossRef]
- Gerloff-Elias, A.; Spijkerman, E.; Pröschold, T. Effect of external pH on the growth, photosynthesis and photosynthetic electron transport of Chlamydomonas acidophila Negoro, isolated from an extremely acidic lake (pH 2.6). Plant Cell Environ. 2005, 28, 1218–1229. [Google Scholar] [CrossRef]
- Hoham, R.W.; Filbin, R.W.; Frey, F.M.; Pusack, T.J.; Ryba, J.B.; Mcdermott, P.D.; Fields, R.A. The optimum pH of the green snow algae, Chloromonas tughillensis and Chloromonas chenangoensis, from upstate New York. Arct. Antarct. Alp. Res. 2007, 39, 65–73. [Google Scholar] [CrossRef]
- Spijkerman, E.; Barua, D.; Gerloff-Elias, A.; Kern, J.; Gaedke, U.; Heckathorn, S.A. Stress responses and metal tolerance of Chlamydomonas acidophila in metalenriched lake water and artificial medium. Extremophiles 2007, 11, 551–562. [Google Scholar] [CrossRef]
- Ňancucheo, I.; Johnson, B.D. Acidophilic algae isolated from mine-impacted environments and their roles in sustaining heterotrophic acidophiles. Front. Microbiol. 2012, 3, 325. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.R.; Senhorinho, G.N.A.; Scott, J.A. Microalgae under Environmental Stress as a Source of Antioxidants. Algal Res. 2020, 52, 102104. [Google Scholar] [CrossRef]
