Calmodulin-Domain Protein Kinase PiCDPK1 Interacts with the 14-3-3-like Protein NtGF14 to Modulate Pollen Tube Growth
Abstract
:1. Introduction
2. Results
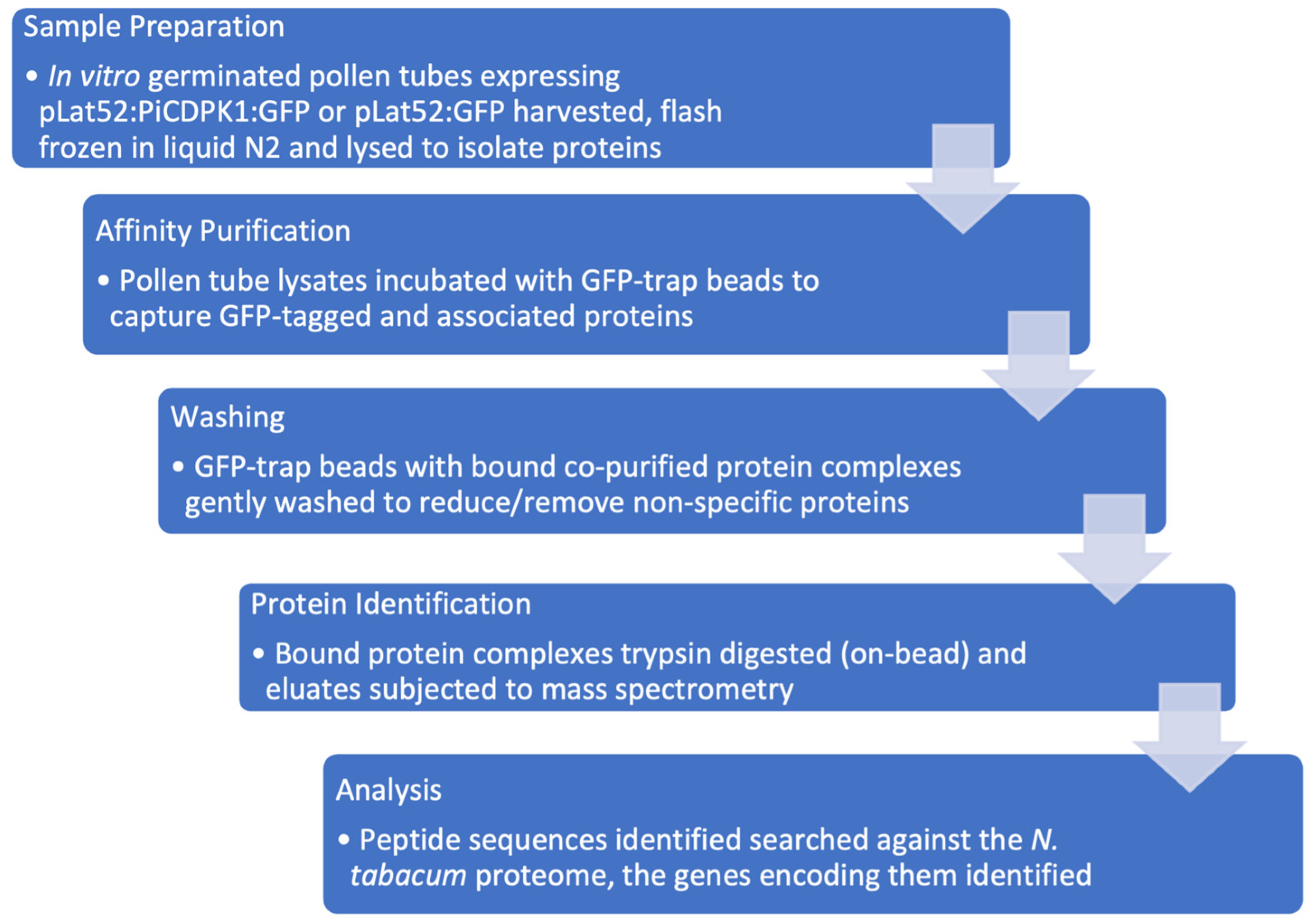
2.1. Affinity Purification and Mass Spectrometry Analysis Experimental Design
2.2. Identification of Putative PiCDPK1 Substrates
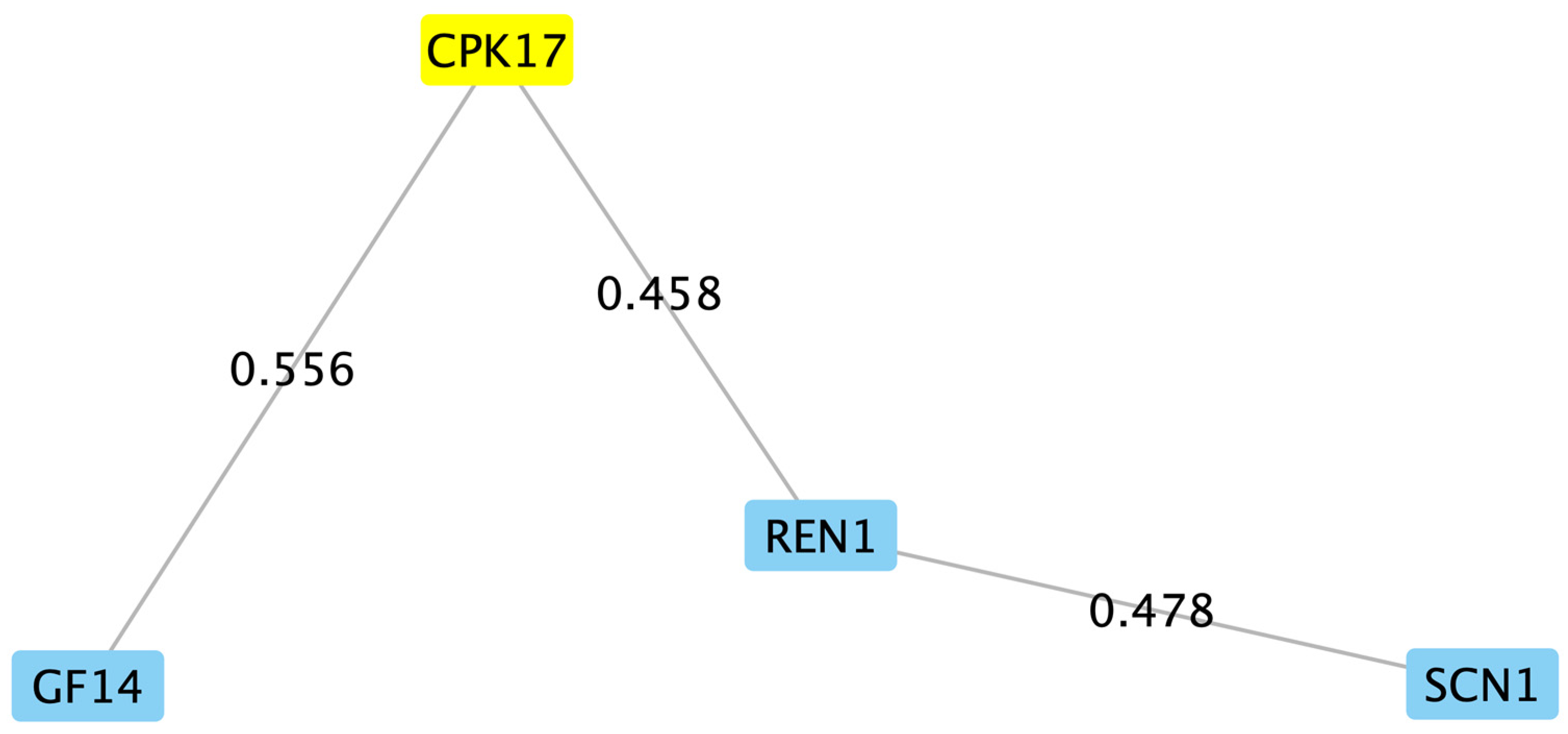
2.3. Bioinformatic Analysis of Filtered Putative PiCDPK1 Substrates
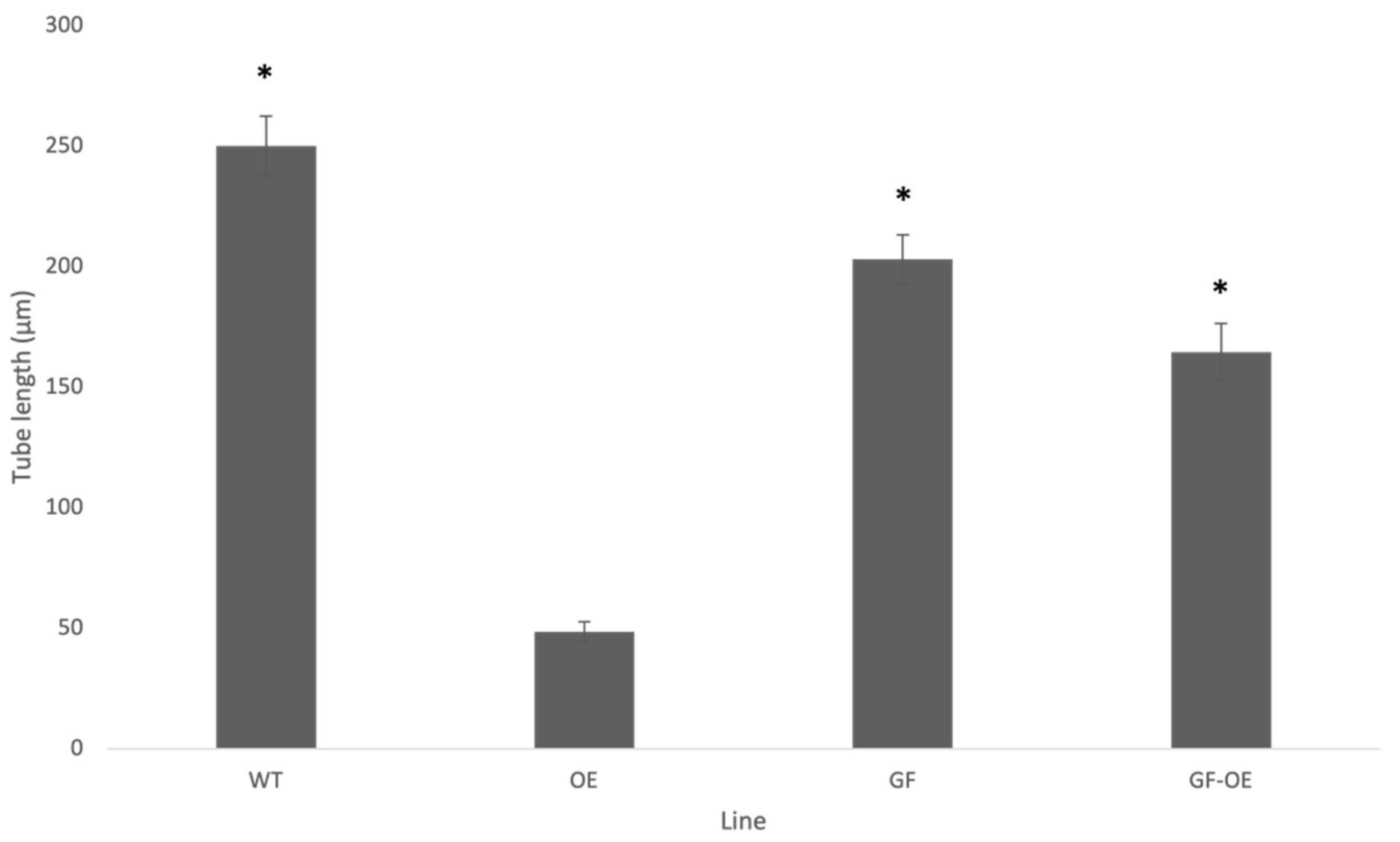
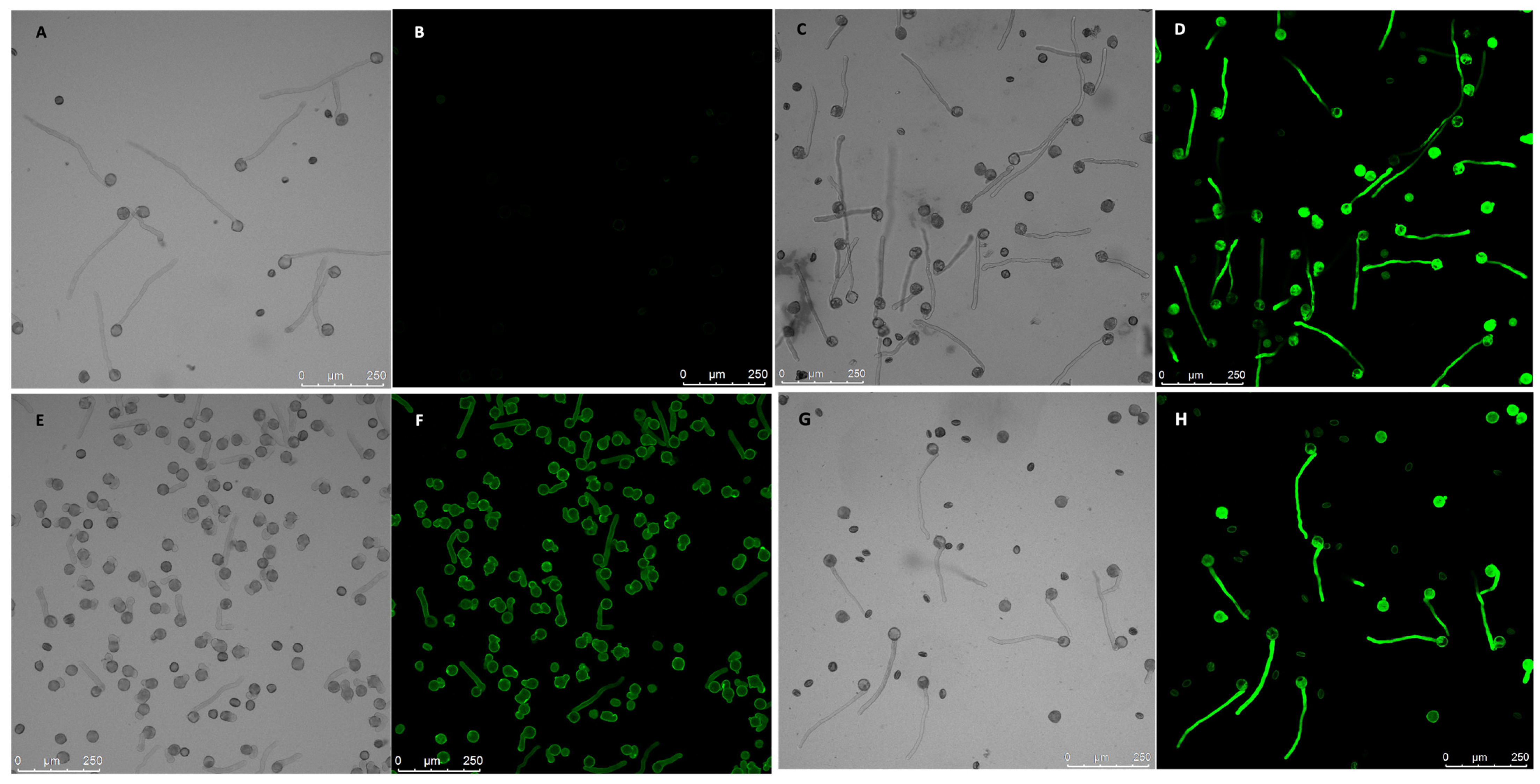
2.4. Stable Over-Expression of NtGF14 in Tobacco Pollen Tubes
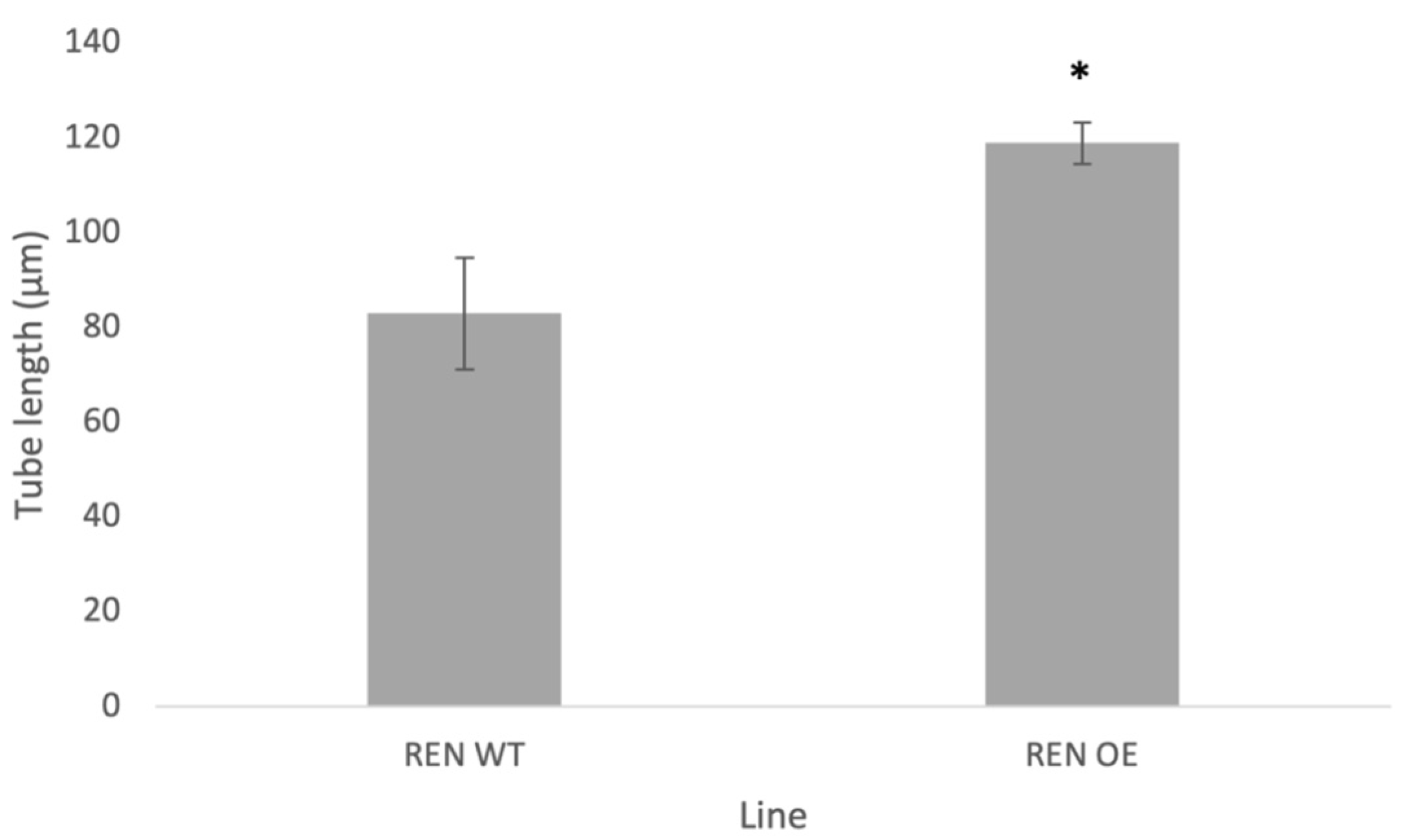
2.5. Stable Over-Expression of NtREN1 in Tobacco Pollen Tubes
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions and Pollen Tube Germination
4.2. Protein Extraction and Affinity Purification
4.3. Sample Preparation for Proteomics Analysis by Liquid Chromatography-Mass Spectrometry
4.4. Proteomics Analysis by Liquid Chromatography-Mass Spectrometry
4.5. Stable Over-Expression of NtGF14 and NtREN1 in Tobacco
4.6. Analysis of Transformed Pollen Tubes
4.7. Computational Analysis
4.8. Quantitative RT-PCR
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheible, N.; McCubbin, A. Signaling in Pollen Tube Growth: Beyond the Tip of the Polarity Iceberg. Plants 2019, 8, 156. [Google Scholar] [CrossRef]
- Pierson, E.S.; Miller, D.D.; Callaham, D.A.; Van Aken, J.; Hackett, G.; Hepler, P.K. Tip-Localized Calcium Entry Fluctuates during Pollen Tube Growth. Dev. Biol. 1996, 174, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Feijo, J.A.; Malho, R.; Obermeyer, G. Ion Dynamics and Its Possible Role during In Vitro Pollen Germination and Tube Growth. Protoplasma 1995, 187, 155–167. [Google Scholar] [CrossRef]
- Holdaway-Clarke, T.L.; Feijo, J.A.; Hackett, G.R.; Kunkel, J.G.; Hepler, P.K. Pollen Tube Growth and the Intracellular Cytosolic Calcium Gradient Oscillate in Phase While Extracellular Calcium Influx Is Delayed. Plant Cell 1997, 9, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Scholz, P.; Anstatt, J.; Krawczyk, H.E.; Ischebeck, T. Signalling Pinpointed to the Tip: The Complex Regulatory Network That Allows Pollen Tube Growth. Plants 2020, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lan, W.; Chen, B.; Fang, W.; Luan, S. A Calcium Sensor-Regulated Protein Kinase, Calcineurin B-like Protein-Interacting Protein Kinase19, Is Required for Pollen Tube Growth and Polarity. Plant Physiol. 2015, 167, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fu, Y.; Yang, Z. A Genome-wide Functional Characterization of Arabidopsis Regulatory Calcium Sensors in Pollen Tubes. JIPB 2009, 51, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Suwińska, A.; Wasąg, P.; Zakrzewski, P.; Lenartowska, M.; Lenartowski, R. Calreticulin Is Required for Calcium Homeostasis and Proper Pollen Tube Tip Growth in Petunia. Planta 2017, 245, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; You, C.; Yang, S.; Zhang, Y.; Yang, F.; Li, X.; Chen, N.; Luo, Y.; Hu, X. The Role of Calcium/Calcium-Dependent Protein Kinases Signal Pathway in Pollen Tube Growth. Front. Plant Sci. 2021, 12, 633293. [Google Scholar] [CrossRef]
