New Garden Rose (Rosa × hybrida) Genotypes with Intensely Colored Flowers as Rich Sources of Bioactive Compounds
Abstract
1. Introduction
2. Results
2.1. Morphological Traits
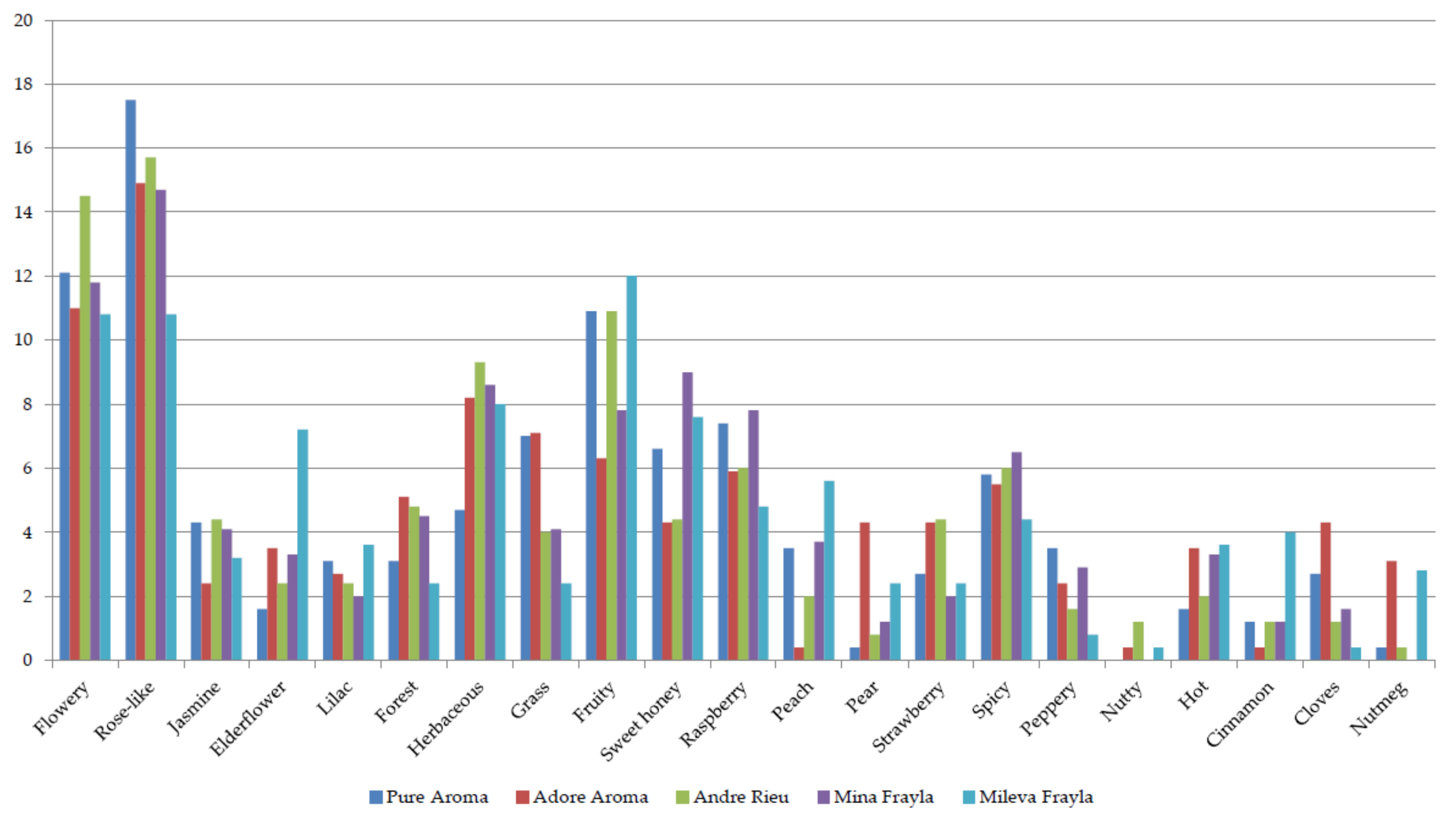
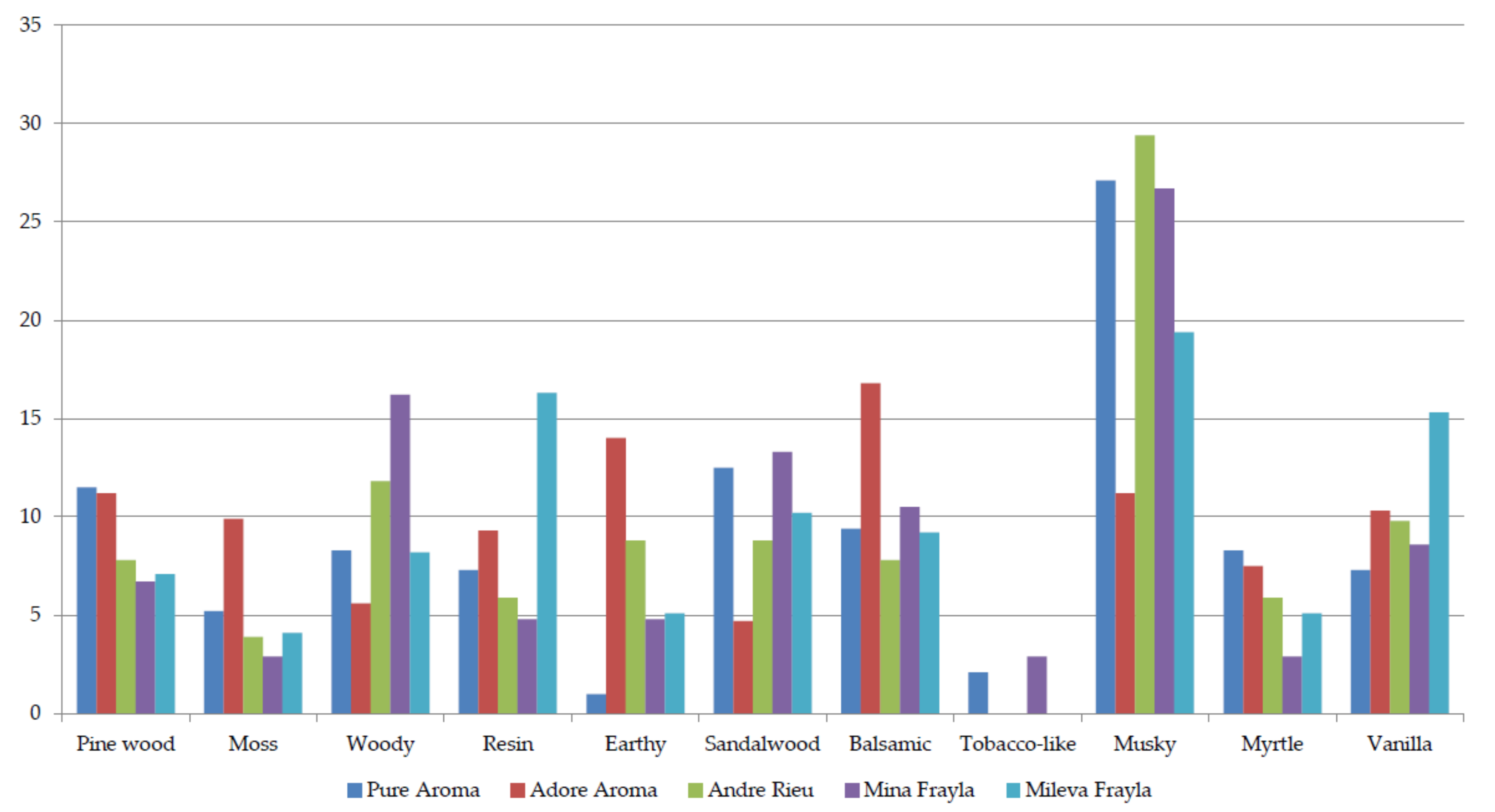
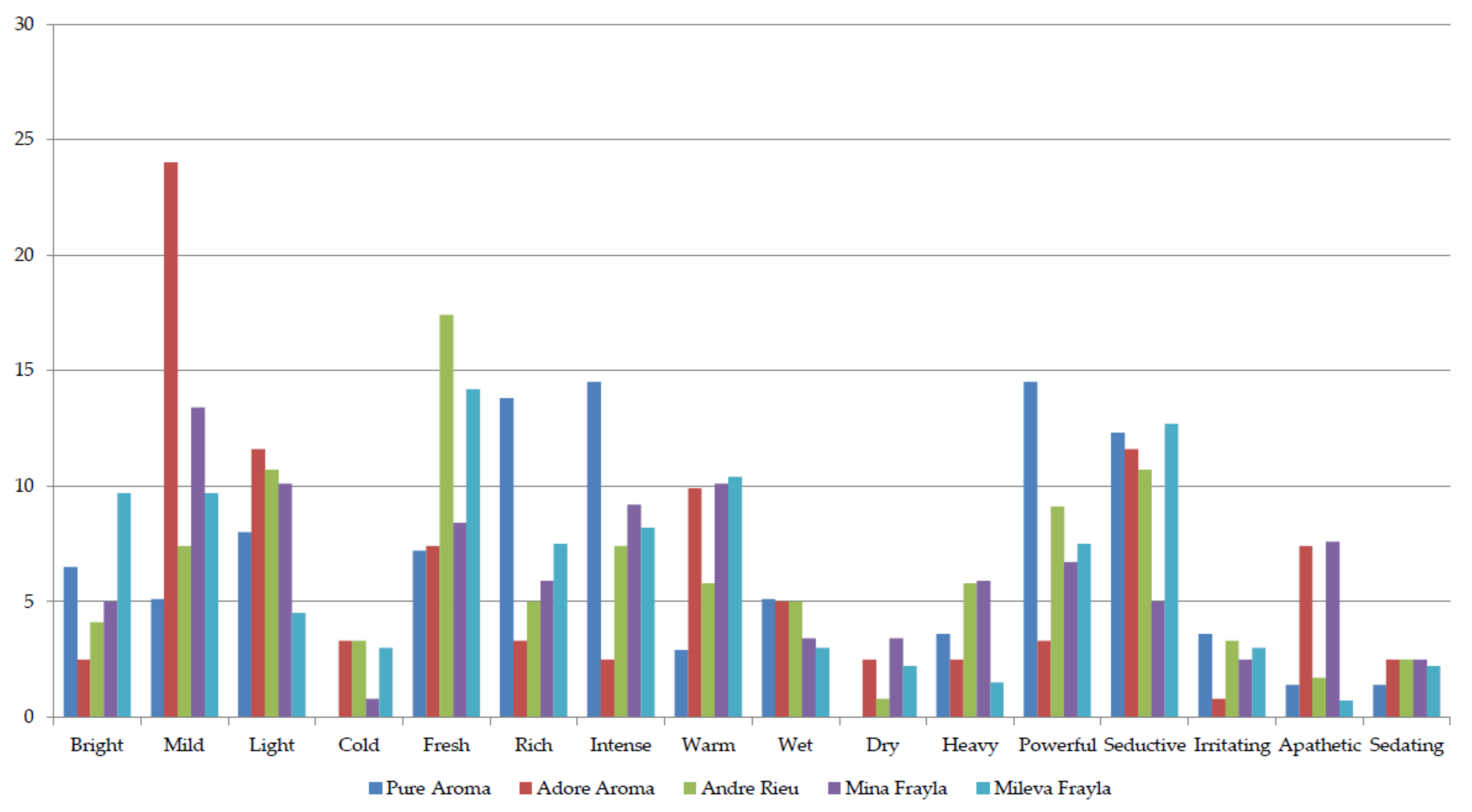
2.2. Sensory Evaluation
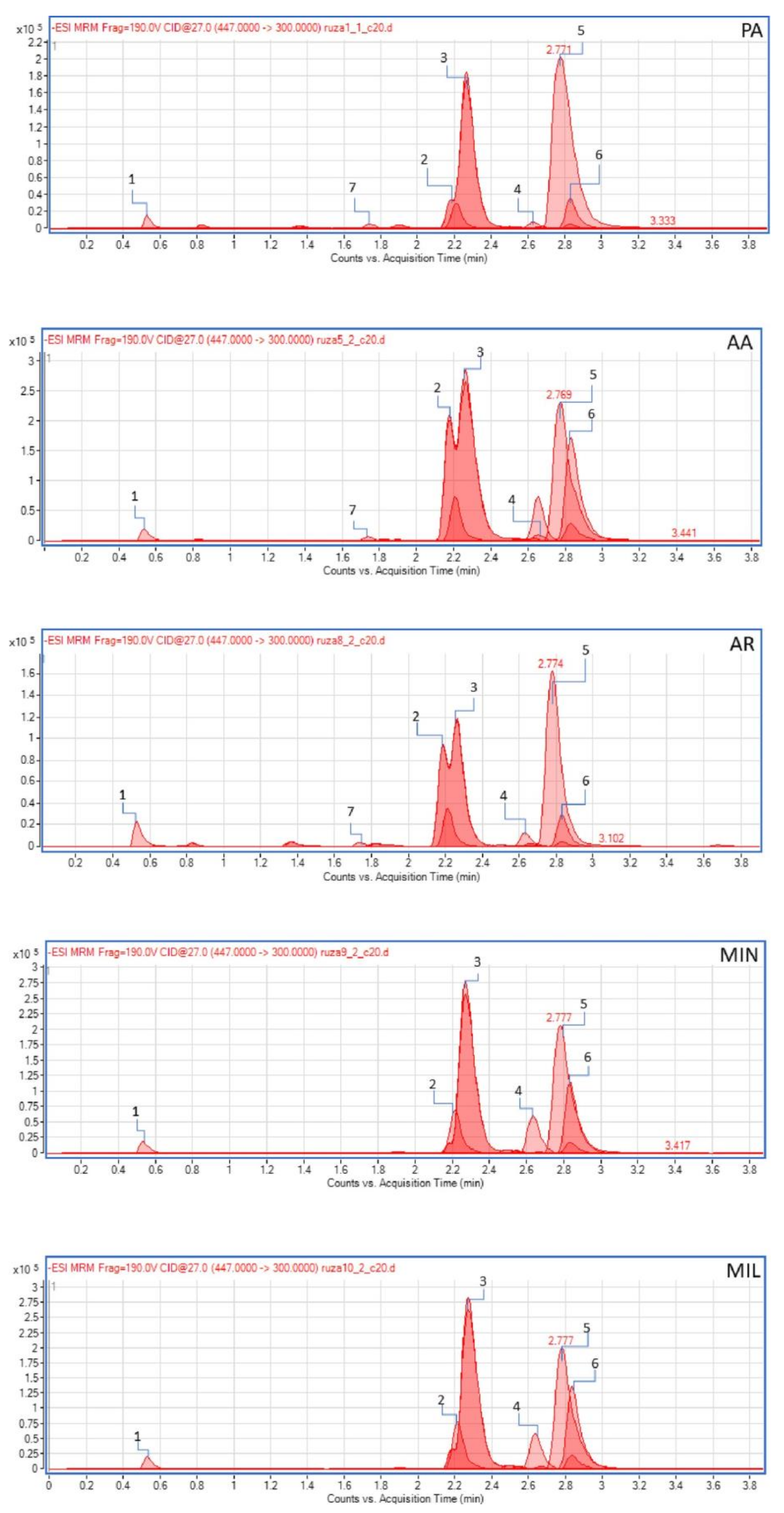
2.3. Chemical Profile of Methanol Extracts of Rose Petals
2.4. Methanol Extracts’ Antioxidant Activityof Rose Petals
2.5. Methanol Extracts’ Neuroprotective Activity of Rose Petals
2.6. Methanol Extracts’ Antidiabetic Activity of Rose Petals
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Morphological Traits
4.3. Sensory Analysis
4.4. Preparation of Methanol Extracts
4.5. Chemical Characterization of Rose Petal Methanol Extracts
4.5.1. Determination of Total Phenolic Content
4.5.2. Determination of Total Flavonoid Content
4.5.3. Determination of Total Monomeric Anthocyanin Content
4.5.4. Quantitative Analysis of Selected Compounds
4.6. Antioxidant Potential
DPPH and FRAP Assays
4.7. Neuroprotective Activity
4.8. Antidiabetic Activity
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, P.; Raju, D.V.S.; Prasad, K.V.; Saha, S.; Panwar, S.; Paul, S.; Banyal, N.; Bains, A.; Chawla, P.; Fogarasi, M.; et al. Characterization of anthocyanins and their antioxidant activities in Indian rose varieties (Rosa × hybrida) using HPLC. Antioxidants 2022, 11, 2032. [Google Scholar] [CrossRef]
- Patel, S. Rose hip as an underutilized functional food: Evidence-based review. Trends Food Sci. Technol. 2017, 63, 29–38. [Google Scholar] [CrossRef]
- Simin, N.; Lesjak, M.; Živanović, N.; Božanić Tanjga, B.; Orčić, D.; Ljubojević, M. Morphological Characters, Phytochemical Profile and Biological Activities of Novel Garden Roses Edible Cultivars. Horticulturae 2023, 9, 1082. [Google Scholar] [CrossRef]
- Vukosavljev, M.; Stranjanac, I.; van Dongen, B.W.P.; Voorrips, R.E.; Miric, M.; Bozanic Tanjga, B.; Arens, P.; Smulders, M.J.M. A novel source of food—Garden rose petals. Acta Hortic. 2023, 1362, 165–171. [Google Scholar] [CrossRef]