- Soru, S.; Malavasi, V.; Caboni, P.; Concas, A.; Cao, G. Behavior of the extremophile green alga Coccomyxa melkonianii SCCA 048 in terms of lipids production and morphology at different pH values. Extremophiles 2019, 23, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Moss, B. The influence of environmental factors on the distribution of freshwater algae: An experimental study: II. The role of pH and the carbon dioxide-bicarbonate system. J. Ecol. 1973, 61, 157–177. [Google Scholar] [CrossRef]
- Novis, P. Taxonomy of Klebsormidium (Klebsormidales, Chlorophyceae) in New Zealand streams, and the significance of low pH habitats. Phycologia 2006, 45, 209–301. [Google Scholar] [CrossRef]
- Xing, R.; Ma, W.; Shao, Y.; Cao, X.; Chen, L.; Jiang, A. Factors that affect the growth and photosynthesis of the filamentous green algae, Chaetomorpha valida, in static sea cucumber aquaculture ponds with high salinity and high pH. PeerJ 2019, 7, e6468. [Google Scholar] [CrossRef]
- Mayo, A.W. Effects of temperature and pH on the kinetic growth of unialga Chlorella vulgaris cultures containing bacteria. Water Environ. Res. 1997, 69, 64–72. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Karemore, A.; Pal, R.; Sen, R. Strategic enhancement of algal biomass and lipid in Chlorococcum infusionum as bioenergy feedstock. Algal Res. 2013, 2, 113–121. [Google Scholar] [CrossRef]
- Xiang, W.-Z.; Wu, H.-L.; Xie, K.; He, H.; Xiao, W. Extreme features of Chlorococcum sp. and rapid induction of ataxantin. J. Trop. Oceanogr. 2007, 26, 50–54. [Google Scholar]
