Bioreactor Systems for Plant Cell Cultivation at the Institute of Plant Physiology of the Russian Academy of Sciences: 50 Years of Technology Evolution from Laboratory to Industrial Implications
Abstract
:1. Introduction
2. Bioreactor Types for Plant Cell Cultivation and Their Specifics
- -
- The large size and vacuoles make plant cells particularly sensitive to physical and mechanical stresses;
- -
- The specifically high requirements for maintaining aseptic conditions during long cultivation due to the relatively low growth rate and long cultivation cycle of plant cell cultures compared to microbial and animal cell cultures;
- -
- The high requirements of uniform mixing due to the high sedimentation rate of cell aggregates and the increasing viscosity of cell suspensions at the high concentrations of cell biomass;
- -
- The intensive foaming and adhesion of cell biomass to the walls of a bioreactor;
- -
- The complex mechanisms of regulating the cell growth and biosynthesis of target metabolites.
2.1. Bioreactor Classification Based on Their Design
- -
- Bioreactors where mixing is performed by compressed air supply;
- -
- Bioreactors with mechanical stirring;
- -
- Wave bioreactors.
2.2. Bioreactors of the Experimental Biotechnological Facility of the IPPRAS
3. Cultivation Regimes
3.1. Cultivation Regimes Suitable for Plant Cell Cultures
- -
- A reduced risk of contamination and cell mutations due to the relatively short cultivation cycle compared to other regimes;
- -
- The high degree of substrate utilization;
- -
- The relatively low cost (compared to the cost of continuous cultivation).
- -
- The production of the cell biomass or compound of interest with predetermined and reproducible characteristics due to the stable and thoroughly controlled cultivation conditions;
- -
- The possibility to shift the composition of the cell population and their metabolic activity by manipulating the oxygen supply and nutrient components;
- -
- The possibility to regulate the growth rate of the culture and the concentration of cell biomass within a wide range by changing the flow rate of the nutrient medium.
- -
- Difficulties to control the production of secondary metabolites that are not directly correlated with the growth of the cell population;
- -
- Difficulties in providing stable cultivation conditions for cell cultures with high aggregation level and viscosity;
- -
- The risk of losing the culture strain due to cell mutation or due to the auto-selection of cells with a high proliferation rate;
- -
- The high cost and complexity of controlling and automation systems;
- -
- The increased risk of contamination due to long cultivation cycles and the use of additional equipment.
- -
- The continuous operation of the system without the problem of cell washout;
- -
- The separated cells are protected from shear stress;
- -
- The possibility of achieving high cell concentrations, up to 30–40 g L−1 medium;
- -
- The intercellular contacts are increased in closed cultivation systems.
- -
- The high chances of cell viability reduction caused by cell separation from the culture fluid or immobilization;
- -
- The difficulties in controlling the growth and biosynthetic parameters of the cell population;
- -
- The significant gradients of nutrients and oxygen within the system in case of cell immobilization or sedimentation;
- -
- The high cost and complexity of the additional equipment.
- -
- Multiple options to control and optimize cultivation conditions depending on the phase of the growth cycle, productivity, or culture age;
- -
- Reduced risk of mutations, contamination, or cell washout during cultivation;
- -
- A high degree of substrate utilization;
- -
- The duration of subcultivations may be varied depending on the physiological requirements of the cell population;
- -
- No time-consuming preparation of equipment and inoculum for each new subcultivation cycle.
3.2. The Use of Different Cultivation Regimes at the Experimental Biotechnological Facility of the IPPRAS

| Species | Bioreactor * | Cultivation Cycle (Days) | Maximum Biomass Accumulation (gDW L−1), Cell Viability (%) | Maximum Metabolites Content Achieved | Operating Conditions | Reference |
|---|---|---|---|---|---|---|
| Periodic (batch) cultivation regime | ||||||
| Dioscorea deltoidea | Bubble-type bioreactors (no. 1) | 21 | 10.0–11.5 g L−1 | ND | 27 °C, daylight, air flow 0.5–1.0 L min−1 | [42] |
| 15–28 | 9.5–10.0 g L−1 | ND | 27 °C, darkness, air flow 0.4 L min−1 | [45,79,81] | ||
| MF-107 (no. 5) | 21 | 10.0–11.5 g L−1 | Diosgenin 7.4–13.7 mg gDW−1 | 27 °C, daylight, stirring rate 350–500 rpm, air flow 0.5–1.0 L min−1 | [42] | |
| 14–15 | 9.0–9.5 g L−1 | Diosgenin 6.2–6.3 mg gDW−1 | 26 °C, darkness, stirring rate 300–500 rpm, pO2 70–90% of saturation volume | [43] | ||
| Polyscias filicifolia | Bubble-type bioreactors (no. 1) | 18 | 11.0–16.0 g L−1 | ND | 26 °C, darkness, pO2–ND | [83] |
| MF-107 (no. 5) | 24–30 | 12.8–17.4 g L−1 | ND | 26 °C, darkness, pO2–ND | ||
| Stephania glabra | Bubble-type bioreactors (no. 2) | 21 | 8.0–16.0 g L−1, 75–90% | Stepharin 0.05–0.16%DW | 26 °C, darkness, pO2 10–40% of saturation volume | [119] |
| 75 L tank bioreactor (no. 6) | 14 | 7.0–9.0 g L−1, 65–90% | Stepharin, traces | 26 °C, darkness, stirring rate 30–65 rpm, pO2 10–40% of saturation volume (single point sparger) | ||
| Alhagi persarum | Bubble-type bioreactors (no. 2) | 16 | 13.71 ± 1.84 g L−1, 74.1 ± 2.16% | ND | 26 °C, darkness, pO2 10–40% of saturation volume | [122] |
| Polyscias filicifolia | 75 L tank bioreactor (no. 6) | 22 | 9.3–13.7 g L−1, 77–85% | ND | 26 °C, darkness, pO2 10–40% of saturation volume (ring-type gas distributor) | [89] |
| Continuous cultivation regime | ||||||
| Dioscorea deltoidea | MF-107 (no. 5) | 115 | ~12.6 g L−1, 52–90% | Total furostanol glycosides 3.2–4.0%DW | 26 °C, darkness, stirring rate 100–360 rpm, dilution rates (D) 0.14–0.23 day−1 | [82] |
| Panax japonicus var. repens | Bubble-type bioreactors (no. 2) | 86 | 4.9–7.8 g L−1, 77–84% | Total ginsenosides 2.5–3.0%DW | 26 °C, darkness, pO2 10–40% of saturation volume, D 0.11–0.22 day−1 | [120] |
| Closed continuous cultivation regime | ||||||
| Dioscorea deltoidea | Fermus-apparatus (no. 3) | 57 | ~14.0 g L−1, 60–70% | Total furostanol glycosides 2.0–3.0%DW | 26 °C, darkness, pO2 20–60% of saturation volume, stirring rate 20–250 rpm, D 0.15 day−1 (days 20–30 and 46–57) ** | [85] |
| AK-210 (no. 4) | 19 | 15.0–15.5 g L−1, 60–80% | Total furostanol glycosides 4.0–6.0%DW | 26 °C, darkness, pO2 20–60% of saturation volume, stirring rate 20–250 rpm, D 0.15 day−1 (days 7 to 19) ** | ||
| 20 | 30–32 g L−1, 62–84% | Total furostanol glycosides 9.5%DW | Same as above, with ×2 medium concentration | [123] | ||
| Semi-continuous cultivation regime | ||||||
| Stephania glabra | Bubble-type bioreactors (no. 2) | Multicycle 40–60 | 11.0–16.0 g L−1, 78–92% | Stepharin 0.06–0.16%DW | 26 °C, darkness, pO2 10–40% of saturation volume *** | [119] |
| Dioscorea deltoidea | Bubble-type bioreactors (no. 2) | Multicycle 182 | 8.50–12.50 g L−1, 80–85% | Total furostanol glycosides 4.2–8.0%DW | 26 °C, darkness, pO2 10–40% of saturation volume *** | [124] |
| 630 L tank bioreactor (no 7) | Multicycle 170 | 8.87–11.13 g L−1, 79–86% | Total furostanol glycosides 7.7–13.9%DW | |||
| Polyscias filicifolia | 630 L tank bioreactor (no. 7) | Multicycle 112 | 10.8–16.2 g L−1, 79–87% | ND | 26 °C, darkness, pO2 10–40% of saturation volume *** | [89] |
| Taxus wallichiana | Bubble-type bioreactors (no. 2) | Multicycle 75 | 10.5–17.5 g L−1, ~90% | Yunnanxane 0.08–0.36 mg gDW−1 taxuyunnanine C 0.09–0.34 mg gDW−1 paclitaxel 0.06–0.15 mg gDW−1 | 26 °C, darkness, pO2 10–40% of saturation volume *** | [88] |
| 75 L tank bioreactor (no. 6) | Multicycle 140 | 9.5–13.0 g L−1, ~90% | Synenxane C ~0.55 mg gDW−1 yunnanxane ~0.1 mg gDW−1 | |||
| Polyscias fruticosa | Bubble-type bioreactors (no. 2) | Multicycle 204 | 6.31–7.31 g L−1, 70–90% | Ladyginoside A 0.66–0.79 mg gDW−1 PFS 0.78–1.03 mg gDW−1 | 26 °C, darkness, pO2 10–40% of saturation volume *** | [86] |
| Panax japonicus | 630 L tank bioreactor (no. 7) | Multicycle 115 | 8.7–10.2 g L−1, 86–90% | Total ginsenosides ~7.5%DW | 26 °C, darkness, pO2 10–40% of saturation volume *** | [87] |
- -
- The dissolved oxygen concentration (pO2) should remain above 10–15%;
- -
- The stirrer rotation speed should be adjusted to aeration intensity to avoid any “dead” zones in the bioreactor.
