Abstract
Plants face multiple stresses in their natural habitats. WRKY transcription factors (TFs) play an important regulatory role in plant stress signaling, regulating the expression of multiple stress-related genes to improve plant stress resistance. In this study, we analyzed the expression profiles of 25 BnWRKY genes in three stages of ramie growth (the seedling stage, the rapid-growth stage, and the fiber maturity stage) and response to abiotic stress through qRT-PCR. The results indicated that 25 BnWRKY genes play a role in different growth stages of ramie and were induced by salt and drought stress in the root and leaf. We selected BnWRKY49 as a candidate gene for overexpression in Arabidopsis. BnWRKY49 was localized in the nucleus. Overexpression of BnWRKY49 affected root elongation under drought and salt stress at the Arabidopsis seedling stage and exhibited increased tolerance to drought stress. Further research found that BnWRKY49-overexpressing lines showed decreased stomatal size and increased cuticular wax deposition under drought compared with wild type (WT). Antioxidant enzyme activities of SOD, POD, and CAT were higher in the BnWRKY49-overexpressing lines than the WT. These findings suggested that the BnWRKY49 gene played an important role in drought stress tolerance in Arabidopsis and laid the foundation for further research on the functional analysis of the BnWRKYs in ramie.
1. Introduction
The harsh environmental conditions such as drought, salinity, cold, and heat can have a devastating impact on plant growth and yield under field conditions [1], becoming one of the important factors restricting agricultural development. To cope with abiotic stress, plants undergo extensive molecular reprogramming at the transcriptional and post-transcriptional levels to enhance their adaptability to the environment. During this process, TFs play an important role [2]. WRKY-TFs are involved in plant growth and development and play important roles in response to abiotic and biotic stresses [3,4]. WRKY genes are divided into three groups (I, II, and III) according to the number of WRKY domains and the pattern of zinc-finger motifs. Members of group I usually have two WRKY domains containing a single C2H2 zinc-finger motif. Group II members contain a WRKY domain and a C2H2 zinc-finger motif. Members of group III have a WRKY domain, but they have different zinc-finger motifs, and the structure is C2HC [5]. At present, WRKY TFs have been identified at the genomic and transcriptome levels in 234 species, including lower plants, higher plants, protozoa, etc. [6]. The expression profiles of AtWRKY25, AtWRKY26, and AtWRKY33 genes under abiotic stress were analyzed using Northern blot analysis, they responded to stresses including heat, NaCl, abscisic acid (ABA), and osmotic stress [7]. Recent research has shown that AtWRKY20, as a member of the WRKY I group, is a sucrose-enhanced TF that plays a role upstream of sucrose-responsive genes. Thereby, AtWRKY20 affects various developmental and metabolic processes in plants [8]. In the phloem of plants infected with Begomovirus, AtWRKY20 could be bound to the begomoviral βC1 protein. This viral hijacking of AtWRKY20 spatiotemporally redeployed plant chemical immunity within the leaf [9]. OsWRKY53 was induced by abscisic acid (ABA), jasmonate (JA), salt, drought, darkness, and low temperatures but inhibited by gibberellic acid (GA) and salicylic acid (SA) [10]. The WRKY-TFs in various woody plants were widely involved in responses to biotic and abiotic stresses, as well as in plant growth and development [11]. PeWRKY41 belongs to Class III WRKY and interacts with casein kinase. Overexpression of PeWRKY41 improved the insect resistance and salt tolerance of transgenic poplar [12]. Drought stress leads to osmotic and oxidative stresses due to cellular dehydration, ROS production is mainly regulated by NADPH oxidase, and RBOH is involved as a major regulatory gene in the regulation of plant responses to adversity stress [13]. The prolonged exposure to ROS leads to impaired cellular function, affecting plant growth and development. To cope with the sudden increase in ROS, plants have adopted various strategies for ROS detoxification and maintenance of cellular redox status [14]. Antioxidant enzymes such as CAT (catalase), SOD (superoxide dismutase), POD (Peroxidase), and GPX (Glutathione peroxidase) regulate the cellular redox status [15]. Compared with the WT, TaGPX1-D-overexpressing plants exhibit enhanced tolerance to salinity and osmotic stress, increased antioxidant enzyme activity and proline, and decreased levels of H2O2 and MDA (Malondialdehyde) [16]. WRKY played a key role in defense responses by regulating ROS homeostasis. In addition, cuticular wax was deposited on the surface of plants to reduce water loss and resist drought stress. CitWRKY28 promoted the accumulation of epidermal wax in Arabidopsis leaves [17].
Ramie, which is also known as “China grass”, originated in China and is a perennial herbaceous plant of the Urticaceae family [18]. Ramie leaves are a rich source of proteins, minerals, vitamins, dietary fiber, phenolics, and flavonoids [19]. The fiber made from stem bast is a good textile material. With the acceleration of urbanization and industrialization in China, the per capita arable land area is gradually decreasing, and cultivation of ramie on mountain slopes or uncultivated land has become an option. However, the growth of ramie is affected by drought stress. It has therefore become urgent to improve the drought tolerance of ramie. In a previous study, twelve TFs from the AP2, MYB, NAC, HD-Zip, bHLH, Dof, C2H2L, ARF, and GRAS gene families were further verified to exhibit activity in response to drought stress [20], and from a transcriptome database, eight WRKY genes with intact, conserved domains were screened [21]. In another study, 25 TFs from the AP2, MYB, NAC, zinc-finger, bZIP, and WRKY families were reported to be potentially associated with drought stress [22]. The BnWRKY gene family has been identified, and the response of 12 BnWRKYs to cadmium stress has been studied [23]. Although the function of most Arabidopsis WRKY-TFs is known, the related research on the WRKY gene family in ramie is still limited.