- Shubert, E.; Rusu, L.E.; Bartok, A.-M.; Moncrieff, C.B. Distribution and abundance of edaphic algae adopted to higly acidic, metal rich soils. Nova Hedwig. Beih. 2001, 123, 411–425. [Google Scholar]
- Agrawal, S.C. Factors affecting spore germination in algae—Review. Folia Microbiol. 2009, 54, 273–302. [Google Scholar] [CrossRef] [PubMed]
- Sinetova, M.A.; Sidorov, R.A.; Medvedeva, A.A.; Starikov, A.Y.; Markelova, A.G.; Allakhverdiev, S.I.; Los, D.A. Effect of salt stress on physiological parameters of microalgae Vischeria punctata strain IPPAS H-242, a superproducer of eicosapentaenoic acid. J. Biotechnol. 2021, 331, 63–73. [Google Scholar] [CrossRef] [PubMed]
- She, Y.; Gao, X.; Jing, X.; Wang, J.; Dong, Y.; Cui, J.; Xue, H.; Li, Z.; Zhu, D. Effects of nitrogen source and NaCl stress on oil production in Vischeria sp. WL1 (Eustigmatophyceae) isolated from dryland biological soil crusts in China. J. Appl. Phycol. 2022, 34, 1281–1291. [Google Scholar] [CrossRef]
- Lukavský, J.; Kopecký, J.; Kubácˇ, D.; Kvíderová, J.; Procházková, L.; Řezanka, T. The alga Bracteacoccus bullatus (Chlorophyceae) isolated from snow, as a source of oil comprising essential unsaturated fatty acids and carotenoids. J. Appl. Phycol. 2023, 35, 649–660. [Google Scholar] [CrossRef]
- Remis, D.; Treffny, B.; Gimmler, H. Light-induced H+ transport across the plasma membrane of the acid-resistant green alga Dunaliella acidophila. Plant Physiol. Biochem. 1994, 32, 75–84. [Google Scholar]