4. Strategies for Upscaling the Cultivation Process
4.1. Mixing
- -
- Providing mass transfer between the gas, liquid, and solid phases of the suspension;
- -
- Maintaining homogeneous chemical and physical conditions in the system for a uniform distribution of the nutrients and gases, heat transfer, and dispersion of cell biomass.
4.2. Aeration
4.3. Oxygen Mass Transfer Coefficient (KLα)
4.4. Scale-Up Technologies at the Experimental Biotechnological Facility of the IPPRAS
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harnischfeger, G. Proposed Guidelines for Commercial Collection of Medicinal Plant Material. J. Herbs. Spices Med. Plants 2000, 7, 43–50. [Google Scholar] [CrossRef]
- Misra, A. Studies on Biochemical and Physiological Aspects in Relation to Phytomedicinal Qualities and Efficacy of the Active Ingredients during the Handling, Cultivation and Harvesting of the Medicinal Plants. J. Med. Plants Res. 2009, 3, 1140–1146. [Google Scholar]
- Verma, N.; Shukla, S. Impact of Various Factors Responsible for Fluctuation in Plant Secondary Metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Alamgir, A.N.M. Biotechnology, In Vitro Production of Natural Bioactive Compounds, Herbal Preparation, and Disease Management (Treatment and Prevention). In Therapeutic Use of Medicinal Plants and their Extracts; Springer: Cham, Switzerland, 2018; Volume 2, pp. 585–664. [Google Scholar]
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-Based, Biological-Active Surfactants from Plants. In Application and Characterization of Surfactants; InTechOpen: London, UK, 2017. [Google Scholar]
- Seca, A.; Pinto, D. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.; Ivanov, K.; Delattre, C.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S. Plant Adaptogens—History and Future Perspectives. Nutrients 2021, 13, 2861. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, H.A.; Najmi, A.; Javed, S.A.; Sultana, S.; Al Bratty, M.; Makeen, H.A.; Meraya, A.M.; Ahsan, W.; Mohan, S.; Taha, M.M.E.; et al. Medicinal Plants and Isolated Molecules Demonstrating Immunomodulation Activity as Potential Alternative Therapies for Viral Diseases Including COVID-19. Front. Immunol. 2021, 12, 637553. [Google Scholar] [CrossRef] [PubMed]
- Sellami, M.; Slimeni, O.; Pokrywka, A.; Kuvačić, G.; D Hayes, L.; Milic, M.; Padulo, J. Herbal Medicine for Sports: A Review. J. Int. Soc. Sports Nutr. 2018, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Nature: A Vital Source of Leads for Anticancer Drug Development. Phytochem. Rev. 2009, 8, 313–331. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Plant Cell Culture Strategies for the Production of Natural Products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef]
- Vasil, I.K. A History of Plant Biotechnology: From the Cell Theory of Schleiden and Schwann to Biotech Crops. Plant Cell Rep. 2008, 27, 1423–1440. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Weber, J.; Maciuk, A. Bioprocessing of Plant Cell Cultures for Mass Production of Targeted Compounds. Appl. Microbiol. Biotechnol. 2009, 83, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Wawrosch, C.; Zotchev, S.B. Production of Bioactive Plant Secondary Metabolites through in Vitro Technologies—Status and Outlook. Appl. Microbiol. Biotechnol. 2021, 105, 6649–6668. [Google Scholar] [CrossRef] [PubMed]
- Kreis, W. Exploiting Plant Cell Culture for Natural Product Formation. J. Appl. Bot. Food Qual. 2019, 92, 216–225. [Google Scholar] [CrossRef]
- Kieran, P.M. Bioreactor Design for Plant Cell Suspension Cultures. In Multiphase Bioreactor Design; Cabral, J., Mota, M., Tramper, J., Eds.; Taylor and Francis: London, UK, 2001; pp. 391–426. [Google Scholar]
- Chattopadhyay, S.; Farkya, S.; Srivastava, A.K.; Bisaria, V.S. Bioprocess Considerations for Production of Secondary Metabolites by Plant Cell Suspension Cultures. Biotechnol. Bioprocess Eng. 2002, 7, 138–149. [Google Scholar] [CrossRef]
- Werner, S.; Maschke, R.W.; Eibl, D.; Eibl, R. Bioreactor Technology for Sustainable Production of Plant Cell-Derived Products. In Bioprocessing of Plant In Vitro Systems. Reference Series in Phytochemistry; Pavlov, A., Bley, T., Eds.; Springer: Cham, Switzerland, 2018; pp. 413–432. [Google Scholar]
- Titova, M.V.; Popova, E.V.; Konstantinova, S.V.; Kochkin, D.V.; Ivanov, I.M.; Klyushin, A.G.; Titova, E.G.; Nebera, E.A.; Vasilevskaya, E.R.; Tolmacheva, G.S.; et al. Suspension Cell Culture of Dioscorea deltoidea—A Renewable Source of Biomass and Furostanol Glycosides for Food and Pharmaceutical Industry. Agronomy 2021, 11, 394. [Google Scholar] [CrossRef]
- Yuorieva, N.; Sinetova, M.; Messineva, E.; Kulichenko, I.; Fomenkov, A.; Vysotskaya, O.; Osipova, E.; Baikalova, A.; Prudnikova, O.; Titova, M.; et al. Plants, Cells, Algae, and Cyanobacteria In Vitro and Cryobank Collections at the Institute of Plant Physiology, Russian Academy of Sciences—A Platform for Research and Production Center. Biology 2023, 12, 838. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, R.; van der Heijden, R.; ten Hoopen, H.; Memelink, J. Metabolic Engineering of Plant Secondary Metabolite Pathways for the Production of Fine Chemicals. Biotechnol. Lett. 1999, 21, 467–479. [Google Scholar] [CrossRef]
- Smetanska, I. Production of Secondary Metabolites Using Plant Cell Cultures. In Food Biotechnology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 187–228. [Google Scholar]
- Doran, P.M. Foreign Protein Production in Plant Tissue Cultures. Curr. Opin. Biotechnol. 2000, 11, 199–204. [Google Scholar] [CrossRef]
- Huang, T.-K.; McDonald, K.A. Bioreactor Engineering for Recombinant Protein Production in Plant Cell Suspension Cultures. Biochem. Eng. J. 2009, 45, 168–184. [Google Scholar] [CrossRef]
- Gamborg, O.L. Plant Tissue Culture. Biotechnology. Milestones. Vitr. Cell. Dev. Biol. Plant Plant 2002, 38, 84–92. Available online: http://www.jstor.org/stable/20065017 (accessed on 28 November 2023). [CrossRef]
