Abstract
Chickpea (Cicer arietinum L.), encompassing the desi and kabuli varieties, is a beloved pulse crop globally. Its cultivation spans over fifty countries, from the Indian subcontinent and southern Europe to the Middle East, North Africa, the Americas, Australia, and China. With a rich composition of carbohydrates and protein, constituting 80% of its dry seed mass, chickpea is also touted for its numerous health benefits, earning it the title of a ‘functional food’. In the past two decades, research has extensively explored the rhizobial diversity associated with chickpea and its breeding in various countries across Europe, Asia, and Oceania, aiming to understand its impact on the sustainable yield and quality of chickpea crops. To date, four notable species of Mesorhizobium—M. ciceri, M. mediterraneum, M. muleiense, and M. wenxiniae—have been reported, originally isolated from chickpea root nodules. Other species, such as M. amorphae, M. loti, M. tianshanense, M. oportunistum, M. abyssinicae, and M. shonense, have been identified as potential symbionts of chickpea, possibly acquiring symbiotic genes through lateral gene transfer. While M. ciceri and M. mediterraneum are widely distributed and studied across chickpea-growing regions, they remain absent in China, where M. muleiense and M. wenxiniae are the sole rhizobial species associated with chickpea. The geographic distribution of chickpea rhizobia is believed to be influenced by factors such as genetic characteristics, competitiveness, evolutionary adaptation to local soil conditions, and compatibility with native soil microbes. Inoculating chickpea with suitable rhizobial strains is crucial when introducing the crop to new regions lacking indigenous chickpea rhizobia. The introduction of a novel chickpea variety, coupled with the effective use of rhizobia for inoculation, offers the potential not only to boost the yield and seed quality of chickpeas, but also to enhance crop productivity within rotation and intercropped systems involving chickpea and other crops. Consequently, this advancement holds the promise to drive forward the cause of sustainable agriculture on a global scale.
1. Rationale of Chickpea
1.1. The Origin of Chickpea, Types, and Distribution
Chickpea, scientifically known as Cicer arietinum L., is a member of the Cicereae tribe within the Fabaceae family, specifically belonging to the Papilionaceae subfamily [1]. This ancient pulse crop holds significant importance as one of the world’s most vital legume crops [2,3]. Across various cultures and languages, chickpea is embraced with a plethora of names, such as garbanzo in Spanish, pois chiche in French, kichar or chicher in German, chana in Hindi, and gram or Bengal gram in English [1]. Its global presence is further reflected in its Turkish name ‘nakhut’ and Romanian, Bulgarian, Afghan, and Russian name ‘nohut’ [4]. Archaeological excavations in Middle Eastern countries, the birthplace of wild chickpeas like C. judaicum Boiss., C. pinnatifidum Jaub et Spach, and C. bijugum Rech, have uncovered carbonized chickpea seeds. Moreover, two additional wild chickpea species, C. echinospermum Davis and C. reticulatum Ladiz [2], were discovered in South-East Turkey, further enriching the diversity of this remarkable legume.
Desi and Kabuli represent two widely cultivated chickpea cultivars worldwide. Morphologically distinct, Desi (microsperma) is characterized by its pink flowers and a seed coat that is both colored and notably thick. Conversely, Kabuli (macrosperma) features white flowers and seeds that are either white or beige, bearing a distinct ram’s head shape, thin seed coat, and a seed surface that is smooth to the touch [5]. In India, there is also recognition of an intermediate type known for its pea-shaped seeds of local significance [6]. Seed weights vary, with the Desi cultivar weight ranging from 0.1 to 0.3 g and Kabuli types weighing between 0.2 and 0.6 g. Approximately 80–85% of chickpeas planted in Asia and Africa belong to the Desi cultivar, while in West Asia, North Africa, North America, and Europe, the Kabuli cultivar holds greater prominence [6].
Chickpea cultivation spans over 50 countries, encompassing the Indian subcontinent, North Africa, the Middle East, southern Europe, the Americas, and Australia [6]. On a global scale, it stands as one of the most widely grown pulses, with impressive production figures of 14.2 million tons and an average yield of 0.96 tons per hectare (FAO, 2014). As an affordable protein source, chickpea holds immense significance for low-income consumers worldwide, especially in developing nations where access to animal-based protein is limited for large segments of the population [7]. Remarkably, chickpea demonstrates resilience in regions characterized by climate variability, drought, and limited fertilizer usage, which often lead to reduced agricultural productivity [8]. India takes the lead in chickpea production, followed by Pakistan, Turkey, Australia, Myanmar, Ethiopia, Iran, Mexico, Canada, and the USA as other notable contributors [6]. Worth mentioning is China’s emergence as an Asian chickpea producer, with Xinjiang Province in Northwest China as the primary planting hub [9].
1.2. Nutritional Quality of Chickpea Seeds
Chickpea is an excellent source of both carbohydrates and protein, which account for 80% of the total dry mass of chickpea [10]. Chickpea has been and continues to be consumed by humans since ancient times owing to its good nutritional properties [6]. It is used as food in different styles in different countries [11], such as chickpea four for making snacks in India [12], and chickpea is used in stews and soups/salads in Asia and Africa [13]. The diverse cooking styles make chickpea appeal to consumers worldwide [6].
1.2.1. Classification of Carbohydrates
Chickpea stands out for its rich carbohydrate content, specifically monosaccharides like ribose and glucose, disaccharides such as sucrose, and oligosaccharides including stachyose and ciceritol [14]. Studies reveal that glucose concentrations in chickpea are 0.7% (w/w) [15], with maltose and sucrose being the most prevalent free disaccharides [16]. Notably, α-galactosides are the second most abundant carbohydrates in chickpea [14,17], comprising two significant groups: the raffinose family of oligosaccharides (RFOs), which includes raffinose, stachyose, and verbascose [14], and galactosyl cyclitols, with ciceritol being a prime example [18]. Reports indicate that ciceritol and stachyose make up 36–43% and 25% of total sugars, respectively, in chickpea seeds [15,19]. Interestingly, chickpea contains lower levels of absolute flatulent α-galactosides compared to other legume seeds, such as white beans, lentils, or pinto beans [16].
Polysaccharides, i.e., high-molecular-weight monosaccharide polymers, are found in chickpea as storage carbohydrates (like starch) [16] or structural carbohydrates (like cellulose) [10], providing structural support. Starch synthesis and storage are primary functions of chickpea, accounting for the majority of carbon storage in pulse seeds. The starch content in chickpea ranges from 41 to 50% of total carbohydrates [20,21,22], with the kabuli variety containing more soluble sugars than the desi type [22]. Notably, chickpea seeds boast a high starch content of approximately 525 g/kg dry matter, with 35% being resistant starch (RS) and the remaining 65% as available starch [19,23]. The in vitro starch digestibility values (ISDVs) of chickpea range between 37% and 60% [24,25], which is relatively high compared to other pulses [26].
1.2.2. Dietary Fiber and Protein Content
Dietary fiber (DF), encompassing both soluble and insoluble forms, represents the portion of plant food that remains undigested in the human small intestine. Chickpea stands out for its high DF content, ranging from 18 to 22 g per 100 g [19,27]. Specifically, raw chickpea seeds contain approximately 4–8 g of soluble DF and 10–18 g of insoluble DF per 100 g [20,28]. However, compared to other legumes, the fiber content of chickpea hulls on a dry weight basis is lower, at 75%, than that of lentils (87%) and peas (89%) [20].
Interestingly, the desi variety of chickpea boasts a higher content of total DF and insoluble DF compared to the kabuli variety. This difference can be attributed to the thicker hulls and seed coat in desi, which account for 11.5% of the total seed weight, compared with only 4.3–4.4% in kabuli. This variation underscores the diverse characteristics and nutritional profiles within the chickpea family, making it a versatile and valuable addition to a balanced diet [28].
