Onion Fusarium Basal Rot Disease Control by Arbuscular Mycorrhizal Fungi and Trichoderma harzianum
Abstract
1. Introduction
2. Results
2.1. Isolation and Characterization of Pathogenic Isolates
2.2. Pathogenicity Tests of Susceptible Onion Variety
2.3. Greenhouse Disease Biocontrol Experiment
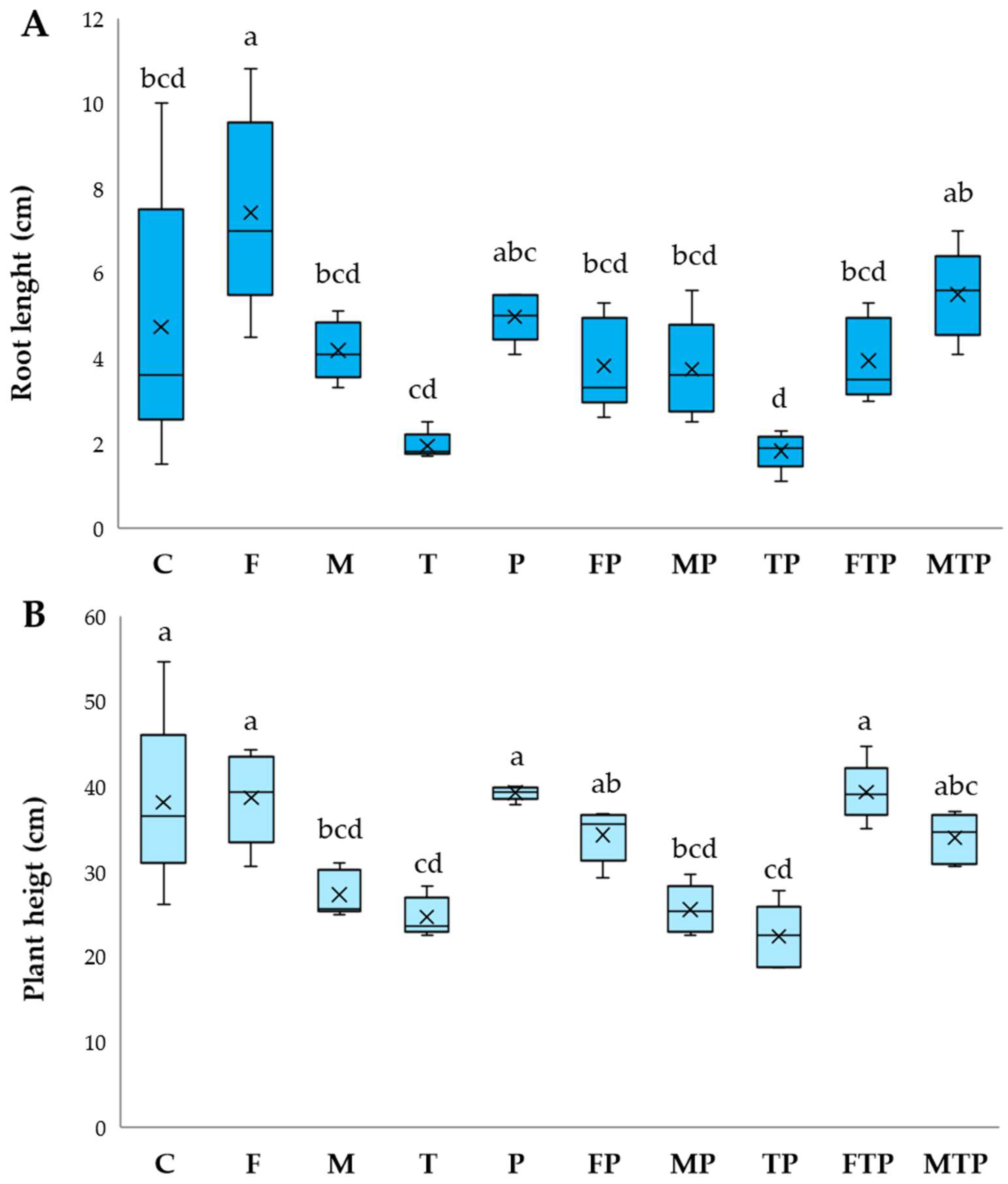
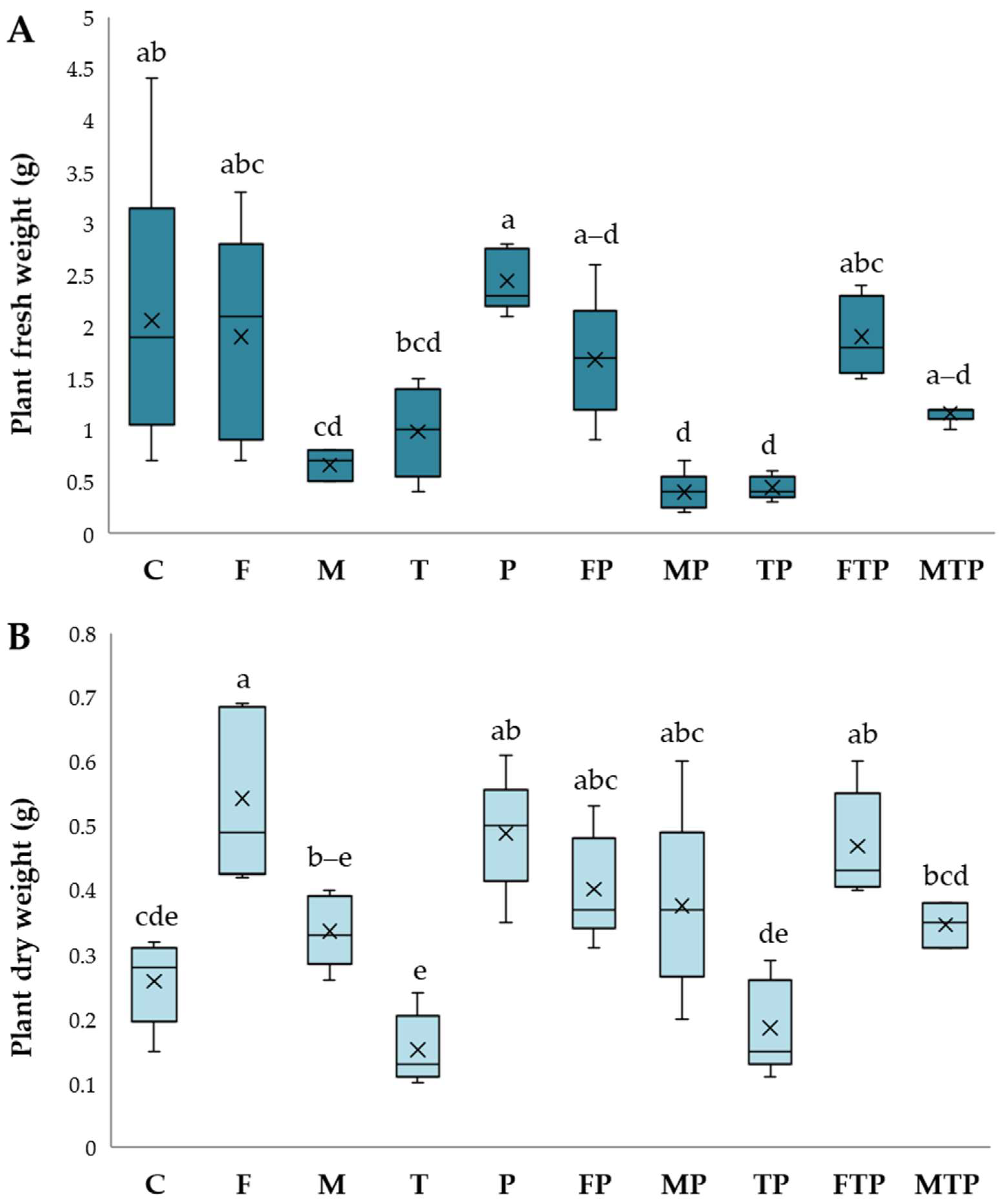
