Abstract
Dwarfing is one of the common phenotypic variations in asexually reproduced progeny of banana, and dwarfed banana is not only windproof and anti-fallout but also effective in increasing acreage yield. As a key gene in the strigolactone signalling pathway, DWARF53 (D53) plays an important role in the regulation of the height of plants. In order to gain insight into the function of the banana D53 gene, this study conducted genome-wide identification of banana D53 gene based on the M. acuminata, M. balbisiana and M. itinerans genome database. Analysis of MaD53 gene expression under high temperature, low temperature and osmotic stress based on transcriptome data and RT-qPCR was used to analyse MaD53 gene expression in different tissues as well as in different concentrations of GA and SL treatments. In this study, we identified three MaD53, three MbD53 and two MiD53 genes in banana. Phylogenetic tree analysis showed that D53 Musa are equally related to D53 Asparagales and Poales. Both high and low-temperature stresses substantially reduced the expression of the MaD53 gene, but osmotic stress treatments had less effect on the expression of the MaD53 gene. GR24 treatment did not significantly promote the height of the banana, but the expression of the MaD53 gene was significantly reduced in roots and leaves. GA treatment at 100 mg/L significantly promoted the expression of the MaD53 gene in roots, but the expression of this gene was significantly reduced in leaves. In this study, we concluded that MaD53 responds to GA and SL treatments, but “Yinniaijiao” dwarf banana may not be sensitive to GA and SL.
1. Introduction
The D53 (DWARF53) gene was originally discovered in short stem dominant mutant rice, in which the D53 gene was dominantly mutated, and the mutated protein could not be degraded by strigolactone, resulting in a dwarf multiple tiller phenotype [1]. With increasing research, the D53 gene is considered to be a key gene in strigolactones (SLs) signalling. In rice, the D53 protein interacts with transcription factors, as well as the transcriptional co-repressor proteins TOPLESS (TPL) and TPL RELATED (TPR), to repress the expression of downstream genes when SLs are deficient or at very low concentrations [1]. Whereas, when the concentration of SLs is sufficient to activate the SL signalling pathway, the D14 protein recognises and binds SLs and recruits the SCF (ASK1-CULLIN-F-BOX) complex and D3 protein to further form an SCF–D14 complex. The D3 protein specifically recognises and binds to the SLs signalling inhibitor D53, forming the D53–D14–SCFD3 protein complex. D53 is modified by ubiquitin-conjugating enzyme E2 and degraded by the 26S proteasome to activate the expression of downstream target genes [2]. In dicotyledonous plants, such as Arabidopsis thaliana, it was found that the D53 gene (the immediate homologue of SMXL6/7/8 in Arabidopsis) not only acts as a repressor to inhibit the expression of downstream genes, but SMXL6 can also directly bind to the promoter of SMXL6/7/8 and regulate its transcription, thus acting as a transcription factor in SL signalling [3]. D53/SMXLs genes have been identified and functionally analysed in a variety of species, such as rice [1], maise [4], Arabidopsis [5], cotton [6] and poplar [7], but no systematic identification has been conducted in banana.
Both endogenous and exogenous signals regulate plant growth and development, and hormones, which are one of the major endogenous signals in plants, can respond rapidly to external stimuli [8]. Strigolactones (SLs) are carotenoid-derived terpenoid lactones that are produced in plant roots and transported up the stem to various parts of the plant, regulating multiple stages of plant growth and development [9]. In recent decades, tremendous progress has been made in research on the biosynthesis, signalling and biological functions of SLs. SLs were first found in the seeds of striga hermonthica parasiticum in the roots of cotton and have been shown to play important roles in promoting seed germination, mycelial branching of arbuscular mycorrhizal fungi [10,11], regulation of plant branching [12], flowering period and secondary growth and enhancement of drought and salt tolerance in plants [13]. Among them, five major enzymes are involved in the biosynthesis of SLs, namely D27 (DWARF27), CDD7, CCD8, MAX1 and LOB, and three enzymes are involved in signal transduction, namely Clp proteins D53 (DWARF53)/SMXL6/7/8, D14 (DWARF14) and D3 (DWARF3)/MAX2 [14]. The rice D10 gene encodes the carotenoid cleavage dioxygenase CCD8, a direct homologue of Arabidopsis MAX4 [15], and when this gene is mutated, plants exhibit reduced plant height and increased tillering [16]. In addition, D17 encodes the carotenoid cleavage dioxygenase CCD7, whose partial loss of function leads to dwarfing, multiple tillering and increased yield in rice [17]. D14 is the SL receptor, and d14 mutants have increased tillering and reduced plant height [18]. D3 is involved in the degradation of inhibitory proteins in the SL signalling pathway, and Arabidopsis d3 (max2) mutants exhibit plant dwarfing and reduced adventitious roots [19].
Gibberellic acid (GA) is also a widely available phytohormone that plays an important role in promoting seed germination, flowering and fruit growth, as well as regulating plant flower development and plant height [20]. Plants defective in GA synthesis or signalling are characterised by plant dwarfism, dark green leaves, stunted growth and reduced seed production [21]. Several key genes for GA signalling have been identified. The binding of GAs to the GA receptor GID1 drives the interaction between GID1 and DELLA, which ultimately leads to the degradation of the DELLA protein by the 26S proteasome [22]. A total of three GID1 genes were identified in Arabidopsis, and it was found that GID1 single mutants did not have a significant GA-insensitive phenotype, but triple mutants exhibited plant dwarfism and impaired growth and development [23]. DELLA belongs to the GARS family of transcription factors [24], and when the structure of the protein is altered so that it cannot sense GA signals, resulting in an inability to be degraded, it leads to shortened internodes and plant dwarfism. F-box proteins can interact with target proteins to ubiquitinate them and then degrade them via the 26S proteasome pathway. It was found that DELLA proteins increased, and plants were dwarfed when the Arabidopsis AtSLY1 gene was functionally deficient [25], whereas DELLA proteins were significantly reduced when the AtSLY1 gene was overexpressed, and some of the plant heights of the plants were rescued [26]. The GID2 mutation in rice also results in plant dwarfism accompanied by insensitivity to GAs and reduced fertility [27]. GA-induced degradation of DELLA protein is considered to be a key step in the GA signalling pathway.
The molecular mechanisms of GA and SL signalling are very similar, both acting after hormone-induced protein hydrolysis, and both hormone receptors D14 and GID1 belong to the α/β hydrolase family [28]. It is, therefore, speculated that there may be an interaction between GA and SL. It has been reported that in rice, GAs negatively regulate SL synthesis in plants, that SL levels are higher in GA-insensitive mutants [29] and that D14 can be combined with SLR1 in the presence of SLs [30]. However, studies in pea indicated that the effect of SLs in promoting internode elongation was not affected by GAs [31]. Whether D53, an important gene in the SL signalling pathway, responds to GA in banana is unclear.
Banana (Musa spp.) is a monocotyledonous plant of the genus Musa in the family Musaceae, which is popularly known for its nutrition and taste and is an important economic and food crop worldwide [32]. In the production process, due to the banana plant’s tall, heavy crown and poor wind resistance, it is prone to serious breakage and collapse when it encounters typhoons or tropical storms, causing the banana industry to suffer significant losses [33]. Dwarf varieties of banana plants are short and strong, which not only improves wind resistance but also facilitates cultivation and management [34]. At the same time, it can shorten the growth cycle and effectively improve the mu yield of bananas [35]. Therefore, it is important to study the dwarfing mechanism of banana for the long-term development of the banana industry. D53/SMXLs, as a key repressor of the SL signal transduction pathway, plays an important role in SL-mediated plant dwarfing. In order to further investigate the mechanism of the D53 gene in dwarf banana, this study was based on genomic data from three species of M. acuminata, M. balbisiana and M. itinerans, and the genome-wide identification of the banana D53 gene. We also investigated the effects of plant height-related hormones GR24 (synthetic SL analogue) and GA on the plant height of “Yinniaijiao” dwarf banana (Musa spp. AAA group, a dwarf cultivar from variation via tissue culture) and the expression of its MaD53 gene, and to provide a reference for further investigation of the function of banana D53 gene and the mechanism of banana dwarfing.
2. Results and Analysis
2.1. Identification of D53 Gene and Analysis of the Protein in Banana
Through protein Blast and structural domain prediction analysis, 3 MaD53, 3 MbD53 and 2 MiD53 genes were finally identified in the genomes of Musa acuminata, M. balbisiana and M. itinerans, respectively. Based on the position information of each member in the genome, the three MaD53 were named MaD53-1–MaD53-3, and the naming principles of MbD53 and MiD53 were consistent with those of MaD53.