- Datta, S.K. Breeding of new ornamental varieties: Rose. Curr. Sci. 2018, 114, 1194–1206. [Google Scholar] [CrossRef]
- Mileva, M.; Ilieva, Y.; Jovtchev, G.; Gateva, S.; Zaharieva, M.M.; Georgieva, A.; Dimitrova, L.; Dobreva, A.; Angelova, T.; Vilhelmova-Ilieva, N.; et al. Rose flowers—A delicate perfume or a natural healer? Biomolecules 2021, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, B.; Jaimand, K. Chemical variation in the essential oil of Iranian Rosa damascena landraces under semi-arid and cool conditions. Int. J. Hortic. Sci. Technol. 2018, 5, 81–92. [Google Scholar]
- Baser, K.H.C.; Kurkcuoglu, M.; Ozek, T. Turkish rose oil: Recent results. Perfum. Flavorist 2003, 28, 34–42. [Google Scholar]
- Tanjga, B.B.; Lončar, B.; Aćimović, M.; Kiprovski, B.; Šovljanski, O.; Tomić, A.; Travičić, V.; Cvetković, M.; Raičević, V.; Zeremski, T. Volatile profile of garden rose (Rosa hybrida) hydrosol and evaluation of its biological activity in vitro. Horticulturae 2022, 8, 895. [Google Scholar] [CrossRef]
- Bayhan, G.I.; Gumus, T.; Alan, B.; Savas, I.K.; Cam, S.A.; Sahin, E.A.; Arslan, S.O. Influence of Rosa damascena hydrosol on skin flora (contact culture) after hand-rubbing. GMS Hyg. Infect. Control 2020, 15, Doc21. [Google Scholar]
- Popović-Djordjević, J.; Špirović-Trifunović, B.; Pećinar, I.; de Oliveira, L.F.C.; Krstić, Đ.; Mihajlović, D.; Akšić, M.F.; Simal-Gandara, J. Fatty acids in seed oil of wild and cultivated rosehip (Rosa canina L.) from different locations in Serbia. Ind. Crops Prod. 2023, 191, 115797. [Google Scholar] [CrossRef]
- Mawarni, E.; Ginting, C.N.; Chiuman, L.; Girsang, E.; Handayani, R.A.S.; Widowati, W. Antioxidant and elastase inhibitor potential of petals and receptacle of rose rlower (Rosa damascena). Pharm. Sci. Res. (PSR) 2020, 7, 105–113. [Google Scholar]
- Boskabady, M.H.; Shafei, M.N.; Saberi, Z.; Amini, S. Pharmacological effects of Rosa damascena. Iran. J. Basic. Med. Sci. 2011, 14, 295–307. [Google Scholar]