- Wu, J.; Zhong, J.-J. Production of Ginseng and Its Bioactive Components in Plant Cell Culture: Current Technological and Applied Aspects. J. Biotechnol. 1999, 68, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-W.; Yao, H.; Zhong, J.-J. Improvement of Panax notoginseng Cell Culture for Production of Ginseng Saponin and Polysaccharide by High Density Cultivation in Pneumatically Agitated Bioreactors. Biotechnol. Prog. 2001, 17, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Dixit, V.K. Yield Enhancement Strategies for Artemisinin Production by Suspension Cultures of Artemisia annua. Bioresour. Technol. 2008, 99, 4609–4614. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.B.; Abranches, R.; Fischer, R.; Sack, M.; Holland, T. Putting the Spotlight Back on Plant Suspension Cultures. Front. Plant Sci. 2016, 7, 297. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ge, X.; Dolan, M.C. Towards High-Yield Production of Pharmaceutical Proteins with Plant Cell Suspension Cultures. Biotechnol. Adv. 2011, 29, 278–299. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, J.; Raven, N.; Boes, A.; Roberts, J.L.; Russell, S.; Treffenfeldt, W.; Fischer, R.; Schinkel, H.; Schiermeyer, A.; Schillberg, S. Monoclonal Tobacco Cell Lines with Enhanced Recombinant Protein Yields Can Be Generated from Heterogeneous Cell Suspension Cultures by Flow Sorting. Plant Biotechnol. J. 2012, 10, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant Cell Culture Technology in the Cosmetics and Food Industries: Current State and Future Trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, B.; Wiesner, W.; Arens, H. Large-Scale Production of Rosmarinic Acid from Plant Cell Cultures of Coleus blumei Benth. In Primary and Secondary Metabolism of Plant Cell Cultures; Springer: Berlin/Heidelberg, Germany, 1985; pp. 293–303. [Google Scholar]
- Tabata, M.; Fujita, Y. Production of Shikonin by Plant Cell Cultures. In Biotechnology in Plant Science; Zaitlin, M., Day, P., Hollaender, A., Eds.; Academic Press: New York, NY, USA, 1985; pp. 207–218. [Google Scholar]
- Kobayashi, Y.; Akita, M.; Sakamoto, K.; Liu, H.; Shigeoka, T.; Koyano, T.; Kawamura, M.; Furuya, T. Large-Scale Production of Anthocyanin by Aralia cordata Cell Suspension Cultures. Appl. Microbiol. Biotechnol. 1993, 40, 215–218. [Google Scholar] [CrossRef]
- Nosov, A.M. Application of Cell Technologies for Production of Plant-Derived Bioactive Substances of Plant Origin. Appl. Biochem. Microbiol. 2012, 48, 609–624. [Google Scholar] [CrossRef]
- Nosov, A.M.; Popova, E.V.; Kochkin, D.V. Isoprenoid Production via Plant Cell Cultures: Biosynthesis, Accumulation and Scaling-Up to Bioreactors. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Springer: Dordrecht, The Netherlands, 2014; pp. 563–623. [Google Scholar]
- Yazaki, K. Lithospermum erythrorhizon Cell Cultures: Present and Future Aspects. Plant Biotechnol. 2017, 34, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Popova, E.V.; Nosov, A.V.; Titova, M.V.; Kochkin, D.V.; Fomenkov, A.A.; Kulichenko, I.E.; Nosov, A.M. Advanced Biotechnologies: Collections of Plant Cell Cultures As a Basis for Development and Production of Medicinal Preparations. Russ. J. Plant Physiol. 2021, 68, 385–400. [Google Scholar] [CrossRef]
- Butenko, R.G.; Lipsky, A.K.; Paukov, V.N.; Davidova, I.M. Applying Different Types of Bioreactors for Cultivation of Plant Cell Cultures (Kultivatoren Zur Zuchtung von Pflanzenzellen). In Entwicklung von Laborfermentoren. VI. Reinhardsbrunner Symposium veranstaltet von der Sektion Mikrobiologie der Biologischen Gesellschaft der DDR und dem Institut für Technische Chemie der Akademie der Wissenschaften der DDR vom 21.–27. Mai 1978; Ringpfeil, M., Dimter, L., Eds.; De Gruyter: Berlin, Germany, 1979; pp. 193–198. [Google Scholar]
- Nosov, A.M.; Paukov, N.V.; Butenko, R. Steroid Compounds of D. deltoidea Wall. during Incubation of Culture in a Microbiological Fermentor. Appl. Biochem. Microbiol. 1984, 20, 119. [Google Scholar]
- Butenko, R.G. The Tissue Culture of Medical Plants and Its Possible Use in Pharmacy. Probl. Pharmacog. 1967, 21, 184–191. [Google Scholar]
- Lipsky, A.K.; Chernyak, N.D.; Butenko, R.G. Estimation of Biomass Amounts during the Submerged Cultivation of Dioscorea deltoidea Wall. by an Optical Method. Appl. Biochem. Microbiol. 1983, 19, 624–631. [Google Scholar]
- Lara, A.R.; Galindo, E.; Ramírez, O.T.; Palomares, L.A. Living with Heterogeneities in Bioreactors: Understanding the Effects of Environmental Gradients on Cells. Mol. Biotechnol. 2006, 34, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Varley, J.; Birch, J. Reactor Design for Large Scale Suspension Animal Cell Culture. Cytotechnology 1999, 29, 177–205. [Google Scholar] [CrossRef]
- Padilla-Córdova, C.; Mongili, B.; Contreras, P.; Fino, D.; Tommasi, T.; Díaz-Barrera, A. Productivity and Scale-up of Poly(3-hydroxybutyrate) Production under Different Oxygen Transfer Conditions in Cultures of Azotobacter vinelandii. J. Chem. Technol. Biotechnol. 2020, 95, 3034–3040. [Google Scholar] [CrossRef]
- Liu, W.-C.; Inwood, S.; Gong, T.; Sharma, A.; Yu, L.-Y.; Zhu, P. Fed-Batch High-Cell-Density Fermentation Strategies for Pichia pastoris Growth and Production. Crit. Rev. Biotechnol. 2019, 39, 258–271. [Google Scholar] [CrossRef]
- Ha, S.-J.; Kim, S.-Y.; Seo, J.-H.; Oh, D.-K.; Lee, J.-K. Optimization of Culture Conditions and Scale-up to Pilot and Plant Scales for Coenzyme Q10 Production by Agrobacterium tumefaciens. Appl. Microbiol. Biotechnol. 2007, 74, 974–980. [Google Scholar] [CrossRef]
- Kieran, P.; MacLoughlin, P.; Malone, D. Plant Cell Suspension Cultures: Some Engineering Considerations. J. Biotechnol. 1997, 59, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.I.; Weber, J. Bioreactors for Plant Cells: Hardware Configuration and Internal Environment Optimization as Tools for Wider Commercialization. Biotechnol. Lett. 2014, 36, 1359–1367. [Google Scholar] [CrossRef]
- Scragg, A.H. The Problems Associated with High Biomass Levels in Plant Cell Suspensions. Plant Cell. Tissue Organ Cult. 1995, 43, 163–170. [Google Scholar] [CrossRef]
- Verdú-Navarro, F.; Moreno-Cid, J.A.; Weiss, J.; Egea-Cortines, M. The Advent of Plant Cells in Bioreactors. Front. Plant Sci. 2023, 14, 1310405. [Google Scholar] [CrossRef]
- Eibl, R.; Eibl, D. Design of Bioreactors Suitable for Plant Cell and Tissue Cultures. Phytochem. Rev. 2008, 7, 593–598. [Google Scholar] [CrossRef]
- Motolinía-Alcántara, E.A.; Castillo-Araiza, C.O.; Rodríguez-Monroy, M.; Román-Guerrero, A.; Cruz-Sosa, F. Engineering Considerations to Produce Bioactive Compounds from Plant Cell Suspension Culture in Bioreactors. Plants 2021, 10, 2762. [Google Scholar] [CrossRef]
- Kantarci, N.; Borak, F.; Ulgen, K.O. Bubble Column Reactors. Process Biochem. 2005, 40, 2263–2283. [Google Scholar] [CrossRef]
- de Jesus, S.S.; Moreira Neto, J.; Maciel Filho, R. Hydrodynamics and Mass Transfer in Bubble Column, Conventional Airlift, Stirred Airlift and Stirred Tank Bioreactors, Using Viscous Fluid: A Comparative Study. Biochem. Eng. J. 2017, 118, 70–81. [Google Scholar] [CrossRef]
- Nickell, L.G.; Tulecke, W. Submerged Growth of Cells of Higher Plants. J. Biochem. Microbiol. Technol. Eng. 1960, 2, 287–297. [Google Scholar] [CrossRef]