- Yoon, G.M.; Dowd, P.E.; Gilroy, S.; McCubbin, A.G. Calcium-Dependent Protein Kinase Isoforms in Petunia Have Distinct Functions in Pollen Tube Growth, Including Regulating Polarity. Plant Cell 2006, 18, 867–878. [Google Scholar] [CrossRef]
- Scheible, N.; Yoon, G.M.; McCubbin, A.G. Calmodulin Domain Protein Kinase PiCDPK1 Regulates Pollen Tube Growth Polarity through Interaction with RhoGDI. Plants 2022, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lin, Y.; Heath, R.M.; Zhu, M.X.; Yang, Z. Control of Pollen Tube Tip Growth by a Rop GTPase-Dependent Pathway That Leads to Tip-Localized Calcium Influx. Plant Cell 1999, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Deng, Y.; Chen, P.; Feng, F.; Chen, W.; Zhou, X.; Wang, Y. A Calcium-Dependent Protein Kinase, ZmCPK32, Specifically Expressed in Maize Pollen to Regulate Pollen Tube Growth. PLoS ONE 2018, 13, e0195787. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.F.; Breton, G.; Harmon, A. Decoding Ca2+ signals Through plant protein kinases. Annu. Rev. Plant Biol. 2004, 55, 263–288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-N.; Shen, L.-K.; Zhang, W.-Z.; Zhang, W.; Wang, Y.; Wu, W.-H. Ca2+-Dependent Protein Kinase11 and 24 Modulate the Activity of the Inward Rectifying K+ Channels in Arabidopsis Pollen Tubes. Plant Cell 2013, 25, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Gutermuth, T.; Lassig, R.; Portes, M.-T.; Maierhofer, T.; Romeis, T.; Borst, J.-W.; Hedrich, R.; Feijo, J.A.; Konrad, K.R. Pollen Tube Growth Regulation by Free Anions Depends on the Interaction between the Anion Channel SLAH3 and Calcium-Dependent Protein Kinases CPK2 and CPK20. Plant Cell 2013, 25, 4525–4543. [Google Scholar] [CrossRef] [PubMed]
- Gutermuth, T.; Herbell, S.; Lassig, R.; Brosché, M.; Romeis, T.; Feijó, J.A.; Hedrich, R.; Konrad, K.R. Tip-localized Ca2+-permeable Channels Control Pollen Tube Growth via Kinase-dependent R- and S-type Anion Channel Regulation. New Phytol. 2018, 218, 1089–1105. [Google Scholar] [CrossRef] [PubMed]
- Allende, J.E.; Allende, C.C. Protein Kinase CK2: An Enzyme with Multiple Substrates and a Puzzling Regulation. FASEB J. 1995, 9, 313–323. [Google Scholar] [CrossRef]
- Xue, L.; Wang, P.; Cao, P.; Zhu, J.; Tao, W.A. Identification of Extracellular Signal-Regulated Kinase 1 (ERK1) Direct Substrates Using Stable Isotope Labeled Kinase Assay-Linked Phosphoproteomics. Mol. Cell. Proteom. 2014, 13, 3199–3210. [Google Scholar] [CrossRef]
- Brymora, A.; Valova, V.A.; Robinson, P.J. Protein-Protein Interactions Identified by Pull-Down Experiments and Mass Spectrometry. Curr. Protocol. Cell Biol. 2004, 22, 17.5.1–17.5.51. [Google Scholar] [CrossRef]
- Free, R.B.; Hazelwood, L.A.; Sibley, D.R. Identifying Novel Protein-Protein Interactions Using Co-Immunoprecipitation and Mass Spectroscopy. CP Neurosci. 2009, 5, 5–28. [Google Scholar] [CrossRef]