The analysis of the proteins revealed that MaD53, MbD53 and MiD53 proteins had small differences in the number of amino acids (Table 1). Their mean average values are 1175, 1177 and 1057, respectively, with molecular weights ranging from 115,061.28 to 130,219.95 Da and isoelectric points ranging from 5.79 to 6.12. In addition, the instability coefficients of banana D53 proteins were all greater than 40, and the average hydrophilicity coefficients were all negative, indicating that all were unstable hydrophilic proteins. Subcellular localisation prediction showed that all of them were localised to the nucleus, except MaD53-3 and MbD53-3, which were localised to the chloroplasts.

Table 1.
The basic information of D53 gene in banana.
2.2. Phylogenetic Analysis of D53
Calculation of the similarity between the D53 sequences of the three genomes of banana, as well as the D53 similarity between banana and other monocotyledonous plants, showed that the similarity between MaD53, MbD53 and MiD53 sequences ranged from 49.7648 to 97.1477 (Table S1). The similarity between banana D53 and other monocotyledon D53 sequences ranged from 22.2123 to 42.8164, with MiD53-1 and DcD53 having the highest similarity of 42.8164.
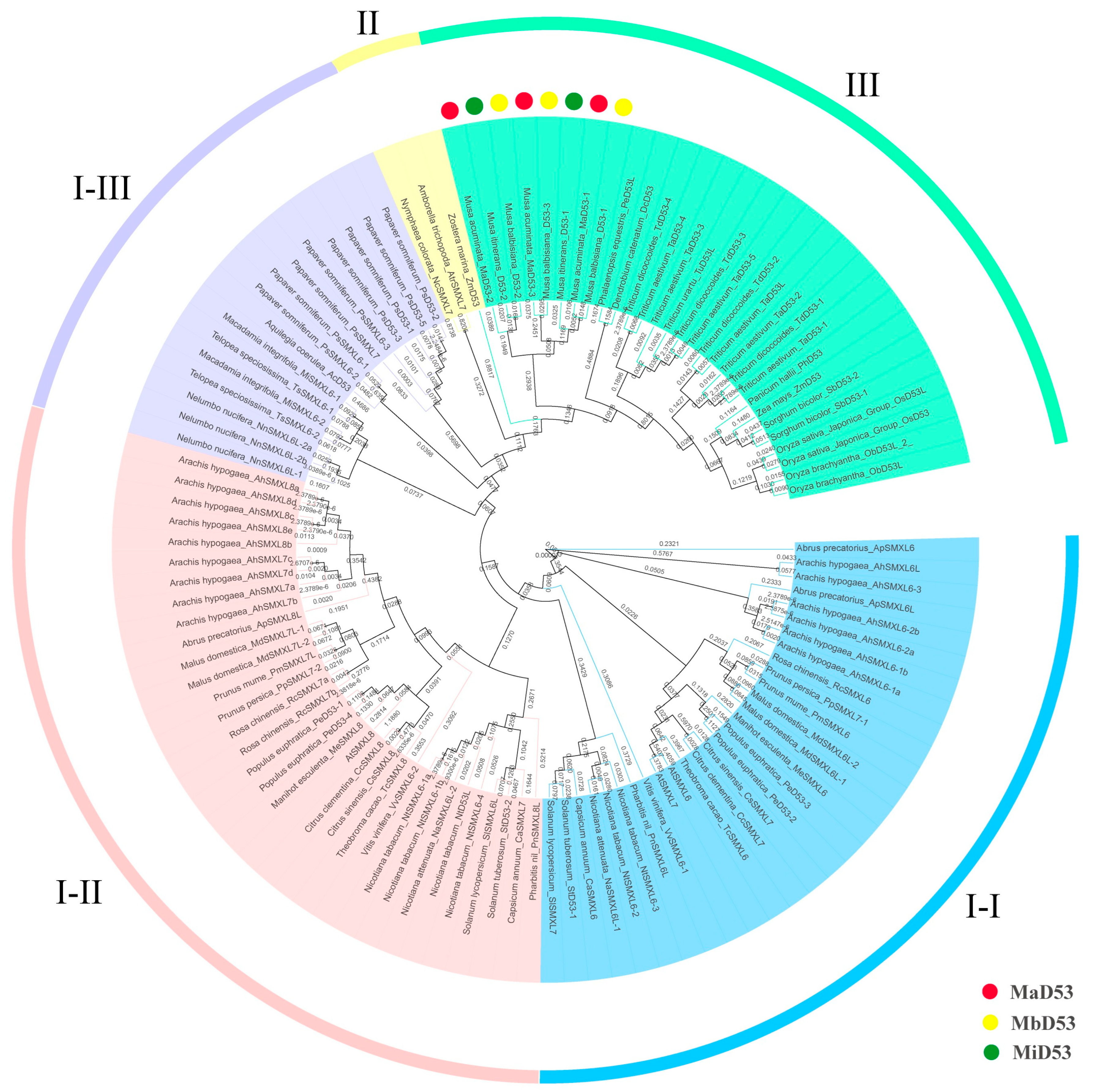
To investigate the phylogenetic relationship of the D53 gene, a phylogenetic tree was constructed by MEGA6.06 for D53 proteins of 40 species, including banana, rice, Arabidopsis, maise and so on (Figure 1). The D53 proteins of the three genomes in banana clustered into a single unit with an equal evolutionary relationship to the D53 proteins of the Asparagales and Poales, such as Phalaenopsis equestris PeD53, Dendrobium catenatum DcD53, wheat (TaD53, TdD53, TuD53), sorghum SbD53, maise ZmD53 and rice OsD53. Currently, the D53 gene has been found only in angiosperms, not in algae, mosses, ferns or gymnosperms, and only the paralogous homologues of D53, such as SMAX1 and SMXL2, have been found. It suggested that the D53 gene originated in angiosperms and evolved by doubling genes such as SMXL1, SMXL2 and so on.
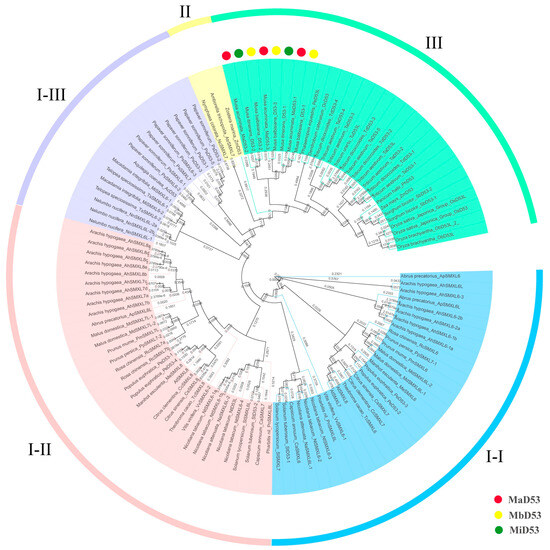
Figure 1.
Phylogenetic tree of D53 proteins. Colour blocks I-I, I-II and I-III are all dicot D53 proteins. Colour block II is a basal angiosperm D53 protein, and colour block III is a monocot D53 protein.
Two or more D53 genes are commonly found in dicotyledons, which cluster into two evolutionary branches each. In contrast, D53 genes in monocotyledons are clustered into only one branch, and only one D53 gene has been found in the basal angiosperms Zostera marina (annotated D53), Amborella trichopoda and Nymphaea colorata (annotated SMXL7). It suggested that at an early stage, the D53 gene underwent a gene doubling event only in the dicot ancestor but not in the monocot ancestor. However, two or more D53 genes were found in some monocotyledonous plants, such as rice, wheat, sorghum and Musa acuminata Colla, suggesting that some monocotyledonous plants underwent a recent D53 doubling event within the family or genus.