- Son, S.H.; Choi, S.M.; Lee, Y.H.; Choi, K.B.; Yun, S.R.; Kim, J.K.; Park, H.J.; Kwon, O.W.; Noh, E.W.; Seon, J.H.; et al. Large-Scale Growth and Taxane Production in Cell Cultures of Taxus cuspidata (Japanese Yew) Using a Novel Bioreactor. Plant Cell Rep. 2000, 19, 628–633. [Google Scholar] [CrossRef]
- Shim, K.-M.; Murthy, H.N.; Park, S.-Y.; Rusli, I.; Paek, K.-Y. Production of Biomass and Bioactive Compounds from Cell Suspension Cultures of Eurycoma longifolia in Balloon Type Bubble Bioreactors. Hortic. Sci. Technol. 2015, 33, 251–258. [Google Scholar] [CrossRef]
- Thanh, N.T.; Murthy, H.N.; Paek, K.Y. Optimization of Ginseng Cell Culture in Airlift Bioreactors and Developing the Large-Scale Production System. Ind. Crop. Prod. 2014, 60, 343–348. [Google Scholar] [CrossRef]
- Reinhard, E.; Kreis, W.; Barthlen, U.; Helmbold, U. Semicontinuous Cultivation of Digitalis lanata Cells: Production of Β-methyldigoxin in a 300-L Airlift Bioreactor. Biotechnol. Bioeng. 1989, 34, 502–508. [Google Scholar] [CrossRef]
- Navia-Osorio, A.; Garden, H.; Cusidó, R.M.; Palazón, J.; Alfermann, A.W.; Piñol, M.T. Taxol® and Baccatin III Production in Suspension Cultures of Taxus baccata and Taxus wallichiana in an Airlift Bioreactor. J. Plant Physiol. 2002, 159, 97–102. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Prediction of Gas-liquid Mass Transfer Coefficient in Sparged Stirred Tank Bioreactors. Biotechnol. Bioeng. 2005, 92, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Golbabaei, F.; Mehrnia, M.R.; Neghab, M.; Mohammad, K.; Nikpey, A.; Pourmand, M.R. Oxygen Mass Transfer in a Stirred Tank Bioreactor Using Different Impeller Configurations for Environmental Purposes. Iranian J. Environ. Health Sci. Eng. 2013, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Doran, P.M. Design of Mixing Systems for Plant Cell Suspensions in Stirred Reactors. Biotechnol. Prog. 1999, 15, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H. Technological Problems in Cultivation of Plant Cells at High Density. Biotechnol. Bioeng. 2000, 67, 775–790. [Google Scholar] [CrossRef]
- Rittershaus, E.; Ulrich, J.; Weiss, A.; Westphal, K. Large Scale Industrial Fermentation of Plant Cells: Experiences in Cultivation of Plant Cells in a Fermentation Cascade up to a Volume of 75,000 L. BioEngineering 1989, 5, 28–34. [Google Scholar]
- Wang, J.; Gao, W.-Y.; Zhang, J.; Zuo, B.-M.; Zhang, L.-M.; Huang, L.-Q. Production of Ginsenoside and Polysaccharide by Two-Stage Cultivation of Panax quinquefolium L. Cells. In Vitro Cell. Dev. Biol. Plant 2012, 48, 107–112. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Zhong, J.-J. Scale-up of Centrifugal Impeller Bioreactor for Hyperproduction of Ginseng Saponin and Polysaccharide by High-Density Cultivation of Panax notoginseng Cells. Biotechnol. Prog. 2004, 20, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Mathur, A.K.; Masood, N.; Luqman, S.; Shanker, K. Tryptophan Over-Producing Cell Suspensions of Catharanthus roseus (L) G. Don and Their up-Scaling in Stirred Tank Bioreactor: Detection of a Phenolic Compound with Antioxidant Potential. Protoplasma 2013, 250, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Srivastava, A.K.; Bhojwani, S.S.; Bisaria, V.S. Production of Podophyllotoxin by Plant Cell Cultures of Podophyllum hexandrum in Bioreactor. J. Biosci. Bioeng. 2002, 93, 215–220. [Google Scholar] [CrossRef]
- Prakash, G.; Srivastava, A.K. Modeling of Azadirachtin Production by Azadirachta indica and Its Use for Feed Forward Optimization Studies. Biochem. Eng. J. 2006, 29, 62–68. [Google Scholar] [CrossRef]
- Zhong, J.-J. Recent Advances in Bioreactor Engineering. Korean J. Chem. Eng. 2010, 27, 1035–1041. [Google Scholar] [CrossRef]
- Eibl, R.; Eibl, D. Design and Use of the Wave Bioreactor for Plant Cell Culture. In Plan Tissue Culture Engineering. Focus on Biotechnology; Guptta, S.D., Ibaraki, Y., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 6, pp. 203–227. [Google Scholar]
- Terrier, B.; Courtois, D.; Hénault, N.; Cuvier, A.; Bastin, M.; Aknin, A.; Dubreuil, J.; Pétiard, V. Two New Disposable Bioreactors for Plant Cell Culture: The Wave and Undertow Bioreactor and the Slug Bubble Bioreactor. Biotechnol. Bioeng. 2007, 96, 914–923. [Google Scholar] [CrossRef]
- Ducos, J.-P.; Terrier, B.; Courtois, D. Disposable Bioreactors for Plant Micropropagation and Mass Plant Cell Culture. In Disposable Bioreactors. Advances in Biochemical Engineering/Biotechnology; Eibl, R., Eibl, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 115, pp. 89–115. [Google Scholar]
- Lipsky, A.K.; Chernyak, N.D. Carbon Dioxide Requirement during Submerged Cultivation of Dioscorea deltoidea Wall. Cells. Sov. Plant Physiol. Transl. 1983, 30, 591–596. [Google Scholar]
- Lipsky, A.K. Problems of Optimisation of Plant Cell Culture Processes. J. Biotechnol. 1992, 26, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Butenko, R.G.; Lipsky, A.K.; Chernyak, N.D.; Arya, H.C. Changes in Culture Medium PH by Cell Suspension Cultures of Dioscorea deltoidea. Plant Sci. Lett. 1984, 35, 207–212. [Google Scholar] [CrossRef]
- Kandarakov, O.F.; Vorobev, A.S.; Nosov, A.M. Biosynthetic Characteristics of Dioscorea deltoidea Cell Population Grown in Continuous Culture. Russ. J. Plant Physiol. 1994, 41, 805–809. [Google Scholar]
- Klyushin, A.G.; Oreshnikov, A.V.; Cherniak, N.D.; Nosov, A.M. The Characteristics of Growth of the Suspension Culture of Polyscias filicifolia Bailey Cells in Bioreactors. Biotekhnologiya 2000, 3, 67–72. [Google Scholar]
- Orlova, L.V.; Globa, E.B.; Chernyak, N.D.; Demidova, E.V.; Titova, M.V.; Solovchenko, A.E.; Sergeev, R.V.; Nosov, A.M. Growth and Bioartificial Characteristics of Taxus baccata Suspension Culture (Cultivation in Flasks and Bioreactor). Vestn. Volga State Univ. Technol. Ser. For. Ecol. Nat. Manag. 2014, 3, 86–97. [Google Scholar]
- Oreshnikov, A.V.; Nosov, A.M.; Manakov, M.N. Characterization of Dioscorea deltoidea Cells Grown in Closed Continuous Culture. Russ. J. Plant Physiol. 1994, 41, 810–814. [Google Scholar]
- Titova, M.V.; Kochkin, D.V.; Sukhanova, E.S.; Gorshkova, E.N.; Tyurina, T.M.; Ivanov, I.M.; Lunkova, M.K.; Tsvetkova, E.V.; Orlova, A.; Popova, E.V.; et al. Suspension Cell Culture of Polyscias fruticosa (L.) Harms in Bubble-Type Bioreactors—Growth Characteristics, Triterpene Glycosides Accumulation and Biological Activity. Plants 2023, 12, 3641. [Google Scholar] [CrossRef]
- Titova, M.V.; Popova, E.V.; Ivanov, I.M.; Fomenkov, A.A.; Nebera, E.A.; Vasilevskaya, E.R.; Tolmacheva, G.S.; Kotenkova, E.A.; Klychnikov, O.I.; Metalnikov, P.S.; et al. Toxicological Evaluation of Ginsenoside-Rich Cell Culture Biomass of Panax japonicus Produced in a Large-Scale Bioreactor System. Ind. Crop. Prod. 2024, 208, 117761. [Google Scholar] [CrossRef]