Chickpea stands out for its protein content, which can range from 17–22% of the dry seed [29,30]. Notably, the quality of chickpea protein is superior to that of some other pulse crops, such as Vigna mungo L. [31], Vigna radiata L., and Cajanus cajan L. However, variations in crude protein concentration exist between the kabuli [K] and desi [D] types, with some studies reporting significant differences (241 g Kg−1 in K versus 217 g kg−1 in D) while others do not show such disparities (217 g kg−1 in K versus 215 g kg−1 in D) [28]. This inconsistency highlights the importance of considering specific varieties and growing conditions when evaluating chickpea’s nutritional profile.
Furthermore, chickpea seeds contain a total of 18 different amino acids, emphasizing their nutritional completeness [32,33,34]. Interestingly, there are no significant differences in amino acid contents between the kabuli and desi varieties [32,33], indicating that both types offer similar benefits in terms of protein quality and amino acid composition. This consistency in amino acid profiles among chickpea varieties adds to their value as a reliable source of plant-based protein, suitable for various dietary needs and preferences.
1.2.3. Fatty Acid Profile
Wood and Grusak [16] reported a fat content ranging from 3.40–8.83% in kabuli and 2.90–7.42% in desi chickpea varieties, surpassing other pulses like lentils, red kidney beans, mung beans, and pigeon peas, as well as wheat and rice (http://www.nal.usda.gov/fnic/foodcomp/search/). Chickpea fatty acid composition consists of approximately 66% polyunsaturated fatty acids (PUFA), 19% monounsaturated fatty acids, and 15% saturated fatty acids. Notably, kabuli types tend to have higher amounts of oleic acid, while desi types have higher amounts of linoleic acid. Chickpea stands out as a rich source of nutritionally essential PUFA, specifically linoleic acid (51.2%; LA) and monounsaturated oleic acid (32.6%; OA), outpacing other edible pulses [32]. Additionally, chickpea’s fatty acid profile is predominantly linoleic acid, followed by oleic and palmitic acids.
1.2.4. Minerals and Vitamins
Chickpea offers consumers a plethora of essential vitamins and minerals [35,36], including iron, zinc, magnesium, and calcium [35]. Selenium is also present in chickpea seeds, making it an even more valuable addition to a balanced diet (http://www.nal.usda.gov/fnic/foodcomp/search/) [35]. A mere 100 g of raw chickpea seeds can provide approximately 5.0 mg of iron, 4.1 mg of zinc, 138 mg of magnesium, and 160 mg of calcium per 100 g. Impressively, just 100 g of chickpea seeds can fulfill the daily dietary requirements for iron (1.05 mg/day for males and 1.46 mg/day for females) and zinc (4.2 mg/day and 3.0 mg/day), while 200 g can meet magnesium needs (260 mg/day and 220 mg/day) (FAO 2002). Interestingly, there are no significant differences in mineral content between the kabuli and desi varieties, except for calcium [32,37]. Additionally, chickpea contains various elements such as aluminum, chromium, nickel, lead, and cadmium [38]. It is also rich in folic acid [39] and provides moderate amounts of water-soluble vitamins like riboflavin (B2), pantothenic acid (B5), and pyridoxine (B6) [40].
1.3. Health Benefits of Chickpea
Chickpea’s consumption offers various physiological perks, making it a potential candidate for the classification of ‘functional food’, beyond its widely recognized role in protein and fiber provision [6].
1.3.1. Diabetes and Blood Pressure
Chickpeas boast a high content of resistant starch and amylose [41]. With a greater degree of polymerization, amylose offers increased resistance to digestion in the small intestine, thus leading to a slower conversion into glucose [41,42]. This results in a delayed glucose entrance into the bloodstream, subsequently reducing insulin requirements, lowering the glycemic index (GI), and mitigating the insulinemic postprandial response [43,44]. These collective effects are instrumental in decreasing both the occurrence and intensity of type II diabetes [45]. Moreover, linoleic acid plays a pivotal role in prostaglandin production, which is crucial in regulating blood pressure levels [46].
1.3.2. Reduce the Risk of Cancer
Butyrate is known to hinder cell proliferation [47] and trigger apoptosis [48], collectively diminishing the likelihood of colorectal cancer. Prior studies indicate that incorporating β-sitosterol (chickpea’s primary phytosterol) in a rat’s diet can curtail the development of colonic tumors induced by N-methyl-N-nitrosourea (a carcinogen) [49]. Moreover, the presence of lycopene in chickpea seeds may offer protection against prostate cancer [50]. In a different investigation, an extract of chickpea isoflavones was discovered to impede epithelial tumor growth while leaving healthy cells unaffected [51].
1.3.3. Control of Weight
Chickpea supplementation has been observed to hinder the escalation of body weight and epididymal adipose tissue mass in rats [52]. Over the course of the experiment, rats fed a high-fat diet (HFD) reached 654 g, whereas those given an HFD with chickpea supplementation (HFD + CP) weighed 562 g. Notably, the ratio of epididymal fat pad weight to total body weight was considerably higher in rats on a pure HFD (0.032 g/g) compared to those on HFD + CP (0.023 g/g) [52]. This underscores chickpea’s potential as a low-GI food that could be a valuable addition to weight loss regimens. Additionally, chickpea consumption has been found to mitigate fat accumulation in individuals with obesity. Incorporating chickpea into the diet leads to increased feelings of satiation and fullness [53]. In a study, forty-two participants adopted a chickpea-enriched diet (averaging 104 g/day) for twelve weeks, sandwiching this period with their usual diet for four weeks each [53].
1.3.4. Gut Health
In an experiment with 19 healthy persons, there was a significant increase (18%) in DF through the consumption of 140 g/day chickpea and chickpea flour for six weeks [54]. Also, in the test conducted by Murty et al. [53], there was an overall improvement in bowel health such as an increased frequency of defecation, ease of defecation, and softer stool consistency in those fed a chickpea diet compared to a habitual diet. The DF played a positive role in promoting bowel function [6].
1.4. Breeding of Chickpea
Globally, chickpea cultivation spans over 13.2 million hectares, with an annual yield of 13.1 million tons, yet its productivity remains below 1 ton per hectare, despite having the potential to reach 6 tons per hectare [55]. The primary challenges to chickpea production encompass both biotic (such as Helicoverpa, Bruchus, Aphidoidea, and Ascochyta) and abiotic (including drought, heat, salt, and cold) stresses, leading to a 10% decrease in yield [56]. However, to boost chickpea productivity, it is imperative to tackle these biotic and abiotic stresses [57]. Consequently, chickpea breeders worldwide are concentrating on developing cultivars with multiple resistances to both biotic and abiotic factors [58]. Additionally, advanced chickpea genotypes with exceptional yield have been developed through the integration of genes offering resistance to drought, cold, salinity, fungi, and pod borers [59]. A range of strategies, including traditional breeding methods, molecular breeding, and modern plant breeding techniques, are being employed to address these challenges [56].
Breeders have extensively utilized conventional technologies such as introduction, selection, hybridization, and mutation [60]. Techniques involving single, multiple, and three-way crosses have been employed in breeding chickpeas [61,62]. Apart from these conventional methods, novel technologies like genetic modification have been utilized to cultivate chickpeas with desirable traits. Additionally, biotechnological techniques have been employed to develop transgenic chickpeas with enhanced resistance to multiple stresses [56]. Worldwide, over 100 gene banks maintain approximately 100,000 chickpea accessions [63], offering breeders valuable genetic resources to develop improved chickpea varieties [57]. However, a sequence of ‘genetic bottleneck’ events has led to a limited genetic base for cultivated chickpeas [64,65].