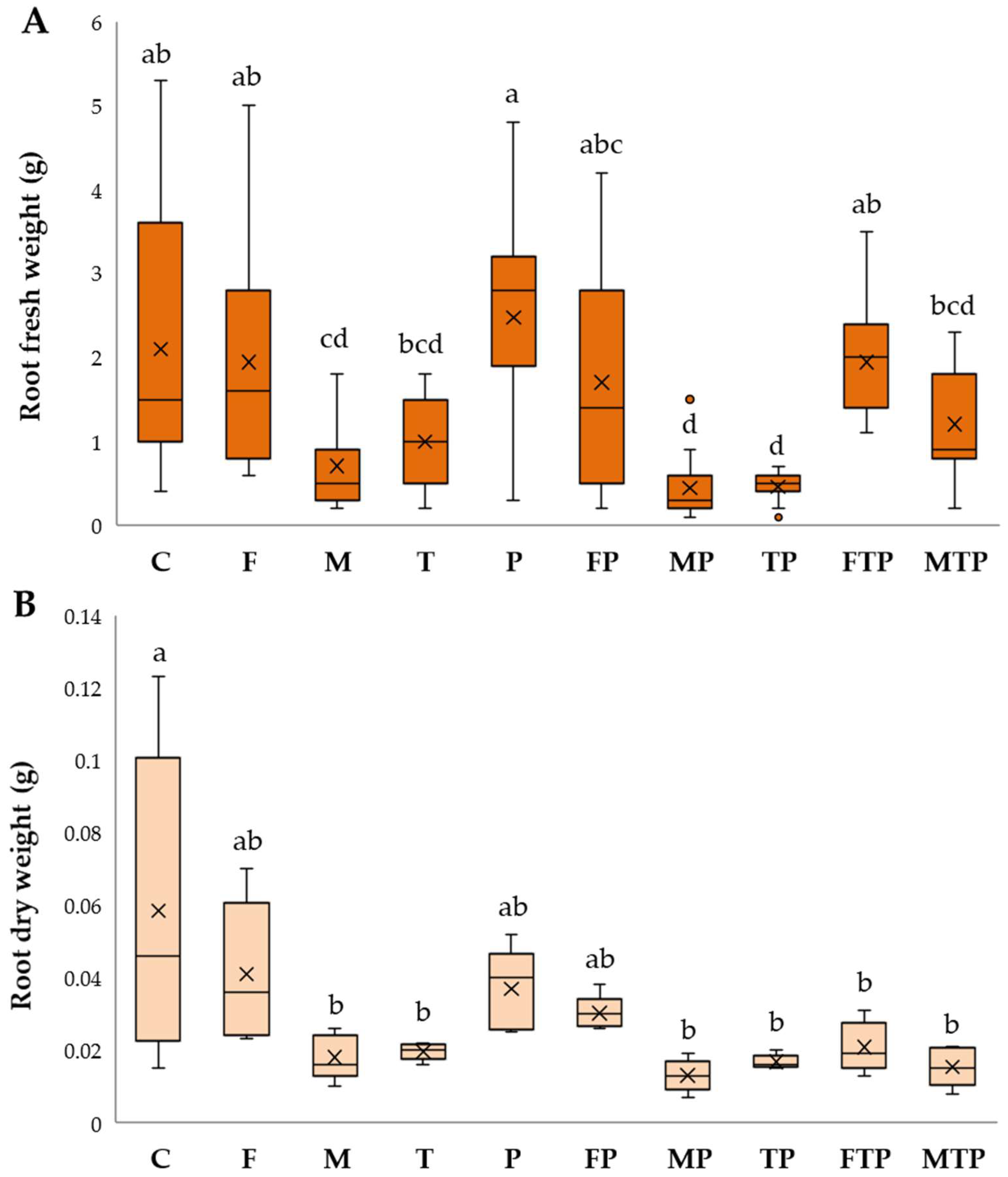
2.3.1. Disease Evaluations
2.3.2. Analyses of Plant Material
3. Discussion
4. Materials and Methods
4.1. Plant and Beneficial Fungi
4.2. Isolation and Morphological Characterization of Pathogenic Isolates
4.3. Pathogenic Isolates Molecular Characterization
4.4. Isolates Pathogenicity Tests and Susceptibility of Onion Varieties
- Healthy plant.
- Brown on 1/4 of the roots.
- Brown on 2/4 of the roots; slight rot.
- Brown on 3/4 of the roots; moderate rot.
- Completely browned or rotten roots.
4.5. Greenhouse Disease Biocontrol Experiment
- Control (C);
- F. mosseae (F);
- ERS (M);
- T22 (T);
- FOC (P);
- F. mosseae + FOC (FP);
- ERS + FOC (MP);
- T22 + FOC (TP);
- F. mosseae + T22 + FOC (FTP);
- ERS + FOC + T22 (MTP).