- Li, H.; Hu, J.; Pang, J.; Zhao, L.; Yang, B.; Kang, X.; Wang, A.; Xu, T.; Yang, Z. Rho GTPase ROP1 Interactome Analysis Reveals Novel ROP1-Associated Pathways for Pollen Tube Polar Growth in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 7033. [Google Scholar] [CrossRef] [PubMed]
- Luzarowski, M.; Kosmacz, M.; Sokolowska, E.; Jasińska, W.; Willmitzer, L.; Veyel, D.; Skirycz, A. Affinity Purification with Metabolomic and Proteomic Analysis Unravels Diverse Roles of Nucleoside Diphosphate Kinases. J. Exp. Bot. 2017, 68, 3487–3499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lin, Y.-H. Whole-Cell Protein Identification Using the Concept of Unique Peptides. Genom. Proteom. Bioinform. 2010, 8, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ranish, J.A.; Hahn, S.; Lu, Y.; Yi, E.C.; Li, X.; Eng, J.; Aebersold, R. Identification of TFB5, a New Component of General Transcription and DNA Repair Factor IIH. Nat. Genet. 2004, 36, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Greco, T.M.; Seeholzer, S.H.; Mak, A.; Spruce, L.; Ischiropoulos, H. Quantitative Mass Spectrometry-Based Proteomics Reveals the Dynamic Range of Primary Mouse Astrocyte Protein Secretion. J. Proteome Res. 2010, 9, 2764–2774. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, B.M.; McDonald, W.H.; Dungrawala, H.; Badu-Nkansah, A.; Kavanaugh, G.M.; Chen, Y.; Tabb, D.L.; Cortez, D. Identification of Proteins at Active, Stalled, and Collapsed Replication Forks Using Isolation of Proteins on Nascent DNA (iPOND) Coupled with Mass Spectrometry. J. Biol. Chem. 2013, 288, 31458–31467. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.M.; Bejide, M.; Trinkle-Mulcahy, L.; Côté, J. Identification of the PRMT1v1 and PRMT1v2 Specific Interactomes by Quantitative Mass Spectrometry in Breast Cancer Cells. Proteomics 2015, 15, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Snel, B. STRING: A Web-Server to Retrieve and Display the Repeatedly Occurring Neighbourhood of a Gene. Nucleic Acids Res. 2000, 28, 3442–3444. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.; Romanowsky, S.M.; Barron, Y.D.; Garg, S.; Azuse, C.L.; Curran, A.; Davis, R.M.; Hatton, J.; Harmon, A.C.; Harper, J.F. Calcium-Dependent Protein Kinases Regulate Polarized Tip Growth in Pollen Tubes. Plant J. 2009, 59, 528–539. [Google Scholar] [CrossRef]
- Dwyer, M.E.; Hangarter, R.P. Light-Dependent Phosphorylation of THRUMIN1 Regulates Its Association with Actin Filaments and 14-3-3 Proteins. Plant Physiol. 2021, 187, 1445–1461. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, M.E.; Hangarter, R.P. Light-Induced Displacement of PLASTID MOVEMENT IMPAIRED1 Precedes Light-Dependent Chloroplast Movements. Plant Physiol. 2022, 189, 1866–1880. