Abstract
The addition of biostimulants to nutrient solutions of hydroponically grown crops to speed up plant growth and improve plant yield and quality has been attracting more and more attention. This study investigated the effects of wood distillate (WD) addition to hydroponically grown lettuce (Lactuca sativa L.) plants. Two concentrations of WD, 0.2% and 0.5%, were added to the nutrient solution, and biometric (i.e., leaf fresh weight, root fresh weight, root length and root surface area), photosynthetic (i.e., chlorophyll a, chlorophyll b, and carotenoid content) and biochemical (i.e., electrolyte leakage, total polyphenols, total flavonoids, and total antioxidant power content) parameters were evaluated. The effects of WD were hormetic, as the 0.2% concentration stimulated biometric and biochemical parameters, while the 0.5% concentration inhibited plant growth. Based on these results, it can be suggested that the addition of 0.2% WD to the nutrient solution has a stimulating effect on the growth of lettuce plants, and could be a successful strategy to boost the yield of crops grown hydroponically.
1. Introduction
Hydroponics has gained attention for its numerous advantages over traditional soil-based farming methods. One of the primary benefits of hydroponics lies in its precise control over the delivery of nutrients [1], which are dissolved in water and delivered directly to the plant roots. This controlled approach ensures that plants receive exactly the nutrients they require, leading to faster growth and higher yields [2] compared to their soil-grown counterparts [3,4].
A further key advantage of hydroponics is the higher water efficiency [5] compared to the conventional farming, which often requires considerable water supply. These systems are designed to be highly water efficient [5], with recirculation of water and nutrients, reducing overall consumption and waste and making hydroponics an eco-friendly solution.
Hydroponics also stands out for its space efficiency. These systems can be installed in small areas, making them ideal for urban agriculture and regions with limited land [6,7]. Vertical hydroponic systems maximize space utilization, allowing a variety of crops to be grown in defined spaces [8].
These systems are also crucial given that the estimated loss of cultivated land in Europe is approximately 280,000 ha per year [9]. This means that by 2030, the EU could lose another 4.2 million ha of cultivated land, bringing the total of abandoned areas to 5.6 million ha. Furthermore, the Food and Agriculture Organization of the United Nations (FAO) estimated that by 2050, agricultural production will have to increase by 60% to adequately meet the food needs of the world’s population, which is why the use of vertical hydroponic systems can be a very promising solution [10].
Hydroponic systems minimize the risk of pests and diseases that commonly affect soil-based plants [11], leading to healthier plants and potentially decreasing the need for pesticides, contributing to more sustainable and chemical-free agriculture. Finally, hydroponic systems allow efficient resource utilization and consistent year-round production [12,13].
To date, many different nutrient solutions have been proposed for hydroponic systems, most of them containing only inorganic ions from soluble salts of elements essential for higher plants. However, there is a growing interest towards the addition of biostimulants to the hydroponic solution, aiming at increasing crop yield and quality. Although the combination of hydroponics and biostimulants is considered as a promising eco-friendly production strategy for vegetables grown in greenhouses [14], studies regarding the direct addition of biostimulants to hydroponic solutions are still limited, and their effects on crop production still need verification.
The utilization of various natural, chemical, and physical agents to improve the quality of crops is a widely recognized and prevalent practice in the field of horticulture [15], though its impact on plants is also affected by genotype and environment [16]. Wood distillate (WD), often referred to as pyroligneous acid or wood vinegar, is one of the most interesting biostimulants available on the market. Recently, it has gained attention for its use on crops via foliar application or fertirrigation [17,18]. Wood distillate has potential applications also in hydroponics, but only few studies investigated its effects, particularly on the photosynthetic parameters of crops [19,20].
One of the primary areas of interest in the use of WD in hydroponics is its capacity to enhance nutrient uptake by plant roots [21]. This is attributed to the large presence of organic compounds within WD, such as acetic acid and various phenolic compounds [22]. These substances have been shown to potentially improve the absorption of essential nutrients by plants, leading to increased yields and quality of crops [23,24]. The precise mechanisms through which WD influences nutrient uptake are still under investigation, but early findings suggest that WD works as an eustressor, activating various defense mechanisms and physiological adaptations to cope with the stress, ultimately leading to improved resilience and growth [25]. Furthermore, WD possesses the ability to stimulate root development, induce flowering and strengthen plant resilience in the face of various environmental stressors [26,27,28]. However, it is crucial to emphasize that the effects of WD on plants are hormetic, i.e., they depend on the dosage and are also species-specific [29,30] and that excessive concentrations of WD can potentially be detrimental to plants due to its inherent acidity [31]. Consequently, it is crucial to determine the appropriate concentration of WD that maximizes its beneficial effects while avoiding any negative impact on plant growth.
Therefore, the aim of this work was to test the effects of the addition of two different concentration of WD [0.2% (v/v) and 0.5% (v/v)] to the nutrient solutions of an hydroponic system on lettuce growth by evaluating both biometric (leaf fresh weight, root fresh weight, root length, root surface area), photosynthetic (chlorophyll a, chlorophyll b, and carotenoid content), and biochemical (electrolyte leakage, total polyphenols, total flavonoids, and total antiradical activity content) parameters.
2. Results
The PERMANOVA analysis showed a significant effect of ‘time’, ‘treatment’, and ‘time per treatment’ on the parameters leaf fresh weight, root fresh weight, root length, root surface area, chlorophyll a, TPC, TFC, and ARA content. For the parameter chlorophyll b, the analysis showed significance of ‘time’ and ‘treatment’, while for the parameters electrolyte leakage and carotenoids it only showed significance for ‘time’ (Table 1).

Table 1.
Results of the PERMANOVA analyses on leaf fresh weight, root fresh weight, root length, root area, electrolyte leakage (EL), chlorophyll a, chlorophyll b, carotenoids, leaf total polyphenol content (TPC), root TPC, leaf total flavonoid content (TFC), root TFC, leaf antiradical activity content (ARA), and root ARA.