In this study, we selected 25 BnWRKY genes from three subgroups of BnWRKY genes and analyzed the transcription patterns of the BnWRKY genes at three stages of ramie growth (the seedling stage, the rapid-growth stage, and the fiber maturity stage) and in response to the abiotic stresses (salt and drought). We further investigated the stress tolerance provided by BnWRKY49 in transgenic Arabidopsis. This study will help us understand the potential molecular response mechanism for drought stress and will provide assistance to the breeding of drought-resistant ramie.
2. Results
2.1. Expression Patterns of the 25 BnWRKY Genes in Ramie
The expression patterns of these genes in four tissues were examined, including leaf tissue and three parts of stem bark tissue (the top, middle, and bottom parts) (Figure 1). The three samples of stem bark represented the different fiber growth stages: from the top to bottom parts, the fiber cell gradually thickened. At the seedling stage, more than half of the 25 BnWRKYs showed similar transcript levels between the young stems and leaves, and most of the 25 BnWRKYs exhibited the higher relative expression in the stem bark. Nine genes (BnWRKY1, 4, 9, 17, 21, 26, 28, 30, 43) exhibited low expression in the stem bark of the rapid growth stage. At the fiber maturation stage, compared to the stem bark, most of the 25 BnWRKY genes had higher expression levels in the leaves.
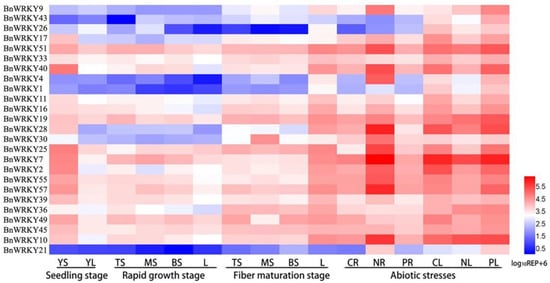
Figure 1.
Expression pattern analysis of BnWRKYs. Relative expression of 25 BnWRKYs in the seedling stage (YS: stem bark; YL: leaves), rapid growth stage (TS: top of stem bark; MS: middle of stem bark; BS: bottom of stem bark; L: leaves), fiber maturation stage (TS: top of stem bark; MS: middle of stem bark; BS: bottom of stem bark; L: leaves), and under abiotic stress (CR, NR, and PR represent roots under control, NaCl, and PEG treatments, respectively; CL, NL, and PL represent leaves under control, NaCl, and PEG stresses, respectively).
To study whether the expression pattern of the 25 BnWRKYs responded to abiotic stresses, qRT-PCR analyses were performed on the roots and leaves of hydroponic ramie seedlings (Figure 1). The results showed that all genes intensively responded to NaCl stress except BnWRKY45 and BnWRKY7. Among these genes, the expression of BnWRKY49 was downregulated in the roots under stress, while the transcripts of the others were highly promoted. In contrast, the expression of a majority of salt-responsive BnWRKYs (16 out of a total of 25 genes) was downregulated in the leaves, while only four genes had a greater expression. With respect to PEG stress, the relative expression level of seven genes showed no significance in either the root or leaf tissue. However, eight BnWRKYs presented transcript accumulation in the roots, five of which were obviously downregulated. Additionally, the expression of thirteen BnWRKYs increased in the leaves, while only three genes (BnWRKY2, 17, and 49) exhibited distinctly reduced expression levels. These stress-responsive genes can provide a better understanding of the BnWRKY genes related to abiotic stress.
2.2. Identification and Subcellular Localization of BnWRKY49
The BnWRKY49 gene consisted of six exons and five introns (Figure 2a). Homologs in six other species were found and used to construct the phylogenetic tree. BnWRKY49 was most closely related to XP_010097277.1 from Morus_notabilis (Figure 2b). BnWRKY49 and its homolog amino acid sequences contained two WRKYGQK motifs and two C2H2 zinc-finger structures (Figure 2c), it belonged to group I, and its domains were highly conserved. The prediction of the tertiary structure model of the BnWRKY49 protein showed that it contains five β-sheet structures (Figure 2d).
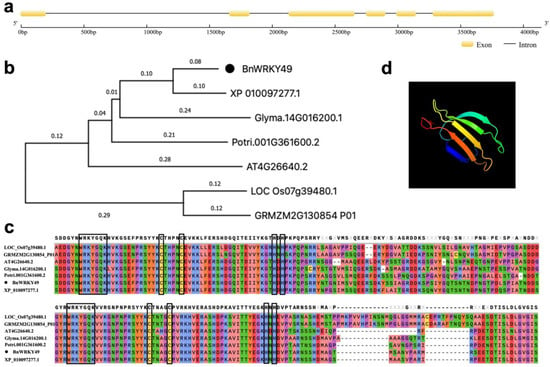
Figure 2.
Bioinformatics alignment of BnWRKY49. (a) Gene structure of BnWRKY49. (b) Phylogenetic tree of BnWRKY49 and homologous genes in other species. (c) WRKY domains and conserved domains within proteins of the BnWRKY49 with homologous gene sequences in other species. Two WRKYGQK motifs and two C2H2 zinc-finger structures were labeled with black boxes. (d) Tertiary structure prediction model of the BnWRKY49 protein.
The coding sequence of BnWRKY49 gene fused in frame with GFP under the control of the CaMV 35S promoter was shown in Figure 3a. In Figure 3b, the GFP signals of the BnWRKY49-GFP fusion protein were detected only in the nucleus, suggesting that the BnWRKY49-GFP fusion protein was targeted to the nucleus and possibly functions as a TF.