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-U.; Vernoud, V.; Szumlanski, A.; Nielsen, E.; Yang, Z. A Tip-Localized RhoGAP Controls Cell Polarity by Globally Inhibiting Rho GTPase at the Cell Apex. Curr. Biol. 2008, 18, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Cummins, I.; Landrum, M.; Steel, P.G.; Edwards, R. Structure Activity Studies with Xenobiotic Substrates Using Carboxylesterases Isolated from Arabidopsis thaliana. Phytochemistry 2007, 68, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, J.; Hao, R.; Guo, Y. Activation of Catalase Activity by a Peroxisome-Localized Small Heat Shock Protein Hsp17.6CII. J. Genet. Genom. 2017, 44, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Kumasaki, S.; Soga, K.; Wakabayashi, K.; Hashimoto, T.; Hoson, T. Gravity-Induced Modifications to Development in Hypocotyls of Arabidopsis Tubulin Mutants. Plant Physiol. 2010, 152, 918–926. [Google Scholar] [CrossRef]
- Bai, Y.; Tian, D.; Chen, P.; Wu, D.; Du, K.; Zheng, B.; Shi, X. A Pectate Lyase Gene Plays a Critical Role in Xylem Vascular Development in Arabidopsis. Int. J. Mol. Sci. 2023, 24, 10883. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yang, S.-L.; Xie, L.-F.; Puah, C.S.; Zhang, X.-Q.; Yang, W.-C.; Sundaresan, V.; Ye, D. VANGUARD1 Encodes a Pectin Methylesterase That Enhances Pollen Tube Growth in the Arabidopsis Style and Transmitting Tract. Plant Cell 2005, 17, 584–596. [Google Scholar] [CrossRef]
- Survila, M.; Davidsson, P.R.; Pennanen, V.; Kariola, T.; Broberg, M.; Sipari, N.; Heino, P.; Palva, E.T. Peroxidase-Generated Apoplastic ROS Impair Cuticle Integrity and Contribute to DAMP-Elicited Defenses. Front. Plant Sci. 2016, 7, 1945. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Y.; Gu, D.; Nan, J.; Chen, S.; Li, H. Overexpression of S-Adenosyl-l-Methionine Synthetase 2 from Sugar Beet M14 Increased Arabidopsis Tolerance to Salt and Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 847. [Google Scholar] [CrossRef]
- Heinze, L.; Freimuth, N.; Rößling, A.-K.; Hahnke, R.; Riebschläger, S.; Fröhlich, A.; Sampathkumar, A.; McFarlane, H.E.; Sauer, M. EPSIN1 and MTV1 Define Functionally Overlapping but Molecularly Distinct Trans-Golgi Network Subdomains in Arabidopsis. Proc. Natl. Acad. Sci. USA 2020, 117, 25880–25889. [Google Scholar] [CrossRef]
- Rottmann, T.; Zierer, W.; Subert, C.; Sauer, N.; Stadler, R. STP10 Encodes a High-Affinity Monosaccharide Transporter and Is Induced under Low-Glucose Conditions in Pollen Tubes of Arabidopsis. J. Exp. Bot. 2016, 67, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Conn, S.J.; Conn, V.; Tyerman, S.D.; Kaiser, B.N.; Leigh, R.A.; Gilliham, M. Magnesium Transporters, MGT2/MRS2-1 and MGT3/MRS2-5, Are Important for Magnesium Partitioning within Arabidopsis thaliana Mesophyll Vacuoles. New Phytol. 2011, 190, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.-D.; Tian, L.-F.; Li, L.-G.; Chen, J.; Deng, P.-Y.; Li, D.-P.; Luan, S. AtMGT7: An Arabidopsis Gene Encoding a Low-Affinity Magnesium Transporter. J. Integr. Plant Biol. 2008, 50, 1530–1538. [Google Scholar] [CrossRef]