2.1. Temporal Trends
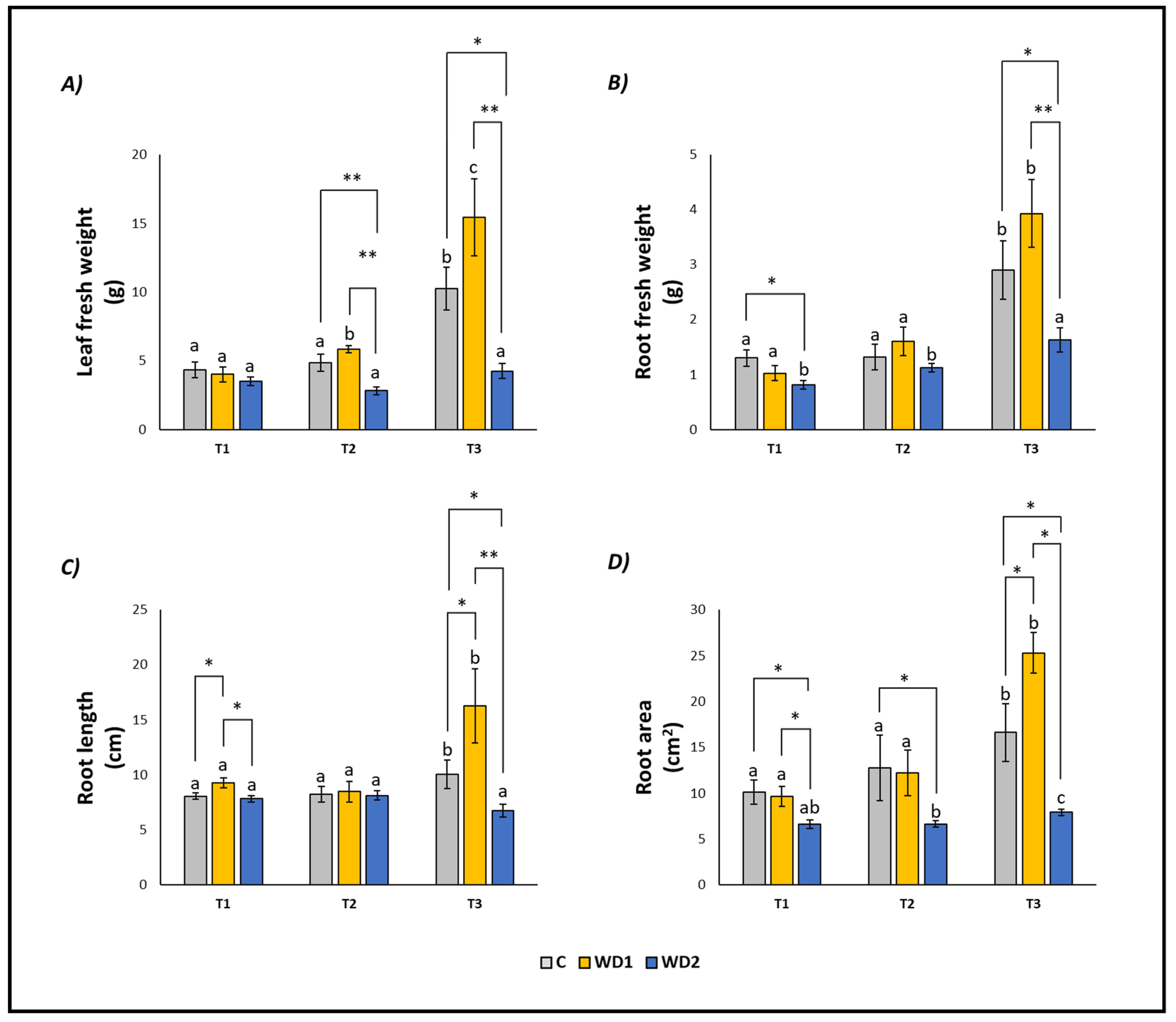
Leaf fresh weight, root fresh weight, root length, and root area showed similar trends (Figure 1). As expected, all the parameters in control samples (C) increased over time, following the growth of the plants, and showing significant differences at T3 (Figure 1). The same was observed in WD1 plants, with only leaf fresh weight increasing also at T2 (Figure 1). WD2 plants showed a different trend, with all parameters remaining constant (leaf fresh weight and root length) or slightly increasing at T3 (root fresh weight and root area) (Figure 1).
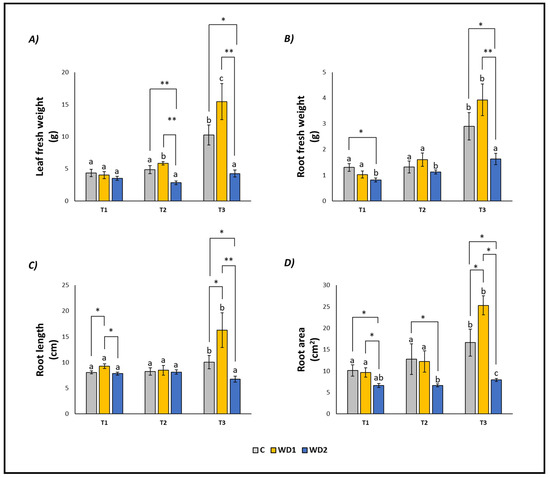
Figure 1.
Leaf fresh weight (A), root fresh weight (B), root length (C), root area (D) of lettuce plants. C = 0% wood distillate; WD1 = 0.2% wood distillate; WD2 = 0.5% wood distillate. T1 = first harvest (three days after the start of the experiment); T2 = second harvest (nine days after the start of the experiment); T3 = third harvest (eighteen days after the start of the experiment). Different letters indicate statistically significant differences in the same treatment at different times. Asterisk (*) = indicates statistically significant differences in treatments at the same time. * = p < 0.05; ** = p < 0.01.