4.5.1. AMF and Trichoderma Harzianum Inoculation
4.5.2. Disease Induction
4.5.3. Disease Evaluations
4.5.4. Analyses of Plant Material
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alemu, D.; Kitila, C.; Garedew, W.; Jule, L.; Badassa, B.; Nagaprasad, N.; Seenivasan, V.; Saka, A.; Ramaswamy, K. Growth, Yield, and Yield Variables of Onion (Allium cepa L.) Varieties as Influenced by Plantspacing at DambiDollo, Western Ethiopia. Sci. Rep. 2022, 12, 20563. [Google Scholar] [CrossRef]
- Griffiths, G.; Trueman, L.; Crowther, T.; Thomas, B.; Smith, B. Onions—A Global Benefit to Health. Phytother. Res. 2002, 16, 603–615. [Google Scholar] [CrossRef]
- Dhananivetha, M.; Amnullah, M.M.; Arthanari, P.M.; Mariappan, S. Weed Management in Onion: A Review. Agric. Rev. 2017, 38, 76–80. [Google Scholar] [CrossRef]
- Poursakhi, S.; Asadi-Gharneh, H.A.; Nasr-Esfahani, M.; Abbasi, Z.; Khankahdani, H.H. Identification of Novel Associations of Candidate Marker Genes with Resistance to Onion-Fusarium Basal Rot Interaction Pathosystem. Plant Gene 2024, 37, 100440. [Google Scholar] [CrossRef]
- Armitage, A.D.; Taylor, A.; Sobczyk, M.K.; Baxter, L.; Greenfield, B.P.J.; Bates, H.J.; Wilson, F.; Jackson, A.C.; Ott, S.; Harrison, R.J.; et al. Characterisation of Pathogen-Specific Regions and Novel Effector Candidates in Fusarium oxysporum f. sp. Cepae. Sci. Rep. 2018, 8, 13530. [Google Scholar] [CrossRef] [PubMed]
- Southwood, M.J.; Viljoen, A.; Mostert, L.; Rose, L.J.; McLeod, A. Phylogenetic and Biological Characterization of Fusarium oxysporum Isolates Associated with Onion in South Africa. Plant Dis. 2012, 96, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Nakahara, K.; Tanaka, S.; Shigyo, M.; Ito, S.I. Genetic and Pathogenic Variability of Fusarium oxysporum f. sp. Cepae Isolated from Onion and Welsh Onion in Japan. Phytopathology 2015, 105, 525–532. [Google Scholar] [CrossRef]
- Taylor, A.; Vágány, V.; Jackson, A.C.; Harrison, R.J.; Rainoni, A.; Clarkson, J.P. Identification of Pathogenicity-Related Genes in Fusarium oxysporum f. sp. Cepae. Mol. Plant Pathol. 2016, 17, 1032–1047. [Google Scholar] [CrossRef]
- Özer, N.; Köycü, N.D. Seed-Borne Fungal Diseases of Onion, and Their Control. In Fruit and Vegetable Diseases; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 281–306. [Google Scholar]
- Cramer, C.S. Breeding and Genetics of Fusarium Basal Rot Resistance in Onion. Euphytica 2000, 115, 159–166. [Google Scholar] [CrossRef]
- Le, D.; Audenaert, K.; Haesaert, G. Fusarium Basal Rot: Profile of an Increasingly Important Disease in Allium spp. Trop. Plant Pathol. 2021, 46, 241–253. [Google Scholar] [CrossRef]