- Ross, S.; Giglione, C.; Pierre, M.; Espagne, C.; Meinnel, T. Functional and Developmental Impact of Cytosolic Protein N-Terminal Methionine Excision in Arabidopsis. Plant Physiol. 2005, 137, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Posé, D.; Castanedo, I.; Borsani, O.; Nieto, B.; Rosado, A.; Taconnat, L.; Ferrer, A.; Dolan, L.; Valpuesta, V.; Botella, M.A. Identification of the Arabidopsis Dry2/Sqe1-5 Mutant Reveals a Central Role for Sterols in Drought Tolerance and Regulation of Reactive Oxygen Species. Plant J. 2009, 59, 63–76. [Google Scholar] [CrossRef]
- Ferguson, C.; Teeri, T.T.; Siika-aho, M.; Read, S.M.; Bacic, A. Location of Cellulose and Callose in Pollen Tubes and Grains of Nicotiana tabacum. Planta 1998, 206, 452–460. [Google Scholar] [CrossRef]
- Chen, W.; Gong, P.; Guo, J.; Li, H.; Li, R.; Xing, W.; Yang, Z.; Guan, Y. Glycolysis Regulates Pollen Tube Polarity via Rho GTPase Signaling. PLoS Genet. 2018, 14, e1007373. [Google Scholar] [CrossRef]
- Henning, P.M.; Shore, J.S.; McCubbin, A.G. Transcriptome and Network Analyses of Heterostyly in Turnera Subulata Provide Mechanistic Insights: Are S-Loci a Red-Light for Pistil Elongation? Plants 2020, 9, 713. [Google Scholar] [CrossRef]
- Twell, D.; Klein, T.M.; Fromm, M.E.; McCormick, S. Transient Expression of Chimeric Genes Delivered into Pollen by Microprojectile Bombardment. Plant Physiol. 1989, 91, 1270–1274. [Google Scholar] [CrossRef]
- Pignocchi, C.; Doonan, J.H. Interaction of a 14-3-3 Protein with the Plant Microtubule-Associated Protein EDE1. Ann. Bot. 2011, 107, 1103–1109. [Google Scholar] [CrossRef]
- Latz, A.; Becker, D.; Hekman, M.; Müller, T.; Beyhl, D.; Marten, I.; Eing, C.; Fischer, A.; Dunkel, M.; Bertl, A.; et al. TPK1, a Ca2+-Regulated Arabidopsis Vacuole Two-Pore K+ Channel Is Activated by 14-3-3 Proteins: TPK1 Activation by 14-3-3. Plant J. 2007, 52, 449–459. [Google Scholar] [CrossRef]
- Li, H.; Luo, N.; Wang, W.; Liu, Z.; Chen, J.; Zhao, L.; Tan, L.; Wang, C.; Qin, Y.; Li, C.; et al. The REN4 Rheostat Dynamically Coordinates the Apical and Lateral Domains of Arabidopsis Pollen Tubes. Nat. Commun. 2018, 9, 2573. [Google Scholar] [CrossRef]
- Paul, A.-L.; Sehnke, P.C.; Ferl, R.J. Isoform-Specific Subcellular Localization among 14-3-3 Proteins in Arabidopsis Seems to Be Driven by Client Interactions. Mol. Biol. Cell 2005, 16, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Taoka, K.; Ohki, I.; Tsuji, H.; Furuita, K.; Hayashi, K.; Yanase, T.; Yamaguchi, M.; Nakashima, C.; Purwestri, Y.A.; Tamaki, S.; et al. 14-3-3 Proteins Act as Intracellular Receptors for Rice Hd3a Florigen. Nature 2011, 476, 332–335. [Google Scholar] [CrossRef]