The enhancement of genetic traits, whether through traditional or molecular techniques, faces constraints not only due to limited genomic resources but also because of the narrow genetic diversity within the elite gene pool [66]. To elucidate the genetic foundations of various agronomic characteristics for yield enhancement at the molecular level, having an accurate chickpea genome assembly is paramount for both fundamental and applied research [67]. Over the past decade, the widespread adoption of NGS (Next-Generation Sequencing) technology has transformed chickpea from an orphan crop to one with abundant genetic, genomic, and transcriptomic resources [68,69,70,71,72,73,74]. To gain novel insights into genome organization, evolution, domestication, and genetic diversity and to expedite molecular breeding for enriching chickpea’s genetic traits, the initial whole-genome shotgun sequencing of the kabuli chickpea cultivar was completed [75]. Subsequently, by analyzing the WGS (Whole Genome Sequencing) data of 3366 chickpea germplasm accessions, a comprehensive map of genetic variations in chickpea was reported, which in turn suggested three genomic breeding strategies for chickpea [76].
To expedite agricultural advancement, innovative crop enhancement practices are continuously emerging worldwide [77]. For instance, chickpeas with stress resilience have been developed through transgenic technology and genome editing techniques. In research on chickpea’s biological resistance, transgenic chickpeas containing α-AI1 demonstrated the inhibition of Callosobruchus maculatus and C. chinensis in insect tests [78]. In another study, chickpeas resistant to aphids were created through the expression of a novel insecticidal lectin [79]. Moreover, transgenic breeding programs have yielded chickpeas expressing chimeric cry1Aabc, effective against the gram pod borer [80]. Although numerous chickpea varieties resistant to H. armigera have been developed in India, only a few have been successfully implemented in agricultural settings [81]. Regarding abiotic stress, research indicates that the DREB1A transcription factor enhances root and shoot growth, leading to improved transpiration efficiency, thereby maintaining drought resistance in transgenic chickpeas [82]. Additionally, chickpeas harboring the P5CS gene exhibit strong salt tolerance [83]. To cultivate chickpea varieties with enhanced abiotic stress resistance, further research in molecular breeding, particularly gene editing, is essential [84].
2. Chickpea Rhizobia and the Inoculation Effects on Chickpea Production
2.1. Diversity, Geographic Distribution, and Natural Succession of Chickpea Rhizobia
Throughout the past two decades, the global research community has delved into the vast diversity of chickpea rhizobia, with investigations spanning Europe [85,86], Asia [87], and Oceania [88]. To date, studies have documented the primary isolation of Mesorhizobium ciceri [85], Mesorhizobium mediterraneum [86], Mesorhizobium muleiense [89], and Mesorhizobium wenxiniae [90], specifically from the root nodules of chickpeas. Furthermore, research has also uncovered various Mesorhizobium species, such as M. amorphae, M. loti, M. tianshanense, M. oportunistum, M. abyssinicae, and M. shonense, as symbiotic partners of chickpeas [91,92,93]. This exploration emphasizes the widespread presence and significance of these rhizobia in the agricultural ecosystem, particularly in relation to chickpea cultivation.
M. ciceri and M. mediterraneum are widespread across numerous countries, including Spain, Portugal, Morocco, Tunisia, and India (as depicted in Figure 1). However, their presence is conspicuously absent in China, where instead, M. muleiense and M. wenxiniae have been discovered and identified [87,89,90,94,95]. Notably, M. muleiense exhibits a broader distribution in the Xinjiang, Gansu, and Ningxia regions of northwestern China [9,87,89,94,95], whereas M. wenxiniae is restricted to Gansu Province [90]. When chickpeas are introduced into a new region lacking native chickpea rhizobia, it becomes imperative to inoculate specific rhizobia during the introduction and planting process [96]. Remarkably, in China, none of the globally prevalent chickpea rhizobia, such as M. ciceri, have been detected [9]. This absence suggests a long history of chickpea introduction in China, leading to the emergence of unique Chinese chickpea rhizobial species through the planting process. During this time, rhizobia may have been co-transported with chickpea seeds, potentially allowing native Chinese rhizobia to acquire symbiotic capabilities from introduced chickpea rhizobia or even evolve directly to nodulate with chickpeas through co-evolutionary processes [87,96].
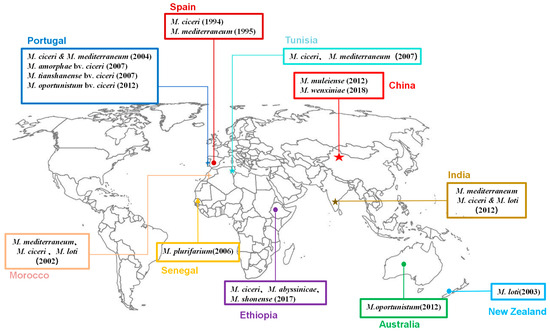
Figure 1.
Geographic distribution of rhizobial species associated with chickpea worldwide.
Zhang et al. [9] conducted a pivotal study on the natural evolution of chickpea rhizobia, focusing on M. muleiense—the sole chickpea rhizobial species in Xinjiang Province in northwestern China. Over a seven-year period spanning from 2009 to 2016, the research delved into the recA genotypes of this species, revealing a total of 28 genotypes, illustrative of the natural succession of M. muleiense. Throughout the sampling years and locations, four primary genotypes exhibited consistency, while certain genotypes appeared exclusively in specific years or at specific sites, pointing to a time-geographic succession model. Additionally, despite the consistent presence of these four main genotypes, their quantitative prevalence varied between sampling years, potentially influenced by differences in agricultural practices. Moreover, the study identified soil pH values and the potassium content as key non-biological factors impacting the natural evolution of M. muleiense, a distinct species of chickpea rhizobium in China.
2.2. Effects of Chickpea Rhizobial Inoculation on Chickpea
Rhizobia are pivotal players in diverse soil biochemical processes, fostering host plant growth and enriching soil quality [97]. The symbiotic alliance between legumes and rhizobia stands as the preeminent N2-fixing mechanism in agricultural systems [98]. Additionally, rhizobia possess the capability of phosphate solubilization, thereby enhancing growth in certain legumes [99], and mitigating plant ethylene levels through ACC deaminase activity [100]. Biological nitrogen fixation (BNF) sustains sustainable agriculture by supplementing chemical fertilizers, ensuring optimal crop yields. Under optimal circumstances, this symbiotic N2 fixation can meet up to 85% of the nitrogen demands in legumes [101].
2.2.1. Effect of Rhizobial Inoculation on the Composition and Diversity of the Rhizosphere Microorganisms of Chickpea
M. ciceri is widely distributed globally, except in China where M. muleiense is the primary species associated with chickpea [96]. In the unsterilized soils of Xinjiang, the local species of M. muleiense displays stronger competitiveness compared to the introduced M. ciceri. However, in sterilized soils, M. ciceri outcompetes M. muleiense [102]. Notably, M. ciceri demonstrates a higher competitive ability in soils from new chickpea cultivation regions compared to M. muleiense [102]. The introduction of different rhizobial species can impact the chickpea rhizosphere microbiota in soils from various planting areas. In the rhizosphere of chickpeas grown in Xinjiang’s traditional soils and newly introduced zones, eight dominant phyla with 34 dominant genera and 10 dominant phyla with 47 dominant genera were identified after inoculation with M. muleiense and M. ciceri rhizobia, respectively. Proteobacteria and Actinobacteria were among the dominant phyla present in all soil rhizospheres. Interestingly, the genus Pseudomonas was significantly enriched after inoculation with M. muleiense in Xinjiang soils, but not in newer chickpea cultivation areas. This leads to the speculation that Pseudomonas could be the key microorganism influencing the competitive nodulation of various chickpea rhizobia in different regions [103].