2.3. Chromosomal Localisation and Collinearity Analysis of Banana D53 Gene
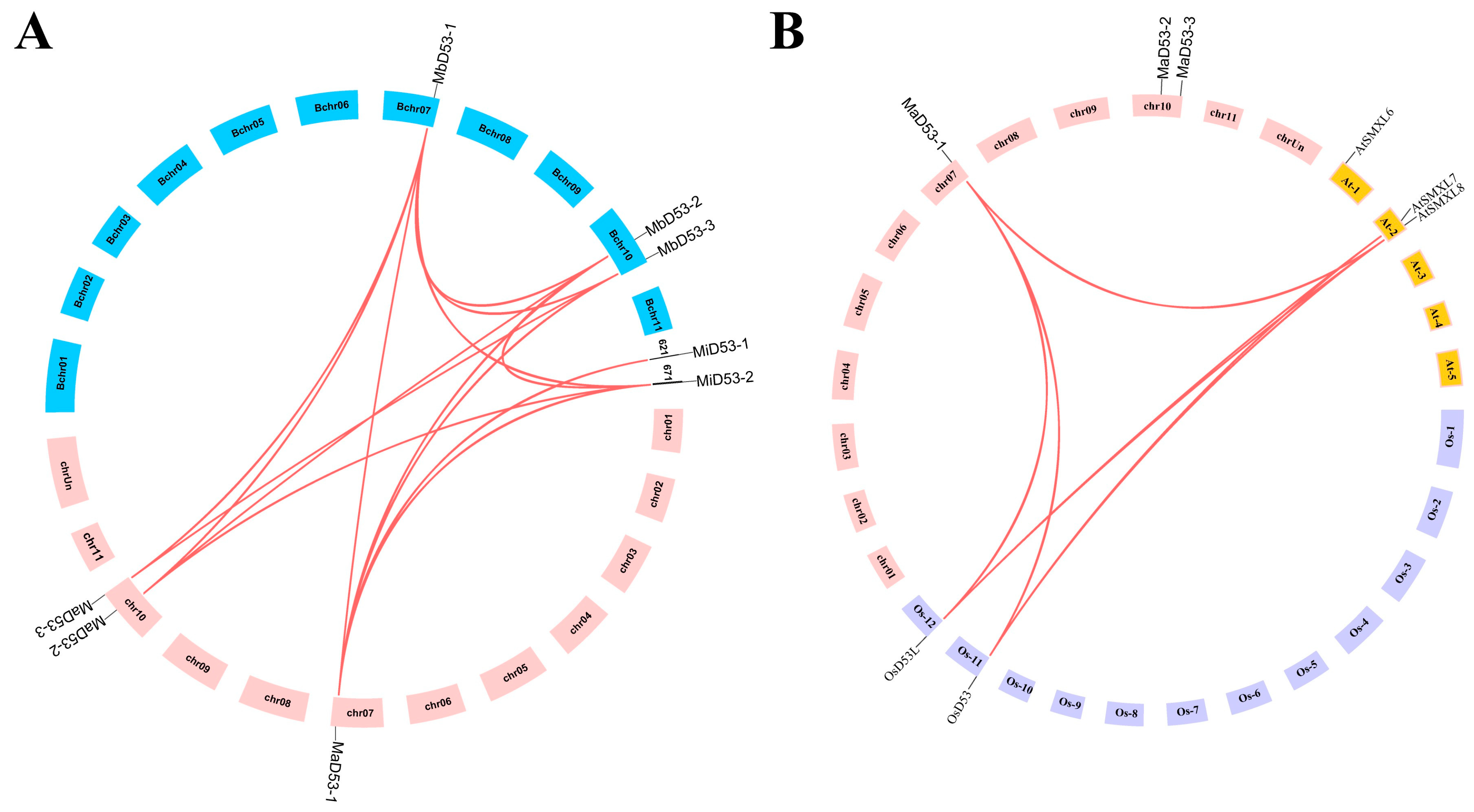
The chromosomal localisation of the banana D53 gene is shown in Figure 2A. Both MaD53 and MbD53 genes are localised on two chromosomes, chr 7 and 10, where MaD53-1 and MbD53-1 are located on chr 7, and MaD53-2, MaD53-3, MbD53-2 and MbD53-3 are all located on chr 10. M. itinerans assembled only to the scaffold (S) level, with MiD53-1 and MiD53-2 localised on S621 and S671, respectively.
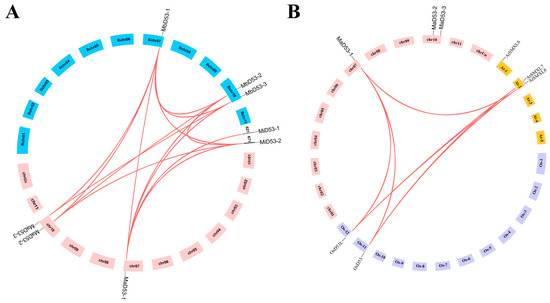
Figure 2.
The co-linear distributions of D53 gene in banana. (A) The co-linear distribution of banana MaD53, MbD53 and MiD53 genes. (B) The co-linear distribution of banana, rice and Arabidopsis D53 genes. The line indicates the segmental replication gene pair of D53 in banana.
To investigate the gene duplication events of banana D53, the D53 genes of banana, rice and Arabidopsis were analysed for collinearity. The results showed that no collinearity existed in both banana MaD53 and MiD53, but collinearity existed between MbD53 genes with two homologous gene pairs. The collinearity analysis of D53 genes in all three genomes of banana revealed that MaD53 found homologous genes in MbD53, and MiD53 found homologous genes in MaD53. There were seven homologous gene pairs between MaD53 and MbD53, three homologous gene pairs between MaD53 and MiD53 and two homologous gene pairs between MbD53 and MiD53, suggesting that the D53 gene was conserved in banana. In addition, MaD53 also has homologous genes in Arabidopsis thaliana and rice, and MaD53-1 has collinearity with two OsD53 and one AtD53 gene, respectively (Figure 2B).
The nonsynonymous substitution (Ka) and synonymous substitution (Ks) values of the homologous gene pairs of banana D53 were calculated to analyse whether the gene was subjected to natural selection pressure during the evolutionary process and to trace its duplication time (Table 2). The Ka/Ks values of the segmentally duplicated gene pairs in the banana D53 gene were all less than 1, indicating that the banana D53 gene was subjected to purifying selection during the evolutionary process. The prediction of the replication time of the banana D53 gene showed that the MiD53-2 and MaD53-1 genes had the earliest replication time of about 57.2744 million years ago, followed by MiD53-2 and MbD53-1, with a replication time of about 57.0194 million years ago. It was hypothesised that the MaD53-1 and MbD53-1 genes might have both been generated by MiD53-2 gene duplication.

Table 2.
D53 gene replication events in banana.
2.4. Gene Structure and Protein Structural Domain Analysis of D53 in Banana
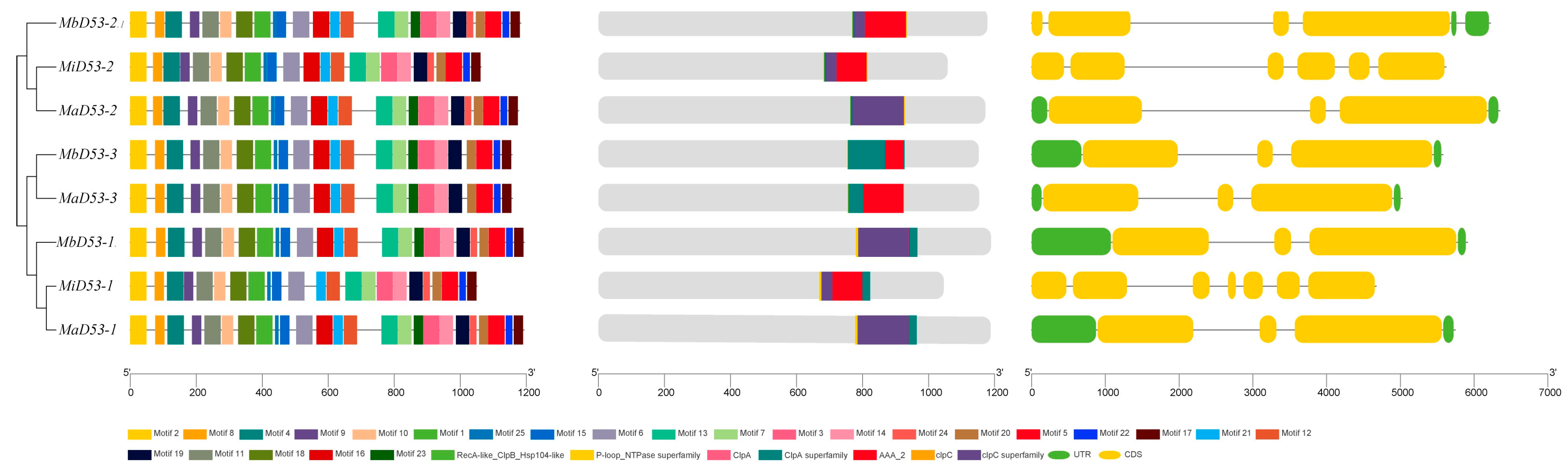
The exon–intron structure of the banana D53 gene was analysed, and it was found that the MiD53 gene had the highest number of exons and introns (Figure 3), about twice as many as MaD53 and MbD53. MaD53 and MbD53 had only three exons and two introns, except for MbD53-2, which had four exons and three introns.
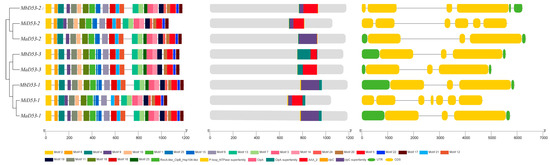
Figure 3.
The analysis of gene structures and protein domain of D53 genes.