- Choi, J.K.; Lee, Y.B.; Lee, K.H.; Im, H.C.; Kim, Y.B.; Choi, E.K.; Joo, S.S.; Jang, S.K.; Han, N.S.; Kim, C.H. Extraction conditions for phenolic compounds with antioxidant activities from white rose petals. J. Appl. Biol. Chem. 2015, 58, 117–124. [Google Scholar] [CrossRef]
- Ghavam, M.; Afzali, A.; Manconi, M.; Bacchetta, G.; Manca, M.L. Variability in chemical composition and antimicrobial activity of essential oil of Rosa × damascena Herrm. from mountainous regions of Iran. Chem. Biol. Technol. Agric. 2021, 8, 22. [Google Scholar] [CrossRef]
- Dagli, R.; Avcu, M.; Metin, M.; Kiymaz, S.; Ciftci, H. The effects of aromatherapy using rose oil (Rosa damascena Mill.) on preoperative anxiety: A prospective randomized clinical trial. Eur. J. Integr. Med. 2019, 26, 37–42. [Google Scholar] [CrossRef]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Hegde, A.S.; Gupta, S.; Sharma, S.; Srivatsan, V.; Kumari, P. Edible rose flowers: A doorway to gastronomic and nutraceutical research. Food Res. Int. 2022, 162, 111977. [Google Scholar] [CrossRef]
- Delgado, C.; Crisosto, G.M.; Heymann, H.; Crisosto, C.H. Determining the primary drivers of liking to predict consumers’ acceptance of fresh nectarines and peaches. J. Food Sci. 2013, 78, S605–S614. [Google Scholar] [CrossRef] [PubMed]
- Wendin, K.; Pálsdóttir, A.M.; Spendrup, S.; Mårtensson, L. Odor Perception and Descriptions of Rose-Scented Geranium Pelargonium graveolens ‘Dr. Westerlund’—Sensory and Chemical Analyses. Molecules 2023, 28, 4511. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Plaskova, A.; Jurikova, T.; Sochor, J.; Baron, M.; Ercisli, S. Chemical, Nutritional and Sensory Characteristics of Six Ornamental Edible Flowers Species. Foods 2021, 10, 2053. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Faller, A.L.K.; Fialho, E. Polyphenol content and antioxidant capacity in organic and conventional plant foods. J. Food Comp. Anal. 2010, 23, 561–568. [Google Scholar] [CrossRef]