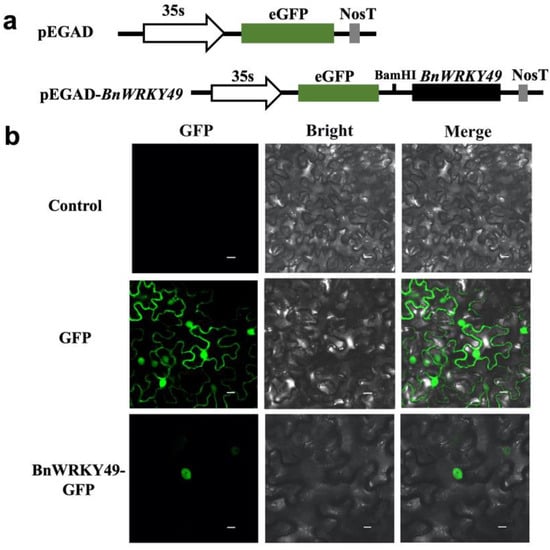
Figure 3.
Subcellular localization of four BnWRKY49 proteins in tobacco leaves. (a) The structures of pEGAD-35S::BnWRKY49:GFP fusion vector and pEGAD-35S::GFP. (b) Green fluorescence images, bright field images, and the merge images overlay of bright and GFP were captured. The untreated tobacco leaves were used as a negative control to exclude autofluorescence. Tobacco leaves that were treated with an empty pEGAD vector were named GFP as a positive control. Bars, 20 μm.
2.3. Overexpression of BnWRKY49 Affects Root Elongation under Drought and Salt Stress
BnWRKY49-overexpressing transgenic plants formed longer primary roots than the WT in cultures supplemented with PEG and NaCl, and the root lengths of the three transgenic lines were significantly longer than the WT (Figure 4a,b).
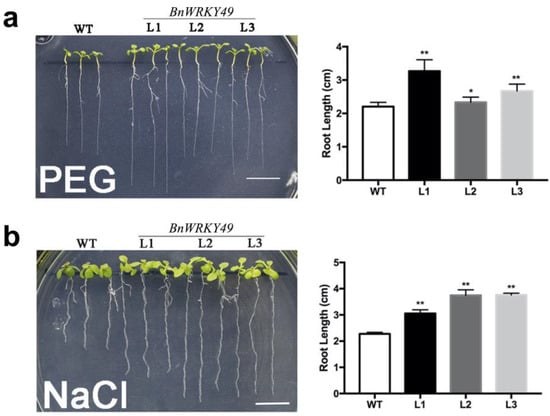
Figure 4.
Assay of the root length of BnWRKY49 overexpression Arabidopsis seedlings under PEG and NaCl stress treatment. (a) The root growth of Arabidopsis seedlings in the final water potential of the PEG-infused plate (−0.75 MPa) for 7 d. (b) The root growth of Arabidopsis seedlings under 125 mM NaCl stress for 7 d. From left to right are WT and three BnWRKY49 overexpressing lines L1, L2, and L3, respectively. The error bars represent the mean (SD) of ten biological replicates (* p < 0.05, ** p < 0.01).
2.4. Overexpression of BnWRKY49 Enhances Drought Stress Tolerance in Arabidopsis
Three BnWRKY49-overexpressing lines (L1, L2, and L3) under drought stress were evaluated to investigate the function of the BnWRKY49. At 7 days of drought stress, both WT plants and overexpression lines were subjected to drought stress and grew slowly, there was no significant difference between them. After 14 days of drought stress, WT exhibited more severe water-loss-related symptoms compared with the overexpression lines. When the plants were rewatered 7 days later, most BnWRKY49-overexpressing lines recovered growth, whereas WT plants did not. Three overexpression lines showed significantly higher recovery rates (Figure 5a). Therefore, under drought stress, the overexpression lines showed better growth and higher survival rates than the WT. We also determined the antioxidant enzyme activities of leaves from plants treated with drought for 7 days. The POD enzyme activities in the leaves of three BnWRKY49-overexpressing lines were higher than that of WT, but the difference was not significant. The activities of CAT and SOD enzymes of three overexpression lines were significantly higher than in WT (Figure 5b–d). The antioxidant enzyme activities were higher in the BnWRKY49-overexpressing lines under drought stress. The above results indicated that overexpression of BnWRKY49 in Arabidopsis could improve drought resistance.
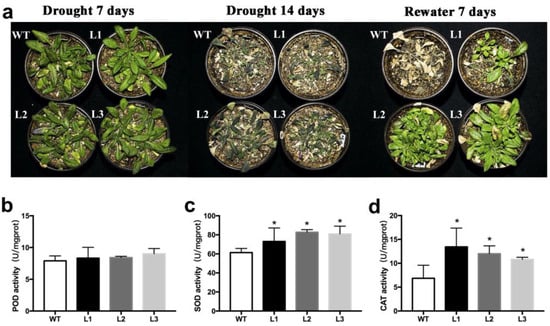
Figure 5.
The phenotype of BnWRKY49-overexpressing Arabidopsis that were exposed to drought stress. (a) The phenotype of WT and three BnWRKY49-overexpressing lines (L1, L2, L3) exposed to drought stress for 7 and 14 days and rewatered for 7 days. (b) POD activity. (c) SOD activity. (d) CAT activity. The error bars represent the mean (SD) of three biological replicates (* p < 0.05).
2.5. BnWRKY49 Induced Stomatal Closure and Promoted Cuticular Wax Accumulation under Drought Stress
By phenotypic observation, the BnWRKY49-overexpressing lines showed increased tolerance to drought stress. From this, we speculated that the difference may be caused by the difference in stomatal closure under drought stress (Figure 6a). Compared with WT plants, the stomatal openings of transgenic plants were shorter and narrower, and the aspect ratio was larger (Figure 6b,c). We also analyzed the cuticular wax of the WT plants and transgenic plants under drought stress with a scanning electron microscope. The results showed that, compared with WT plants, the BnWRKY49-overexpressing lines had more wax crystals on the stem surface (Figure 6d).