The results of protein structural domain prediction showed that MaD53, MbD53 and MiD53 proteins all contain RecA-like_ClpB_Hsp104-like, P-loop_NTPase superfamily, ClpA, ClpA superfamily and AAA_2 structural domains. It was also found that clpC and clpC superfamily structural domains were present in other D53 proteins besides MaD53-1 and MbD53-1.
Analysis of the 25 conserved motifs of the banana D53 protein revealed that MaD53-1, MaD53-2, MbD53-1 and MbD53-2 all contain 25 motifs. In addition, MaD53-3 and MbD53-3 were missing motif 24, MiD53-1 was missing motif 16 and motif 23 and MiD53-2 was missing motif 23. The high sequence and structural similarity of banana D53 proteins suggested that they may be functionally conserved.
2.5. Analysis of Promoter Cis-Acting Elements and Transcription Factor Binding Sites of D53 Gene in Banana
2.5.1. Analysis of Promoter Cis-Acting Elements
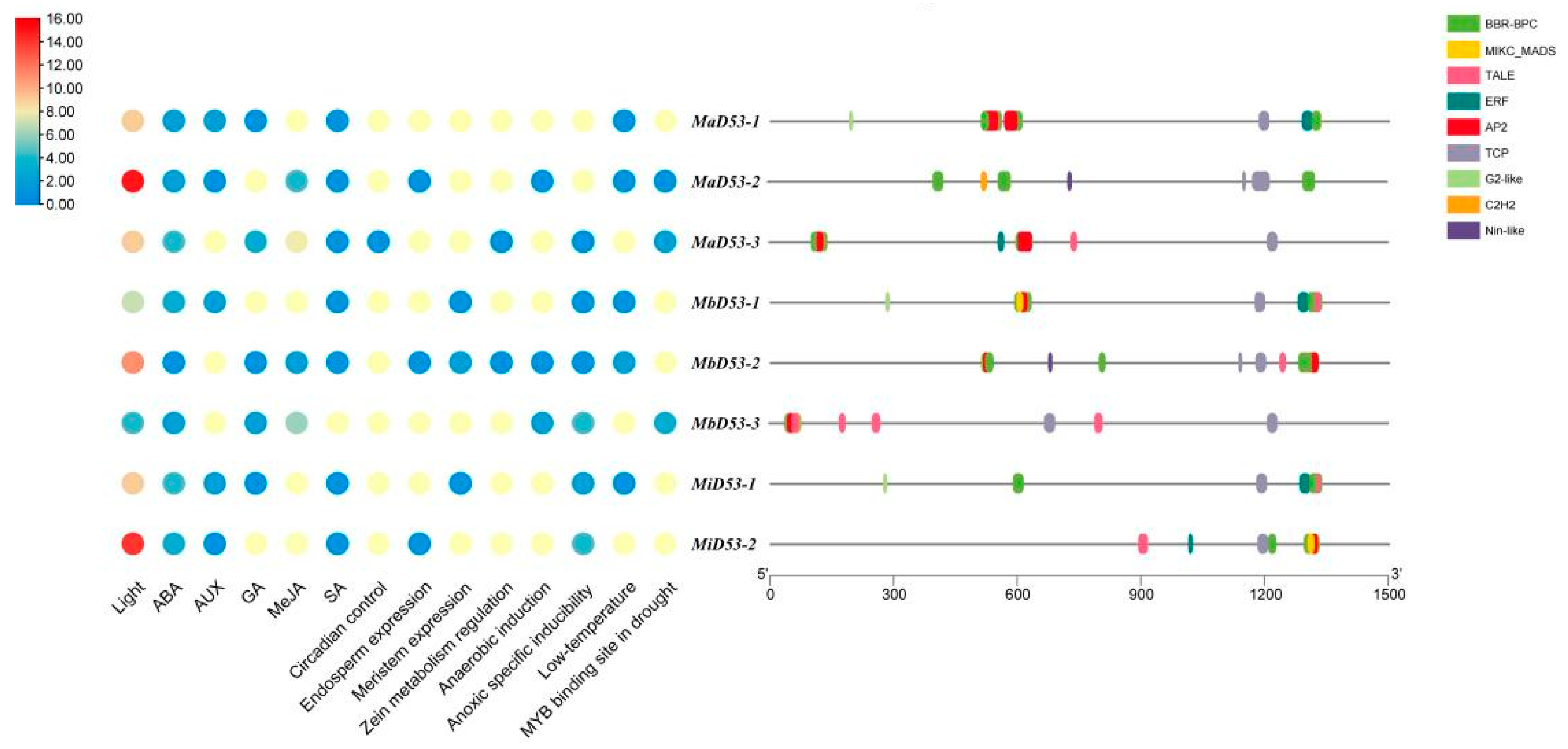
Analysis of cis-acting elements in the 1500 bp region of the banana D53 gene promoter, classification and counting of cis-acting elements with different functions and their site numbers and the results are shown in Figure 4. Multiple light-responsive elements, hormone-responsive elements and response elements related to stress or plant growth and development are present in the banana D53 gene promoter. It indicates that the D53 gene is regulated by various factors and may be involved in the regulation of a variety of physiological processes during banana growth and development.
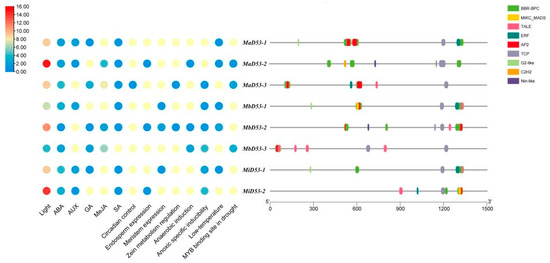
Figure 4.
Prediction of cis-acting elements (left) and transcription factor binding sites (right) in the promoters of D53 in banana.
The banana D53 gene has the highest number of light-responsive elements in the promoter, ranging from 4 to 15, and the highest number was found in D53-2 in all three genomes. The banana D53 gene contains five hormone response elements, abscisic acid (ABA), growth hormone (AUX), gibberellin (GA), methyl jasmonate (MeJA) and salicylic acid (SA). Each D53 gene has an ABA response element, and all contain at least three hormone response elements, suggesting that the D53 genes in bananas may be interlinked with other hormones and work together to regulate plant growth and development. Some growth and development-related response elements were also found in the D53 genes, such as endosperm expression (GCN4_motif), circadian control (circadian), meristem expression (CAT-box) and zeatin metabolism regulation (O2-site). It suggested that the banana D53 genes may be involved in the regulation of circadian rhythm, protein metabolism and other life processes. A variety of adversity stress response elements were also present in the promoter of banana D53 gene, including anaerobic-induced (ARE), anaerobic-specific inducibility (GC-motif), low-temperature (LTR), and MYB-binding sites in drought, which suggested that D53 gene may play a role in abiotic stress processes. In addition, more stress-responsive elements were present in the MbD53 and MiD53 genes compared with the MaD53 gene, indicating that the MbD53 and MiD53 genes may play a more important role in banana’s resistance to stresses.
2.5.2. Transcription Factor Binding Site Analysis
In order to investigate the interaction of transcription factors with banana D53 gene, the transcription factor binding sites (TFBS) within the 1500 bp region of its promoter were categorised and counted. A total of nine transcription factor (TF) families (BBR-BPC, AP2, C2H2, ERF, G2-like, MIKC_MADS, Nin-like, TALE, TCP) were found in the promoter of the banana D53 gene. Of these, seven are MaD53-1, MbD53-1 and MiD53-1; six are MaD53-3, MbD53-2 and MiD53-2; and five are MaD53-2 and MbD53-3. BBR-BPC had the highest number of binding sites, with 134, 90 and 43 in the MaD53, MbD53 and MiD53 genes, respectively, present in each banana D53 gene. This was followed by MIKC_MADS and TALE, with TALE present in every D53 gene and MIKC_MADS present in all D53 genes except MaD53-2. The type, number and distribution of transcription factors on different D53 genes in banana were also different, such as G2-like, which was only present in MaD53-1, MbD53-1 and MiD53-1 genes; Nin-like, which was only present in MaD53-2 and MbD53-2 genes; C2H2, which was only present in MaD53-2 gene. The largest type and number of transcription factors in banana was the MaD53 gene, followed by MbD53, and MiD53 was the least. Therefore, it is hypothesised that banana D53 genes play multiple roles in the growth, development and fruiting process of banana, and different D53 genes may play different roles.