- Kumar, N.; Bhandari, P.; Singh, B.; Shamsher, S.B. Antioxidant activity and ultra-performance LC-electrospray ionization-quadrupole time-of-flight mass spectrometry for phenolics-based fingerprinting of Rose species: Rosa damascena, Rosa bourboniana and Rosa brunonii. Food Chem. Toxicol. 2009, 47, 361–367. [Google Scholar] [CrossRef]
- Yang, H.; Shin, Y. Antioxidant compounds and activities of edible roses (Rosa hybrida spp.) from different cultivars grown in Korea. Appl. Biol. Chem. 2017, 60, 129–136. [Google Scholar] [CrossRef]
- Lutz, M.; Hernández, J.; Henríquez, C. Phenolic content and antioxidant capacity in fresh and dry fruits and vegetables grown in Chile. CyTA—J. Food 2015, 13, 541–547. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Mikanagi, Y.; Yokoi, M.; Ueda, Y.; Saito, N. Flower flavonol and anthocyanin distribution in subgenus Rosa. Biochem. Syst. Ecol. 1995, 23, 183–200. [Google Scholar] [CrossRef]
- Beara, I.; Torović, L.J.; Pintać, D.; Majkić, T.; Orčić, D.; Mimica-Dukić, N.; Lesjak, M. Polyphenolic profile, antioxidant and neuroprotective potency of grape juices and wines from Fruška Gora region (Serbia). Int. J. Food Prop. 2017, 20, S2552–S2568. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Kusumawati, I.; Indrayanto, G. Natural Antioxidants in Cosmetics. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 40, pp. 485–505. [Google Scholar]
- Chermahini, S.H.; Adibah, F.; Majid, A.; Sarmidi, M.R. Cosmeceutical value of herbal extracts as natural ingredients and novel technologies in anti-aging. J. Med. Plants Res. 2011, 5, 3074–3077. [Google Scholar]
- Simin, N.; Orcic, D.; Cetojevic-Simin, D.; Mimica-Dukic, N.; Anackov, G.; Beara, I.; Mitic-Culafic, D.; Bozin, B. Phenolic profile, antioxidant, anti-inflammatory and cytotoxic activities of small yellow onion (Allium flavum L. subsp. flavum, Alliaceae). LWT—Food Sci. Technol. 2013, 54, 139–146. [Google Scholar] [CrossRef]