- Pan, S.; Sehnke, P.C.; Ferl, R.J.; Gurley, W.B. Specific Interactions with TBP and TFIIB in Vitro Suggest That 14-3-3 Proteins May Participate in the Regulation of Transcription When Part of a DNA Binding Complex. Plant Cell 1999, 11, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Ottmann, C.; Marco, S.; Jaspert, N.; Marcon, C.; Schauer, N.; Weyand, M.; Vandermeeren, C.; Duby, G.; Boutry, M.; Wittinghofer, A.; et al. Structure of a 14-3-3 Coordinated Hexamer of the Plant Plasma Membrane H+-ATPase by Combining X-Ray Crystallography and Electron Cryomicroscopy. Mol. Cell 2007, 25, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Sottocornola, B.; Visconti, S.; Orsi, S.; Gazzarrini, S.; Giacometti, S.; Olivari, C.; Camoni, L.; Aducci, P.; Marra, M.; Abenavoli, A.; et al. The Potassium Channel KAT1 Is Activated by Plant and Animal 14-3-3 Proteins. J. Biol. Chem. 2006, 281, 35735–35741. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, J.D.; Folta, K.M.; Paul, A.-L.; Ferl, R.J. The 14-3-3 Proteins μ and υ Influence Transition to Flowering and Early Phytochrome Response. Plant Physiol. 2007, 145, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.; Kusano, M.; Sulpice, R.; Araki, M.; Redestig, H.; Saito, K.; Stitt, M.; Shin, R. Determining Novel Functions of Arabidopsis14-3-3 Proteins in Central Metabolic Processes. BMC Syst. Biol. 2011, 5, 192. [Google Scholar] [CrossRef]
- Dou, Y.; Liu, X.; Yin, Y.; Han, S.; Lu, Y.; Liu, Y.; Hao, D. Affinity Chromatography Revealed Insights into Unique Functionality of Two 14-3-3 Protein Species in Developing Maize Kernels. J. Proteom. 2015, 114, 274–286. [Google Scholar] [CrossRef]
- Zhang, Z.-T.; Zhou, Y.; Li, Y.; Shao, S.-Q.; Li, B.-Y.; Shi, H.-Y.; Li, X.-B. Interactome Analysis of the Six Cotton 14-3-3s That Are Preferentially Expressed in Fibres and Involved in Cell Elongation. J. Exp. Bot. 2010, 61, 3331–3344. [Google Scholar] [CrossRef]
- Grønlund, A.L.; Dickinson, J.R.; Kille, P.; Harwood, J.L.; Herbert, R.J.; Francis, D.; Rogers, H.J. Plant WEE1 Kinase Interacts with a 14-3-3 Protein, GF14ω but a Mutation of WEE1 at S485 Alters Their Spatial Interaction. Open J. Plant Sci. 2009, 3, 40–48. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Yu, H.; Peng, J.; Hu, Z.; Chen, L. The Role of 14-3-3 Proteins in Plant Growth and Response to Abiotic Stress. Plant Cell Rep. 2022, 41, 833–852. [Google Scholar] [CrossRef]
- Jones, D.H.; Ley, S.; Aitken, A. Isoforms of 14-3-3 Protein Can Form Homo- and Heterodimers in Vivo and in Vitro: Implications for Function as Adapter Proteins. FEBS Lett. 1995, 368, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Camoni, L.; Visconti, S.; Aducci, P.; Marra, M. 14-3-3 Proteins in Plant Hormone Signaling: Doing Several Things at Once. Front. Plant Sci. 2018, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Aitken, A. 14-3-3 Proteins: A Historic Overview. Semin. Cancer Biol. 2006, 16, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Shin, R.; Alvarez, S.; Burch, A.Y.; Jez, J.M.; Schachtman, D.P. Phosphoproteomic Identification of Targets of the Arabidopsis Sucrose Nonfermenting-like Kinase SnRK2.8 Reveals a Connection to Metabolic Processes. Proc. Natl. Acad. Sci. USA 2007, 104, 6460–6465. [Google Scholar] [CrossRef] [PubMed]