- Dimant, E.; Degani, O. Molecular Real-Time PCR Monitoring of Onion Fusarium Basal Rot Chemical Control. J. Fungi 2023, 9, 809. [Google Scholar] [CrossRef]
- Degani, O.; Dimant, E.; Gordani, A.; Graph, S.; Margalit, E. Prevention and Control of Fusarium spp., the Causal Agents of Onion (Allium cepa) Basal Rot. Horticulturae 2022, 8, 1071. [Google Scholar] [CrossRef]
- Venkataramanamma, K.; Bhaskara Reddy, B.V.; Jayalakshmi, R.S.; Jayalakshmi, V.; Prasad, K.V.H. Integrated Disease Management of Fusarium Wilt (Fusarium oxysporum f. sp. Ciceris) of Chickpea. Indian Phytopathol. 2023, 76, 497–509. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Xie, M.-M.; Hashem, A.; Abd-Allah, E.F.; Wu, Q.-S. Arbuscular Mycorrhizal Fungi and Rhizobia Synergistically Promote Root Colonization, Plant Growth, and Nitrogen Acquisition. Plant Growth Regul. 2023, 100, 691–701. [Google Scholar] [CrossRef]
- Mitra, D.; Djebaili, R.; Pellegrini, M.; Mahakur, B.; Sarker, A.; Chaudhary, P.; Khoshru, B.; Del Gallo, M.; Kitouni, M.; Barik, D.P.; et al. Arbuscular Mycorrhizal Symbiosis: Plant Growth Improvement and Induction of Resistance under Stressful Conditions. J. Plant Nutr. 2021, 44, 1993–2028. [Google Scholar] [CrossRef]
- Schouteden, N.; De Waele, D.; Panis, B.; Vos, C.M. Arbuscular Mycorrhizal Fungi for the Biocontrol of Plant-Parasitic Nematodes: A Review of the Mechanisms Involved. Front. Microbiol. 2015, 6, 1280. [Google Scholar] [CrossRef] [PubMed]
- Schüssler, A.; Walker, C. The Glomeromycota: A Species List with New Families and New Genera; The Royal Botanic Garden Kew, Botanische Staatssammlung Munich, and Oregon State University: Gloucester, UK, 2010. [Google Scholar]
- Alrajhi, K.; Bibi, S.; Abu-Dieyeh, M. Diversity, Distribution, and Applications of Arbuscular mycorrhizal Fungi in the Arabian Peninsula. Saudi J. Biol. Sci. 2024, 31, 103911. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Urbano, M.; Goicoechea, N.; Velasco, P.; Poveda, J. Development of Agricultural Bio-Inoculants Based on Mycorrhizal Fungi and Endophytic Filamentous Fungi: Co-Inoculants for Improve Plant-Physiological Responses in Sustainable Agriculture. Biol. Control 2023, 182, 105223. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, L.; Guo, N.; Cai, B. Transcriptomic Analyses Revealed the Effect of Funneliformis mosseae on Genes Expression in Fusarium oxysporum. PLoS ONE 2020, 15, e0234448. [Google Scholar] [CrossRef]