- Conti Filho, C.E.; Loss, L.B.; Marcolongo-Pereira, C.; Rossoni Junior, J.V.; Barcelos, R.M.; Chiarelli-Neto, O.; da Silva, B.S.; Passamani Ambrosio, R.; Castro, F.C.A.Q.; Teixeira, S.F.; et al. Advances in Alzheimer’s disease’s pharmacological treatment. Front. Pharmacol. 2023, 14, 1101452. [Google Scholar] [CrossRef]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Oboh, G.; Ogunsuyi, O.B.; Ogunbadejo, M.D.; Adefegha, S.A. Influence of gallic acid on α-amylase and α-glucosidase inhibitory properties of acarbose. J. Food Drug Anal. 2016, 24, 627–634. [Google Scholar] [CrossRef]
- Ghani, U. Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: Finding needle in the haystack. Eur. J. Med. Chem. 2015, 103, 133–162. [Google Scholar] [CrossRef] [PubMed]
- Gholamhoseinian, A.; Fallah, H.; Sharififar, F. Inhibitory effect of methanol extract of Rosa damascena Mill. flowers on α-glucosidase activity and postprandial hyperglycemia in normal and diabetic rats. Phytomedicine 2009, 16, 935–941. [Google Scholar] [CrossRef]
- UPOV. Guidelines for the Conduct of Tests Distinctness, Uniformity and Stability—Rosa L.; International Union for The Protection of New Varieties of Plants: Geneva, Switzerland, 2010. [Google Scholar]
- Lesjak, M.M.; Beara, I.N.; Orčić, D.Z.; Anačkov, G.T.; Balog, K.J.; Francišković, M.M.; Mimica-Dukić, N.M. Juniperus sibirica Burgsdorf. as a novel source of antioxidant and anti-inflammatory agents. Food Chem. 2011, 124, 850–856. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Pintać, D.; Četojević-Simin, D.; Berežni, B.; Orčić, D.; Mimica-Dukić, N.; Lesjak, M. Investigation of the chemical composition and biological activity of edible grapevine (Vitis vinifera L.) leaf varieties. Food Chem. 2019, 286, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Huang, M.Z.; Jin, Y.S.; Sun, L.N.; Song, Y.; Chen, H.S. Phenolics from Bidens bipinnata and their amylase inhibitory properties. Fitoterapia 2012, 83, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, U.D.; Ling, L.T.; Manaharan, T.; Appleton, D. Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycemic activity. Food Chem. 2011, 127, 21–27. [Google Scholar] [CrossRef]



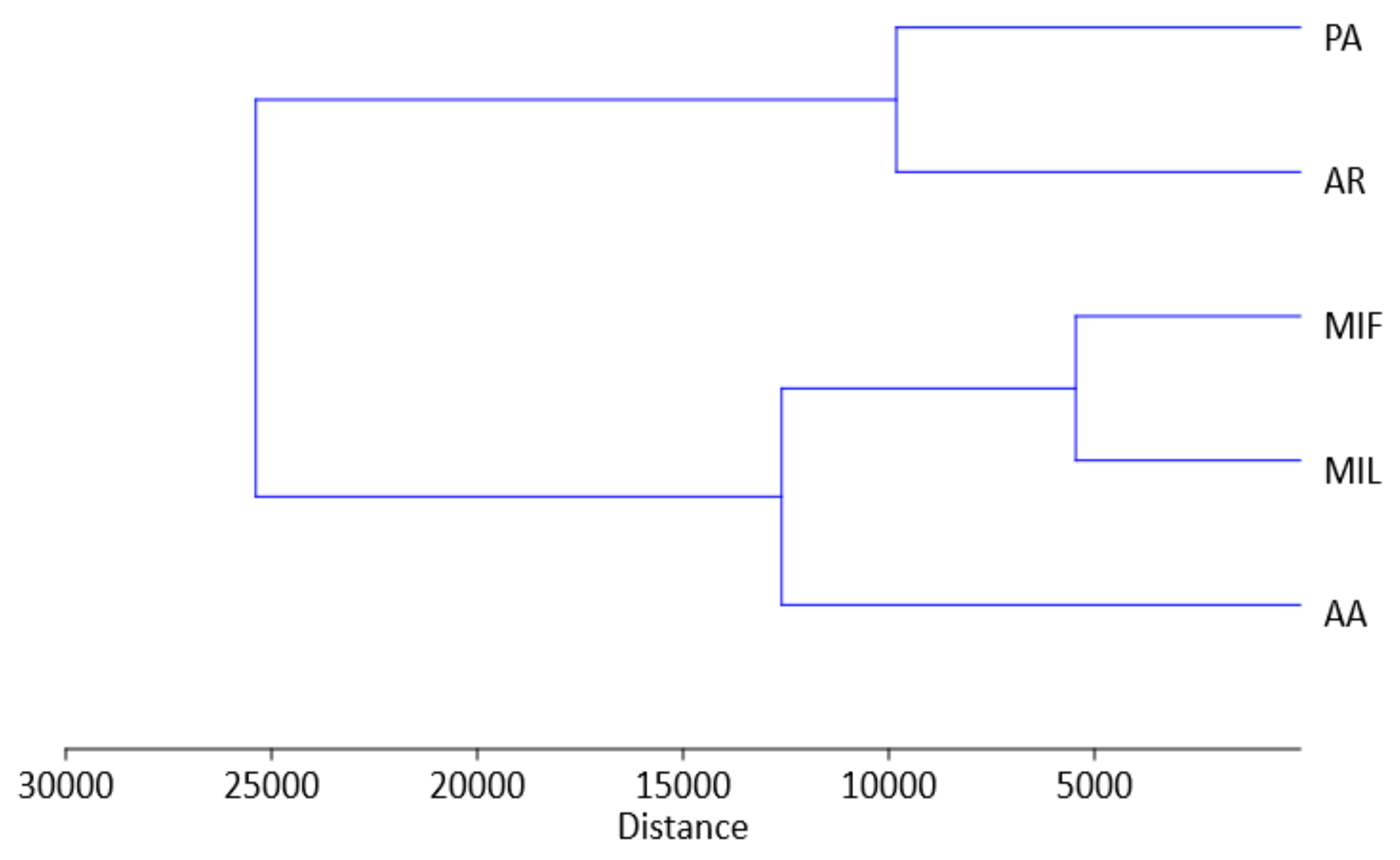
| Cultivar/Trait | PA | AA | AR | MIF | MIL |