2.2.2. Effect of Rhizobial Inoculation on Plant Growth
In Rudresh et al.’s study [104], the positive impact of rhizobium inoculation on chickpea growth was evident, with inoculated plants exhibiting greater plant height, branch count, and biomass compared to uninoculated ones. Elkoca et al. [105] also observed increases in plant height, shoot dry weight, and chlorophyll content in chickpeas inoculated with rhizobia. These inoculated treatments also led to longer roots [106], which expanded the root surface area and subsequently boosted nutrient uptake [107]. Shahzad et al.’s findings [108] concurred, highlighting that rhizobial inoculation fostered greater plant height and shoot biomass. Rhizobium inoculation has the potential to enhance the growth and development of photosynthetic organs, subsequently accelerating the accumulation of photosynthates [109]. In our prior research, USDA 3378 inoculation resulted in a substantial increase in chickpea root dry weight (2.82-fold), shoot dry weight (2.62-fold), and chlorophyll content (2.34-fold) compared to the control group [96]. Co-inoculation with USDA 3378 and CCBAU 83963 also yielded promising symbiotic efficiency, albeit with a slight reduction in shoot dry weight, possibly due to strain competition affecting biological nitrogen fixation [96].
2.2.3. Nutrient Content, Uptake, and Protein
Chickpea is prized for its rich protein content, boasting approximately 16–20% protein in its grains, a trait closely linked to its nitrogen content. Boosting nitrogen levels through chickpea rhizobial inoculation can potentially increase the protein content [101]. Research conducted by Erman et al. [110] revealed that grains with enhanced shoot N and P contents outperformed uninoculated controls. Similarly, Kumar et al. [111] observed higher protein levels in plants inoculated with rhizobium. Field experiments have also shown that chickpea plants treated with rhizobial inoculation exhibit increased N and P uptake in both grains and shoots compared to uninoculated controls [112]. This improvement can likely be attributed to augmented nitrogen fixation [112] and enhanced root growth, leading to improved nutrient acquisition [113].
2.2.4. Selection of the Best Strain for the Inoculation of Chickpea in China
Over the last 15 years, numerous farming inoculation trials have taken place across eastern China, spanning Jilin Province in the northeast, Henan Province in the central region, Shandong Province in the east, and Yunnan Province in the southwest. These selected test locations lacked native chickpea rhizobia in their soil, making inoculant production and application necessary. The chosen strains for the trials were the introduced Mesorhizobium ciceri USDA 3378T and an indigenous strain, Mesorhizobium muleiense CCBAU 83963T. Results revealed that the introduced USDA 3378 strain exhibited a notable competitive edge in nodulation, with nodulation rates ranging between 84.6% and 100% in all newly introduced chickpea soils [96]. Chickpea plants inoculated with USDA 3378 exhibited superior symbiotic performance, evident in plant dry weight, leaf chlorophyll content, and nodule count. Moreover, it formed nodules approximately 2 days sooner than CCBAU 83963. Additionally, USDA 3378 demonstrated higher growth rates in media and stronger adsorption abilities on chickpea roots. Consequently, USDA 3378 was chosen as the preferred strain for developing chickpea rhizobial inoculants in China’s newly introduced chickpea regions. This development holds promise for enhancing soil conditioning and fostering environmentally friendly chickpea production in China [96].
3. Conclusions
The presented information will unveil the origins, dispersal, and nutritional worth of chickpeas, alongside their pivotal role in bolstering consumers’ wellbeing. Chickpea stands as the second most prominent legume globally, enriched with proteins, carbohydrates, minerals, vitamins, dietary fiber, and fatty acids. Prior research indicates that chickpea components may mitigate the risks of diverse chronic ailments, albeit the underlying mechanisms remain elusive. Breeding chickpeas involves a blend of conventional and modern molecular techniques, enabling scientists to cultivate multi-resistant varieties leveraging prior genomic data for optimized growth in challenging environments. Copious evidence underscores that inoculating chickpeas with rhizobia prior to sowing fosters their growth and enhances symbiotic attributes, nutrient uptake, and overall quality. This is attributed to optimized nutrient acquisition.
To reduce cultivation expenses and enhance chickpea quality, combining rhizobial inoculation with inorganic fertilizers offers a promising solution. This approach is particularly vital when introducing chickpeas to new regions lacking a planting history, as these soils often lack native chickpea rhizobia. Studies reveal that various chickpea rhizobial strains exhibit differing adaptation and competitive nodulation capabilities, with Pseudomonas potentially playing a pivotal role in soil biology. Inoculation with high-performance rhizobia not only boosts bio-nitrogen fixation and plant growth but also significantly minimizes chemical nitrogen and pesticide usage, crucial for sustainable agriculture. In conclusion, chickpea’s global significance as a legume is unmistakable, and continuous breeding efforts combined with rhizobial inoculation will undoubtedly benefit chickpea producers, consumers, and agroecologists alike.
Funding
This work was financed by the Project of National Nature Science Foundation of China (project no. 31970006) and Central Plains Youth Top Talent Project (Yuzutong[2023]No.11) from JJ Zhang.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, F.; Diwakar, B. (Eds.) Chickpea Botany and Production Practices; International Crops Research Institute for the Semi-Arid Tropics: Patancheru, India, 1995; p. 1. [Google Scholar]
- Ladizinsky, G. A new Cicer from Turkey. In Notes from the Royal Botanic Garden; Royal Botanic Garden: Edinburgh, UK, 1975; Volume 34, pp. 201–202. [Google Scholar]
- Iqbal, A.; Ateeq, N.; Khalil, I.A.; Perveen, S.; Saleemullah, S. Physicochemical characteristics and amino acid profile of chickpea cultivars grown in Pakistan. J. Foodserv. 2010, 17, 94–101. [Google Scholar] [CrossRef]
- Van der Maesen, L.J.G. Origin, history and taxonomy of chickpea. In The Chickpea; Saxena, M.C., Singh, K.B., Eds.; CAB International: Wallingford, UK, 1987; pp. 11–34. [Google Scholar]