- Demidova, E.; Globa, E.; Klushin, A.; Kochkin, D.; Nosov, A. Effect of Methyl Jasmonate on the Growth and Biosynthesis of C13- and C14-Hydroxylated Taxoids in the Cell Culture of Yew (Taxus wallichiana Zucc.) of Different Ages. Biomolecules 2023, 13, 969. [Google Scholar] [CrossRef]
- Titova, M.V.; Popova, E.V.; Shumilo, N.A.; Kulichenko, I.E.; Chernyak, N.D.; Ivanov, I.M.; Klushin, A.G.; Nosov, A.M. Stability of Cryopreserved Polyscias filicifolia Suspension Cell Culture during Cultivation in Laboratory and Industrial Bioreactors. Plant Cell Tissue Organ Cult. 2021, 145, 591–600. [Google Scholar] [CrossRef]
- Sukhanova, E.S.; Kochkin, D.V.; Titova, M.V.; Nosov, A.M. Growth and Biosynthetic Characteristics of Different Polyscias Plant Cell Culture Strains. Vestn. Volga State Univ. Technol. Ser. For. Ecol. Nat. Manag. 2012, 2, 57–66. [Google Scholar]
- Pirt, S.J. Principles of Microbe and Cell Cultivation; Blackwell Scientific: Oxford, UK, 1975; ISBN 0632081503. [Google Scholar]
- Prakash, G.; Srivastava, A.K. Azadirachtin Production in Stirred Tank Reactors by Azadirachta indica Suspension Culture. Process Biochem. 2007, 42, 93–97. [Google Scholar] [CrossRef]
- Van Gulik, W.M.; Nuutila, A.M.; Vinke, K.L.; Ten Hoopen, H.J.G.; Heijnen, J.J. Effects of Carbon Dioxide, Air Flow Rate, and Inoculation Density on the Batch Growth of Catharanthus roseus Cell Suspensions in Stirred Fermentors. Biotechnol. Prog. 1994, 10, 335–339. [Google Scholar] [CrossRef]
- Han, J.; Zhong, J. High Density Cell Culture of Panax Notoginseng for Production of Ginseng Saponin and Polysaccharide in an Airlift Bioreactor. Biotechnol. Lett. 2002, 24, 1927–1930. [Google Scholar] [CrossRef]
- Hibino, K.; Ushiyama, K. Commercial Production of Ginseng by Plant Tissue Culture Technology. In Plant Cell and Tissue Culture for the Production of Food Ingredients; Springer: Boston, MA, USA, 1999; pp. 215–224. [Google Scholar]
- van Gulik, W.M.; ten Hoopen, H.J.G.; Heijnen, J.J. The Application of Continuous Culture for Plant Cell Suspensions. Enzyme Microb. Technol. 2001, 28, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Hoopen, H.J.G.; Gulik, W.M.; Heijnen, J.J. Continuous Culture of Suspended Plant Cells. In Vitro Cell. Dev. Biol. Plant 1992, 28, 115–120. [Google Scholar] [CrossRef]
- Wilson, G. A Simple and Inexpensive Design of Chemostat Enabling Steady-State Growth of Acer pseudoplatanus L. Cells under Phosphate-Limiting Conditions. Ann. Bot. 1976, 40, 919–932. [Google Scholar] [CrossRef]
- Kurz, W.G.W. A Chemostat for Growing Higher Plant Cells in Single Cell Suspension Cultures. Exp. Cell Res. 1971, 64, 476–479. [Google Scholar] [CrossRef]
- de Gucht, L.P.E.; van der Plas, L.H.W. Growth and Respiration of Petunia hybrida Cells in Chemostat Cultures: A Comparison of Glucose-Limited and Nitrate-Limited Cultures. Biotechnol. Bioeng. 2000, 52, 412–422. [Google Scholar] [CrossRef]
- Meijer, J.J.; ten Hoopen, H.J.G.; van Gameren, Y.M.; Luyben, K.C.A.M.; Libbenga, K.R. Effects of Hydrodynamic Stress on the Growth of Plant Cells in Batch and Continuous Culture. Enzyme Microb. Technol. 1994, 16, 467–477. [Google Scholar] [CrossRef]
- Sahai, O.P.; Shuler, M.L. Multistage Continuous Culture to Examine Secondary Metabolite Formation in Plant Cells: Phenolics from Nicotiana tabacum. Biotechnol. Bioeng. 1984, 26, 27–36. [Google Scholar] [CrossRef]
- Wilson, S.B.; King, P.J.; Street, H.E. Studies on the Growth in Culture of Plant Cells: XII. A Versatile System for the Large Scale Batch or Continuous Culture of Plant Cell Suspensions. J. Exp. Bot. 1971, 22, 177–207. [Google Scholar] [CrossRef]
- Peel, E. Photoautotrophic Growth of Suspension Cultures of Asparagus officinalis L. Cells in Turbidostats. Plant Sci. Lett. 1982, 24, 147–155. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Eibl, R.; Zhong, J.-J. Hosting the Plant Cells in Vitro: Recent Trends in Bioreactors. Appl. Microbiol. Biotechnol. 2013, 97, 3787–3800. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.R.; Qi, N.M.; Wang, Z.M. Application of a Stirrer-Tank Bioreactor for Perfusion Culture and Continuous Harvest of Glycyrrhiza inflata Suspension Cells. Afr. J. Biotechnol. 2010, 9, 347–351. [Google Scholar]
- Su, W.W.; Arias, R. Continuous Plant Cell Perfusion Culture: Bioreactor Characterization and Secreted Enzyme Production. J. Biosci. Bioeng. 2003, 95, 13–20. [Google Scholar] [CrossRef]
- De Dobbeleer, C.; Cloutier, M.; Fouilland, M.; Legros, R.; Jolicoeur, M. A High-Rate Perfusion Bioreactor for Plant Cells. Biotechnol. Bioeng. 2006, 95, 1126–1137. [Google Scholar] [CrossRef]
- Hao, Y.-J.; Ye, W.-Q.; Wang, M.; Liu, L.-L.; Yu, S.; Piao, X.-C.; Lian, M.-L. Selection of Initial Culture Medium in Fed-Batch Bioreactor Culture of Rhodiola sachalinensis Cells. J. Biotechnol. 2022, 346, 15–22. [Google Scholar] [CrossRef]
- Chastang, T.; Pozzobon, V.; Taidi, B.; Courot, E.; Clément, C.; Pareau, D. Resveratrol Production by Grapevine Cells in Fed-Batch Bioreactor: Experiments and Modelling. Biochem. Eng. J. 2018, 131, 9–16. [Google Scholar] [CrossRef]
- Restrepo, M.I.; Atehortúa, L.; Rojas, L.F. Optimization of Fed-Batch Culture for Polyphenol Production from Theobroma Cacao Cell Culture. Acta Hortic. 2023, 1359, 281–288. [Google Scholar] [CrossRef]
- Choi, J.W.; Cho, J.M.; Kim, Y.K.; Park, S.Y.; Kim, I.H.; Park, Y.H. Control of Glucose Concentration in a Fed—Batch Cultivation of Scutellaria baicalensis G. Plant Cells Using a Self—Organizing Fuzzy Logic Controller. J. Microbiol. Biotechnol. 2001, 11, 739–748. [Google Scholar]
- Corbin, J.M.; Hashimoto, B.I.; Karuppanan, K.; Kyser, Z.R.; Wu, L.; Roberts, B.A.; Noe, A.R.; Rodriguez, R.L.; McDonald, K.A.; Nandi, S. Semicontinuous Bioreactor Production of Recombinant Butyrylcholinesterase in Transgenic Rice Cell Suspension Cultures. Front. Plant Sci. 2016, 7, 412. [Google Scholar] [CrossRef]
- Santoyo-Garcia, J.H.; Valdivia-Cabrera, M.; Ochoa-Villarreal, M.; Casasola-Zamora, S.; Ripoll, M.; Escrich, A.; Moyano, E.; Betancor, L.; Halliday, K.J.; Loake, G.J.; et al. Increased Paclitaxel Recovery from Taxus baccata Vascular Stem Cells Using Novel in Situ Product Recovery Approaches. Bioresour. Bioprocess. 2023, 10, 68. [Google Scholar] [CrossRef]
- Kreis, W.; Reinhard, E. Two-Stage Cultivation of Digitalis lanata Cells: Semicontinuous Production of Deacetyllanatoside C in 20-Litre Airlift Bioreactors. J. Biotechnol. 1990, 16, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, M.L.; Arias, C.; Vega, J.; Feria-Velasco, A.; Ramírez, O.T.; Nicasio, P.; Rojas, G.; Quintero, R. Large-Scale Cultivation of Solanum chrysotrichum Cells: Production of the Antifungal Saponin SC-1 in 10-I Airlift Bioreactors. Plant Cell Rep. 1997, 16, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-W.; Kim, Y.-K.; Lee, W.H.; Pedersen, H.; Chin, C.-K. Bioreactor Operating Strategy in Thalictrum rugosum Plant Cell Culture for the Production of Berberine. Biotechnol. Bioprocess Eng. 1999, 4, 138–146. [Google Scholar] [CrossRef]