- Qian, L.; Yu, W.J.; Cui, J.Q.; Jie, W.G.; Cai, B.Y. Funneliformis Mosseae Affects the Root Rot Pathogen Fusarium oxysporum in Soybeans. Acta Agric. Scand. B Soil. Plant Sci. 2015, 65, 321–328. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A Multipurpose, Plant-Beneficial Microorganism for Eco-Sustainable Agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Hatami, M.; Ahangarani, F. Role of Beneficial Fungi in Sustainable Agricultural Systems. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Springer: Singapore, 2016; pp. 397–416. [Google Scholar]
- Xiao, Z.; Zhao, Q.; Li, W.; Gao, L.; Liu, G. Strain Improvement of Trichoderma Harzianum for Enhanced Biocontrol Capacity: Strategies and Prospects. Front. Microbiol. 2023, 14, 1146210. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Bhunjun, C.S.; Phillips, A.J.L.; Jayawardena, R.S.; Promputtha, I.; Hyde, K.D. Importance of Molecular Data to Identify Fungal Plant Pathogens and Guidelines for Pathogenicity Testing Based on Koch’s Postulates. Pathogens 2021, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
- Asan, A. Regional Annotated Mycobiotas New to Www.Mycotaxon.Com. Mycotaxon 2011, 116, 479–480. [Google Scholar] [CrossRef]
- Bayraktar, H.; Dolar, F.S. Molecular Identification and Genetic Diversity of Fusarium Species Associated with Onion Fields in Turkey. J. Phytopathol. 2011, 159, 28–34. [Google Scholar] [CrossRef]
- Reinders, M.; Riemens, M.; Bremmer, J. The Future of Crop Protection in Europe; EU Institutions: Riga, Latvia, 2021. [Google Scholar]
- Weng, W.; Yan, J.; Zhou, M.; Yao, X.; Gao, A.; Ma, C.; Cheng, J.; Ruan, J. Roles of Arbuscular Mycorrhizal Fungi as a Biocontrol Agent in the Control of Plant Diseases. Microorganisms 2022, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of Plant Secondary Metabolites in Defence and Transcriptional Regulation in Response to Biotic Stress. Plant Stress 2023, 8, 100154. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic Biostimulatory Action: Designing the Next Generation of Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Safaie, N.; Mohammadi Goltapeh, E.; Rezaee Danesh, Y.; Khelghatibana, F. Biological Control of Fusarium Basal Rot of Onion Using Trichoderma Harzianum and Glomus Mosseae. J. Crop Prot. 2016, 5, 359–368. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Pascual, J.A.; Lloret, E.; Roldán, A. Interactions between Arbuscular Mycorrhizal Fungi and Trichoderma harzianum and Their Effects on Fusarium Wilt in Melon Plants Grown in Seedling Nurseries. J. Sci. Food Agric. 2009, 89, 1843–1850. [Google Scholar] [CrossRef]
- Szczałba, M.; Kopta, T.; Gąstoł, M.; Sękara, A. Comprehensive Insight into Arbuscular Mycorrhizal Fungi, Trichoderma spp. and Plant Multilevel Interactions with Emphasis on Biostimulation of Horticultural Crops. J. Appl. Microbiol. 2019, 127, 630–647. [Google Scholar] [CrossRef]