2.6. Analysis of Transcriptome Data of MaD53 Gene under Different Treatments
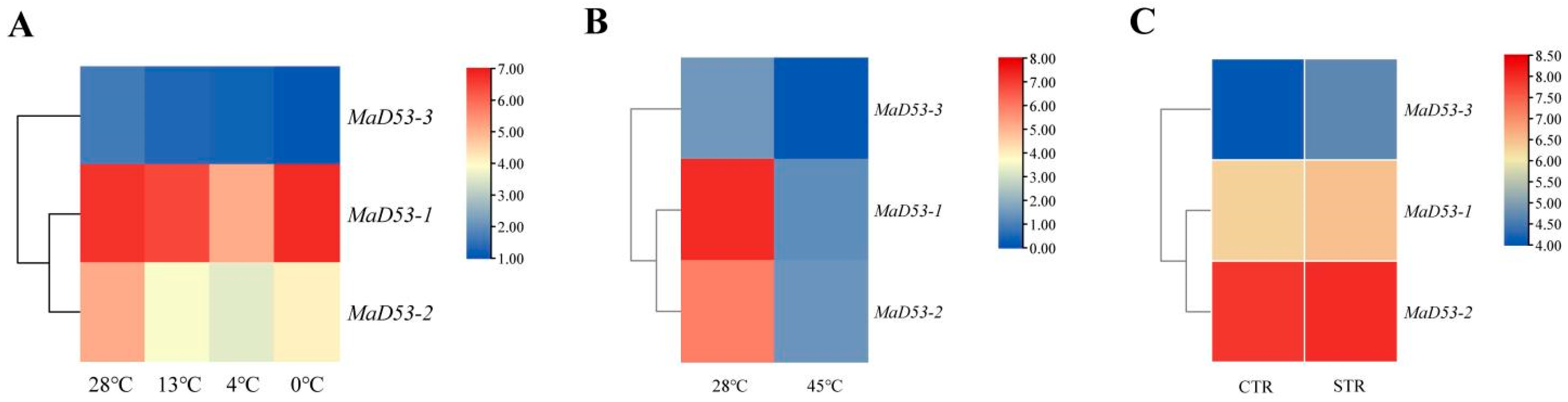
To understand the expression of the MaD53 gene under various abiotic stresses, the FPKM values of the MaD53 gene were extracted from the transcriptomic data of high temperature “Tianbaojiao”, different low temperatures of “Sanmingyeshengjiao” and “Grande Naine” Cavendish osmotic stress to plot a heatmap (Figure 5). Under different low-temperature treatments, the expression of MaD53-1 and MaD53-2 decreased and then increased, reaching a minimum at 4 °C, and the expression of MaD53-3 decreased continuously. Compared with the 28 °C control, the expression of MaD53 was substantially decreased under high-temperature treatments. However, the expression of MaD53 was only slightly up-regulated under osmotic stress. It can be seen that both high and low-temperature stresses have a large effect on the expression of MaD53-1 and MaD53-2, while MaD53-3 may have a smaller effect because of its relatively low expression in the plant.
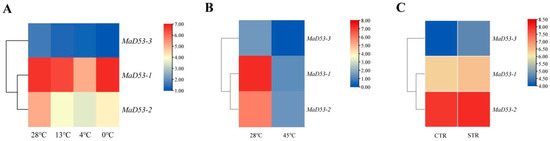
Figure 5.
Heatmap of banana MaD53 gene expression. (A) Transcriptome data of ‘Sanmingyyeshengjiao’ banana leaves from different low-temperature treatments and the control at 28 °C. (B) Transcriptome data of ‘Tianbaojiao’ banana leaves from high-temperature treatments at 45 °C and the control at 28 °C. (C) Transcriptome data of the osmotic stress treatments and the control.
2.7. Expression Analysis of MaD53 Gene in Different Tissue Parts of “Yinniaijiao” Dwarf Banana
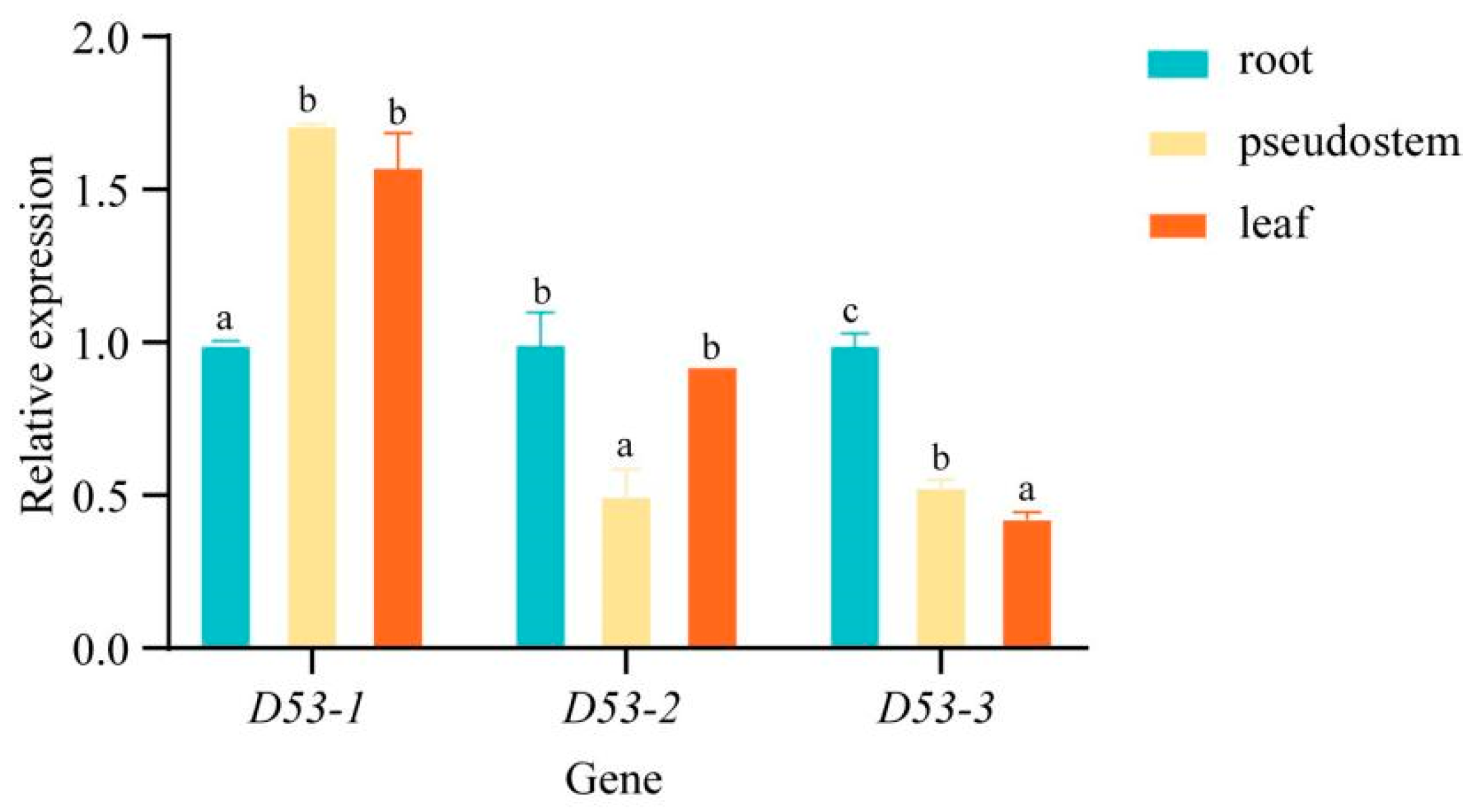
In order to investigate the specific expression pattern of MaD53 genes in “Yinni Dwarf”, the expression of three MaD53 genes was analysed in different tissues using UBQ2 as an internal reference gene (Figure 6). The three MaD53 genes were expressed in banana roots, pseudostems and leaves, and they were widely involved in the growth and development of banana. The expression of MaD53 genes and their expression patterns were different in different parts of banana, among which MaD53-1 was significantly higher in pseudostem and leaves than in roots. The expression of MaD53-2 was highest in roots, followed by leaves and, finally, pseudostems. MaD53-3 was not highly expressed in roots, pseudostems and leaves, and its expression was significantly lower in both pseudostems and leaves than in roots.
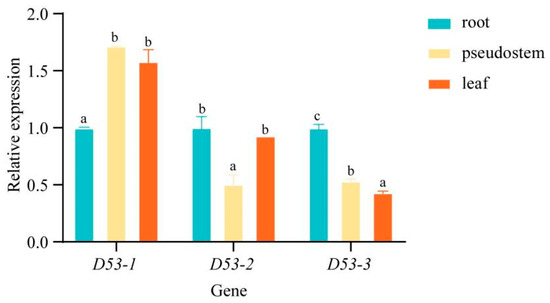
Figure 6.
Relative expression levels of MaD53 genes in different tissues. Lowercase letters indicate significant differences at p < 0.05.