|---|---|---|---|---|---|
| Plant | |||||
| GT | Shrub | Shrub | Shrub | Shrub | Shrub |
| GH | Semi-upright | Upright | Upright | Upright | Semi-upright |
| Height (cm) | 80.8 b | 69.6 d | 75.0 c | 79.8 b | 90.4 a |
| Leaf | |||||
| IGC | Dark | Dark | Medium | Dark | Medium |
| LAC | Present | Present | Present | Present | Present |
| GUS | Weak | Medium | Weak | Medium | Very weak |
| Length (cm) | 4.76 b | 4.24 c | 4.56 b | 3.58 d | 5.68 a |
| Width (cm) | 2.98 b | 2.86 b,c | 3.72 a | 2.66 c | 3.88 a |
| Cultivar/Trait | PA | AA | AR | MIF | MIL |
|---|---|---|---|---|---|
| Flowering Shoot | |||||
| NFS | 0 b | 1.82 a | 0 b | 0 b | 0 b |
| FL | Present | Absent | Present | Present | Present |
| NFL | 1.83 b | 0 d | 1.64 c | 1.62 c | 3.44 a |
| NF/L | 2.94 c | 0 d | 3.86 b | 3.68 b | 8.72 a |
| Flower | |||||
| TP | Double | Double | Double | Double | Double |
| CG | Medium purple | Medium violet | Dark purple | Medium purple red | Medium purple red |
| CC | Red purple | Red purple | Red purple | Red purple | Pink |
| SH | Irregularly rounded | Rounded | Rounded | Irregularly rounded | Rounded |
| PUP | Flat | Flat | Flat | Flat | Flat |
| PLP | Concave | Concave | Concave | Concave | Flat |
| FG | 5 | 5 | 5 | 5 | 5 |
| NOP | 96.2 c | 114.4 a | 107.4 b | 65.8 d | 94.4 c |
| DM (cm) | 5.86 b | 6.54 a | 6.66 a | 6.50 a | 6.56 a |
| PL (cm) | 4.40 b | 4.48 b | 4.84 a | 3.72 c | 3.32 d |
| PW (cm) | 4.24 a | 3.58 b | 3.36 b | 3.39 b | 3.24 b |
| TPC | TFC | TAC | ||||
|---|---|---|---|---|---|---|
| Genotype | mg GAE/g de | mg GAE/g fw | mg QE/g de | mg QE/g fw | mg CE/g de | µg CE/g fw |
| PA | 249 ± 13.0 a a | 26.8 ± 1.40 a | 43.7 ± 2.52 b | 4.70 ± 0.271 c | 9.19 ± 0.193 b | 0.990 ± 0.021 b |
| AA | 148 ± 14.6 c | 14.0 ± 1.31 c | 59.8 ± 3.73 a | 5.65 ± 0.335 b | 7.55 ± 0.317 c | 0.714 ± 0.028 c |
| AR | 260 ± 5.01 a | 25.9 ± 0.50 a | 23.7 ± 0.215 c | 2.36 ± 0.021 e | 13.1 ± 0.615 a | 1.31 ± 0.061 a |
| MIF | 182 ± 15.8 bc | 18.2 ± 1.58 b | 56.8 ± 4.90 a | 5.70 ± 0.492 a | 2.24 ± 0.019 d | 0.225 ± 0.002 d |