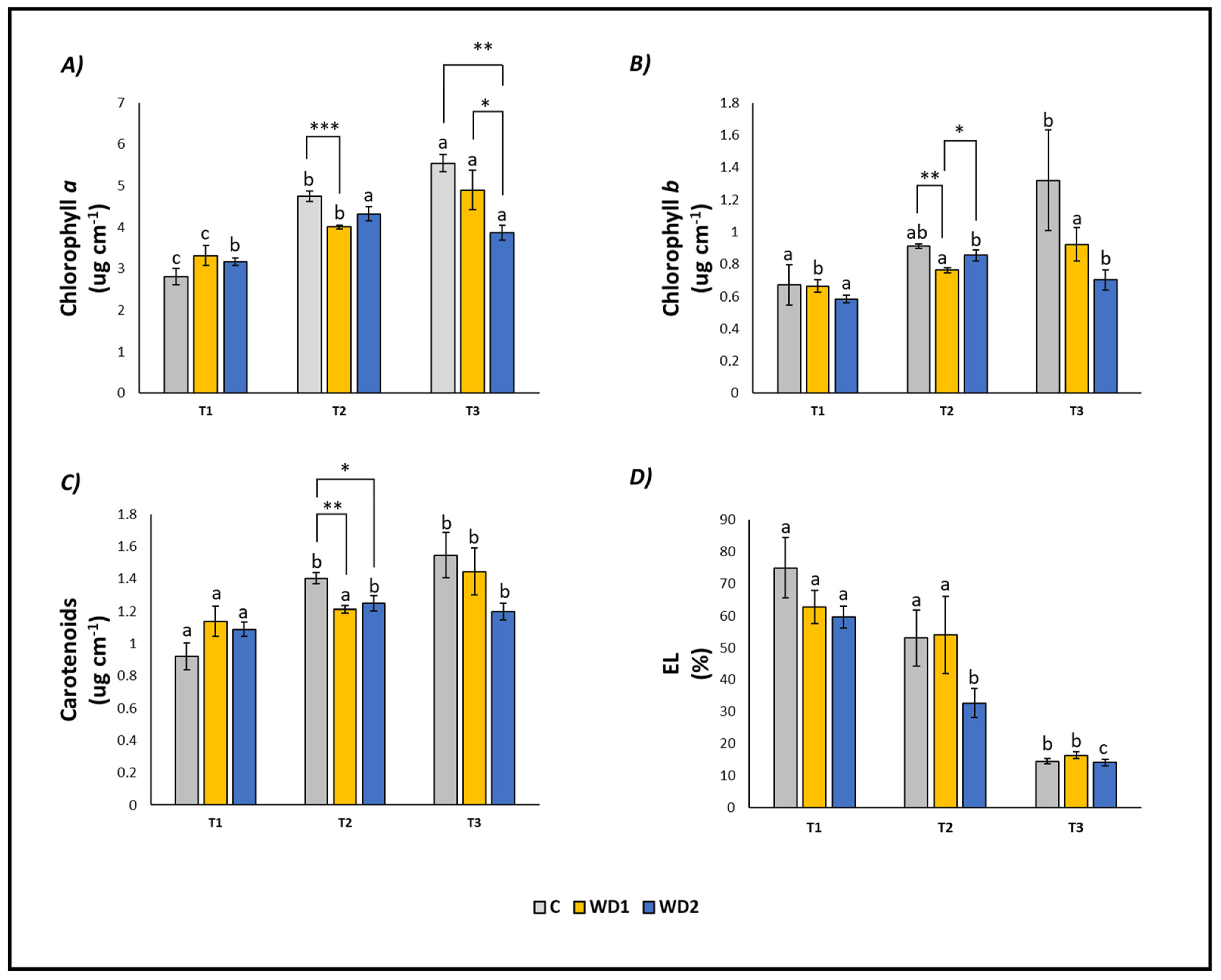
The content of chlorophyll a (T1 < T2 < T3), chlorophyll b (T1 = T2 < T3) and carotenoids (T1 < T2 = T3) increased over time in C plants. Conversely, EL decreased significantly at T3 compared to T1 and T2 (T1 = T2 > T3; Figure 2). Plants treated with WD1 showed a similar trend, with a significant progressive increase in time of chlorophyll a (T1 < T2 < T3), chlorophyll b (T1 < T2 = T3) and carotenoids (T1 = T2 < T3), and a decrease in EL (T1 = T2 > T3; Figure 2). In plants treated with WD2, chlorophyll a, chlorophyll b, and carotenoids showed a significant increase at T2 and T3 compared to T1 (T1 < T2 = T3), while EL showed a significant decrease at all three investigated times (T1 > T2 > T3) (Figure 2).
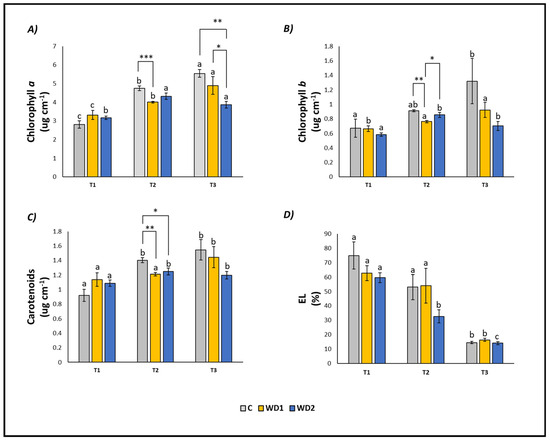
Figure 2.
Chlorophyll a content (A), chlorophyll b content (B), carotenoid content (C), and electrolyte leakage (EL) (D) of lettuce plants. C = 0% wood distillate; WD1 = 0.2% wood distillate; WD2 = 0.5% wood distillate. T1 = first harvest (three days after the start of the experiment); T2 = second harvest (nine days after the start of the experiment); T3 = third harvest (eighteen days after the start of the experiment). Different letters indicate statistically significant differences in the same treatment at different times. Asterisk (*) = indicates statistically significant differences in treatments at the same time. * = p < 0.05; ** = p < 0.01; *** = p < 0.001.
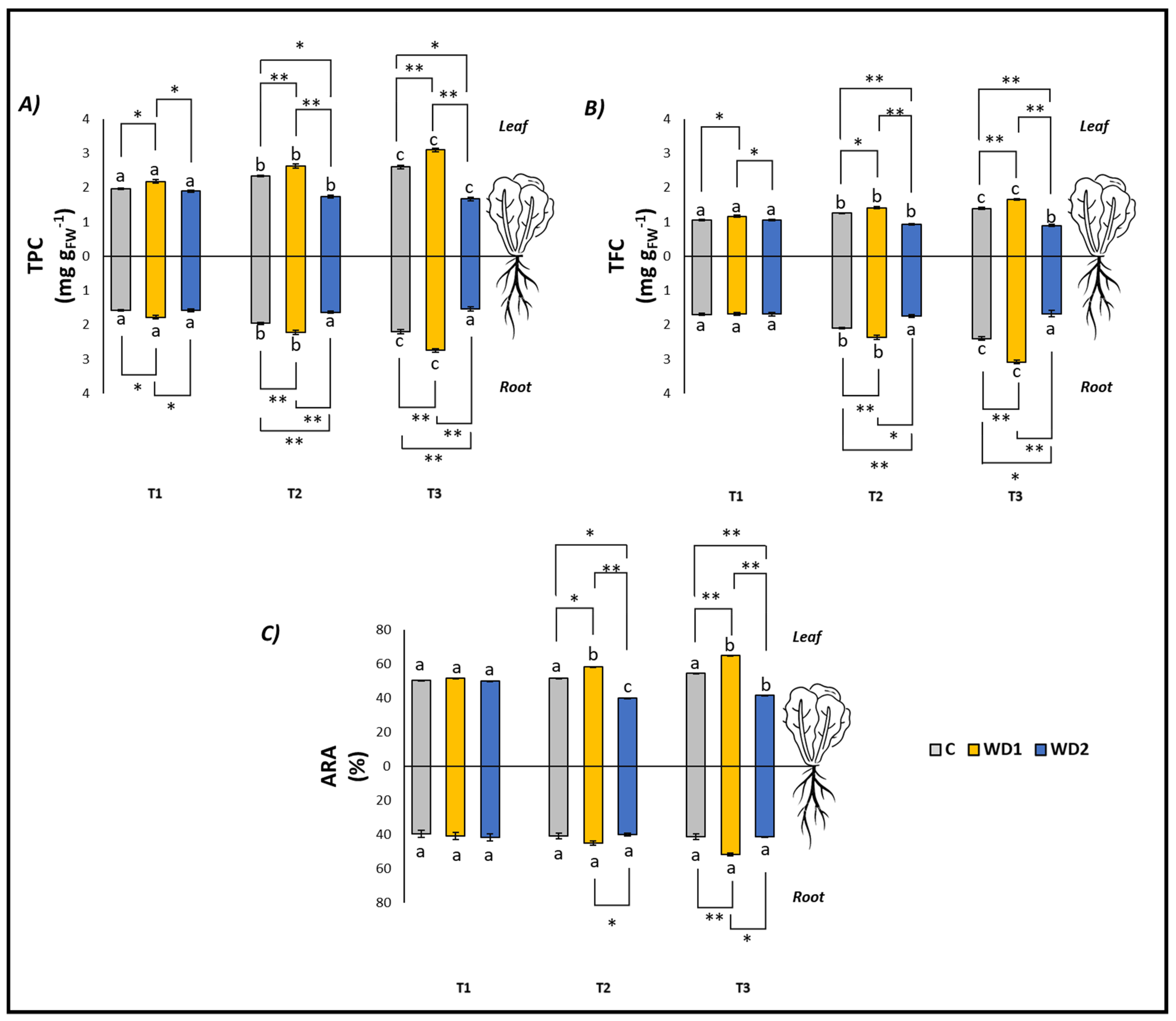
Regarding the antioxidant compounds (TPC and TFC, both in leaves and roots), C plants showed a significant increase at all three investigated times (T1 < T2 < T3), while no difference was found in the ARA content in both leaves and roots (T1 = T2 = T3) (Figure 3). Plants treated with WD1 also showed a significant increase in TPC and TFC (both leaves and roots) at all three investigated times (T1 < T2 < T3). ARA content in the leaves was significantly higher at T2 and T3 with respect to T1 (T1 < T2 = T3), while no differences were found in ARA content in roots over time (T1 = T2 = T3) (Figure 3). In plants treated with WD2, the leaf content of TPC and ARA showed a significant decrease in time (T1 > T2 > T3), the leaf content of TPC showed a significant progressive decrease with time (T1 < T2 < T3), and differences were found for the root content of TPC, TFC, and ARA (T1 = T2 = T3) (Figure 3).
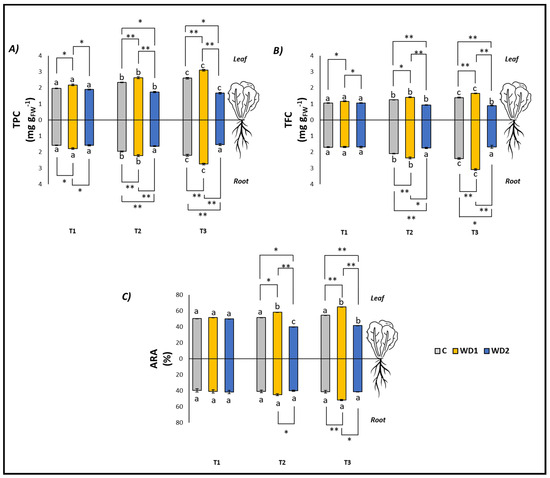
Figure 3.