- Moreno, M.T.; Cubero, J.I. Variation in Cicer arietinum L. Euphytica 1978, 27, 465–485. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108 (Suppl. 1), S11. [Google Scholar] [CrossRef] [PubMed]
- Ramalho Ribeiro, J.; Portugal Melo, I. Composition and nutritive value of chickpea. In Present Status and Future Prospects of Chickpea Crop Production and Improvement in the Mediterranean Countries; Saxena, M., Cubero, J., Wery, J., Eds.; CIHEAM: Zaragoza, Spain, 1990; pp. 107–111. [Google Scholar]
- Oliveira, R.S.; Carvalho, P.; Marques, G.; Ferreira, L.; Nunes, M.; Rocha, I.; Ma, Y.; Carvalho, M.F.; Vosátka, M.; Freitas, H. Increased protein content of chickpea (Cicer arietinum L.) inoculated with arbuscular mycorrhizal fungi and nitrogen-fixing bacteria under water deficit conditions. J. Sci. Food Agric. 2017, 97, 4379–4385. [Google Scholar] [CrossRef]
- Zhang, J.J.; Guo, C.; Chen, W.F.; Shang, Y.M.; de Lajudie, P.; Yang, X.; Mao, P.H.; Zheng, J.Q.; Wang, E.T. Dynamic succession of chickpea rhizobia over years and sampling sites in Xinjiang, China. Plant Soil 2018, 425, 241–251. [Google Scholar] [CrossRef]
- Chibbar, R.N.; Ambigaipalan, P.; Hoover, R. Molecular diversity in pulse seed starch and complex carbohydrates and its role in human nutrition and health. Cereal Chem. 2010, 87, 342–352. [Google Scholar] [CrossRef]
- Ibrikci, H.; Knewtson, S.J.; Grusak, M.A. Chickpea leaves as a vegetable green for humans: Evaluation of mineral composition. J. Sci. Food Agric. 2003, 83, 945–950. [Google Scholar] [CrossRef]
- Chavan, J.K.; Kadam, S.S.; Salunkhe, D.K. Biochemistry and technology of chickpea (Cicer arietinum L.) seeds. CRC Crit. Rev. Food Technol. 1986, 25, 107–158. [Google Scholar]
- Gecit, H. Chickpea utilization in Turkey. In Proceedings of the Consultants Meeting, Andra Pradesh, India, 27–30 March 1989; pp. 69–74. [Google Scholar]
- Han, I.; Baik, B.-K. Oligosaccharide content and composition of legumes and their reduction by soaking, cooking, ultrasound and high hydrostatic pressure. Cereal Chem. 2006, 83, 428–433. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.C.; Peñuela-Teruel, M.J.; Cámara-Hurtado, M.; Díez-Marqués, C.; Torija-Isasa, M.E. Determination of mono-, di-, and oligosaccharides in legumes by high-performance liquid chromatography using an amino-bonded silica column. J. Agric. Food Chem. 1998, 46, 3648–3652. [Google Scholar] [CrossRef]
- Wood, J.; Grusak, M. Nutritional value of chickpea. In Chickpea Breeding and Management; Yadav, S., Redden, R., Chen, W., Sharma, B., Eds.; CAB International: Wallingford, UK, 2007; pp. 101–142. [Google Scholar]
- Jones, D.; DuPont, M.; Ambrose, M. The discovery of compositional variation for the raffinose family of oligosaccharides in pea seeds. Seed Sci. Res. 1999, 9, 305–310. [Google Scholar]
- Bernabe, M.; Fenwick, R.; Frias, J.; Jimenezbarbero, J.; Price, K.; Valverde, S.; Vidalvalverde, C. Determination, by NMR spectroscopy, of the structure of ciceritol, a pseudotrisaccharide isolated from lentils. J. Agric. Food Chem. 1993, 41, 870–872. [Google Scholar] [CrossRef]
- Aguilera, Y.; Martín-Cabrejas, M.A.; Benítez, V.; Mollá, E.; López-Andréu, F.J.; Esteban, R.M. Changes in carbohydrate fraction during dehydration process of common legumes. J. Food Compos. Anal. 2009, 22, 678–683. [Google Scholar] [CrossRef]
- Dalgetty, D.; Baik, B. Isolation and characterization of cotyledon fibres from peas, lentils, and chickpea. Cereal Chem. 2003, 80, 310–315. [Google Scholar] [CrossRef]
- Özer, S.; Karaköy, T.; Toklu, F.; Baloch, F.S.; Kilian, B.; Özkan, H. Nutritional and physicochemical variation in Turkish kabuli chickpea (Cicer arietinum L.) landraces. Euphytica 2010, 175, 237–249. [Google Scholar] [CrossRef]
- Jambunathan, R.; Singh, U. Studies on desi and kabuli chickpea (Cicer arietinum L.) cultivars. 1. Chemical composition. In Proceedings of the International Workshop on Chickpea Improvement, Hyderabad, India, 28 February–2 March 1979; ICRISAT: Andra Pradesh, India, 1980; pp. 61–66. [Google Scholar]
- Aguilera, Y.; Esteban, R.M.; Benitez, V. Starch, Functional properties, and microstructural characteristics in chickpea and Lentil as affected by thermal processing. J. Agric. Food Chem. 2009, 57, 10682–10688. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Iqbal, S.; Ahmad, S.; Imran, M.; Niaz, A.; Bhanger, M.I. Nutritional and compositional study of Desi chickpea (Cicer arietinum L.) cultivars grown in Punjab, Pakistan. Food Chem. 2007, 105, 1357–1363. [Google Scholar] [CrossRef]
- Khalil, A.W.; Zeb, A.; Mahmood, F.; Tariq, S.; Khattak, A.B.; Shah, H. Comparison of sprout quality characteristics of desi and kabuli type chickpea cultivars (Cicer arietinum L.). LWT Food Sci. Technol. 2007, 40, 937–945. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Shah, W.H. Thermal heat processing effects on antinutrients, protein and starch digestibility of food legumes. Food Chem. 2005, 91, 327–331. [Google Scholar] [CrossRef]
- Tosh, S.M.; Yada, S. Dietary fibres in pulse seeds and fractions: Characterization, functional attributes, and applications. Food Res. Int. 2010, 43, 450–460. [Google Scholar] [CrossRef]
- Rincón, F.; Martínez, B.; Ibáñez, M. Proximate composition and antinutritive substances in chickpea (Cicer arietinum L.) as affected by the biotype factor. J. Sci. Food Agric. 1998, 78, 382–388. [Google Scholar] [CrossRef]
- Hulse, J. Nature, composition and utilization of pulses. In Proceedings of the Uses of Tropical Grain Legumes, Proceedings of a Consultants Meeting, Andra Pradesh, India, 27–30 March 1989; pp. 11–27. [Google Scholar]
- Badshah, A.; Khan, M.; Bibi, N.; Khan, M.; Ali, S.; Ashraf Chaudry, M. Quality studies of newly evolved chickpea cultivars. Adv. Food Sci. 2003, 25, 95–99. [Google Scholar]
- Kaur, M.; Singh, N.; Sodhi, N.S. Physicochemical, cooking, textural and roasting characteristics of chickpea (Cicer arietinum L.) cultivars. J. Food Eng. 2005, 69, 511–517. [Google Scholar] [CrossRef]
- Wang, N.; Daun, J. The Chemical Composition and Nutritive Value of Canadian Pulses; Canadian Grain Commission Report; Canadian Grain Commission: Winnipeg, MB, Canada, 2004; pp. 19–29. [Google Scholar]
- Wang, X.; Gao, W.; Zhang, J.; Hua, Z.; Li, J.; He, X.; Hao, M. Subunit, amino acid composition and in vitro digestibility of protein isolates from Chinese kabuli and desi chickpea (Cicer arietinum L.) cultivars. Food Res. Int. 2010, 43, 567–572. [Google Scholar] [CrossRef]