- Barjaktarovic, Z.; Schutz, W.; Madlung, J.; Fladerer, C.; Nordheim, A.; Hampp, R. Changes in the Effective Gravitational Field Strength Affect the State of Phosphorylation of Stress-Related Proteins in Callus Cultures of Arabidopsis Thaliana. J. Exp. Bot. 2009, 60, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Swatek, K.N.; Wilson, R.S.; Ahsan, N.; Tritz, R.L.; Thelen, J.J. Multisite Phosphorylation of 14-3-3 Proteins by Calcium-Dependent Protein Kinases. Biochem. J. 2014, 459, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lachaud, C.; Prigent, E.; Thuleau, P.; Grat, S.; Da Silva, D.; Brière, C.; Mazars, C.; Cotelle, V. 14-3-3-Regulated Ca2+-Dependent Protein Kinase CPK3 Is Required for Sphingolipid-Induced Cell Death in Arabidopsis. Cell Death Differ. 2013, 20, 209–217. [Google Scholar] [CrossRef]
- Klahre, U.; Kost, B. Tobacco RhoGTPase ACTIVATING PROTEIN1 Spatially Restricts Signaling of RAC/Rop to the Apex of Pollen Tubes. Plant Cell 2006, 18, 3033–3046. [Google Scholar] [CrossRef]
- Konagaya, K.; Matsushita, Y.; Kasahara, M.; Nyunoya, H. Members of 14-3-3 Protein Isoforms Interacting with the Resistance Gene Product N and the Elicitor of Tobacco Mosaic Virus. J. Gen. Plant Pathol. 2004, 70, 221–231. [Google Scholar] [CrossRef]
- Rosenquist, M.; Alsterfjord, M.; Larsson, C.; Sommarin, M. Data Mining the Arabidopsis Genome Reveals Fifteen 14-3-3 Genes. Expression Is Demonstrated for Two out of Five Novel Genes. Plant Physiol. 2001, 127, 142–149. [Google Scholar] [CrossRef]
- Paul, A.-L.; Denison, F.C.; Schultz, E.R.; Zupanska, A.K.; Ferl, R.J. 14-3-3 Phosphoprotein interaction networks—Does isoform diversity present functional interaction specification? Front. Plant Sci. 2012, 3, 190. [Google Scholar] [CrossRef]
- Johnson, C.; Crowther, S.; Stafford, M.J.; Campbell, D.G.; Toth, R.; MacKintosh, C. Bioinformatic and Experimental Survey of 14-3-3-Binding Sites. Biochem. J. 2010, 427, 69–78. [Google Scholar] [CrossRef]
- Muslin, A.J.; Tanner, J.W.; Allen, P.M.; Shaw, A.S. Interaction of 14-3-3 with Signaling Proteins Is Mediated by the Recognition of Phosphoserine. Cell 1996, 84, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Lauster, T.; Stöckle, D.; Gabor, K.; Haller, T.; Krieger, N.; Lotz, P.; Mayakrishnan, R.; Späth, E.; Zimmermann, S.; Livanos, P.; et al. Arabidopsis Pavement Cell Shape Formation Involves Spatially Confined ROPGAP Regulators. Curr. Biol. 2022, 32, 532–544.e7. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Guo, L.; Cai, Q.; Ma, F.; Zhu, Q.-Y.; Zhang, Q. Sodmergen Arabidopsis JINGUBANG Is a Negative Regulator of Pollen Germination That Prevents Pollination in Moist Environments. Plant Cell 2016, 28, 2131–2146. [Google Scholar] [CrossRef] [PubMed]
- Tcherkezian, J.; Lamarche-Vane, N. Current Knowledge of the Large RhoGAP Family of Proteins. Biol. Cell 2007, 99, 67–86. [Google Scholar] [CrossRef] [PubMed]
- BLAST® Command Line Applications User Manual 101. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279690/ (accessed on 1 August 2021).