- Gimmler, H. Acidophilic and acidotolerant algae. In Algal Adaptations to Environmental Stresses; Rai, L.C., Gaur, J.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 259–290. [Google Scholar]
- Nixdorf, B.; Fyson, A.; Krumbeck, H. Review: Plant life in extremely acidic water. Environ. Exp. Bot. 2001, 46, 203–211. [Google Scholar] [CrossRef]
- Grzmil, B.; Wronkowski, J. Removal of phosphates and fluorides from industrial wastewater. Desalination 2006, 189, 261–268. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef]
- Nguyen, T.D.P.; Tran, T.N.T.; Le, T.V.A.; Nguyen Phan, T.X.; Show, P.-L.; Chia, S.R. Auto-flocculation through cultivation of Chlorella vulgaris in seafood wastewater discharge: Influence of culture conditions on microalgae growth and nutrient removal. J. Biosci. Bioeng. 2019, 127, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Singh, A.K.; Sikandar, M. Biosorption of Hg (II) from aqueous solution using algal biomass: Kinetics and isotherm studies. Heliyon 2020, 6, e03321. [Google Scholar] [CrossRef]
- Scarcelli, P.G.; Ruas, G.; Lopez-Serna, R.; Leite Serejo, M.; Blanco, S.; Boncz, M.A.; Muñoz, R. Integration of algae-based sewage treatment with anaerobic digestion of the bacterial-algal biomass and biogas upgrading. Bioresour. Technol. 2021, 340, 125552. [Google Scholar] [CrossRef] [PubMed]