2.8. Expression Pattern Analysis of MaD53 under Hormone Treatment
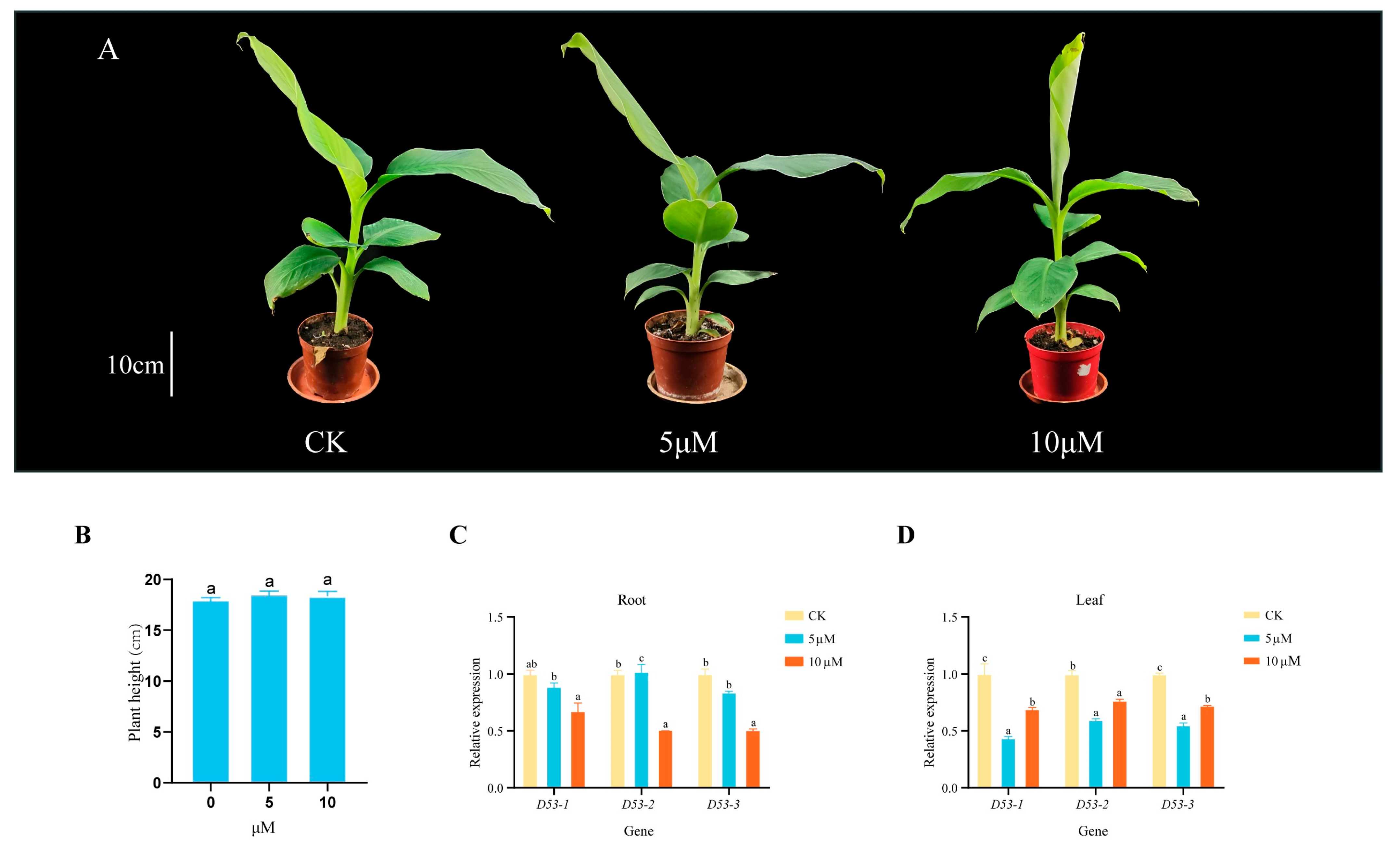
Several studies have shown that the D53 gene in plants responds to SL treatment, but whether MaD53 expression in banana is regulated by SLs has not been reported. Therefore, in this study, different concentrations of GR24 solution were applied to the “Yinniaijiao” dwarf banana to investigate the effect of exogenous GR24 treatment on the height of banana plants and the expression of MaD53 gene (the internal reference gene was CAC). Plant height of banana was not significantly increased by 5 μM and 10 μM GR24 treatments compared to control (p < 0.05) (Figure 7A,B and Table 3). The expression of MaD53 after GR24 treatment was different in leaves and roots (Figure 7C,D), but most of them were lower than the control. In roots, the expression of MaD53-1 and MaD53-3 decreased with the increase in treatment concentration, and the expression of MaD53-2 was highest at 5 μM and significantly lower than the control at 10 μM. In leaves, the expression of all MaD53 genes decreased and then increased, with the highest expression in control and the lowest at 5 μM treatment. The above results indicated that all MaD53 genes responded to GR24 treatment.
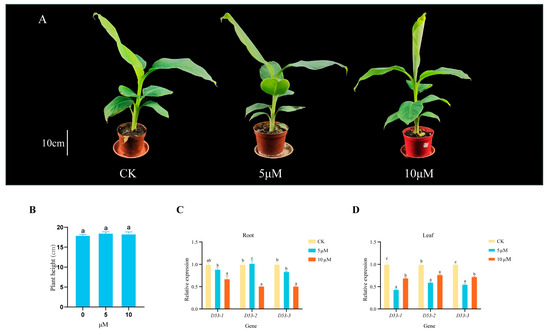
Figure 7.
(A) “Yinniaijiao” dwarf banana treated with different concentrations of GR24. (B–D) Growth rate and expression levels of MaD53 gene in roots and leaves of “Yinniaijiao” dwarf banana under GR24 treatment. Lowercase letters indicate significant differences at p < 0.05.

Table 3.
Effect of different concentrations of exogenous hormone spraying on plant height of “Yinniaijiao” dwarf banana.
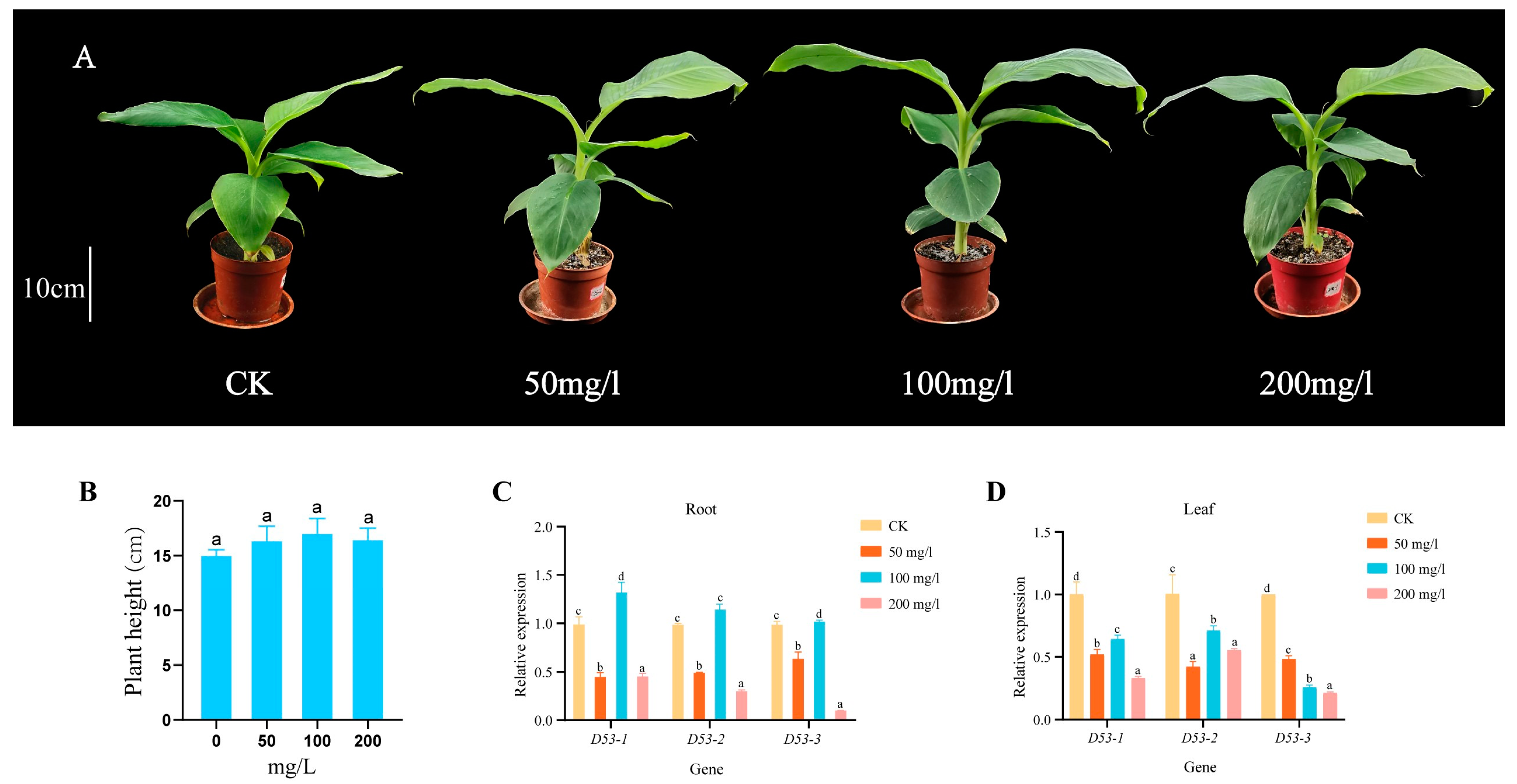
In order to investigate whether GA treatment can affect the plant height and MaD53 gene expression of “Yinniaijiao” dwarf banana, the present study was carried out with different concentrations of GA, and the expression of MaD53 gene was detected (the internal reference gene was UBQ2). Plant height of banana was not significantly increased in different concentrations of GA treatments compared to the control, but the expression of MaD53 was significantly changed (Figure 8A,B and Table 3). The expression of the three MaD53 genes in roots showed the same trend of decreasing (Figure 8C,D), then increasing and then decreasing, with the highest expression at 100 mg/L and a significant decrease at 200 mg/L, which was similar to the trend of plant growth rate. In leaves, the expression of MaD53 genes in the treatment groups was all significantly lower than that in the control group. Among them, the expression of MaD53-1 and MaD53-2 firstly increased and then decreased, while the expression of MaD53-3 continuously decreased. The above results suggested that exogenous GA treatment affected the expression of MaD53, a key gene for SL signalling in banana, and that the effect had tissue and concentration variability.