- Alajaji, S.A.; El-Adawy, T.A. Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. J. Food Compos. Anal. 2006, 19, 806–812. [Google Scholar] [CrossRef]
- Cabrera, C.; Lloris, F.; Giménez, R.; Olalla, M.; López, M.C. Mineral content in legumes and nuts: Contribution to the Spanish dietary intake. Sci. Total Environ. 2003, 308, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Duhan, A.; Khetarpaul, N.; Bishnoi, S. Improvement in starch digestibility (in vitro) of various Pigeonpea cultivars through processing and cooking. Ecol. Food Nutr. 1998, 37, 557–568. [Google Scholar] [CrossRef]
- Quinteros, A.; Farré, R.; Lagarda, M.J. Optimization of iron speciation (soluble, ferrous and ferric) in beans, chickpeas and lentils. Food Chem. 2001, 75, 365–370. [Google Scholar] [CrossRef]
- Guillon, F.; Champ, M.M. Carbohydrate fractions of legumes: Uses in human nutrition and potential for health. Br. J. Nutr. 2002, 88, 293–306. [Google Scholar] [CrossRef]
- Çiftçi, H.; Ozkaya, A.; Çevrimli, B.S.; Bakoğlu, A. Levels of fat-soluble vitamins in some foods. Asian J. Chem. 2010, 22, 1251–1256. [Google Scholar]
- Lebiedzińska, A.L.; Szefer, P. Vitamins B in grain and cereal-grain food, soy-products and seeds. Food Chem. 2006, 95, 116–122. [Google Scholar] [CrossRef]
- Pittaway, J.K.; Ahuja, K.D.K.; Robertson, I.K.; Ball, M.J. Effects of a controlled diet supplemented with chickpeas on serum lipids, glucose tolerance, satiety and bowel function. J. Am. Coll. Nutr. 2007, 26, 334–340. [Google Scholar]
- Muir, J.G.; O’Dea, K. Measurement of resistant starch: Factors affecting the amount of starch escaping digestion in vitro. Am. J. Clin. Nutr. 1992, 56, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.; Emam, A.; Augustin, L.S.; Jenkins, D.J. Resistant starches and health. J. AOAC Int. 2004, 87, 769–774. [Google Scholar] [CrossRef]
- Osorio-Díaz, P.; Agama-Acevedo, E.; Mendoza-Vinalay, M.; Tovar, J.; Bello-Pérez, L.A. Pasta added with chickpea flour: Chemical composition, in vitro starch digestibility and predicted glycemic index. Cienc. Tecnol. Aliment. 2008, 6, 6–12. [Google Scholar]
- Ahmed, R.; Anthony, B.; David, T.; Sarah, B.; Judy, F.; Tina, B.; Behjat, K.H.; Zhongyi, L.; Sadequr, R.; Matthew, M. High-amylose wheat generated by RNA interference improves indices of large-bowel health in rats. Proc. Natl. Acad. Sci. USA 2006, 103, 3546–3551. [Google Scholar]
- Aurand, L.; Woods, A.; Wells, M. Food Composition and Analysis; Van Nostrand Reinhold Company: New York, NY, USA, 1987. [Google Scholar]
- Cummings, J.; Stephen, A.; Branch, W. Implications of dietary fibre breakdown in the human colon. In Banbury Report 7 Gastrointestinal Cancer; Bruce, W., Correa, P., Lipkin, M., Tannenbaum, S., Wilkins, T., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1981; pp. 71–81. [Google Scholar]
- Mathers, J.C. Pulses and carcinogenesis: Potential for the prevention of colon, breast and other cancers. Br. J. Nutr. 2002, 88, 273–279. [Google Scholar]
- Raicht, R.F.; Cohen, B.I.; Fazzini, E.P.; Sarwal, A.N.; Takahashi, M. Protective effect of plant sterols against chemically induced colon tumors in rats. Cancer Res. 1980, 40, 403–405. [Google Scholar] [PubMed]
- Giovannucci, E.; Ascherio, A.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Intake of carotenoids and retinol in relation to risk of prostate cancer. J. Natl. Cancer Inst. 1995, 87, 1767–1776. [Google Scholar] [CrossRef]
- Julio, G.C.; Javier, V.; María, D.M.Y.; Justo, P.; Manuel, A.; Francisco, M. Effect of chickpea aqueous extracts, organic extracts, and protein concentrates on cell proliferation. J. Med. Food 2004, 7, 122–129. [Google Scholar]
- Ying, Y.; Libin, Z.; Yuanjun, G.; Yibo, Z.; Jingfeng, T.; Fengying, L.; Wenbin, S.; Boren, J.; Xiaohua, Y.; Mingdao, C. Dietary chickpeas reverse visceral adiposity, dyslipidaemia and insulin resistance in rats induced by a chronic high-fat diet. Br. J. Nutr. 2007, 98, 720–726. [Google Scholar]
- Murty, C.M.; Pittaway, J.K.; Ball, M.J. Chickpea supplementation in an Australian diet affects food choice, satiety and bowel health. Appetite 2010, 54, 282–288. [Google Scholar]
- Paul, N.; Marja, C.; Andriana, C. Effects of long-term consumption and single meals of chickpeas on plasma glucose, insulin, and triacylglycerol concentrations. Am. J. Clin. Nutr. 2004, 79, 390–395. [Google Scholar]
- Thudi, M.; Chitikineni, A.; Liu, X.; He, W.; Roorkiwal, M.; Yang, W.; Jian, J.; Doddamani, D.; Gaur, P.M.; Rathore, A.; et al. Recent breeding programs enhanced genetic diversity in both desi and kabuli varieties of chickpea (Cicer arietinum L.). Sci. Rep. 2016, 6, 38636. [Google Scholar] [CrossRef]
- Kumar, M.; Yusuf, M.A.; Nigam, M.; Kumar, M. An update on genetic modification of chickpea for increased yield and stress tolerance. Mol. Biotechnol. 2018, 60, 651–663. [Google Scholar] [CrossRef]
- Roorkiwal, M.; Bharadwaj, C.; Barmukh, R.; Dixit, G.P.; Thudi, M.; Gaur, P.M.; Chaturvedi, S.K.; Fikre, A.; Hamwieh, A.; Kumar, S.; et al. Integrating genomics for chickpea improvement: Achievements and opportunities. Theor. Appl. Genet. 2020, 133, 1703–1720. [Google Scholar] [CrossRef] [PubMed]
- Seyedimoradi, H.; Talebi, R.; Kanouni, H.; Naji, A.M.; Karami, E. Genetic diversity and population structure analysis of chickpea (Cicer arietinum L.) advanced breeding lines using whole-genome DArTseq-generated SilicoDArT markers. Braz. J. Bot. 2020, 43, 541–549. [Google Scholar] [CrossRef]
- Li, H.; Rodda, M.; Gnanasambandam, A.; Aftab, M.; Redden, R.; Hobson, K.; Rosewarne, G.; Materne, M.; Kaur, S.; Slater, A.T. Breeding for biotic stress resistance in chickpea: Progress and prospects. Euphytica 2015, 204, 257–288. [Google Scholar]
- Ahmar, S.; Gill, R.A.; Jung, K.-H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef]
- Stefaniak, T.; McPhee, K. Comparison of hybridization techniques in chickpea. Crop Sci. 2017, 57, 843–846. [Google Scholar] [CrossRef]
- Salimath, P.M.; Toker, C.; Sandhu, J.S.; Kumar, J.; Bahl, P.N. Conventional breeding methods. In Chickpea Breeding & Management; CABI: Wallingford, UK, 2007. [Google Scholar]
- Upadhyaya, H.D.; Dwivedi, S.L.; Sharma, S. Managing and discovering agronomically beneficial traits in chickpea germplasm collections. In The Chickpea Genome; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Abbo, S.; Berger, J.; Turner, N.C. Evolution of cultivated chickpea: Four bottlenecks limit diversity and constrain adaptation. Funct. Plant Biol. 2003, 30, 1081–1087. [Google Scholar] [CrossRef]