- Luo, J.; Mei, X.J.; Hu, D.W. Improved Paclitaxel Production by Fed-Batch Suspension Cultures of Taxus chinensis in Bioreactors. Biotechnol. Lett. 2002, 24, 561–565. [Google Scholar] [CrossRef]
- Titova, M.V.; Reshetnyak, O.V.; Osipova, E.A.; Osip’yants, A.I.; Shumilo, N.A.; Oreshnikov, A.V.; Nosov, A.M. Submerged Cultivation of Stephania glabra (Roxb.) Miers Cells in Different Systems: Specific Features of Growth and Accumulation of Alkaloid Stepharine. Appl. Biochem. Microbiol. 2012, 48, 645–649. [Google Scholar] [CrossRef]
- Demidova, E.V. Biosynthesis of Triterpene Glycosides in Suspension Cell Culture of Panax japonicus Var. Repens at Different Cultivation Conditions; K.A. Timiryazev Institute of Plant Physiology of RAS: Moscow, Russia, 2007. [Google Scholar]
- Titova, M.V. Personal Communication; Institute of Plant Physiology of RAS: Moscow, Russia, 2023. [Google Scholar]
- Titova, M.V.; Kochkin, D.V.; Fomenkov, A.A.; Ivanov, I.M.; Kotenkova, E.A.; Kocharyan, G.L.; Dzhivishev, E.G.; Mekhtieva, N.P.; Popova, E.V.; Nosov, A.M. Obtaining and Characterization of Suspension Cell Culture of Alhagi persarum Boiss. et Buhse: A Producer of Isoflavonoids. Russ. J. Plant Physiol. 2021, 68, 652–660. [Google Scholar] [CrossRef]
- Oreshnikov, A.V. Physiology of Dioscorea Deltoidea Cell Population under Conditions of Closed Continuous Cultivation Regime; K.A. Timiryazev Institute of Plant Physiology of Russian Academy of Sciences: Moscow, Russia, 1996. [Google Scholar]
- Titova, M.V.; Shumilo, N.A.; Kulichenko, I.E.; Ivanov, I.M.; Sukhanova, E.S.; Nosov, A.M. Features of Respiration and Formation of Steroidal Glycosides in Dioscorea deltoidea Cell Suspension Culture Grown in Flasks and Bioreactors. Russ. J. Plant Physiol. 2015, 62, 557–563. [Google Scholar] [CrossRef]
- Taticek, R.A.; Moo-Young, M.; Legge, R.L. The Scale-up of Plant Cell Culture: Engineering Considerations. Plant Cell. Tissue Organ Cult. 1991, 24, 139–158. [Google Scholar] [CrossRef]
- Scragg, A.H. Large-Scale Plant Cell Culture: Methods, Applications and Products. Curr. Opin. Biotechnol. 1992, 3, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.M. Equipment Design Considerations for Large Scale Cell Culture. Cytotechnology 2003, 42, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor Scale-up and Oxygen Transfer Rate in Microbial Processes: An Overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-H.; Henderson, K.A.; Rorrer, G.L. Cell Damage and Oxygen Mass Transfer during Cultivation of Nicotiana tabacum in a Stirred-tank Bioreactor. Biotechnol. Prog. 1995, 11, 140–145. [Google Scholar] [CrossRef]
- Wagner, F.; Vogelmann, H. Cultivation of Plant Tissue Cultures in Bioreactors and Formation of Secondary Metabolites. In Plant Tissue Culture and Its Biotechnological Application; Barz, W., Reinhard, E., Zenk, M.H., Eds.; Springer: Berlin, Germany, 1977; pp. 245–255. [Google Scholar]
- Takeda, T.; Seki, M.; Furusaki, S. Hydrodynamic Damage of Cultured Cells of Carthamus tinctorius in a Stirred Tank Reactor. J. Chem. Eng. Jpn. 1994, 27, 466–471. [Google Scholar] [CrossRef]
- Jolicoeur, M.; Chavarie, C.; Carreau, P.J.; Archambault, J. Development of a Helical-ribbon Impeller Bioreactor for High-density Plant Cell Suspension Culture. Biotechnol. Bioeng. 1992, 39, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Nienow, A.W. On Impeller Circulation and Mixing Effectiveness in the Turbulent Flow Regime. Chem. Eng. Sci. 1997, 52, 2557–2565. [Google Scholar] [CrossRef]
- Leckie, F.; Scragg, A.H.; Cliffe, K.C. The Effect of Continuous High Shear Stress on Plant Cell Suspension Cultures. In Progress in Plant Cellular and Molecular Biology. Current Plant Science and Biotechnology in Agriculture; Nijkamp, H.J.J., Van Der Plas, L.H.W., Van Aartrijk, J., Eds.; Springer: Dordrecht, The Netherlands, 1990; Volume 9, pp. 689–693. [Google Scholar]
- Wilson, S.A.; Roberts, S.C. Recent Advances towards Development and Commercialization of Plant Cell Culture Processes for the Synthesis of Biomolecules. Plant Biotechnol. J. 2012, 10, 249–268. [Google Scholar] [CrossRef]
- Pavlov, A.I.; Georgiev, M.I.; Ilieva, M.P. Production of Rosmarinic Acid by Lavandula vera MM Cell Suspension in Bioreactor: Effect of Dissolved Oxygen Concentration and Agitation. World J. Microbiol. Biotechnol. 2005, 21, 389–392. [Google Scholar] [CrossRef]
- Zhong, J.; Fujiyama, K.; Seki, T.; Yoshida, T. A Quantitative Analysis of Shear Effects on Cell Suspension and Cell Culture of Perilla frutescens in Bioreactors. Biotechnol. Bioeng. 1994, 44, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.-J. Biochemical Engineering of the Production of Plant-Specific Secondary Metabolites by Cell Suspension Cultures. In Advances in Biochemical Engineering/Biotechnology; Zhong, J.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 72, pp. 1–26. [Google Scholar]
- Chattopadhyay, S.; Srivastava, A.K.; Bisaria, V.S. Production of Phytochemicals in Plant Cell Bioreactors. In Plant Biotechnology and Molecular Markers; Srivastava, P., Narula, A., Srivastava, S., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 117–128. [Google Scholar]
- Hohe, A.; Winkelmann, T.; Schwenkel, H.G. The Effect of Oxygen Partial Pressure in Bioreactors on Cell Proliferation and Subsequent Differentiation of Somatic Embryos of Cyclamen persicum. Plant Cell Tissue Organ Cult. 1999, 59, 39–45. [Google Scholar] [CrossRef]
- Trung Thanh, N.; Niranjana Murthy, H.; Yu, K.-W.; Seung Jeong, C.; Hahn, E.-J.; Paek, K.-Y. Effect of Oxygen Supply on Cell Growth and Saponin Production in Bioreactor Cultures of Panax ginseng. J. Plant Physiol. 2006, 163, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Amthor, J. The McCree–de Wit–Penning de Vries–Thornley Respiration Paradigms: 30 Years Later. Ann. Bot. 2000, 86, 1–20. [Google Scholar] [CrossRef]
- McDonald, A.E.; Sieger, S.M.; Vanlerberghe, G.C. Methods and Approaches to Study Plant Mitochondrial Alternative Oxidase. Physiol. Plant. 2002, 116, 135–143. [Google Scholar] [CrossRef]
- Del-Saz, N.F.; Ribas-Carbo, M.; Martorell, G.; Fernie, A.R.; Florez-Sarasa, I. Measurements of Electron Partitioning between Cytochrome and Alternative Oxidase Pathways in Plant Tissues. In Plant Respiration and Internal Oxygen. Methods in Molecular Biology; Jagadis Gupta, K., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1670, pp. 203–217. [Google Scholar]