| MIL | 193 ± 18.3 b | 16.4 ± 1.56 bc | 44.0 ± 2.81 b | 3.75 ± 0.239 d | 2.21 ± 0.008 d | 0.188 ± 0.001 e |
| Content [μg/g de] a | |||||
|---|---|---|---|---|---|
| Genotype | PA | AA | AR | MIF | MIL |
| Quinic acid | 16,837 b ± 1684 bc c | 13,380 ± 1338 c | 23,929 ± 2393 a | 14,960 ± 1496 c | 21,541 ± 2154 ab |
| Protocatechuic acid | 43.9 ± 3.51 b | 60.7 ± 4.86 a | 51.6 ± 4.13 b | 15.3 ± 1.22 c | 12.8 ± 1.02 c |
| p-Coumaric acid | 0.209 ± 0.019 b | 2.92 ± 0.262 a | 0.111 ± 0.010 b | 0.137 ± 0.012 b | 0.173 ± 0.016 b |
| Gallic acid | 22.6 ± 2.03 cd | 29.3 ± 2.64 bc | 21.7 ± 1.95 d | 30.4 ± 2.74 ab | 36.4 ± 3.27 a |
| Naringenin | 0.335 ± 0.023 b | 0.717 ± 0.050 a | 0.651 ± 0.046 a | <0.3 d | 0.341 ± 0.024 b |
| Luteolin | 0.520 ± 0.026 a | 0.523 ± 0.026 a | 0.546 ± 0.027 a | 0.520 ± 0.026 a | 0.440 ± 0.022 b |
| Kaempferol | 2.48 ± 0.174 c | 9.52 ± 0.666 a | 1.61 ± 0.113 c | 5.79 ± 0.405 b | 6.41 ± 0.448 b |
| Catechin | 19.2 ± 1.92 b | 144 ± 14.4 a | 133 ± 13.3 a | 30.9 ± 3.09 b | 26.5 ± 2.65 b |
| Epicatechin | 0.969 ± 0.097 c | 1.92 ± 0.192 b | 5.17 ± 0.517 a | / e | / |
| Chrysoeriol | 0.171 ± 0.005 c | < 0.075 | 0.170 ± 0.005 c | 0.960 ± 0.029 b | 2.10 ± 0.063 a |
| Quercetin | 31.4 ± 9.43 ab | 59.2 ± 17.8 a | 53.3 ± 16.0 a | 14.9 ± 4.48 b | 17.1 ± 5.14 b |
| Chlorogenic acid | 0.603 ± 0.030 d | 1.37 ± 0.069 c | 0.502 ± 0.025 d | 3.02 ± 0.151 b | 5.98 ± 0.299 a |
| Apigenin- 7-O-glucoside | <0.075 | 0.131 ± 0.007a | 0.116 ± 0.006 b | <0.075 | <0.075 |
| Vitexin | <0.075 | <0.075 | <0.075 | 0.102 ± 0.005 b | 0.132 ± 0.007 a |
| Kaempferol- 3-O-glucoside | 2348 ± 93.9 d | 23,965 ± 959 a | 1147 ± 45.9 d | 11,454 ± 458 c | 14,275 ± 571 b |
| Luteolin- 7-O-glucoside | 1.10 ± 0.033 a | / | 0.824 ± 0.025 b | / | / |
| Quercitrin | 13,945 ± 837 a | 10,654 ± 639 b | 2978 ± 179 d | 9338 ± 560 bc | 8564 ± 514 c |
| Quercetin- 3-O-glucoside + Quercetin- 3-O-galactoside | 8267 ± 496 d | 19,949 ± 1197 a | 3981 ± 239 e | 11,969 ± 718 c | 14,709 ± 883 b |
| Rutin | 2235 ± 67.0 b | 2193 ± 65.8 b | 703 ± 21.1 c | 2188 ± 65.6 b | 2663 ± 79.9 a |