- Abbo, S.; Shtienberg, D.; Lichtenzveig, J.; Lev-Yadun, S.; Gopher, A. The chickpea, summer cropping, and a new model for pulse domestication in the ancient Near East. Q. Rev. Biol. 2003, 78, 435–448. [Google Scholar] [CrossRef]
- Varshney, R.K.; Thundi, M.; May, G.D.; Jackson, S.A. Legume Genomics and Breeding. In Plant Breeding Reviews; John Wiley & Sons: Hoboken, NJ, USA, 2010; Volume 33. [Google Scholar]
- Jha, U.C. Current advances in chickpea genomics: Applications and future perspectives. Plant Cell Rep. 2018, 37, 947–965. [Google Scholar] [CrossRef]
- Varshney, R.K.; Hiremath, P.J.; Lekha, P.; Kashiwagi, J.; Balaji, J.; Deokar, A.A.; Vadez, V.; Xiao, Y.; Srinivasan, R.; Gaur, P.M.; et al. A comprehensive resource of drought- and salinity-responsive ESTs for gene discovery and marker development in chickpea (Cicer arietinum L.). BMC Genom. 2009, 10, 523. [Google Scholar] [CrossRef]
- Nayak, S.N.; Zhu, H.; Varghese, N.; Datta, S.; Choi, H.-K.; Horres, R.; Juengling, R.; Singh, J.; Kishor, P.B.K.; Sivaramakrishnan, S.; et al. Integration of novel SSR and gene-based SNP marker loci in the chickpea genetic map and establishment of new anchor points with Medicago truncatula genome. Theor. Appl. Genet. 2010, 120, 1415–1441. [Google Scholar] [CrossRef]
- Hiremath, P.J.; Kumar, A.; Penmetsa, R.V.; Farmer, A.; Schlueter, J.A.; Chamarthi, S.K.; Whaley, A.M.; Carrasquilla-Garcia, N.; Gaur, P.M.; Upadhyaya, H.D.; et al. Large-scale development of cost-effective SNP marker assays for diversity assessment and genetic mapping in chickpea and comparative mapping in legumes. Plant Biotechnol. J. 2012, 10, 716–732. [Google Scholar] [CrossRef]
- Thudi, M.; Bohra, A.; Nayak, S.N.; Varghese, N.; Shah, T.M.; Penmetsa, R.V.; Thirunavukkarasu, N.; Gudipati, S.; Gaur, P.M.; Kulwal, P.L. Novel SSR markers from BAC-end sequences, DArT arrays and a comprehensive genetic map with 1,291 marker loci for chickpea (Cicer arietinum L.). PLoS ONE 2011, 6, e27275. [Google Scholar]
- Kudapa, H.; Azam, S.; Sharpe, A.G.; Taran, B.; Li, R.; Deonovic, B.; Cameron, C.; Farmer, A.D.; Cannon, S.B.; Varshney, R.K. Comprehensive Transcriptome Assembly of Chickpea (Cicer arietinum L.) Using sanger and next generation sequencing platforms: Development and applications. PLoS ONE 2014, 9, e86039. [Google Scholar] [CrossRef]
- Agarwal, G.; Garg, V.; Kudapa, H.; Doddamani, D.; Pazhamala, L.T.; Khan, A.W.; Thudi, M.; Lee, S.-H.; Varshney, R.K. Genome-wide dissection of AP2/ERF and HSP90 gene families in five legumes and expression profiles in chickpea and pigeonpea. Plant Biotechnol. J. 2016, 14, 1563–1577. [Google Scholar] [CrossRef]
- Mashaki, K.M.; Garg, V.; Ghomi, A.A.N.; Kudapa, H.; Chitikineni, A.; Nezhad, K.Z.; Yamchi, A.; Soltanloo, H.; Varshney, R.K.; Thudi, M. RNA-Seq analysis revealed genes associated with drought stress response in kabuli chickpea (Cicer arietinum L.). PLoS ONE 2018, 13, e0199774. [Google Scholar] [CrossRef]
- Varshney, R.K.; Song, C.; Saxena, R.K.; Azam, S.; Yu, S.; Sharpe, A.G.; Cannon, S.; Baek, J.; Rosen, B.D.; Tar’an, B.; et al. Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nat. Biotechnol. 2013, 31, 240–246. [Google Scholar] [CrossRef]
- Varshney, R.K.; Roorkiwal, M.; Sun, S.; Bajaj, P.; Chitikineni, A.; Thudi, M.; Singh, N.P.; Du, X.; Upadhyaya, H.D.; Khan, A.W.; et al. A chickpea genetic variation map based on the sequencing of 3366 genomes. Nature 2021, 599, 622–627. [Google Scholar] [CrossRef]
- Zhang, Y.; Massel, K.; Godwin, I.D.; Gao, C. Applications and potential of genome editing in crop improvement. Genome Biol. 2018, 19, 210. [Google Scholar] [CrossRef]
- Sarmah, B.K.; Moore, A.; Tate, W.; Molvig, L.; Morton, R.L.; Rees, D.P.; Chiaiese, P.; Chrispeels, M.J.; Tabe, L.M.; Higgins, T.J.V. Transgenic chickpea seeds expressing high levels of a bean α-amylase inhibitor. Mol. Breed. 2004, 14, 73–82. [Google Scholar]
- Chakraborti, D.; Sarkar, A.; Mondal, H.A.; Das, S. Tissue specific expression of potent insecticidal, Allium sativum leaf agglutinin (ASAL) in important pulse crop, chickpea (Cicer arietinum L.) to resist the phloem feeding Aphis craccivora. Transgenic Res. 2009, 18, 529–544. [Google Scholar] [CrossRef]
- Das, A.; Datta, S.; Thakur, S.; Shukla, A.; Ansari, J.; Sujayanand, G.K.; Chaturvedi, S.K.; Kumar, P.A.; Singh, N.P. Expression of a chimeric gene encoding insecticidal crystal protein Cry1Aabc of Bacillus thuringiensis in chickpea (Cicer arietinum L.) confers resistance to gram pod borer (Helicoverpa armigera Hubner.). Front. Plant Sci. 2017, 8, 1423. [Google Scholar] [CrossRef]
- Kambrekar, D.N. Management of Legume Podborer, Helicoverpa armigera with Host Plant Resistance. J. Farm Sci. 2016, 29, 157–171. [Google Scholar]
- Anbazhagan, K.; Bhatnagar-Mathur, P.; Vadez, V.; Dumbala, S.R.; Kishor, P.B.K.; Sharma, K.K. DREB1A overexpression in transgenic chickpea alters key traits influencing plant water budget across water regimes. Plant Cell Rep. 2015, 34, 199–210. [Google Scholar] [CrossRef]
- Ghanti, S.K.K.; Sujata, K.G.; Kumar, B.M.V.; Karba, N.N.; Reddy, K.J.; Rao, M.S.; Kishor, P.B.K. Heterologous expression of P5CS gene in chickpea enhances salt tolerance without affecting yield. Biol. Plant. 2011, 55, 634–640. [Google Scholar] [CrossRef]
- Razzaq, M.K.; Akhter, M.; Ahmad, R.M.; Cheema, K.L.; Hina, A.; Karikari, B.; Raza, G.; Xing, G.; Gai, J.; Khurshid, M. CRISPR-Cas9 based stress tolerance: New hope for abiotic stress tolerance in chickpea (Cicer arietinum). Mol. Biol. Rep. 2022, 49, 8977–8985. [Google Scholar] [CrossRef] [PubMed]
- Nour, S.M.; Fernandez, M.P.; Normand, P.; Cleyet-Marel, J.C. Rhizobium ciceri sp. nov., consisting of strains that nodulate chickpeas (Cicer arietinum L.). Int. J. Syst. Bacteriol. 1994, 44, 511–522. [Google Scholar]
- Nour, S.M.; Cleyet-Marel, J.C.; Normand, P.; Fernandez, M.P. Genomic heterogeneity of strains nodulating chickpeas (Cicer arietinum L.) and description of Rhizobium mediterraneum sp. nov. Int. J. Syst. Bacteriol. 1995, 45, 640–648. [Google Scholar] [PubMed]
- Zhang, J.J.; Lou, K.; Jin, X.; Mao, P.H.; Wang, E.T.; Tian, C.F.; Sui, X.H.; Chen, W.F.; Chen, W.X. Distinctive Mesorhizobium populations associated with Cicer arietinum L. in alkaline soils of Xinjiang, China. Plant Soil 2012, 353, 123–134. [Google Scholar] [CrossRef]