Total polyphenol content (TPC) (A), total flavonoid content (TFC) (B), antiradical activity (ARA) (C) of lettuce plants. C = 0% wood distillate; WD1 = 0.2% wood distillate; WD2 = 0.5% wood distillate. T1 = first harvest (three days after the start of the experiment); T2 = second harvest (nine days after the start of the experiment); T3 = third harvest (eighteen days after the start of the experiment). Different letters indicate statistically significant differences in the same treatment at different times. Asterisk (*) = indicates statistically significant differences in treatments at the same time. * = p < 0.05; ** = p < 0.01.
2.2. Effects of WD
At T1, plants did not show any statistically significant difference in leaf fresh weight (T1 = T2 = T3); root fresh weight showed a significant difference between C and WD2 (C = WD1 < WD2); root length was significantly higher for WD1 compared to C and WD2 (C = WD2 < WD1); root area was significantly higher in C and WD1 compared to WD2 (C = WD1 < WD2; Figure 1). No significant difference was found for chlorophyll a, chlorophyll b, carotenoid, and EL (C = WD1 = WD2) (Figure 2). Regarding the antioxidant compounds, TPC (leaves and roots) and TFC (leaves) showed significantly higher values for WD1 than for C and WD2 (C = WD1 > WD2); a difference did not emerge for the content of TFC (roots) and ARA (leaves and roots) (C = WD1 = WD2) (Figure 3).
At T2, plants showed statistically significant differences in leaf fresh weight between C and WD2 and WD1 and WD2; significant differences were not found in root fresh weight, root length, and root area (C = WD1 = WD2) (Figure 1). As for the content of chlorophyll a, chlorophyll b, and carotenoids, a statistically significant difference was found between C and WD1; furthermore, for chlorophyll b, a statistically significant difference was found between WD1 and WD2, and for carotenoids between C and WD2; no difference was found for EL between treatments (C = WD1 = WD2) (Figure 2). Regarding the antioxidant compounds, TPC and TFC (leaves and roots) and ARA (leaves), statistically significant differences were found between all treatments with consistently higher values of WD1 (WD1 > C > WD2) (Figure 3).
At T3, plants showed statistically significant differences in leaf fresh weight and root fresh weight between C and WD2 and WD1 and WD2; root length and root area showed statistically significant differences between all treatments with higher values found in WD1 (WD1 > C > WD2) (Figure 1). Regarding the chlorophyll a content, statistically significant differences were found between C and WD2 and WD1 and WD2; differences were not found in the content of chlorophyll b, carotenoids and EL (C = WD1 = WD2; Figure 2). Regarding the antioxidant compounds, TPC and TFC (leaves and roots) and ARA (leaves) showed statistically significant differences among all treatments, with higher values found in WD1 (WD1 > C > WD2); on the other hand, ARA content in roots showed statistically significant differences between C and WD1 and WD1 and WD2 (Figure 3).