- Yousif, Y.I.D.; Mohamed Essam, S.; El-Gendy, A.S. Using Chlorella vulgaris for nutrient removal from hydroponic wastewater: Experimental investigation and economic assessment. Water Sci. Technol. 2022, 85, 3240. [Google Scholar] [CrossRef] [PubMed]
- Cheirsilp, B.; Maneechote, W.; Srinuanpan, S.; Angelidaki, I. Microalgae as tools for bio-circular-green economy: Zero-waste approaches for sustainable production and biorefineries of microalgal biomass. Bioresour. Technol. 2023, 387, 129620. [Google Scholar] [CrossRef]
- Bishoff, H.; Bold, H. Phycological Studies. IV. Some Algae from Enchanted Rock and Related Algae Species. Univ. Texas Publ. 1963, 6318, 91–95. [Google Scholar]
- Jongman, R.H.G.; Ter Braak, C.J.F.; Van Tongeren, O.F.R. Data Analysis in Community and Landscape Ecology; Cambridge University Press: Cambridge, UK, 1996; pp. 29–50. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar] [CrossRef]
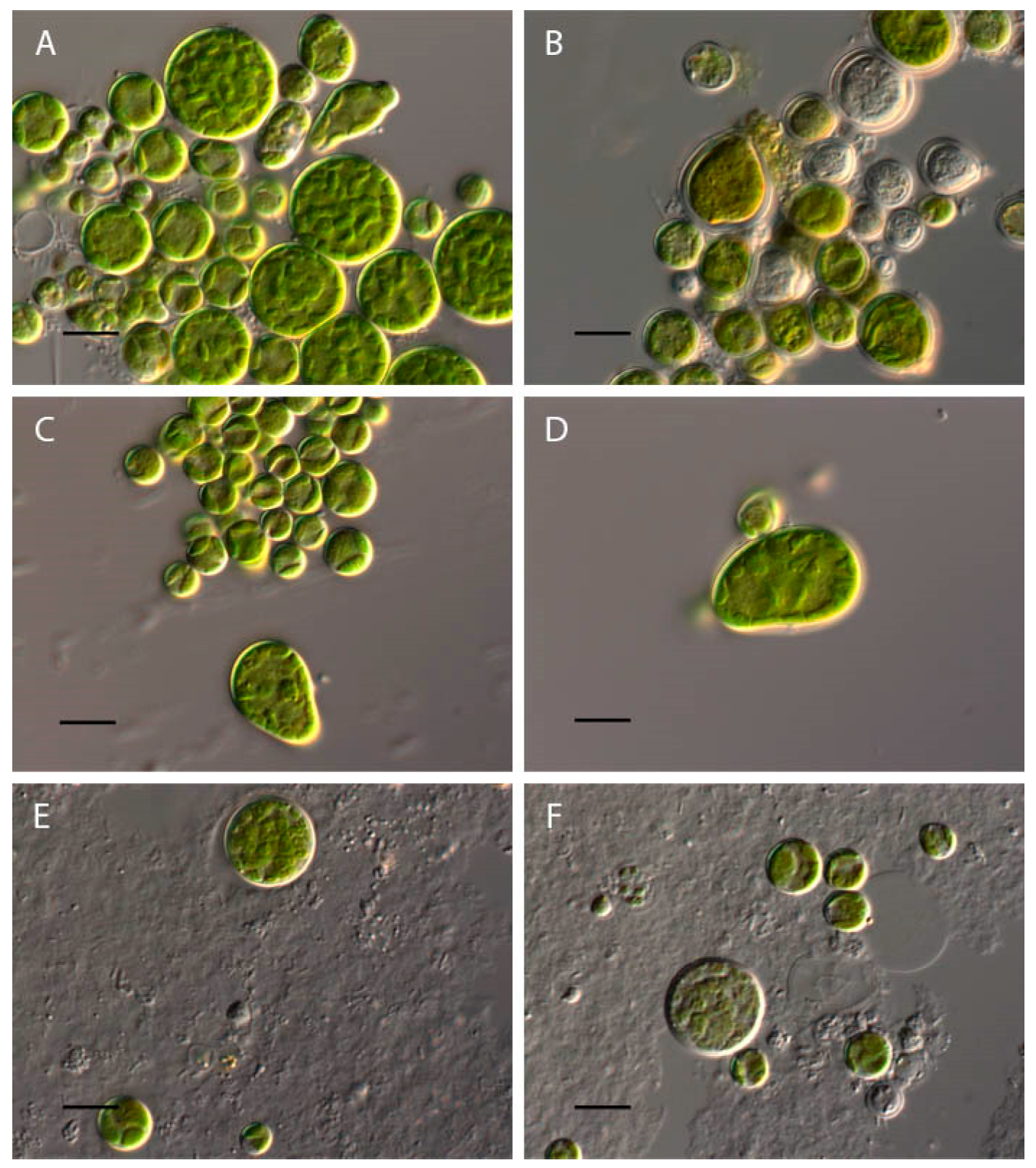
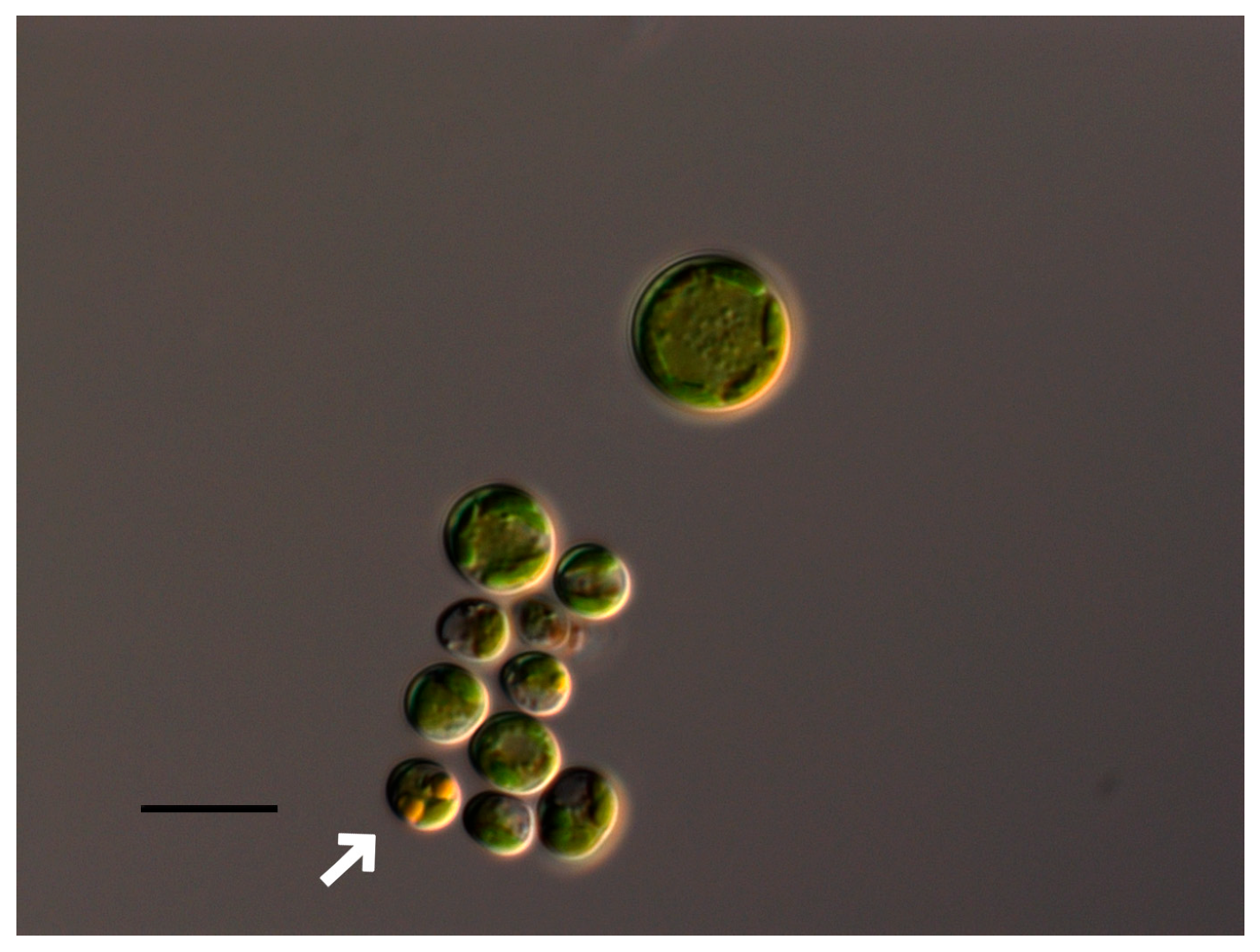
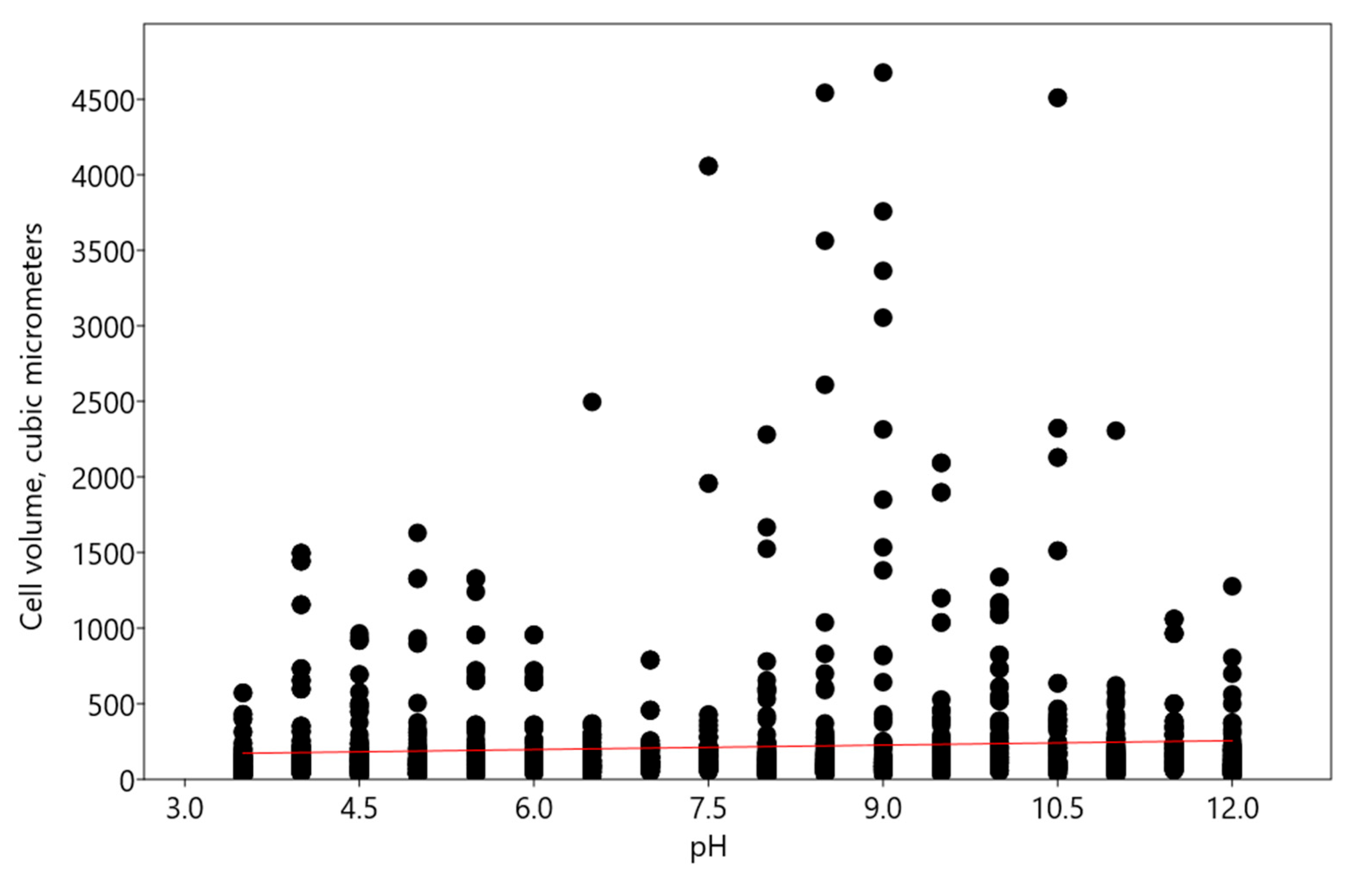
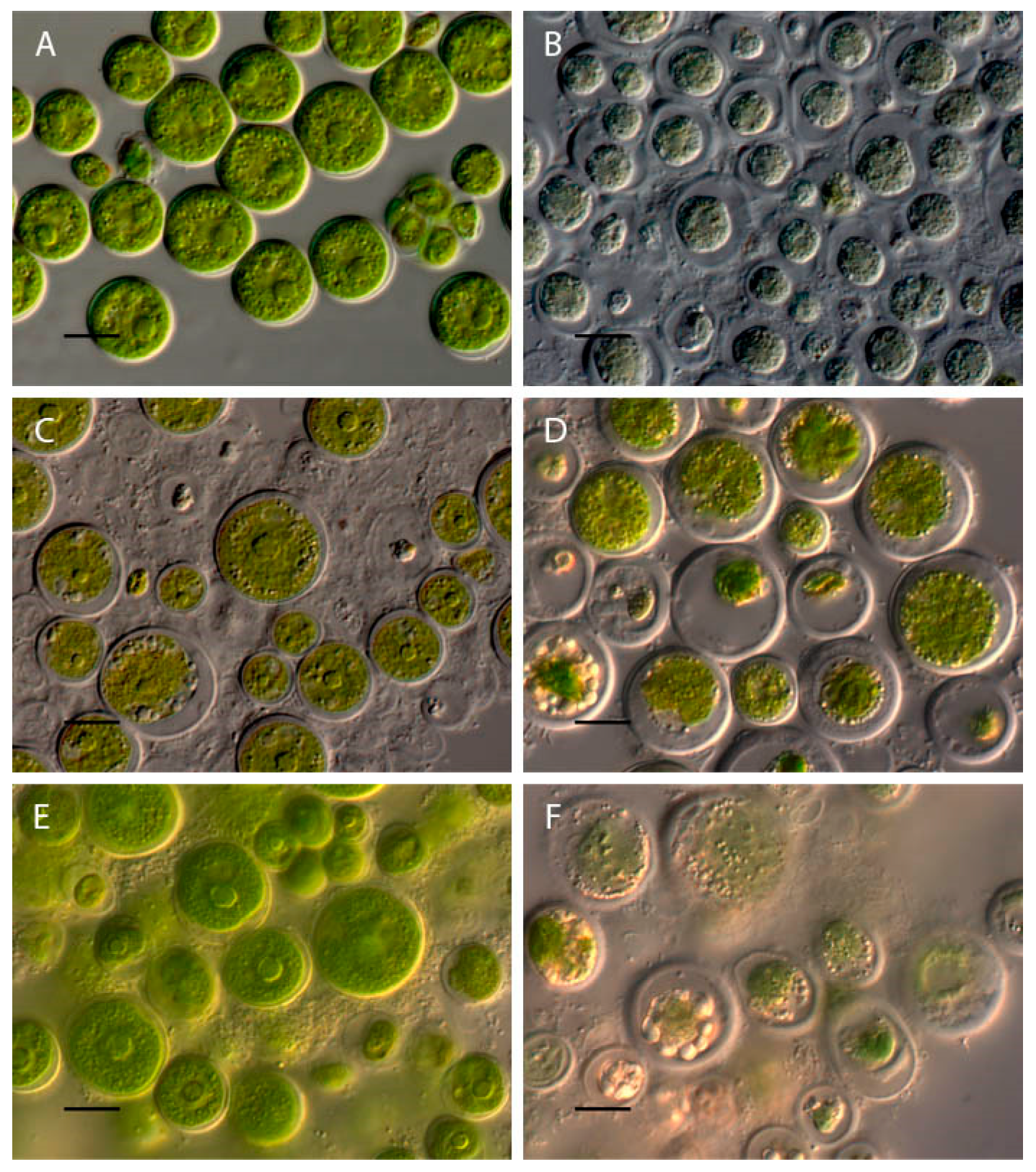
| Taxa | pH Limits | Morphological and Physiological Features Change at pH < 4 | Morphological and Physiological Features at pH > 10 |
|---|---|---|---|
| Bracteacoccusminor | 4–10 | Complete discoloration and destruction of cells; the appearance of orange granules | Discoloration and destruction of cells |