- Schertl, P.; Braun, H.-P. Respiratory Electron Transfer Pathways in Plant Mitochondria. Front. Plant Sci. 2014, 5, 163. [Google Scholar] [CrossRef]
- Moore, A.L.; Albury, M.S.; Crichton, P.G.; Affourtit, C. Function of the Alternative Oxidase: Is It Still a Scavenger? Trends Plant Sci. 2002, 7, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.L.; Arrabaça, J.D.; Vaz-Pinto, V.; Lima-Costa, M.E. Induction of Alternative Oxidase Chain under Salt Stress Conditions. Biol. Plant. 2008, 52, 66–71. [Google Scholar] [CrossRef]
- Noguchi, K.; Taylor, N.L.; Millar, A.H.; Lambers, H.; Day, D.A. Response of Mitochondria to Light Intensity in the Leaves of Sun and Shade Species. Plant. Cell Environ. 2005, 28, 760–771. [Google Scholar] [CrossRef]
- Clifton, R.; Lister, R.; Parker, K.L.; Sappl, P.G.; Elhafez, D.; Millar, A.H.; Day, D.A.; Whelan, J. Stress-Induced Co-Expression of Alternative Respiratory Chain Components in Arabidopsis thaliana. Plant Mol. Biol. 2005, 58, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Soole, K.L.; Wiskich, J.T. Regulation of Respiration in Rotenone-Treated Tobacco Cell Suspension Cultures. Planta 2001, 212, 765–773. [Google Scholar] [CrossRef]
- Duque, P.; Arrabaça, J.D. Respiratory Metabolism during Cold Storage of Apple Fruit. II. Alternative Oxidase Is Induced at the Climacteric. Physiol. Plant. 1999, 107, 24–31. [Google Scholar] [CrossRef]
- Sieger, S.M.; Kristensen, B.K.; Robson, C.A.; Amirsadeghi, S.; Eng, E.W.Y.; Abdel-Mesih, A.; Møller, I.M.; Vanlerberghe, G.C. The Role of Alternative Oxidase in Modulating Carbon Use Efficiency and Growth during Macronutrient Stress in Tobacco Cells. J. Exp. Bot. 2005, 56, 1499–1515. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, W.-Y.; Man, S.; Zuo, B.; Wang, J.; Huang, L.; Xiao, P. Effect of Bioreactor Angle and Aeration Rate on Growth and Hydromechanics Parameters in Bioreactor Culture of Ginseng Suspension Cells. Acta Physiol. Plant. 2013, 35, 1497–1501. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Fukui, H.; Tabata, M. Effect of Oxygen Supply on Berberine Production in Cell Suspension Cultures and Immobilized Cells of Thalictrum minus. Plant Cell Rep. 1989, 8, 255–258. [Google Scholar] [CrossRef]
- Gao, J.; Lee, J.M. Effect of Oxygen Supply on the Suspension Culture of Genetically Modified Tobacco Cells. Biotechnol. Prog. 1992, 8, 285–290. [Google Scholar] [CrossRef]
- Leckie, F.; Scragg, A.H.; Cliffe, K.C. An Investigation into the Role of Initial K L a on the Growth and Alkaloid Accumulation by Cultures of Catharanthus roseus. Biotechnol. Bioeng. 1991, 37, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Çalik, P.; Yilgör, P.; Ayhan, P.; Demir, A.S. Oxygen Transfer Effects on Recombinant Benzaldehyde Lyase Production. Chem. Eng. Sci. 2004, 59, 5075–5083. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Theoretical Prediction of Gas–Liquid Mass Transfer Coefficient, Specific Area and Hold-up in Sparged Stirred Tanks. Chem. Eng. Sci. 2004, 59, 2489–2501. [Google Scholar] [CrossRef]
- Raval, K.N.; Hellwig, S.; Prakash, G.; Ramos-Plasencia, A.; Srivastava, A.; Buchs, J. Necessity of a Two-Stage Process for the Production of Azadirachtin-Related Limonoids in Suspension Cultures of Azadirachta indica. J. Biosci. Bioeng. 2003, 96, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Rechmann, H.; Friedrich, A.; Forouzan, D.; Barth, S.; Schnabl, H.; Biselli, M.; Boehm, R. Characterization of Photosynthetically Active Duckweed (Wolffia australiana) in Vitro Culture by Respiration Activity Monitoring System (RAMOS). Biotechnol. Lett. 2007, 29, 971–977. [Google Scholar] [CrossRef]
- Klöckner, W.; Gacem, R.; Anderlei, T.; Raven, N.; Schillberg, S.; Lattermann, C.; Büchs, J. Correlation between Mass Transfer Coefficient KLa and Relevant Operating Parameters in Cylindrical Disposable Shaken Bioreactors on a Bench-to-Pilot Scale. J. Biol. Eng. 2013, 7, 28. [Google Scholar] [CrossRef]
- García-Salas, S.; Gómez-Montes, E.O.; Ramírez-Sotelo, M.G.; Oliver-Salvador, M. del C. Shear Rate as Scale-up Criterion of the Protein Production with Enhanced Proteolytic Activity by Phosphate Addition in the Jacaratia mexicana Cell Culture. Biotechnol. Biotechnol. Equip. 2021, 35, 1031–1042. [Google Scholar] [CrossRef]
- Votruba, J.; Sobotka, M. Physiological Similarity and Bioreactor Scale-Up. Folia Microbiol. 1992, 37, 331–345. [Google Scholar] [CrossRef]
- Xia, J.; Wang, G.; Fan, M.; Chen, M.; Wang, Z.; Zhuang, Y. Understanding the Scale-up of Fermentation Processes from the Viewpoint of the Flow Field in Bioreactors and the Physiological Response of Strains. Chin. J. Chem. Eng. 2021, 30, 178–184. [Google Scholar] [CrossRef]
- Xia, J.; Wang, G.; Lin, J.; Wang, Y.; Chu, J.; Zhuang, Y.; Zhang, S. Advances and Practices of Bioprocess Scale-Up. In Bioreactor Engineering Research and Industrial Applications II; Springer: Berlin/Heidelberg, Germany, 2015; pp. 137–151. [Google Scholar]
- Lee, S.; Kim, D. Effects of Pluronic F-68 on Cell Growth of Digitalis lanata in Aqueous Wo-Phase Systems. J. Microbiol. Biotechnol. Biotechnol. 2004, 14, 1129–1133. [Google Scholar]
- Zhong, J.-J.; Pan, Z.-W.; Wang, Z.-Y.; Wu, J.; Chen, F.; Takagi, M.; Yoshida, T. Effect of Mixing Time on Taxoid Production Using Suspension Cultures of Taxus chinensis in a Centrifugal Impeller Bioreactor. J. Biosci. Bioeng. 2002, 94, 244–250. [Google Scholar] [CrossRef]
- Pan, Z.-W.; Zhong, J.-J.; Wu, J.-Y.; Takagi, M.; Yoshida, T. Fluid Mixing and Oxygen Transfer in Cell Suspensions of Taxus chinensis in a Novel Stirred Bioreactor. Biotechnol. Bioprocess Eng. 1999, 4, 269–272. [Google Scholar] [CrossRef]
- Titova, M.V.; Shumilo, N.A.; Reshetnyak, O.V.; Glagoleva, E.S.; Nosov, A.M. Physiological Characteristics of Panax japonicus Suspension Cell Culture during Growth Scaling-Up. Biotekhnologiya 2015, 3, 71–80. [Google Scholar] [CrossRef]
- Titova, M.V.; Berkovich, E.A.; Reshetnyak, O.V.; Kulichenko, I.E.; Oreshnikov, A.V.; Nosov, A.M. Respiration Activity of Suspension Cell Culture of Polyscias filicifolia Bailey, Stephania glabra (Roxb.) Miers, and Dioscorea deltoidea Wall. Appl. Biochem. Microbiol. 2011, 47, 87–92. [Google Scholar] [CrossRef]
- Povydysh, M.N.; Titova, M.V.; Ivkin, D.Y.; Krasnova, M.V.; Vasilevskaya, E.R.; Fedulova, L.V.; Ivanov, I.M.; Klushin, A.G.; Popova, E.V.; Nosov, A.M. The Hypoglycemic and Hypocholesterolemic Activity of Dioscorea deltoidea, Tribulus terrestris and Panax japonicus Cell Culture Biomass in Rats with High-Fat Diet-Induced Obesity. Nutrients 2023, 15, 656. [Google Scholar] [CrossRef] [PubMed]