3. Discussion
Our findings provide insights into the dose-dependent effects of wood distillate (WD) on crops grown hydroponically. Only a few studies investigated the addition of WD directly to the nutrient solution of hydroponic systems, and the results obtained are contrasting. We observed a hormetic effect: the concentration of 0.2% WD led to a general improvement of plant growth, while at a concentration of 0.5%, WD had adverse effects on plant development. It is important to note that the biostimulant properties of WD on crops have been widely reported in the literature, typically falling within the range 0.2–0.5% for both foliar applications and fertigation [32,33]. The use of biostimulants in hydroponic systems has a boosting effect not only on plant growth, but also on crop yield and quality [34,35,36]. In our case, it is evident that WD proved to be an excellent solution for increasing the fresh weight of plants, but also the development and growth of the roots. Specifically, the root system plays a fundamental role in the growth of plants in hydroponics, which is even more important than in traditional soil growing [37]. Our results clearly show that the addition of WD to the nutrient solution showed a hormetic effect depending on the concentration used. The plants grown with WD2 showed the first negative symptoms already at T1, which became increasingly evident at T3 (18 days later). On the other hand, the results for the treatment with WD1 are completely opposed, resulting at T3 in a general improvement of all root-related parameters. Consistently with our results, Chen et al. [19] investigated the effects of WD addition in hydroponic solution on the growth of lettuce plants, founding no statistically significant difference between control plants and plants grown in the presence of 0.25% WD in terms of fresh root weight, whereas higher concentrations (0.5% and 1%) showed a statistically significant reduction in this parameter. This effect of WD on plants is commonly attributed to the different organic and inorganic components, including polyphenols, tannins, and alcohols [22]. These compounds have the remarkable capacity to stimulate plant growth by inducing a specific form of stress, known as eustress. This eustress, in turn, activates a variety of antioxidant compounds, which are crucial for the development of primary and secondary metabolic processes within the plant [38].
Antioxidant compounds play a key role in the development and overall health of plants [39]. A primary function is the protection against oxidative stress, as they neutralize harmful reactive oxygen species, safeguarding plant cells and promoting their vitality [40], also boosting the plant’s defense mechanisms. Thus, when exposed to stressors, pathogens or pests, plants often produce more antioxidants as part of their response, contributing to their overall health and resilience [40]. In addition, antioxidant compounds influence various aspects of plant growth and development by inhibiting the development of pathogens and protecting plants from disease [41]. Moreover, they are also involved in secondary metabolism, influencing the production of pigments, alkaloids and other bioactive molecules that have a major impact on plant-environment interactions [42]. They contribute to root development, promoting plant stability and aiding nutrient uptake by protecting root cells from oxidative damage [39]. The increase in the root system growth and antioxidant compound content can enhance yield and crop quality. Increased uptake of nutrients and water, as well as increased resistance to stress, can result in healthier plants [43]. Our results show that there is a positive correlation between biometric parameters and the content of antioxidant compounds (i.e., polyphenols, flavonoids, and total antioxidant power) in the plant. Indeed, the biometric parameters and antioxidant pool are statistically higher in plants treated with WD1 than in control plants, but even more so in plants treated with WD2, where the worst performance has been observed.
An increase in total polyphenol content (TPC) and total flavonoid content (TFC) has been demonstrated to be directly connected with the augmentation in antioxidant activity [44,45]. Flavonoids and polyphenols, which are often present in bio-based products, are physiologically active substances having antioxidant and anti-inflammatory properties [46]. When compared to C and WD2 plants, WD1 plants had an overall rise in both TPC and TFC in roots and leaves. To the best of our knowledge, this is the first report regarding the impact of WD on these two parameters in hydroponically grown plants. The effects of WD on antioxidant component level are limited to plants cultivated in soil. The application of WD at concentrations of 0.2% and 0.4% to tomato plants increased fruit TPC level, while only 0.4% WD increased ARA [47]; however, no increase in TPC was found in the fruits of strawberry (Fragaria ananassa), except for chlorogenic acid, levels of which doubled compared to the control [48].
Concerning the temporal trends observed, there were notable differences overall for all the parameters examined across the various treatments. Under normal growth conditions, plants exhibit growth over time, leading to an increase in biometric parameters (i.e., fresh leaf weight, root fresh weight, length, and area), and biochemical parameters, including the antioxidant compounds in our case. Our findings indicate that the two concentrations of WD demonstrated distinct temporal responses. Specifically, the addition of 0.2% (v/v) WD resulted in a general increase in the different parameters compared to the C plants. Conversely, the addition of 0.5% (v/v) WD led to a general reduction in the different parameters compared to both the C and WD1 plants. Therefore, it is plausible to characterize the hormetic effect of WD based on concentration, presumably attributable to the chemical composition of WD exerting a dose-dependent influence on plant growth [29].
4. Materials and Methods
4.1. Experimental Protocol and Growing Conditions
The research was carried out at the Faculdade de Ciências, of the University of Lisboa, on lettuce (Lactuca sativa L.), in February 2023. The experimental protocol was based on the application of the biostimulant wood distillate at two levels [0.2% (v/v) and 0.5% (v/v)] plus an untreated control (C), and the time of harvest for analytical determination (T1 = 3 days, T2 = 9 days, T3 = 18 days), using an experimental design with 10 replicates × treatment × time. Ninety lettuce seedlings were purchased from a local nursery (Horta do Campo Grande, Lisbon, Portugal). In the laboratory, after washing the roots with tap water, each seedling was placed inside a plastic basket filled with sterilized clay and placed in 15 rectangular plastic containers (22 × 8 × 33 cm) with a volumetric capacity of 8 L filled with a modified Hoagland solution (Table 2) following Cruz et al. [49]. Five of the fifteen containers were filled only with the nutrient solution, while two different treatments with wood distillate were applied in the others: WD was added to a final concentration of 0.2% (v/v) to 5 containers (WD1) and to a final concentration of 0.5% (v/v) to the remaining 5 containers (WD2). The experiment was carried out inside a climatic chamber at a temperature of 20 ± 1 °C, relative humidity (RH) of 70 ± 2% and photosynthetic photon flux density (PPFD) of 300 µmol m−2 s−1 with a 16/8 h day/night circle. At three different times (T1 = 3 days, T2 = 9 days, T3 = 18 days), ten seedlings (statistical replicates) for each treatment were removed from the hydroponic system and once brought to the laboratory were either analyzed or stored for later analyses.

Table 2.
Chemical composition of the base nutrient solution.
Wood Distillate Characteristics
Wood pyrolysis results in two primary residual components: a solid fraction named biochar and WD, a liquid fraction [17]. To maximize the production of WD in comparison to biochar, the most efficient method involves fast pyrolysis carried out within the temperature range 350–500 °C. This process includes rapidly heating woody biomass followed by quick cooling. Consequently, approximately 60–75% of the resulting product constitutes the liquid phase, while the solid component comprises 15–25%, and the gaseous component contributes 10–20% to the overall product. The composition of WD primarily consists of 80–90% water, along with >300 water-soluble organic compounds, including organic acids, alkanes, phenolics, alcohols, and esters [22,50]. It is important to clarify that each WD is different being dependent on the feedstock used and the operating parameters employed during the pyrolytic process, resulting in a final product with different chemical characteristics. The WD used in this experiment (Distillato di Legno, produced by BioDea® (BioDea, Arezzo, Italy) [51]) was derived from the pyrolysis of sweet chestnut (Castanea sativa Mill.) wood sourced from residual materials from forest management and has already been extensively studied for the cultivation of crops in soil [23,25] with important results in terms of yield and nutritional quality.