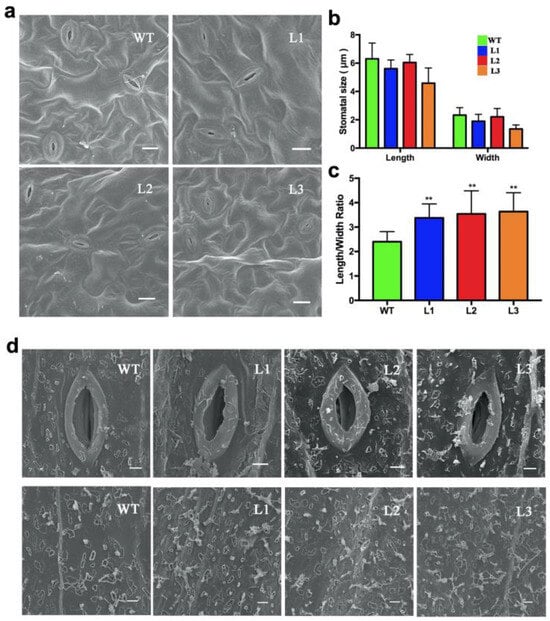
Figure 6.
Stomatal aperture and epicuticular wax crystals of BnWRKY49-overexpressing Arabidopsis under drought stress. (a) Stomatal aperture of the WT plant and transgenic plant leaves. The stomata were observed using SEM; bars, 10 μm. (b) The size of the stomata. (c) The length/width ratio of the stomata. The error bars represent the mean (SD) of at least three biological replicates (** p < 0.01). (d) The picture above shows SEM images of the stomatal aperture and epicuticular wax crystals on the WT plant and transgenic plant stems that were exposed to drought stress. Bars, 2 μm.
3. Discussion
The WRKY TFs, as a widely studied plant defense response protein, play a crucial regulatory role in resisting abiotic stress. The most significant of the various biological functions that WRKY TFs have in plants involves being key players in response to drought and salt stress [24]. Recently, increasing numbers of studies have supported the essential roles of WRKY TFs.
Herein, we cloned a novel WRKY TF, which belonged to the family group I, BnWRKY49. In our study, the transient transformation of the pEGAD-35S::BnWRKY49:GFP fusion vector in tobacco leaves showed a strong GFP signal exclusively in the nucleus. BnWRKY49 was localized in the nucleus, consistent with the results of other reported WRKY-TF [25]. The expression pattern of BnWRKY49 was similar under drought stress and salt stress treatments, with downregulation in both roots and leaves. However, we overexpressed BnWRKY49 in Arabidopsis, which enhanced the drought resistance of the overexpressed lines. The dynamics of BnWRKY expression changed in cadmium stress from 0 to 48 h. At 48 h, BnWRKY10, 27, 40, 44, and 46 showed downregulation of expression in roots, and BnWRKY10 was an upregulated expression that reached its highest value at 6 h of the Cd2+ stress [23]. We analyzed that after abiotic stress treatment, we might not be able to observe the upregulation of BnWRKY49 expression only after 48 h of sampling.
Drought severely affects crop growth, development, and yield. WRKYs enhance the drought tolerance of plants. GmWRKY27 positively regulates drought tolerance [26]. TaWRKY1 and TaWRKY33 overexpressed in Arabidopsis participated in drought tolerance via ABA signaling [27], and the OsWRKY30 gene enhanced the drought tolerance of rice through MAPK signaling [28,29]. In our study, it was found that ectopic overexpression of BnWRKY49 enhanced drought stress tolerance in Arabidopsis. At the molecular level, plants usually respond to abiotic stress with the same signal transduction pathways, leading to the accumulation of ROS and ABA [30]. CAT, SOD, and POD enzymes act as scavengers of ROS to protect cells when plants are exposed to stresses and produce excess ROS. Our results indicated that the BnWRKY49-overexpressing plants exhibited higher CAT, SOD, and POD activities than the WT plants under drought conditions. Drought and salt stresses can lead to dehydration of cells and can affect plant yields and spatial distribution [30]. During long-term evolution, stomatal development (including density, closure, aperture, and size) and cuticle adjustments were the main defense mechanisms against water loss under drought stress [31,32,33,34,35]. Many reports are consistent with our research findings. Heterologous overexpression of the GhWRKY41 gene in tobacco led to tolerance to drought and salt stress by enhancing stomatal closure and modulating clearance scavenging of ROS [36]. AtWRKY70 and AtWRKY54 cooperate as negative regulators of stomatal closure [37]. The SlWRKY81 gene plays a negative role in drought stress by suppressing stomatal closure mediated by guard cells and H2O2 [38]. Moreover, overexpression of GsWRKY20 resulted in decreased stomatal density and water loss rates, suggesting that GsWRKY20 may act as a major transcriptional regulator of cuticular wax biosynthesis and accumulation in response to drought [34]. Under drought treatment, TaWRKY31-overexpressing lines in Arabidopsis lead to a decrease in H2O2 and MDA levels, reducing stomatal opening. In addition, an increase in antioxidant enzyme activity, germination rate, and root length was observed in TaWRKY31 transgenic Arabidopsis [25]. Overexpression of the TaNCL2-A gene in Arabidopsis showed significant cadmium tolerance, salt tolerance, and osmotic stress, with increased calcium accumulation, increased proline content, and antioxidant enzyme activities, and decreased oxidative stress-related molecules such as H2O2 and MDA [39].