- Povydysh, M.N.; Titova, M.V.; Ivanov, I.M.; Klushin, A.G.; Kochkin, D.V.; Galishev, B.A.; Popova, E.V.; Ivkin, D.Y.; Luzhanin, V.G.; Krasnova, M.V.; et al. Effect of Phytopreparations Based on Bioreactor-Grown Cell Biomass of Dioscorea deltoidea, Tribulus terrestris and Panax japonicus on Carbohydrate and Lipid Metabolism in Type 2 Diabetes Mellitus. Nutrients 2021, 13, 3811. [Google Scholar] [CrossRef]
- Glagoleva, E.S.; Konstantinova, S.V.; Kochkin, D.V.; Ossipov, V.; Titova, M.V.; Popova, E.V.; Nosov, A.M.; Paek, K.-Y. Predominance of Oleanane-Type Ginsenoside R0 and Malonyl Esters of Protopanaxadiol-Type Ginsenosides in the 20-Year-Old Suspension Cell Culture of Panax japonicus C.A. Meyer. Ind. Crop. Prod. 2022, 177, 114417. [Google Scholar] [CrossRef]
- Kotin, A.M.; Bichevaya, N.K. Anti-Teratogenic Agent. WO1996002266A1, 11 May 1996. [Google Scholar]
- Kotin, O.A.; Kotin, A.M.; Emel’yanov, M.O. Use of a Preparation of the Plant Polyscias filicifolia for Treating Mineral Deficiencies 2022. WO2022010387A1, 13 January 2022. [Google Scholar]
- Werner, S.; Olownia, J.; Egger, D.; Eibl, D. An Approach for Scale-Up of Geometrically Dissimilar Orbitally Shaken Single-Use Bioreactors. Chem. Ing. Tech. 2013, 85, 118–126. [Google Scholar] [CrossRef]
- de Mello, A.F.M.; de Souza Vandenberghe, L.P.; Herrmann, L.W.; Letti, L.A.J.; Burgos, W.J.M.; Scapini, T.; Manzoki, M.C.; de Oliveira, P.Z.; Soccol, C.R. Strategies and Engineering Aspects on the Scale-up of Bioreactors for Different Bioprocesses. Syst. Microbiol. Biomanuf. 2023. [Google Scholar] [CrossRef]
- Seidel, S.; Mozaffari, F.; Maschke, R.W.; Kraume, M.; Eibl-Schindler, R.; Eibl, D. Automated Shape and Process Parameter Optimization for Scaling Up Geometrically Non-Similar Bioreactors. Processes 2023, 11, 2703. [Google Scholar] [CrossRef]
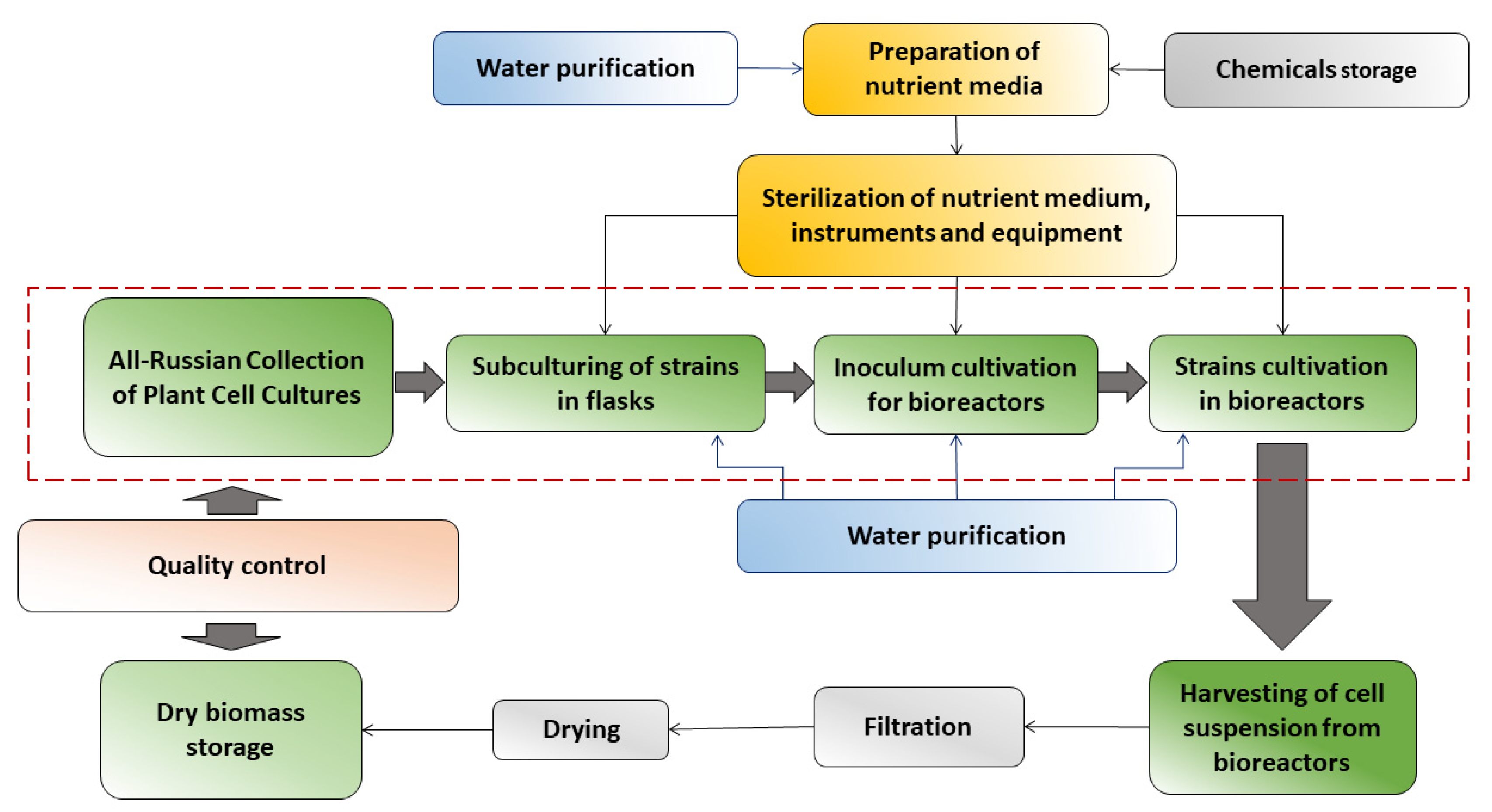
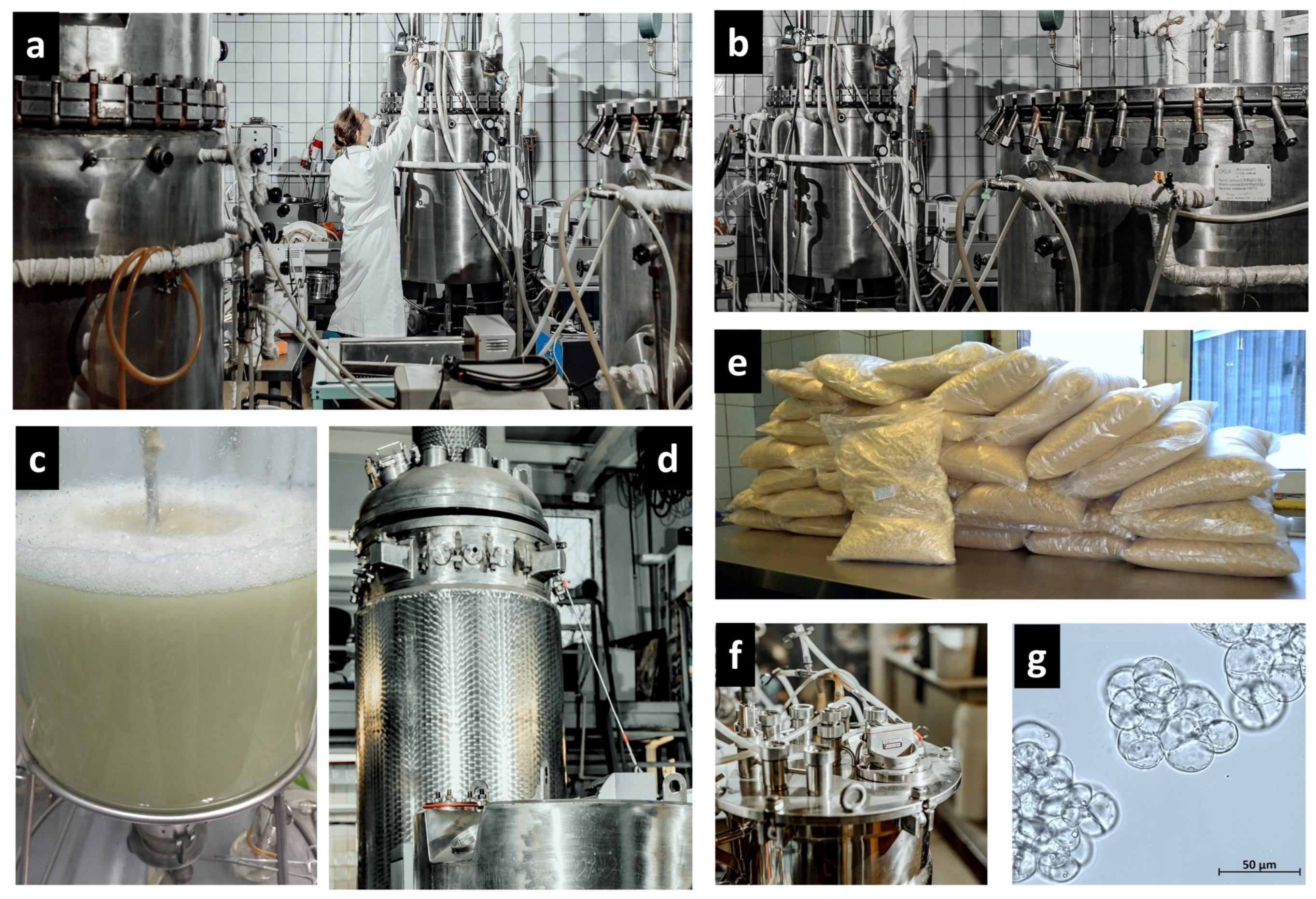

| No. | Bioreactor | Material | Total/Working Volume, L | Mixing | Sparger Type | Impeller Type | Manufacturer | Advantages (A), Disadvantages (D) | Exploitation Period |
|---|---|---|---|---|---|---|---|---|---|
| Laboratory (bench-top) bioreactors | |||||||||
| 1. | Bubble-type bioreactors | Glass | 1.5/1.0, 3.0/2.5 | Aeration | Single point sparger, ∅~2 mm * | n/a | IPPRAS, Moscow, Russia | A: Easy upscaling, simple construction, low cost D: Small volume, intense foaming, non-optimal mass transfer, application is limited to fine-aggregated, non-foaming cell lines | Until 2014 [84] |
| 2. | Glass | 10/7, 20/15 | Single point sparger, ∅~6 mm | Currently in use [86] | |||||
| 3. | Fermus-apparatus | Glass + stainless steel | 8/6 | Magnetic stirrer + aeration | Single point sparger, ∅~4 mm | Open turbine impeller | R&D Center “Bioavtomatika”, N. Novgorod, Russia | A: Highly efficient mass transfer D: Intense shear stress, foaming, non-optimal magnetic drive configuration, limited options for modification, higher chances for contamination due to construction specifics | Until 1995 [85] |
| 4. | AK-210 | Glass + stainless steel | 10/8 | Magnetic stirrer + aeration | Single point sparger, ∅~4 mm | Open turbine impeller | R&D Bureau, Pushchino, Russia | Until 1995 [85] | |
| 5. | MF-107 | Glass + stainless steel | 7/5 | Magnetic stirrer + aeration | Single point sparger, ∅~4 mm | Three-impeller stirrer (two open turbine impellers and one marine-type impeller) | New Brunswick, USA | Until 2000 [83] | |
| Pilot-scale bioreactors | |||||||||
| 6. | Tank bioreactor | Stainless steel | 75/50 | Magnetic stirrer for media sterilization, aeration for cell cultivation | Single point sparger, ∅~6 mm or ring-type gas distributor ∅~200 mm with multiple holes ∅~1 mm | Marine-type impeller | Electrolux, Sweden | A: Highly efficient mass transfer, suitable for viscous cell suspensions D: Intense shear stress, high energy cost due to mechanical agitation | Currently in use [87,88,89] |
| Industrial-scale bioreactors | |||||||||
| 7. | Tank bioreactor | Stainless steel | 630/550 | Magnetic stirrer for media sterilization, aeration for cell cultivation | Ring-type gas distributor ∅~750 mm with multiple holes ∅~1 mm | Marine-type impeller | 1T series, “EBEE” Research & Manufacturing facility, Yoshkar-Ola, Russia | A: Highly efficient mass transfer, suitable for viscous cell suspensions D: Intense shear stress, high energy cost due to mechanical agitation | Currently in use [21,87,89] |
| Suspension Cell Culture | Metabolites Produced | Biological and Pharmacological Activities | Reference |
|---|---|---|---|
| Dioscorea deltoidea, strain DM-05-03 | 25(S)- and 25(R)-deltoside isomers, 25(S)- and 25(R)-protodioscin isomers, dioscin | Bioreactor-produced cell biomass was assessed for elemental composition and toxicology, and it demonstrated positive effects in rats with induced type 2 diabetes mellitus and obesity | [21,124,171,172] |
| Panax japonicus, strain 62 | Ginsenosides: PPD: Rb1, Rc, Rb2/Rb3, Rd; PPT: Rg1, Re, Rf; OA: R0, chikusetsusaponin IVa; malonylated derivatives of ginsenosides | Bioreactor-produced cell biomass was assessed for elemental composition and toxicology and exhibited hypoglycemic and hypocholesterolemic activity in rats with diet-induced obesity | [87,169,173] |
| Polyscias filicifolia, strain BFT-01-95 | Triterpene glycosides of the oleanane type: PFS, ladyginoside A, polysciosides A–E | Bioreactor-produced cell biomass has documented adaptogenic and anti-teratogenic activities and is currently used in commercial food supplements | [89,174,175] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titova, M.; Popova, E.; Nosov, A. Bioreactor Systems for Plant Cell Cultivation at the Institute of Plant Physiology of the Russian Academy of Sciences: 50 Years of Technology Evolution from Laboratory to Industrial Implications. Plants 2024, 13, 430. https://doi.org/10.3390/plants13030430
Titova M, Popova E, Nosov A. Bioreactor Systems for Plant Cell Cultivation at the Institute of Plant Physiology of the Russian Academy of Sciences: 50 Years of Technology Evolution from Laboratory to Industrial Implications. Plants. 2024; 13(3):430. https://doi.org/10.3390/plants13030430
Chicago/Turabian StyleTitova, Maria, Elena Popova, and Alexander Nosov. 2024. "Bioreactor Systems for Plant Cell Cultivation at the Institute of Plant Physiology of the Russian Academy of Sciences: 50 Years of Technology Evolution from Laboratory to Industrial Implications" Plants 13, no. 3: 430. https://doi.org/10.3390/plants13030430
APA StyleTitova, M., Popova, E., & Nosov, A. (2024). Bioreactor Systems for Plant Cell Cultivation at the Institute of Plant Physiology of the Russian Academy of Sciences: 50 Years of Technology Evolution from Laboratory to Industrial Implications. Plants, 13(3), 430. https://doi.org/10.3390/plants13030430