- Shannon, P. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein–Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Pillich, R.T.; Chen, J.; Rynkov, V.; Welker, D.; Pratt, D. NDEx: A Community Resource for Sharing and Publishing of Biological Networks. In Protein Bioinformatics; Wu, C.H., Arighi, C.N., Ross, K.E., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1558, pp. 271–301. ISBN 978-1-4939-6781-0. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| N. tabacum ID | Arabidopsis Homolog | Gene Family | Putative Function | |
|---|---|---|---|---|
| ID | Gene Name | |||
| A0A1S4BR59 | AT5G12180 | CPK 17 | Calcium Dependent Protein Kinase | Transduce Ca2+ signals to modulate pollen tube growth [30] |
| Q75ZD6 | AT1G78300 | GF14 Omega | 14-3-3 protein | Facilitates interaction of actin-bundling plasma membrane proteins [31,32] |
| A0A1S3ZQG5 | AT4G24580 | ROP1 enhancer 1 | Rho GTPase activation protein (RhoGAP) with PH domain | Inhibits activation of ROP1, regulates pollen tube growth [33] |
| A0A024AYA4 | AT3G48700 | Carboxyesterase 13 | Carboxyesterase | Hydrolysis of esters [34] |
| A0A077DBK4 | AT5G12020 | 17.6 kDa class II heat shock protein | Heat-shock protein | Interacts with and activates catalase in peroxisomes [35] |
| A0A0S0N4U0 | AT5G19770 | Tubulin alpha-3 | Tubulin | Maintenance of growth against gravitational force [36] |
| A0A1S3X6C4 | AT1G14420 | Pectate lyase-like 8 | Pectate lyase protein | Pectin synthesis/remodeling and secondary wall formation [37] |
| A0A1S3X7U8 | AT2G47040 | Vanguard 1 | Plant invertase/pectin methylesterase inhibitor superfamily | Enhancer of growth of pollen tube in female floral tissues [38] |
| A0A1S3XSQ7 | AT5G17820 | Peroxidase 57 | Peroxidase superfamily protein | Affects permeability of leaf cuticle [39] |
| A0A1S3XWB5 | AT3G17390 | S-adenosylmethionine synthetase 3 | S-adenosylmethionine synthetase family protein | Involved in salt and H2O2 stress tolerance [40] |
| A0A1S3YE49 | AT3G22530 | Unknown | Hypothetical heat-shock protein | Unknown |
| A0A1S3Z0D6 | AT1G34340 | Unknown | Hypothetical alpha/beta-Hydrolase superfamily protein | Unknown |
| A0A1S3Z6E1 | AT2G32730 | Unknown | Hypothetical 26S proteasome regulatory complex | Unknown |
| A0A1S3Z926 | AT3G59290 | Epsin 3 | Epsin family of endocytic proteins | Accessory proteins that facilitate vesicle biogenesis [41] |
| A0A1S4AFS9 | AT3G19940 | Sugar Transport Protein 10 | Sugar Transport Protein | Glucose uptake in growing pollen tubes [42] |
| A0A1S4AKF1 | AT1G16010 | Magnesium Transporter 2 | Transmembrane magnesium transporter | Tonoplast-targeted transporter for vacuolar accumulation of magnesium [43] |
| A0A1S4C178 | AT5G09690 | Magnesium Transporter 7 | Transmembrane magnesium transporter | Low-affinity Mg2+ transporter [44] |
| A0A1S4CMX7 | AT3G59990 | Methionine aminopeptidase 2b | Methionine Amino-peptidase (MAP) | Required for development at various stages of Arabidopsis life cycle [45] |
| A0A1S4CPM1 | AT4G37760 | Squalene epoxidase 3 | Squalene epoxidase enzyme | Plays a role in sterol biosynthesis in shoots [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheible, N.; Henning, P.M.; McCubbin, A.G. Calmodulin-Domain Protein Kinase PiCDPK1 Interacts with the 14-3-3-like Protein NtGF14 to Modulate Pollen Tube Growth. Plants 2024, 13, 451. https://doi.org/10.3390/plants13030451
Scheible N, Henning PM, McCubbin AG. Calmodulin-Domain Protein Kinase PiCDPK1 Interacts with the 14-3-3-like Protein NtGF14 to Modulate Pollen Tube Growth. Plants. 2024; 13(3):451. https://doi.org/10.3390/plants13030451
Chicago/Turabian StyleScheible, Nolan, Paige M. Henning, and Andrew G. McCubbin. 2024. "Calmodulin-Domain Protein Kinase PiCDPK1 Interacts with the 14-3-3-like Protein NtGF14 to Modulate Pollen Tube Growth" Plants 13, no. 3: 451. https://doi.org/10.3390/plants13030451
APA StyleScheible, N., Henning, P. M., & McCubbin, A. G. (2024). Calmodulin-Domain Protein Kinase PiCDPK1 Interacts with the 14-3-3-like Protein NtGF14 to Modulate Pollen Tube Growth. Plants, 13(3), 451. https://doi.org/10.3390/plants13030451