However, little is known about the role of BnWRKYs in regulating drought response in ramie. The qRT-PCR results showed that the BnPP2C1 and BnPP2C26 genes responded to drought, high levels of salt, and ABA treatments, and there were a large number of stress-responsive elements in the promoter region, which can be used as candidate genes for drought and salt tolerance in ramie [40]. BnbZIP2 gene overexpression in Arabidopsis showed high sensitivity to drought stress at the seed germination stage and higher tolerance to salinity stress than the WT [41]. The molecular mechanism research related to drought resistance in ramie is still limited [42]. In our study, the stomatal openings of the transgenic plants were longer and wider, with a smaller length/width ratio, and less cuticular wax was observed on the surfaces of WT plants compared with the transgenic plants. Thus, BnWRKY49 may positively regulate the expression of genes involved in wax biosynthesis and may regulate stomatal opening. WRKY-TFs are involved in plant signaling pathways (e.g., phytohormones, MAPKs, ROS, Ca2+) and metabolic regulation associated with stress responses [43]. Recently, a novel mechanism of DPY1-SAPK6-mediated drought tolerance was revealed [44]. WRKY-mediated crosstalk between abiotic and biotic stress responses is a common element in plants. WRKYs have high potential applications in crop improvement in the future.
4. Materials and Methods
4.1. Plant Materials and Treatments
Ramie cultivar Huazhu No. 5 was cultivated at the Ramie Germplasm Resources Garden of Huazhong Agricultural University (Wuhan, China). We harvested leaves and stem bark in three growth stages of ramie, including the seedling stage, the rapid-growth stage, and fiber maturity stage. During the seedling stage, we took the young stem bark (YS) and leaves (YL). During the rapid-growth stage and the fiber maturity stage, we adopted the method of Chen et al. [45] for sampling. We took stem bark from the top, middle, and bottom positions of the stem, and young leaves, and named them TS, MS, BS, and L.
For stress treatments, we cut off the young shoot tips (approximately 15 cm) of Huazhu No. 5 from the Ramie Germplasm Resources Garden and cultivated them in the greenhouse (28 °C/16 h, light; 20 °C/8 h, dark). The young shoot tips were soaked in 0.1 g/L KMnO4 for 2 days and then transferred into a conical flask filled with tap water for rooting. After rooting, all plantlets were placed into half-strength Hoagland’s solution and grown for 20 days. Afterward, the plantlets were treated with 150 mM NaCl (representing salt stress), and the roots and leaves were sampled after 20 h for salinity stress. Other plantlets were cultured in 20% PEG 6000 (w/v) (representing drought stress), and the roots and leaves were sampled after 48 h for drought stress. Untreated plants served as controls, and all the treatments were carried out in three biological replicates. All samples were immediately placed in liquid nitrogen and then stored at −80 °C for RNA isolation.
4.2. Total RNA Extraction and Quantitative Real-Time Reverse Transcription PCR (qRT-PCR) Analysis
We extracted the total RNA using the RNAPrep Pure plant kit (Tiangen Biotech, Beijing, China). The complementary DNA was synthesized by the GoScript reverse transcription system (Promega, Madison, WI, USA). qRT-PCR analysis was conducted via an iQ5 multicolor real-time PCR system (Bio-Rad, Hercules, CA, USA). Here we analyzed the expression of 25 BnWRKY genes from three subgroups [23]. The primers (Table S1) applied to qRT-PCR were generated from the Primer3 website (http://primer3.ut.ee/, accessed on 1 June 2015) and were further evaluated by Oligo 7 (Molecular Biology Insights Inc., Cascade, CO, USA). The EF1A gene was selected as a reference gene. Three biological and technical replicates were performed, and the relative expression levels were calculated based on the comparative cycle threshold (2−∆∆Ct) values [46]. Heatmap Illustrator 1.0 was used to generate a comprehensive presentation of gene expression under the different tested conditions.
4.3. Phylogenetic Tree and Sequence Alignment Analyses of BnWRKY49
The young leaves of ramie cultivar Huazhu No. 5 were used for the extraction of genomic DNA with an HP Plant DNA Kit (Omega, Norcross, GA, USA). The cDNA was derived from Section 4.2. The BnWRKY49 CDS sequence obtained from whole-genome analysis were used as templates [24]. We designed three pairs of primers by Oligo 7.0 to clone BnWRKY49 (Table S2), including genomic sequence and CDS sequence. The amplified products were A-T cloned into the pEASY-T5 Zero Vector and sequenced. The intron-exon structure of BnWRKY49 analysis was carried out via GSDS 2.0 (http://gsds.gao-lab.org/, accessed on 15 January 2018). We compared the BnWRKY49 protein with homologous proteins from Populus_trichocarpa (Potri.001G361600.2), Glycine_max (Glyma.14G016200.1), Oryza_sativa (LOC_Os07g39480.1), Zea_mays (GRMZM2G130854_P01), Morus_notabilis (XP_010097277.1), and Arabidopsis (AT4G26640.2) to construct an evolutionary tree via the neighbor-joining (NJ) method using MEGA 7 software, and the parameter of bootstrap repetition was 1000. Multiple sequence alignments were performed using the online multiple sequence alignment of muscle (https://www.omicshare.com/tools/Home/Soft/musclecmp, accessed on 15 December 2023). The tertiary structure prediction model of the BnWRKY49 protein was predicted using the Phyre 2 (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index, accessed on 15 January 2018).
4.4. Subcellular Localization of BnWRKY49 Proteins
To determine the subcellular localization of BnWRKY49 proteins, BnWRKY49:GFP fusion genes controlled by the CaMV 35S promoter were generated. The fusion genes were then transferred into tobacco plants via Agrobacterium-mediated transformation and labeled. After the tobacco plants were incubated at room temperature in the dark for 48 h, the epidermal cells were detected by a laser scanning confocal microscope (Olympus FV1200, Tokyo, Japan) with green fluorescence excited with a 488 nm laser. The untreated tobacco leaves were used as negative controls to exclude autofluorescence [47]. Three biological replicates and the imaging data were processed using Photoshop CC software (Adobe Inc., San Jose, CA, USA).