| Total phenolics (mg/g de) f | 43.76 | 70.45 | 33.01 | 50.01 | 61.86 |
| FRAP | DPPH IC50 | AChE-IP | α-Amylase-IP | α-Glucosidase-IP | |||
|---|---|---|---|---|---|---|---|
| Genotype | mg AAE /g de | μg/mL | ng EE/g de | I (%) | mg ACAE /g de | I (%) | g ACAE /g de |
| PA | 209 ± 10.1a a | 18.6 ± 1.25 c | 18.3 ± 0.561 a | 11.5 ± 1.62 a | 95.6 ± 13.6 a | 87.1 ± 4.11 a | 292 ± 7.20 b |
| AA | 132 ± 7.40 d | 27.8 ± 1.01 a | 10.2 ± 0.117 e | 2.40 ± 0.435 b | 19.5 ± 3.66 b | 47.2 ± 4.35 b | 38.6 ± 6.98 d |
| AR | 198 ± 12.8 ab | 22.7 ± 0.16 b | 16.4 ± 0.405 b | 13.6 ± 1.99 a | 113 ± 16.7 a | 86.3 ± 1.19 a | 339 ± 39.4 a |
| MIF | 175 ± 6.24 c | 24.0 ± 1.19 b | 12.4 ± 0.132 d | 0.94 ± 0.156 c | 7.3 ± 1.32 c | 57.1 ± 5.70 b | 60.7 ± 16.4 c |
| MIL | 177 ± 1.96 bc | 17.7 ± 1.27 c | 14.7 ± 1.16 c | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 54.5 ± 1.01 b | 52.7 ± 2.39 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simin, N.; Živanović, N.; Božanić Tanjga, B.; Lesjak, M.; Narandžić, T.; Ljubojević, M. New Garden Rose (Rosa × hybrida) Genotypes with Intensely Colored Flowers as Rich Sources of Bioactive Compounds. Plants 2024, 13, 424. https://doi.org/10.3390/plants13030424
Simin N, Živanović N, Božanić Tanjga B, Lesjak M, Narandžić T, Ljubojević M. New Garden Rose (Rosa × hybrida) Genotypes with Intensely Colored Flowers as Rich Sources of Bioactive Compounds. Plants. 2024; 13(3):424. https://doi.org/10.3390/plants13030424
Chicago/Turabian StyleSimin, Nataša, Nemanja Živanović, Biljana Božanić Tanjga, Marija Lesjak, Tijana Narandžić, and Mirjana Ljubojević. 2024. "New Garden Rose (Rosa × hybrida) Genotypes with Intensely Colored Flowers as Rich Sources of Bioactive Compounds" Plants 13, no. 3: 424. https://doi.org/10.3390/plants13030424
APA StyleSimin, N., Živanović, N., Božanić Tanjga, B., Lesjak, M., Narandžić, T., & Ljubojević, M. (2024). New Garden Rose (Rosa × hybrida) Genotypes with Intensely Colored Flowers as Rich Sources of Bioactive Compounds. Plants, 13(3), 424. https://doi.org/10.3390/plants13030424