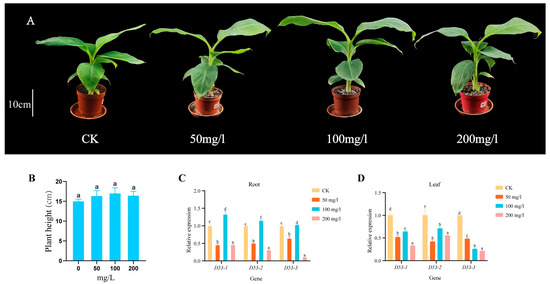
Figure 8.
(A) “Yinniaijiao” dwarf banana treated with different concentrations of gibberellic acid (GA). (B–D) Growth rate and expression levels of MaD53 gene in roots and leaves under different concentrations of GA treatment. Lowercase letters indicate significant differences at p < 0.05.
3. Discussion
3.1. The Banana D53 Gene Evolved with a Gene Doubling Event with a Loss Event
Phylogenetic tree analysis of the D53 protein revealed some important phenomena in the origin and evolution of the D53 gene in the plant kingdom. One D53 gene (annotated as SMXL7) was present in the basal angiosperms Amborella trichopoda and Nymphaea colorata, whereas no D53 gene was found in algae, mosses, ferns and gymnosperms, and there were only its paraphyletic homologous genes SMAX1, SMXL2 and so on. This suggested that the D53 gene originated in angiosperms, presumably as a result of a gene doubling event in the ancestral genes of SMAX1 and SMXL2. This doubled gene was mutated to form the D53 gene, the D53 (SMXL7) gene in early angiosperms, which was retained by natural selection in the evolutionary process. The D53 gene doubling event did not occur in the ancestors of monocotyledonous plants, but some monocotyledonous plants underwent a recent doubling event of the D53 gene within the family or genus. Thus, some monocotyledonous plants have two or even more D53 genes, such as Musa acuminata Colla, Oryza sativa Japonica and wheat. Doubling events of the D53 gene occurred in early dicot ancestors after the separation from the basal angiosperms, so that more than one D53 gene is commonly present in dicotyledons. In addition, some other dicotyledons have also undergone recent doubling events of the D53 gene within the family, such as Arabidopsis thaliana in the Cruciferae, peanut in the Leguminosae, apple in the Rosaceae and tobacco in the Solanaceae. Some of their D53 genes first clustered into a single unit and then collocated with D53 genes in other species.
It has been pointed out that three whole-genome duplication (WGD) events occurred in banana during the evolutionary process, namely the α, β (about 70 million years ago) and γ events (about 100 million years ago) [36]. The duplication event between banana MaD53 and MbD53 genes occurred from 3.3939–56.9012 Mya after the α event, while the A and B genomes diverged at a very close time of about 5.4 Mya [37]. It is hypothesised that the banana A genome underwent a gene-doubling event when it underwent the three WGD events, which resulted in three MaD53 genes in the A genome. The A and B genomes had begun to diverge by the time the MaD53 and MbD53 gene duplications occurred, and therefore, there were also three MbD53 genes in the B genome after complete divergence. Musa itinerans is the same AA-type diploid wild species, and the duplication between MaD53 and MiD53 genes was also after three WGD events, but only two MiD53 genes exist in its genome at present, and it is presumed that the MiD53 gene was doubled and then lost. The same loss of the D53 gene occurred in both monocotyledonous and dicotyledonous plants. Aquilegia coerulea is a tetraploid plant whose ancestral palaeotetraploidisation occurred after the divergence of the columbine from the opium poppy [38]. There are eight D53 genes in the opium poppy, whereas only one D53 gene is found in the columbine, suggesting that a loss event of the D53 gene in the columbine may have occurred, as well.
3.2. MaD53 Gene May Have Evolved Functionally Differently in Banana
The D53 gene plays an important role in SL signalling as a key repressor of the SL signalling pathway. There are three D53 immediate homologs in Arabidopsis, AtSMXL6/7/8, which all function as repressors in the SL pathway but have their own unique functions. In terms of regulating branching, the smxl6/7/8 single mutant and smxl6/7, smxl6/8, smxl7/8 double mutants showed similar phenotypes to the wild type, whereas the number of secondary shoots of the smxl6/7/8 triple mutant was significantly less than that of the wild type. It suggested that SMXL6, SMXL7 and SMXL8 had a redundant function in promoting the growth of axillary buds in Arabidopsis [39]. In addition, the number of branches in the smxl7max2 mutant was much less than that in the smxl6max2 and smxl8max2 mutants, suggesting that although SMXL6/7/8 can regulate Arabidopsis branching, SMXL7 plays a major role, which may be related to the fact that SMXL7 is mainly expressed in axillary branches [5]. Furthermore, it was found that SMXL6, in addition to acting as a repressor of the SL pathway, can also act as a transcript that directly binds to the promoter of AtSMXL6/7/8 and regulates its transcription.
Analysis of MaD53 gene expression in several tissues of “Yinniaijiao” dwarf banana revealed that MaD53-1, MaD53-2 and MaD53-3 were expressed in roots, pseudostems and leaves and were widely involved in the growth and development of banana, but the dominant sites of expression of each gene were different. Among them, the expression of MaD53-1 was higher in leaves and pseudostems and significantly higher than in roots. MaD53-2 was mainly expressed in roots and leaves, with significantly lower expression in pseudostems than in other tissues. The expression of MaD53-3 was the highest in roots and significantly higher than in pseudostems and leaves. MaD53-1 is hypothesised to function mainly in leaves and pseudostems, MaD53-2 mainly in leaves and roots and MaD53-3 mainly in roots. The functions of plant genes are, to some extent, regulated by their promoter cis-acting elements and transcription factor binding sites [40]. It was found that the number of hormone-responsive elements, as well as the types of stress, growth and development-responsive elements in MaD53-1, were much less than those in MaD53-2 and MaD53-3, while the types and numbers of transcription factor binding sites in MaD53-2 were much less than those in MaD53-1 and MaD53-3. Taken together, it is hypothesised that the function of the MaD53 gene has evolved in different directions. In addition, the expression trends of MaD53-2 and MaD53-3 were found to be more similar, with both having the highest expression in roots, which was quite different from MaD53-1, suggesting that the functions of MaD53-2 and MaD53-3 may be more similar. Strigolactones are a class of terpene lactones, which are mainly synthesised in roots and transported up the stem to various parts of the aboveground, and then play a role in multiple stages of plant growth and development. MaD53-1 was mainly expressed in the aboveground part of the plant, which coincided with the function of SLs in the plant, and thus, it was hypothesised that MaD53-1 played the main function in the SL pathway.