4.5. Vector Construction and Transformation
Specific primers (Table S2) were designed, and the cDNA was used as the template for the cloning of the BnWRKY49 CDS sequence. The fragment was then ligated into the pMDC32 plant expression vector at the KpnI and SacI sites under the control of the 2× 35S promoter. The construct was transformed into Agrobacterium tumefaciens GV3101 by electroporation, which was then transformed into Arabidopsis (Col-0) using the floral-dip method [48]. Positive transformants on hygromycin (50 μg/mL) plates were selected. Total DNA was extracted using the cetyltrimethylammonium Ammonium Bromide (CTAB) method and confirmed by PCR using specific primers (Table S3). Abiotic stress tolerance analysis was performed using homozygous T3-generation plants.
4.6. Analyses of Root Growth under Drought and Salt Stresses
The seeds of transgenic plants were placed in 1.5 mL EP tubes, after which 70% ethanol was added for 1 min. Afterward, 10% (v/v) NaClO was added for 8 min, and then the seeds were washed 5 times with water. Half-strength Murashige and Skoog (1/2 MS) medium containing the seeds were incubated in the dark at 4 °C for 3 d and then incubated under 16 h light/8 h dark conditions at 22 °C for 5 d. For root growth measurements, 5-day-old transgenic Arabidopsis and WT seedlings were transferred to 1/2 MS medium containing 125 mM NaCl. Due to PEG cannot be dissolved in agar, we adopted the protocol described by Verslues et al. [49]. The agar medium and PEG covering layer (excluding agar) were prepared separately, the final water potential of the PEG-infused plate having ψw = −0.75 MPa. First, 1/2 MS agar medium was poured into the regular (100 mm diameter) plate, and then the PEG covering layer was placed on the solidified 1/2 MS agar medium. The volume ratio of the agar medium to PEG covering layer was 2:3, and it was left overnight (12–15 h) to allow PEG to diffuse into the agar. Before use, pour out the solution. The seedlings were then grown vertically. Imaging and root length measurements were performed after 7 d.
4.7. Drought Tolerance Assays
For drought tolerance assessments, we mixed the vermiculite and nutrient soil in a 1:1 ratio, filled the pot with the same weight, and absorbed water through the small holes at the bottom to completely moisten the soil. WT and three BnWRKY49 transgenic Arabidopsis lines grown under normal watering for 18 days were subjected to drought treatment. The phenotypes were observed on the 7th and 14th days when watering was stopped. When WT Arabidopsis exhibited a severe dehydration phenotype, watering was resumed and the phenotype was observed 7 days after rewatering.
4.8. Measurements of the Antioxidant Enzyme Activities
Leaves were sampled from 7d drought-treated WT Arabidopsis and three BnWRKY49 overexpression lines to measure the antioxidant enzyme activities. The peroxidase (POD) [50], superoxide dismutase (SOD), and catalase (CAT) [51] were measured according to the manufacturers’ instructions of the assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Three biological and technical replicates were performed.
4.9. Stomatal Aperture and Cuticular Wax
Leaves and stems during the same growth period and at the same position were sampled from 7d drought-treated WT Arabidopsis and three BnWRKY49 overexpression lines, put into a buffer, and then subjected to a vacuum to allow the buffer to infiltrate the leaves. The stomata of the leaves and stems were viewed using scanning electron microscopy (SEM). For statistical analysis, the length and width of the stomata were measured by ImageJ software (National Institutes of Health, Bethesda, MD, USA) [52], and the stomatal length/width ratio was calculated and used as a measurement of stomatal closure [53]. The epicuticular waxes of the stem were also observed via SEM. Three biological replicates were performed.
4.10. Statistical Analysis
The data were analyzed by analysis of variance (ANOVA), and the means values were compared by t-tests using SPSS 11.5 (SPSS, Chicago, IL, USA). Figures of the bar charts were produced by Prism 7 (GraphPad Software Inc., San Diego, CA, USA).
5. Conclusions
In conclusion, the present study suggested that BnWRKY49, as a type I WRKY TF, played an important role in abiotic stress. The gene structure analysis and phylogenetic analysis revealed its conserved nature. The expression profiling suggested the involvement of BnWRKY49 proteins in plant development and abiotic stress responses. BnWRKY49 showed increased root length in BnWRKY49-overexpressing Arabidopsis under NaCl and PEG treatment, as well as enhanced drought resistance by the stomatal closure and the density of surface wax. We speculated that it played a positive regulatory role in the resistance to drought stress. This study will provide ideas for ramie breeders to research and develop drought-resistant ramie varieties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13030379/s1, Table S1. List of qRT PCR primers for BnWRKYs and BnEF1A genes; Table S2. Primers used for BnWRKY49 gene clone and the sequences; Table S3. Primers for positive plant tests.