- Perez-Alvarez, R.; Nault, B.A.; Poveda, K. Effectiveness of Augmentative Biological Control Depends on Landscape Context. Sci. Rep. 2019, 9, 8664. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed.; Leslie, J.F., Summerell, B.A., Eds.; Blackwell Publishing: Ames, IA, USA, 2006; ISBN 9780813819198. [Google Scholar]
- Pellegrini, M.; Ercole, C.; Gianchino, C.; Bernardi, M.; Pace, L.; Del Gallo, M. Fusarium oxysporum f. Sp. Cannabis Isolated from Cannabis Sativa L.: In Vitro and In Planta Biocontrol by a Plant Growth Promoting-Bacteria Consortium. Plants 2021, 10, 2436. [Google Scholar] [CrossRef] [PubMed]
- Ellis, W.O.; Smith, J.P.; Simpson, B.K.; Khanizadeh, S.; Oldham, J.H. Control of Growth and Aflatoxin Production of Aspergillus flavus under Modified Atmosphere Packaging (MAP) Conditions. Food Microbiol. 1993, 10, 9–21. [Google Scholar] [CrossRef]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; APS Pres: St. Paul, MN, USA, 1972. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A Simple Method for Estimating Evolutionary Rates of Base Substitutions through Comparative Studies of Nucleotide Sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Şavur, O.B. The Effects of Arbuscular Mycorrhizal Fungi (AMF) and Salicylic Acid Applications against Crown and Rot Root Disease (Fusarium oxysporum f. Sp. Radicis-Lycopersici Jarvis & Shoemaker) on the Some of Growth and Yield Parameters and Disease Severity of Tomato (Lycopersicum esculantum L.) Plant; Graduate School of Natural and Applied Sciences, Department of Plant Protection, Yüzüncü Yıl University: Van, Turkey, 2015; 145p. [Google Scholar]
- Boyno, G.; Demir, S.; Danesh, Y.R. Effects of Some Biological Agents on the Growth and Biochemical Parameters of Tomato Plants Infected with Alternaria solani (Ellis & Martin) Sorauer. Eur. J. Plant Pathol. 2022, 162, 19–29. [Google Scholar]
- Feller, C.; Bleiholder, H.; Buhr, L.; Hack, H.; Hess, M.; Klose, R.; Meier, U.; Stauss, R.; Boom, T.v.d.; Weber, E. Phanologische Entwicklungsstadien von Gemusepflanzen II. Fruchtgemuse Und Hulsenfruchte. Nachr. Dtsch. Pflanzenschutzd. 1995, 47, 217–232. [Google Scholar]
- Phillips, C.W. The Antonine Wall. Geogr. J. 1970, 136, 158. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in Roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of Mycorrhizal Endogone Species Extracted from Soil by Wet Sieving and Decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Declerck, S.; Plenchette, C.; Strullu, D.G. Mycorrhizal Dependency of Banana (Musa acuminata, AAA Group) Cultivar. Plant Soil. 1995, 176, 183–187. [Google Scholar] [CrossRef]