4.2. Plant Analysis
4.2.1. Biometric Parameters
Both the leaves and the roots were initially weighed and subsequently promptly frozen at −80 °C. Photos of the roots of each sample were taken with a digital camera against a black background (Iphone 14, Apple Inc., Cupertino, CA, USA). Subsequently, the root length and the root area were assessed with the tools (i.e., Segmented line and Threeshold) available within the Fiji/ImageJ software (v. 1.54 h) calibrating the image scale to the relative pixels, equivalent to 1 mm.
4.2.2. Pigment Content
Foliar pigments were extracted from three leaf discs (Ø 2 mm) placed in 2 mL of pure methanol kept at +4 °C for 24 h in the dark. After incubation, the absorbance of 200 μL of the sample at 470 nm, 652.4 nm, 665.2 nm, and 700 nm were measured using a UV–Vis plate reader spectrophotometer. The pigment content was calculated using the equations by Lichtenthaler and Buschmann [52]:
4.2.3. Electrolyte Leakage
Electrolyte leakage from leaf tissue was assessed using the method described by Sunkar et al. [53]. Fresh, young lettuce leaves were carefully cleansed with distilled water (dH2O). Subsequently, uniform pieces of leaf tissue (Ø 5 mm) were taken and soaked in 20 mL of dH2O at room temperature for two hours. After this soaking period, the initial electrical conductivity (EC1) of the solutions was recorded using a conductivity-meter. Following this measurement, the solutions were subjected to a heating process at 90 °C for 25 min. Afterwards, the solutions were allowed to cool down to room temperature before a subsequent electrical conductivity measurement (EC2) was carried out. The electrical leakage of the samples was quantified as a percentage, and calculated using the formula:
4.2.4. Antioxidant Compounds
For the quantification of the total antiradical activity, 500 mg of freeze-dried lettuce leaves were subjected to homogenization in 2 mL of 80% (v/v) ethanol, followed by centrifugation at 15,000 rpm for 5 min. An aliquot of the supernatant (200 µL) was mixed with 1 mL of a solution containing 2,2-Diphenyl-1-picrylhydrazyl (DPPH), which had been previously prepared by dissolving 1.85 mg of the compound in 50 mL of 80% methanol (v/v). To facilitate a comparative analysis of the antioxidant potential, a blank and a control were prepared: the former consisting of 200 µL of 80% (v/v) ethanol in 1 mL of 80% (v/v) methanol, and the latter involving 200 µL of 80% (v/v) ethanol in 1 mL of the DPPH solution. After 1 h of dark incubation, the absorbance was read at 517 nm using a UV–Vis spectrophotometer (Agilent 8453, Santa Clara, CA, USA). Results were expressed as a percentage of antiradical activity (ARA%) using the formula [54]:
The total polyphenol content (TPC) was measured using the method proposed by Lamaro et al. [55]. Briefly, 500 mg of freeze-dried lettuce leaves were homogenized in 4 mL of 70% (v/v) acetone, followed by centrifugation at 4000 rpm for 5 min. The supernatant (0.5 mL) was mixed with 3 mL of dH2O, 0.125 mL of Folin-Denis’ reagent (Sigma-Aldrich, St. Louis, MO, USA), 0.750 mL of saturated Na2CO3, and finally 0.950 mL of dH2O. After a 30-min incubation at 37 °C, the samples were centrifuged again at 4000 rpm for 5 min, after which the supernatant was taken and finally the absorbance was measured at 765 nm using a UV–Vis spectrophotometer (Agilent 8453; Santa Clara, CA, USA). Quantification was made through referencing the absorbance of the samples to a calibration curve (ranging from 5 to 20 µg mL−1) made with gallic acid (Sigma-Aldrich) used as a standard. Results were expressed as milligrams of gallic acid equivalent per gram of dry weight (mgGAE g−1 dw).
The total flavonoid content (TFC) was determined following the method proposed by Heimler et al. [56]. In brief, 500 mg of freeze-dried lettuce leaves were homogenized in 2 mL of 80% ethanol, then centrifuged at 15,000 rpm for 5 min. The resulting supernatant (300 µL) was mixed with 45 µL of a 10% AlCl3 solution, 300 µL of a 1M NaOH solution, and 300 µL of dH2O. Samples were read at 510 nm using a UV–Vis spectrophotometer (Agilent 8453). Quantification was made through a calibration curve (ranging from 5 to 200 µg mL−1) made with quercetin (Sigma-Aldrich) as a standard, and the results were presented as milligrams of quercetin equivalent per gram of dry weight (mgQE g−1 dw).
4.3. Statistical Analysis
To test for significant effects of WD application, we carried out Permutational Uni- and Multivariate Analyses of Variance (PERMANOVA) on the responses of time and WD treatment, respectively. Univariate analyses were based on Euclidean distance matrices, while multivariate ones were based on Bray–Curtis dissimilarity matrices, calculated on untransformed data. The following settings were used for all tests: 999 unrestricted permutations of raw data, α = 0.05. Significant terms were then investigated using posterior pairwise comparisons with the PERMANOVA t statistic and 999 permutations to test for significant differences between the treatments. All the analyses were performed using the PERMANOVA routine in the program PRIMER v.6 [57], including the add-on package PERMANOVA+ [58]. PERMANOVA correctly calculates an appropriate pseudo-F statistic for each term in the model for uni- and multivariate datasets. Moreover, the permutation approach is free from many of the assumptions of parametric statistics [57].
5. Conclusions
This study suggests that the effect of wood distillate in plants grown in hydroponic systems is hormetic and thus depends on the specific dosage employed. While a too high concentration can have a detrimental impact on plant growth, causing partial inhibition, a proper dose can boost root and foliar development, providing an economically and environmentally sustainable replacement of traditional fertilizers. Given the pressing global need for sustainable solutions to address the escalating human population and its impact on food production, wood distillate represents a viable option to enhance both crop yield and quality in hydroponic cultivation.
Author Contributions
Conceptualization, R.F. and S.M.; methodology, R.F.; formal analysis, R.F. and S.M.; investigation, R.F. and S.M.; resources, C.C. and S.L.; data curation, R.F.; writing—original draft preparation, R.F.; writing—review and editing, R.F., C.C., S.L. and S.M.; supervision, C.C., S.L. and S.M.; funding acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work received support from the grant UIBD/00329/2020 for the Centre for Ecology, Evolution and Environmental Changes (cE3c) from FCT.