Author Contributions
Conceptualization, B.W., Y.B. and Y.L.; validation, Y.B., Y.L. and L.D.; formal analysis, Y.Z.; data curation, Y.Z. and Y.L.; writing—original draft preparation, Y.B, Y.Z. and Y.L.; writing—review and editing, X.A. and X.H.; supervision, B.W. and D.P.; funding acquisition, B.W., L.L. and Y.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the “National Natural Science Foundation of China (grant numbers 31171594, 31671736)”, the “China Agriculture Research System of MOF and MARA”, and “Talent Introduction Research Fund of Guizhou University (Guizhou University Talent Fund Cooperation Project [2020] No.21).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Guo, X.; Ullah, A.; Siuta, D.; Kukfisz, B.; Iqbal, S. Role of WRKY Transcription Factors in Regulation of Abiotic Stress Responses in Cotton. Life 2022, 12, 1410. [Google Scholar] [CrossRef]
- Birkenbihl, R.; Liu, S.; Somssich, I. Transcriptional events defining plant immune responses. Curr. Opin. Plant Biol. 2017, 38, 1–9. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.; Robatzek, S.; Somssich, I. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Song, H.; Cao, Y.; Zhao, L.; Zhang, J.; Li, S. Review: WRKY transcription factors: Understanding the functional divergence. Plant Sci. 2023, 334, 111770. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.T.; Yu, D.Q. Expression profiles of AtWRKY25, AtWRKY26 and AtWRKY33 under abiotic stresses. Yi Chuan 2010, 32, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cho, L.H.; Tun, W.; Jeon, J.S.; An, G. Sucrose signaling in higher plants. Plant Sci. 2021, 302, 110703. [Google Scholar] [CrossRef]
- Zhao, P.; Yao, X.; Cai, C.; Li, R.; Du, J.; Sun, Y.; Wang, M.; Zou, Z.; Wang, Q.; Kliebenstein, D.J.; et al. Viruses mobilize plant immunity to deter nonvector insect herbivores. Sci. Adv. 2019, 5, eaav9801. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.; Zhang, R.; Wu, B.; Xiao, G. Expression identification of three OsWRKY genes in response to abiotic stress and hormone treatments in rice. Plant Signal Behav. 2023, 18, 2292844. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Gu, L.; Wang, S.; Cai, H.; Wu, J.; Wang, J.; Yang, M. Progress in the understanding of WRKY transcription factors in woody plants. Int. J. Biol. Macromol. 2023, 242 Pt 1, 124379. [Google Scholar] [CrossRef]
- Yu, X.; Bin, L.; Dong, Y.; Li, Y.; Yang, M. Cloning and functional identification of PeWRKY41 from Populus × euramericana. Ind. Crops Prod. 2022, 175, 114279. [Google Scholar] [CrossRef]
- Sharma, Y.; Ishu; Shumayla; Dixit, S.; Singh, K.; Upadhyay, S.K. Decoding the features and potential roles of respiratory burst oxidase homologs in bread wheat. Curr. Plant Biol. 2024, 37, 100315. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Liu, N.; Lin, Z.; Guan, L.; Gaughan, G.; Lin, G. Antioxidant enzymes regulate reactive oxygen species during pod elongation in Pisum sativum and Brassica chinensis. PLoS ONE 2014, 9, e87588. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Shumayla; Sharma, Y.; Madhu; Sharma, A.; Pandey, A.; Singh, K.; Upadhyay, S.K. TaGPX1-D overexpression provides salinity and osmotic stress tolerance in Arabidopsis. Plant Sci. 2023, 337, 111881. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhu, Z.; Zhang, M.; Li, X.; Xu, R.; Zhu, F.; Xu, J.; Deng, X.; Cheng, Y. CitWRKY28 and CitNAC029 promote the synthesis of cuticular wax by activating CitKCS gene expression in citrus fruit. Plant Cell Rep. 2022, 41, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Lu, H. Bast-Fiber Crops Cultivation Science in China; Agri Press: Beijing, China, 1992. [Google Scholar]
- Lee, Y.R.; Nho, J.W.; Hwang, I.G.; Kim, W.J.; Jeong, H.S. Chemical composition and antioxidant activity of ramie leaf (Boehmeria nivea L.). Food Sci. Biotechnol. 2009, 18, 1096–1099. [Google Scholar]
- Liu, T.; Zhu, S.; Tang, Q.; Yu, Y.; Tang, S. Identification of drought stress-responsive transcription factors in ramie (Boehmeria nivea L. Gaud). BMC Plant Biol. 2013, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Liu, T.; Zhu, S.; Tang, Q.; Tang, S. Sequence Analysis of WRKY Transcription Factor in Ramie. Plant Fiber Sci. China 2013, 35, 1671–3532. [Google Scholar]
- An, X.; Chen, J.; Zhang, J.; Liao, Y.; Dai, L.; Wang, B.; Liu, L.; Peng, D. Transcriptome Profiling and Identification of Transcription Factors in Ramie (Boehmeria nivea L. Gaud) in Response to PEG Treatment, Using Illumina Paired-End Sequencing Technology. Int. J. Mol. Sci. 2015, 16, 3493–3511. [Google Scholar] [CrossRef]
- Feng, X.; Abubakar, A.S.; Yu, C.; Zhu, A.; Chen, J.; Chen, K.; Gao, G.; Wang, X.; Mou, P.; Shao, D.; et al. Analysis of WRKY Resistance Gene Family in Boehmeria nivea (L.) Gaudich: Crosstalk Mechanisms of Secondary Cell Wall Thickening and Cadmium Stress. Front. Plant Sci. 2022, 13, 812988. [Google Scholar] [CrossRef]
- Bai, Y.; Sunarti, S.; Kissoudis, C.; Visser, R.; van der Linden, C. The Role of Tomato WRKY Genes in Plant Responses to Combined Abiotic and Biotic Stresses. Front. Plant Sci. 2018, 9, 2645–2647. [Google Scholar] [CrossRef]