- Barton, C.J. Determination of Phosphorus as Molybdovanadophosphoric Acid. Anal. Chem. 1948, 20, 1068. [Google Scholar] [CrossRef]
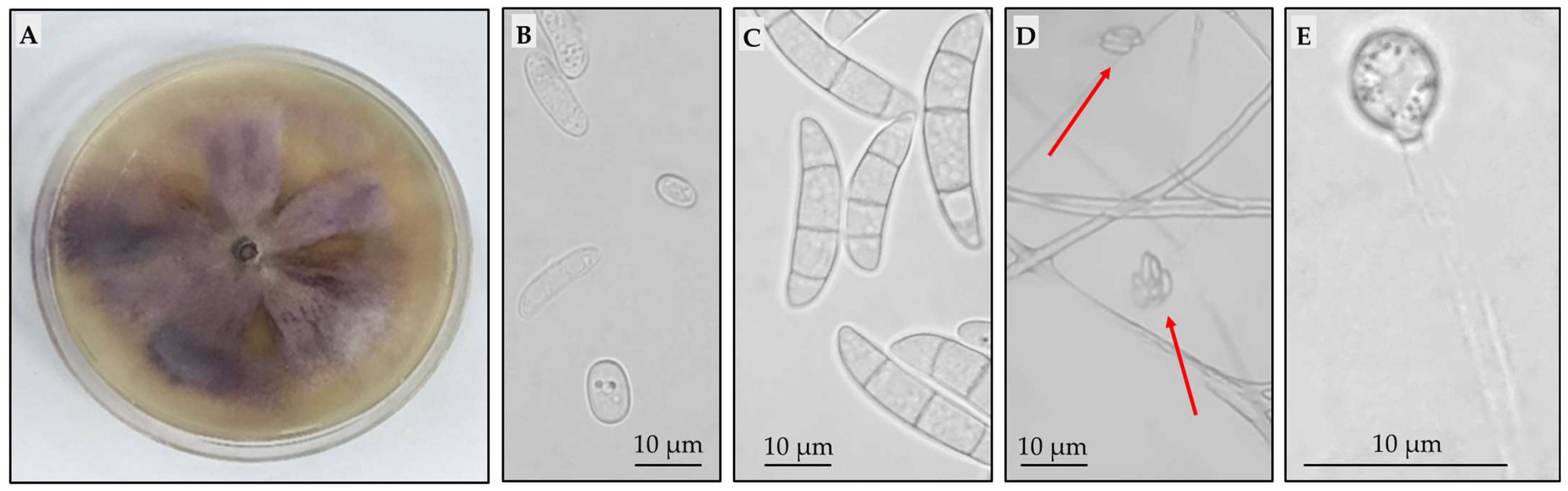
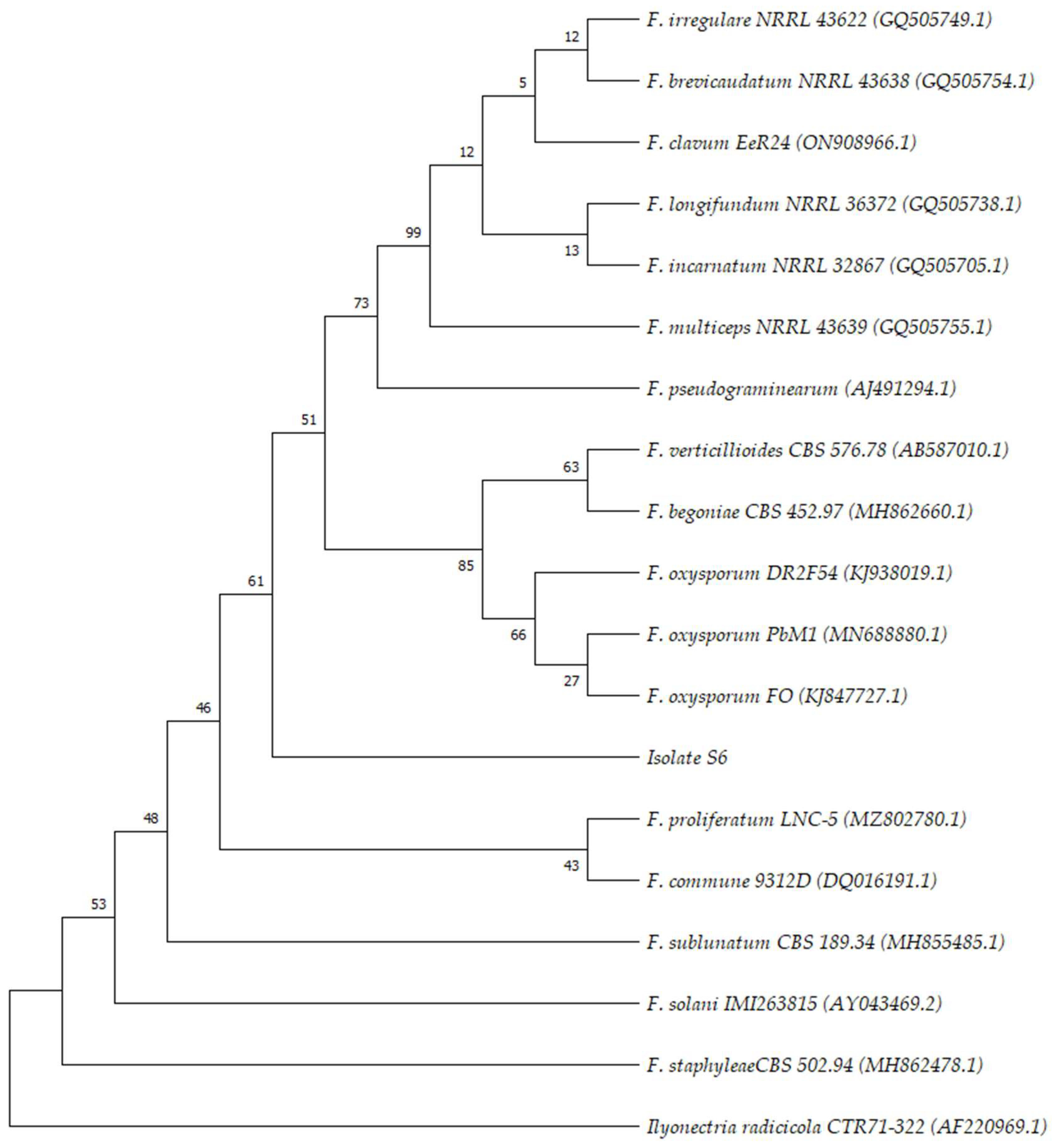

| Variety | Disease Severity (%) |
|---|---|
| Gence | 70.7 ± 5.0 a |
| Seç | 42.7 ± 1.6 b |
| Şampiyon | 26.7 ± 2.1 c |
| Treatment | Disease Severity (%) | Disease Suppression Rate (%) |
|---|---|---|
| P | 90 ± 4.45 a | |
| FP | 68.88 ± 5.73 b | 23.55 |
| MP | 75.55 ± 4.17 ab | 16.05 |
| TP | 77.77 ± 8.62 ab | 13.58 |
| FTP | 70.55 ± 2.43 ab | 21.61 |
| MTP | 71.10 ± 2.73 ab | 21.00 |
| Treatment | Colonization Rate (%) | Mycorrhizal Dependency (%) | Spore Density (Spores 10 g−1) |
|---|---|---|---|
| F | 20.85 ± 2.150 ab | 51.98 ± 4.25 a | 50.40 ± 1.36 a |
| M | 20.86 ± 1.66 ab | 23.58 ± 5.58 bc | 17.2 ± 1.07 c |
| FP | 24.078 ± 0.96 a | 36.02 ± 4.69 abc | 34.2 ± 2.94 b |
| MP | 14.60 ± 1.87 bc | 18.33 ± 3.24 c | 21.8 ± 1.68 c |
| FTP | 24.09 ± 0.69 a | 44.39 ± 6.69 ab | 48.6 ± 2.32 a |
| MTP | 8.91 ± 0.68 c | 23.50 ± 4.39 bc | 16.4 ± 1.50 c |
| Treatment | Phosporous Content (mg Kg−1) |
|---|---|
| C | 0.78 ± 0.19 |
| F | 0.71 ± 0.06 |
| M | 0.88 ± 0.10 |
| T | 0.71 ± 0.06 |
| P | 0.61 ± 0.08 |
| FP | 1.01 ± 0.09 |
| MP | 0.94 ± 0.12 |
| TP | 0.81 ± 0.18 |
| FTP | 0.78 ± 0.06 |
| MTP | 0.74 ± 0.16 |
| Tukey post hoc test | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yağmur, A.; Demir, S.; Canpolat, S.; Rezaee Danesh, Y.; Farda, B.; Djebaili, R.; Pace, L.; Pellegrini, M. Onion Fusarium Basal Rot Disease Control by Arbuscular Mycorrhizal Fungi and Trichoderma harzianum. Plants 2024, 13, 386. https://doi.org/10.3390/plants13030386
Yağmur A, Demir S, Canpolat S, Rezaee Danesh Y, Farda B, Djebaili R, Pace L, Pellegrini M. Onion Fusarium Basal Rot Disease Control by Arbuscular Mycorrhizal Fungi and Trichoderma harzianum. Plants. 2024; 13(3):386. https://doi.org/10.3390/plants13030386
Chicago/Turabian StyleYağmur, Abdulaziz, Semra Demir, Sirel Canpolat, Younes Rezaee Danesh, Beatrice Farda, Rihab Djebaili, Loretta Pace, and Marika Pellegrini. 2024. "Onion Fusarium Basal Rot Disease Control by Arbuscular Mycorrhizal Fungi and Trichoderma harzianum" Plants 13, no. 3: 386. https://doi.org/10.3390/plants13030386
APA StyleYağmur, A., Demir, S., Canpolat, S., Rezaee Danesh, Y., Farda, B., Djebaili, R., Pace, L., & Pellegrini, M. (2024). Onion Fusarium Basal Rot Disease Control by Arbuscular Mycorrhizal Fungi and Trichoderma harzianum. Plants, 13(3), 386. https://doi.org/10.3390/plants13030386