| Chlorococcum infusionum | 4–9.5 | Discoloration and destruction of cells; the appearance of large granules in cytoplasm | Discoloration and destruction of cells, appearance of large granules in cytoplasm |
| Chlorella vulgaris | 4–11.5 | Discoloration of cells | Discoloration of cells |
| Pseudococcomyxasimplex | 4–11.5 | Discoloration and “wrinkling” of cells; appearance of almost round cells | Discoloration of cells |
| Vischeriamagna | 3.5–11 | Discoloration and appearance of the orange granules in cells; maximal zoospores production at pH 3.5; increase in cell volume | Discoloration of cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaysina, L.A. Influence of pH on the Morphology and Cell Volume of Microscopic Algae, Widely Distributed in Terrestrial Ecosystems. Plants 2024, 13, 357. https://doi.org/10.3390/plants13030357
Gaysina LA. Influence of pH on the Morphology and Cell Volume of Microscopic Algae, Widely Distributed in Terrestrial Ecosystems. Plants. 2024; 13(3):357. https://doi.org/10.3390/plants13030357
Chicago/Turabian StyleGaysina, Lira A. 2024. "Influence of pH on the Morphology and Cell Volume of Microscopic Algae, Widely Distributed in Terrestrial Ecosystems" Plants 13, no. 3: 357. https://doi.org/10.3390/plants13030357
APA StyleGaysina, L. A. (2024). Influence of pH on the Morphology and Cell Volume of Microscopic Algae, Widely Distributed in Terrestrial Ecosystems. Plants, 13(3), 357. https://doi.org/10.3390/plants13030357