- Ge, M.; Tang, Y.; Guan, Y.; Lv, M.; Zhou, C.; Ma, H.; Lv, J. TaWRKY31, a novel WRKY transcription factor in wheat, participates in regulation of plant drought stress tolerance. BMC Plant Biol. 2024, 24, 27. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Li, Q.; Wei, W.; Li, W.; Zhang, W.; Ma, B.; Bi, Y.; Lai, Y.; Liu, X. GmWRKY27 interacts with GmMYB174 to reduce expression of GmNAC29 for stress tolerance in soybean plants. Plant J. 2015, 83, 224–236. [Google Scholar] [CrossRef]
- He, G.; Xu, J.; Wang, Y.; Liu, J.; Li, P.; Chen, M.; Ma, Y.; Xu, Z. Drought-responsive WRKY transcription factor genes TaWRKY1 and TaWRKY33 from wheat confer drought and/or heat resistance in Arabidopsis. BMC Plant Biol. 2016, 16, 116. [Google Scholar] [CrossRef]
- Rushton, P.; Somssich, I.; Ringler, P.; Shen, Q. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Shen, H.; Liu, C.; Zhang, Y.; Meng, X.; Zhou, X.; Chu, C.; Wang, X. OsWRKY30 is activated by MAP kinases to confer drought tolerance in rice. Plant Mol. Biol. 2012, 80, 241–253. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Sirichandra, C.; Wasilewska, A.; Vlad, F.; Valon, C.; Leung, J. The guard cell as a single-cell model towards understanding drought tolerance and abscisic acid action. J. Exp. Bot. 2009, 60, 1439–1463. [Google Scholar] [CrossRef] [PubMed]
- Buschhaus, C.; Jetter, R. Composition differences between epicuticular and intracuticular wax substructures: How do plants seal their epidermal surfaces? J. Exp. Bot. 2011, 62, 841–853. [Google Scholar] [CrossRef]
- Seo, P.; Lee, S.; Suh, M.; Park, M.J.; Go, Y.; Park, C. The MYB96 Transcription Factor Regulates Cuticular Wax Biosynthesis under Drought Conditions in Arabidopsis. Plant Cell 2011, 23, 1138–1152. [Google Scholar] [CrossRef]
- Luo, X.; Bai, X.; Sun, X.; Zhu, D.; Liu, B.; Ji, W.; Cai, H.; Cao, L.; Wu, J.; Hu, M.; et al. Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signalling. J. Exp. Bot. 2013, 64, 2155–2169. [Google Scholar] [CrossRef]
- Xiong, L.; Schumaker, K.; Zhu, J. Cell Signaling during Cold, Drought, and Salt Stress. Plant Cell 2002, 14 (Suppl. 1), S165–S183. [Google Scholar] [CrossRef]
- Chu, X.; Wang, C.; Chen, X.; Lu, W.; Li, H.; Wang, X.; Hao, L.; Guo, X. The Cotton WRKY Gene GhWRKY41 Positively Regulates Salt and Drought Stress Tolerance in Transgenic Nicotiana benthamiana. PLoS ONE 2015, 10, e0143022-21. [Google Scholar] [CrossRef]
- Li, J.; Besseau, S.; Törönen, P.; Sipari, N.; Kollist, H.; Holm, L.; Palva, E.T. Defense-related transcription factors WRKY70 and WRKY54 modulate osmotic stress tolerance by regulating stomatal aperture in Arabidopsis. New Phytol. 2013, 200, 457–472. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Yang, Y.; Liu, C.; Zhou, G.; Wan, H.; Cheng, Y. Tomato WRKY81 acts as a negative regulator for drought tolerance by modulating guard cell H2O2-mediated stomatal closure. Environ. Exp. Bot. 2020, 171, 103960. [Google Scholar] [CrossRef]
- Shumayla; Tyagi, S.; Sharma, Y.; Madhu; Sharma, A.; Pandey, A.; Singh, K.; Upadhyay, S.K. Expression of TaNCL2-A ameliorates cadmium toxicity by increasing calcium and enzymatic antioxidants activities in Arabidopsis. Chemosphere 2023, 329, 138636. [Google Scholar]
- Chen, Y.; Zhao, H.; Wang, Y.; Qiu, X.; Gao, G.; Zhu, A.; Chen, P.; Wang, X.; Chen, K.; Chen, J.; et al. Genome-Wide Identification and Expression Analysis of BnPP2C Gene Family in Response to Multiple Stresses in Ramie (Boehmeria nivea L.). Int. J. Mol. Sci. 2023, 24, 15282. [Google Scholar] [CrossRef]
- Huang, C.; Zhou, J.; Jie, Y.; Xing, H.; Zhong, Y.; Yu, W. A ramie bZIP transcription factor BnbZIP2 is involved in drought, salt, and heavy metal stress response. DNA Cell Biol. 2016, 35, 776–786. [Google Scholar] [CrossRef]
- Rasheed, A.; Jie, Y.; Nawaz, M.; Jie, H.; Ma, Y.; Shah, A.N.; Hassan, M.U.; Gillani, S.F.A.; Batool, M.; Aslam, M.T.; et al. Improving Drought Stress Tolerance in Ramie (Boehmeria nivea L.) Using Molecular Techniques. Front. Plant Sci. 2022, 13, 911610. [Google Scholar] [CrossRef]
- Javed, T.; Gao, S.J. WRKY transcription factors in plant defense. Trends Genet. 2023, 39, 787–801. [Google Scholar] [CrossRef]
- Shekhawat, J.; Upadhyay, S.K. DPY1 as an osmosensor for drought signaling. Trends Plant Sci. 2023. [Google Scholar] [CrossRef]
- Chen, J.; Pei, Z.; Dai, L.; Wang, B.; Liu, L.; An, X.; Peng, D. Transcriptome profiling using pyrosequencing shows genes associated with bast fiber development in ramie (Boehmeria nivea L.). BMC Genom. 2014, 15, 919. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]
- Zhang, X.; Henriques, R.; Lin, S.; Niu, Q.; Chua, N. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef]
- Verslues, P.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef]
- Li, H.X.; Xiao, Y.; Cao, L.L.; Yan, X.; Li, C.; Shi, H.Y.; Wang, J.W.; Ye, Y.H. Cerebroside C increases tolerance to chilling injury and alters lipid composition in wheat roots. PLoS ONE 2013, 8, e73380. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, H.R.; Luo, Y. Variation in Antioxidant Enzyme Activities of Two Strawberry Cultivars with Short-term Low Temperature Stress. World J. Agric. Sci. 2008, 4, 458–462. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ren, X.; Chen, Z.; Liu, Y.; Zhang, H.; Zhang, M.; Liu, Q.; Hong, X.; Zhu, J.; Gong, Z. ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. Plant J. 2020, 63, 417–429. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).