- Elias, N.V.; Herridge, D.F. Naturalised populations of mesorhizobia in chickpea (Cicer arietinum L.) cropping soils: Effects on nodule occupancy and productivity of commercial chickpea. Plant Soil 2015, 387, 233–249. [Google Scholar]
- Zhang, J.J.; Liu, T.Y.; Chen, W.F.; Wang, E.T.; Sui, X.H.; Zhang, X.X.; Li, Y.; Li, Y.; Chen, W.X. Mesorhizobium muleiense sp. nov., nodulating with Cicer arietinum L. Int. J. Syst. Evol. Microbiol. 2012, 62, 2737–2742. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, C.; Chen, W.; De, L.P.; Zhang, Z.; Shang, Y.; Wang, E.T. Mesorhizobium wenxiniae sp. nov., isolated from chickpea (Cicer arietinum L.) in China. Int. J. Syst. Evol. Microbiol. 2018, 68, 1930–1936. [Google Scholar] [PubMed]
- Tena, W.; Wolde-Meskel, E.; Degefu, T.; Walley, F. Genetic and phenotypic diversity of rhizobia nodulating Chickpea (Cicer arietinum L.) in soils from southern and central Ethiopia. Can. J. Microbiol. 2017, 63, 690–707. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, A.; Brigido, C.; Laranjo, M.; Rodrigues, S.; Oliveira, S. Survey of Chickpea Rhizobia diversity in Portugal reveals the predominance of species distinct from Mesorhizobium ciceri and Mesorhizobium mediterraneum. Microb. Ecol. 2009, 58, 930–941. [Google Scholar] [CrossRef]
- Laranjo, M.; Machado, J.; Young, J.P.; Oliveira, S. High diversity of chickpea Mesorhizobium species isolated in a Portuguese agricultural region. FEMS Microbiol. Ecol. 2004, 48, 101–107. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yang, X.; Guo, C.; de Lajudie, P.; Singh, R.P.; Wang, E.T.; Chen, W.F. Mesorhizobium muleiense and Mesorhizobium gsp. nov. are symbionts of Cicer arietinum L. in alkaline soils of Gansu, Northwest China. Plant Soil 2017, 410, 103–112. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, S.; Shang, Y.; Brunel, B.; Li, S.; Zhao, Y.; Liu, Y.; Chen, W.; Wang, E.; Singh, R.P.; et al. Genomic diversity of chickpea-nodulating rhizobia in Ningxia (north central China) and gene flow within symbiotic Mesorhizobium muleiense populations. Syst. Appl. Microbiol. 2020, 43, 126089. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Wang, N.; Chen, W.; Feng, X.; Jia, B.; Zhao, Y.; Yang, T.; Zong, X. The introduced strain Mesorhizobium ciceri USDA 3378 is more competitive than an indigenous strain in nodulation of chickpea in newly introduced areas of China. Lett. Appl. Microbiol. 2022, 75, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Handbook of Agriculture; Indian Council of Agricultural Research: New Delhi, India, 2014. [Google Scholar]
- Unkovich, M.; Herridge, D.; Peoples, M.; Cadisch, G.; Boddey, B.; Giller, K.; Alves, B.; Chalk, P. Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; Australian Centre for International Agricultural Research: Canberra, Australia, 2008; p. 16. [Google Scholar]
- Sridevi, M.; Mallaiah, K.V. Phosphate solubilization by Rhizobium strains. Indian J. Microbiol. 2009, 49, 98. [Google Scholar]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar]
- Singh, Z.; Singh, G. Role of Rhizobium in chickpea (Cicer arietinum) production—A review. Agric. Rev. 2018, 39, 31–39. [Google Scholar]
- Zhang, J.J.; Yu, T.; Lou, K.; Mao, P.H.; Wang, E.T.; Chen, W.F.; Chen, W.X. Genotypic alteration and competitive nodulation of Mesorhizobium muleiense against exotic chickpea rhizobia in alkaline soils. Syst. Appl. Microbiol. 2014, 37, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, N.; Li, S.; Wang, J.; Feng, Y.; Wang, E.; Li, Y.; Yang, T.; Chen, W. The effect of different rhizobial symbionts on the composition and diversity of rhizosphere microorganisms of chickpea in different soils. Plants 2023, 2023, 3421. [Google Scholar] [CrossRef]
- Rudresh, D.L.; Shivaprakash, M.K.; Prasad, R.D. Effect of combined application of Rhizobium, phosphate solubilizing bacterium and Trichoderma spp. on growth, nutrient uptake and yield of chickpea (Cicer aritenium L.). Appl. Soil Ecol. 2005, 28, 139–146. [Google Scholar]
- Elkoca, E.; Kantar, F.; Sahin, F. Influence of nitrogen fixing and phosphorus solubilizing bacteria on the nodulation, plant growth, and yield of chickpea. J. Plant Nutr. 2008, 31, 157–171. [Google Scholar]
- Solaiman, A.; Talukder, M.S.; Rabbani, M.G. Influence of some Rhizobium strains on chickpea: Nodulation, dry matter yield and nitrogen uptake. Bangladesh J. Microbiol. 2011, 27, 61–64. [Google Scholar] [CrossRef]
- Yadav, J.; Verma, J.P. Effect of seed inoculation with indigenous Rhizobium and plant growth promoting rhizobacteria on nutrients uptake and yields of chickpea (Cicer arietinum L.). Eur. J. Soil Biol. 2014, 63, 70–77. [Google Scholar] [CrossRef]
- Shahzad, S.M.; Khalid, A.; Arif, M.S.; Riaz, M.; Ashraf, M.; Iqbal, Z.; Yasmeen, T. Co-inoculation integrated with P-enriched compost improved nodulation and growth of Chickpea (Cicer arietinum L.) under irrigated and rainfed farming systems. Biol. Fertil. Soils 2014, 50, 1–12. [Google Scholar] [CrossRef]
- Uddin, M.; Hussain, S.; Khan, M.; Akhtar, M.; Hashmi, N.; Idrees, M.; Naeem, M.; Dar, T.A. Use of N and P biofertilizers together with phosphorus fertilizer improves growth and physiological attributes of chickpea. Glob. J. Agric. Sci. 2014, 2, 168–174. [Google Scholar]
- Erman, M.; Demir, S.; Ocak, E.; Tüfenkçi, Ş.; Oğuz, F.; Akköprü, A. Effects of Rhizobium, arbuscular mycorrhiza and whey applications on some properties in chickpea (Cicer arietinum L.) under irrigated and rainfed conditions 1—Yield, yield components, nodulation and AMF colonization. Field Crops Res. 2011, 122, 14–24. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, P.; Sharma, S. Co-inoculation of Mesorhizobium sp. and plant growth promoting rhizobacteria Pseudomonas sp. as bio-enhancer and bio-fertilizer in chickpea (Cicer arietinum L.). Legume Res. 2015, 38, 367–374. [Google Scholar] [CrossRef]
- Sahai, P.; Chandra, R. Performance of Liquid and Carrier-based Inoculants of Mesorhizobium ciceri and PGPR (Pseudomonas diminuta) in Chickpea (Cicer arietinum L.) on Nodulation, Yield and Soil properties. J. Indian Soc. Soil Sci. 2011, 59, 263–267. [Google Scholar]
- Das, S.; Pareek, N.; Raverkar, K.; Chandra, R.; Kaustav, A. Effectiveness of micronutrient application and Rhizobium inoculation on growth and yield of chickpea. Int. J. Agric. Environ. Biotechnol. 2012, 5, 445–452. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).