Data Availability Statement
Data are available on reasonable request from the corresponding author.
Acknowledgments
Thanks are due to Ana Correa and Juliana Melo for support in the laboratory and to Simona Maccherini for the help with the statistical analysis. We also thank Francesco Barbagli [BioDea (Arezzo, Italy) and BioEsperia (Arezzo, Italy)] for providing the wood distillate used for the experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sheikh, B.A. Hydroponics: Key to sustain agriculture in water stressed and urban environment. Pak. J. Agric. Agric. Eng. Vet. Sci. 2006, 22, 53–57. [Google Scholar]
- Hemathilake, D.M.K.S.; Gunathilake, D.M.C.C. High-productive agricultural technologies to fulfill future food demands: Hydroponics, aquaponics, and precision/smart agriculture. In Future Foods; Academic Press: Cambridge, MA, USA, 2022; pp. 555–567. [Google Scholar]
- Gashgari, R.; Alharbi, K.; Mughrbil, K.; Jan, A.; Glolam, A. Comparison between growing plants in hydroponic system and soil based system. In Proceedings of the 4th World Congress on Mechanical, Chemical, and Material Engineering, Madrid, Spain, 16–18 August 2018; Volume 18, pp. 1–7. [Google Scholar]
- Tocquin, P.; Corbesier, L.; Havelange, A.; Pieltain, A.; Kurtem, E.; Bernier, G.; Périlleux, C. A novel high efficiency, low maintenance, hydroponic system for synchronous growth and flowering of Arabidopsis thaliana. BMC Plant Biol. 2003, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364–371. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Prac. Pol. 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Romeo, D.; Vea, E.B.; Thomsen, M. Environmental Impacts of Urban Hydroponics in Europe: A Case Study in Lyon. Proc. CIRP 2018, 69, 540–545. [Google Scholar] [CrossRef]
- Despommier, D. The Rise of Vertical Farms. Sci. Am. 2009, 301, 80–87. [Google Scholar] [CrossRef] [PubMed]
- European Commision. 2018. Available online: https://joint-research-centre.ec.europa.eu/publications/agricultural-land-abandonment-eu-within-2015-2030_en#:~:text=The%20incremental%20abandonment%20within%202015-2030%20is%20nevertheless%20projected,the%20equivalent%20of%203%25%20of%20total%20agricultural%20land (accessed on 8 November 2023).
- FAO. 2012. Available online: https://www.fao.org/fileadmin/user_upload/esag/docs/AT2050_revision_summary.pdf (accessed on 8 November 2023).
- Raviv, M.; Krasnovsky, A.; Medina, S.; Reuveni, R. Assessment of various control strategies for recirculation of greenhouse effluents under semi-arid conditions. J. Hortic. Sci. Biotechnol. 1998, 73, 485–491. [Google Scholar] [CrossRef]
- Dutta, M.; Gupta, D.; Sahu, S.; Limkar, S.; Singh, P.; Mishra, A.; Mutlu, R. Evaluation of Growth Responses of Lettuce and Energy Efficiency of the Substrate and Smart Hydroponics Cropping System. Sensors 2023, 23, 1875. [Google Scholar] [CrossRef]
- Knox, J.; Hess, T.; Daccache, A.; Wheeler, T. Climate change impacts on crop productivity in Africa and South Asia. Environ. Res. Lett. 2012, 7, 034032. [Google Scholar] [CrossRef]
- Vernieri, P.; Borghesi, E.; Ferrante, A.; Magnani, G. Application of biostimulants in floating system for improving rocket quality. Int. J. Environ. Sci. Technol. 2005, 3, 86–88. [Google Scholar]
- Marchica, A.; Ascrizzi, R.; Flamini, G.; Cotrozzi, L.; Tonelli, M.; Lorenzini, G.; Pellegrini, E. Ozone as eustress for enhancing secondarymetabolites and bioactive properties in Salvia officinalis. Ind. Prod. 2021, 170, 113730. [Google Scholar] [CrossRef]
- Brezeanu, C.; Brezeanu, P.M.; Stoleru, V.; Irimia, L.M.; Lipșa, F.D.; Teliban, G.C.; Murariu, O.C. Nutritional value of new sweet pepper genotypes grown in organic system. Agriculture 2022, 12, 1863. [Google Scholar] [CrossRef]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Vannini, A.; Fedeli, R.; Guarnieri, M.; Loppi, S. Foliar Application of Wood Distillate Alleviates Ozone-Induced Damage in Lettuce (Lactuca Sativa L.). Toxics 2022, 10, 17. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.H.; Si, H.P.; Lin, K.Y. Effects of adding wood vinegar to nutrient solution on the growth, photosynthesis, and absorption of mineral elements of hydroponic lettuce. J. Plant Nutr. 2016, 39, 456–462. [Google Scholar] [CrossRef]
- Singh, H.; Dunn, B.; Payton, M. Hydroponic pH modifiers affect plant growth and nutrient content in leafy greens. J. Hortic. Res. 2019, 27, 31–36. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, Y.; Wang, X.; Liu, G. Effect of adding biochar with wood vinegar on the growth of cucumber. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 3rd International Conference on Energy Materials and Environment Engineering, Bangkok, Thailand, 10–12 March 2017; IOP Publishing: Bristol, UK, 2017; Volume 61, p. 012149. [Google Scholar]
- Wei, Q.; Ma, X.; Dong, J. Preparation, chemical constituents and antimicrobial activity of pyroligneous acids from walnut tree branches. J. Anal. Appl. Pyrolysis 2010, 87, 24–28. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Celletti, S.; Maresca, V.; Munzi, S.; Cruz, C.; Loppi, S. Foliar application of wood distillate boosts plant yield and nutritional parameters of chickpea. Ann. Appl. Biol. 2023, 182, 57–64. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Guarnieri, M.; Monaci, F.; Loppi, S. Bio-based solutions for agriculture: Foliar application of wood distillate alone and in combination with other plant-derived corroborants results in different effects on lettuce (Lactuca sativa L.). Biology 2022, 11, 404. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Grattacaso, M.; Loppi, S. Wood distillate (pyroligneous acid) boosts nutritional traits of potato tubers. Ann. Appl. Biol. 2023, 183, 135–140. [Google Scholar] [CrossRef]
- Dissatian, A.; Sanitchon, J.; Pongdontri, P.; Jongrungklang, N.; Jothityangkoon, D. Potential of wood vinegar for enhancing seed germination of three upland rice varieties by suppressing malondialdehyde production. AGRIVITA J. Agric. Sci. 2018, 40, 371–380. [Google Scholar]
- Mungkunkamchao, T.; Kesmala, T.; Pimratch, S.; Toomsan, B.; Jothityangkoon, D. Wood vinegar and fermented bioextracts: Natural products to enhance growth and yield of tomato (Solanum lycopersicum L.). Sci. Hortic. 2013, 154, 66–72. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Song, Q.; Wang, S.; Wang, Y.; Ge, Y. Root proteomics reveals the effects of wood vinegar on wheat growth and subsequent tolerance to drought stress. Int. J. Mol. Sci. 2019, 20, 943. [Google Scholar] [CrossRef]
- Fedeli, R.; Fiaschi, T.; Angiolini, C.; Maccherini, S.; Loppi, S.; Fanfarillo, E. Dose-Dependent and Species-Specific Effects of Wood Distillate Addition on the Germination Performance of Threatened Arable Plants. Plants 2023, 12, 3028. [Google Scholar] [CrossRef] [PubMed]
- Maresca, V.; Fedeli, R.; Vannini, A.; Munzi, S.; Corrêa, A.; Cruz, C.; Loppi, S. Wood distillate enhances seed germination of chickpea, lettuce, and basil. Appl. Sci. 2024, 14, 631. [Google Scholar] [CrossRef]
- de Lima, G.G.; Mendes, C.; de Marchi, G.; Vicari, T.; Cestari, M.M.; Gomes, M.F.; Leme, D.M. The evaluation of the potential ecotoxicity of pyroligneous acid obtained from fast pyrolysis. Ecotoxicol. Environ. Saf. 2019, 180, 616–623. [Google Scholar] [CrossRef]
- Fedeli, R.; Marotta, L.; Frattaruolo, L.; Panti, A.; Carullo, G.; Fusi, F.; Saponara, S.; Gemma, S.; Butini, S.; Cappello, A.R.; et al. Nutritionally-enriched tomatoes (Solanum lycopersicum L.) grown with wood distillate: Chemical and biological characterization for quality assessment. J. Food Sci. 2023, 88, 5324–5338. [Google Scholar] [CrossRef] [PubMed]
- Ofoe, R.; Qin, D.; Gunupuru, L.R.; Thomas, R.H.; Abbey, L. Effect of pyroligneous acid on the productivity and nutritional quality of greenhouse tomato. Plants 2022, 11, 1650. [Google Scholar] [CrossRef]
- Admane, N.; Cavallo, G.; Hadjila, C.; Cavalluzzi, M.M.; Rotondo, N.P.; Salerno, A.; Sanzani, S.M. Biostimulant Formulations and Moringa oleifera Extracts to Improve Yield, Quality, and Storability of Hydroponic Lettuce. Molecules 2023, 28, 373. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Quaggiotti, S. A novel biostimulant, belonging to protein hydrolysates, mitigates abiotic stress effects on maize seedlings grown in hydroponics. Agronomy 2019, 9, 28. [Google Scholar] [CrossRef]
- Kalozoumis, P.; Vourdas, C.; Ntatsi, G.; Savvas, D. Can Biostimulants Increase Resilience of Hydroponically-Grown Tomato to Combined Water and Nutrient Stress? Horticulturae 2021, 7, 297. [Google Scholar] [CrossRef]
- Lobanov, V.; Keesman, K.J.; Joyce, A. Plants dictate root microbial composition in hydroponics and aquaponics. Front. Microbiol. 2021, 13, 848057. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Manda, G.; Nechifor, M.T.; Neagu, T.M. Reactive oxygen species, cancer and anti-cancer therapies. Curr. Chem. Biol. 2009, 3, 22–46. [Google Scholar] [CrossRef]
- Barreca, D. Mechanisms of plant antioxidants action. Plants 2020, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Galindo, F.S.; Pagliari, P.H.; Fernandes, G.C.; Rodrigues, W.L.; Boleta, E.H.M.; Jalal, A.; Céu, E.G.O.; Lima, B.H.D.; Lavres, J.; Teixeira Filho, M.C.M. Improving Sustainable Field-Grown Wheat Production with Azospirillum brasilense Under Tropical Conditions: A Potential Tool for Improving Nitrogen Management. Front. Environ. Sci. 2022, 10, 821628. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Chedea, V.S.; Pop, R.M. Total polyphenols content and antioxidant DPPH assays on biological samples. In Polyphenols in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 169–183. [Google Scholar]
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Benzon, H.R.L.; Lee, S.C. Potential of wood vinegar in enhancing fruit yield and antioxidant capacity in tomato. Korean J. Plant Resour. 2016, 29, 704–711. [Google Scholar] [CrossRef]
- Kårlund, A.; Salminen, J.P.; Koskinen, P.; Ahern, J.R.; Karonen, M.; Tiilikkala, K.; Karjalainen, R.O. Polyphenols in strawberry (Fragaria× ananassa) leaves induced by plant activators. J. Agric. Food Chem. 2014, 62, 4592–4600. [Google Scholar] [CrossRef]
- Cruz, C.; Bio, A.F.M.; Domínguez-Valdivia, M.D.; Aparicio-Tejo, P.M.; Lamsfus, C.; Martins-Louçao, M.A. How does glutamine synthetase activity determine plant tolerance to ammonium? Planta 2006, 223, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Celletti, S.; Fedeli, R.; Ghorbani, M.; Aseka, J.M.; Loppi, S. Exploring sustainable alternatives: Wood distillate alleviates the impact of bioplastic in basil plants. Sci. Total Environ. 2023, 900, 166484. [Google Scholar] [CrossRef] [PubMed]
- BioDea. Available online: https://biodea.bio/bio-wood-distillate/?lang=en (accessed on 1 December 2023).
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Sunkar, R. Plant stress tolerance. Methods Mol. Biol. 2010, 639, 401. [Google Scholar]
- Fedeli, R.; Celletti, S.; Loppi, S.; Vannini, A. Comparison of the Effect of Solid and Liquid Digestate on the Growth of Lettuce (Lactuca sativa L.) Plants. Agronomy 2023, 13, 782. [Google Scholar] [CrossRef]
- Lamaro, G.P.; Tsehaye, Y.; Girma, A.; Vannini, A.; Fedeli, R.; Loppi, S. Evaluation of Yield and Nutraceutical Traits of Orange-Fleshed Sweet Potato Storage Roots in Two Agro-Climatic Zones of Northern Ethiopia. Plants 2023, 12, 1319. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid tests to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J. Agric. Food Chem. 2005, 53, 3053–3056. [Google Scholar] [CrossRef]
- Anderson, M. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; Primer-E Limited.: Auckland, New Zealand, 2008. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: USersManual/Tutorial PRIMER-E; Plymouth Marine Laboratory: Plymouth, UK, 2006. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).