3.3. The MaD53 Gene in “Yinniaijiao” Dwarf Banana Responds to GA and SL but Might Not Regulate Plant Height
Strigolactone has an important role in regulating the formation of aboveground plant phenotypes, including the number of branches, leaf shape, leaf colour, the height of the main stem and thickness, and has been investigated in a variety of plant species. Plant height, one of the important agronomic traits of crops, dwarfing the plant under the premise of ensuring the biological yield of the crop can not only effectively improve the ability of resistance to collapse [41] but also make full use of the land space, improve the utilisation of light energy and increase the yield per unit area [42]. Studies in recent years have shown that GA and SL biosynthesis and signal transduction are usually related to plant height [43]. Studies have shown that spraying GA3 significantly promotes kale plant growth [44]. In rice, the “green revolution” gene sd1 regulates rice plant height by participating in GA biosynthesis [45], and the wheat semi-dwarfing genes Rh1 and Rh2 are involved in GA signalling [46]. The SL synthesis pathway mutant d10 and d17 and the signalling pathway mutants d13, d4 and d53 all showed dwarfing and multiple tillering traits, and the application of GR24 alleviated the dwarfing and multiple tillering phenotypes of d10 and d17 but not those of d3, d14 and d53 [16,47,48], and significantly down-regulated the expression of their D53 gene [2]. In apple, lower concentrations of GA did not promote plant height and internode elongation in A1d (dwarf plants formed by a GA signalling mutation), but it responded to high concentrations of GA [49]. In this experiment, GA and GR24 were sprayed on “Yinniaijiao” dwarf banana, and it was found that GA and GR24 could not significantly increase plant height (p < 0.05), but the expression of MaD53 gene was mainly down-regulated in roots and leaves after the treatments. This suggested that although the MaD53 gene responded to GA and SL treatments, “Yinniaijiao” dwarf banana may not be sensitive to GA or GR24. In this regard, the following speculations were made: (i) The concentration of the hormone used was not high enough to reach the threshold for significant plant height promotion. (ii) The GA or SL signalling genes were mutated, resulting in their inhibitory proteins not being degraded and the dwarfing phenotype not being alleviated. In addition, 100 mg/L GA treatment promoted the expression of the MaD53 gene in roots, but the expression of this gene was significantly reduced in leaves. The D53 gene is a repressor of the SL signalling pathway, and elevated expression of this gene inhibits plant growth. It is, therefore, hypothesised that the rise in MaD53 gene expression in roots at this concentration inhibited root growth and thus resulted in failure to promote plant height.
Since the molecular mechanisms of GA and SL signalling are very similar [50], and the receptor proteins both belong to the α/β-hydrolase family [51], several studies have indicated the existence of interactions between GA and SL, but their interactions seem to be different in different species. In jatropha, SL treatment up-regulated the expression of NAC genes, contrary to the results of GA treatment [52]. In rice, GA treatment decreased the expression of SL synthesis genes, and thus SL levels in vivo, and the regulation was induced through the GID-DELLA signalling pathway [53]. GR24 treatment not only binds D14 and D53, the key genes of the SL signalling pathway but also binds D14 and the DELLA protein SLR [30]. In cucumber, the expression of the CsD14 gene in roots and leaves was suppressed under low-concentration GA treatment and significantly increased under high-concentration GA treatment. In contrast, the expression of the CsD14 gene was significantly increased by both high and low-concentration treatments in stem and leaf axils [54]. This suggested that the regulation of SL signalling pathways by GAs was tissue- and concentration-specific and varies in different tissues. In this study, we found that the expression of MaD53, a key gene of the SL pathway, was significantly changed in both roots and leaves under different concentrations of GA treatment, suggesting an interaction between GA and SL in banana.
4. Materials and Methods
4.1. Material Handling
One-month-old “Yinniaijiao” dwarf banana (Musa spp. AAA group, a dwarf cultivar from variation via tissue culture) transplants with uniform growth were selected for GR24 (SL analogue) and GA treatments. GR24 concentrations were set at 5 μM and 10 μM, GA concentrations were set at 50 mg/L, 100 mg/L and 200 mg/L by spraying method until the leaves and pseudostems were fully wetted, but no droplets fell, and the control group (CK) was sprayed with equal amount of water. Spray every 5 days for a total of 3 sprays. The plant heights were measured after the treatments, and the mean values were taken for statistical analysis. All the treatments were carried out in three biological replications. The first leaf (unfolded leaf), root and different tissue parts (leaf, pseudostem and root) of the control plants were taken and placed in liquid nitrogen for quick freezing and stored in an ultra-low temperature refrigerator at −80 ℃ for total RNA extraction, qRT-PCR expression analysis.
4.2. Methods
4.2.1. Identification of Banana D53 Gene and Phylogenetic Tree Construction
Whole genome files, annotation files and protein files of banana M. acuminata var. DH-Pahang [55], M. balbisiana var. DH PKW [37] and M. itinerans were downloaded from the Banana Genome Database (http://banana-genome-hub.southgreen.fr, accessed on 10 December 2021). D53 protein sequences of other species downloaded from the NCBI database (https://www.ncbi.nlm.nih.gov/, accessed on 15 June 2022).
Homologous blast of banana protein sequences with rice OsD53 and Arabidopsis thaliana SMXL6/7/8 protein sequences by TBtools-II v2.042 [56], respectively, with the parameter threshold E-value ≤ 1 × 10−5 and other parameters as default values. The banana D53 sequence was initially screened by two-way Blast of the obtained candidate sequences using the NCBI website. Combined with NCBI-CDD [57] (https://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml, accessed on 3 September 2023) and SMART [58] (http://smart.embl-heidelberg.de/, accessed on 3 September 2023) predictive analyses of protein structural domains, banana D53 genes finally identified and named with reference to the nomenclature of rice and Arabidopsis thaliana.
Phylogenetic trees were constructed using the maximum likelihood (ML) method of the software MEGA6.06 for D53 proteins of several species, including banana and Arabidopsis thaliana. The sequence alignment method was Muscle. The running model was Jones–Taylor–Thornton (JTT) + Gamma Distributed (G), with the Bootstrap value set to 1000, and other parameter values were defaulted. Similarity of D53 protein sequences was calculated by DNAMAN 6.0 (Lynnon Biosoft) software.
4.2.2. Chromosomal Localisation and Collinearity Analysis of Banana D53 Gene
Based on the genome files and annotation files of banana, rice and Arabidopsis thaliana, TBtools-II v2.042 was used to map the diagram for Chromosomal (chr) localisation and collinearity of the banana D53 gene and the values of nonsynonymous substitutions (Ka) and synonymous substitutions (Ks) were calculated for D53 replicated genes in banana. The gene duplication occurrence time (T) was calculated as T = Ks/2λ, where the evolutionary rate (λ) of Musa was 4.5 × 10−9 [59].
4.2.3. Structure and Protein Analysis of Banana D53 Gene
ExPASy’s [60] online tool ProtParam (https://web.expasy.org/protparam/, accessed on 15 September 2023) were used to predict the amino acids, molecular weight, theoretical isoelectric point, instability coefficient and average hydrophilicity coefficient of banana D53 protein, and similarity of D53 protein sequences was calculated by DNAMAN 6.0 (Lynnon Biosoft) software.
Subcellular localisation of banana D53 protein was predicted using WoLF PSORT [61] (https://wolfpsort.hgc.jp/, accessed on 15 September 2023). The conserved motifs and structural domains of banana D53 protein were analysed by MEME [62] (https://meme-suite.org/meme/, accessed on 3 September 2023) and NCBI-CDD, respectively, with the number of conserved motif identifications set to 25. The resulting files were submitted to TBtools for visual mapping.
4.2.4. Prediction of Cis-Acting Elements and Transcription Factor Binding Sites in the Promoter of the Banana D53 Gene
TBtools was used to extract 1500 bp upstream of the transcription start site of the D53 gene from the banana genome file and submitted to PlantCARE [63] (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 4 September 2023) and PlantTFDB [64] (http://planttfdb.cbi.pku.edu.cn/, accessed on 5 September 2023) online websites (with parameters set to p-value ≤ 1 × 10−6) for prediction of its promoter cis-acting elements and transcription factor binding sites. The results obtained were collated and plotted visually using TBtools.
4.2.5. Analysis of Banana MaD53 Gene Expression Pattern
FPKM (fragments per kilobases per million mapped) values of MaD53 gene were extracted from the transcriptome data of high temperature in “Tianbaojiao”, low temperature in “Sanmingyeshengjiao” [65] (A wild banana from Fujian), and osmotic stress in “Grande Naine” Cavendish [66], and submitted to TBtools for heatmap plotting and expression analysis.
Primers were designed using Primer Premier 6 for the MaD53 sequences with target fragment sizes of 80–200 bp, and the primer sequences and annealing temperatures are shown in Table 4. Total RNA was extracted from roots, pseudostems, and leaves of “Yinniaijiao” dwarf banana treated with water (CK), GA, and GR24, respectively, according to the instructions of the FastPure Universal Plant Total RNA Isolation Kit (Vazyme, Nanjing, China). The concentration, purity and integrity of the extracted RNA were determined by 1.0% non-denaturing agarose gel electrophoresis and UV spectrophotometer. The RNA was reverse transcribed into cDNA for qPCR using the Thermo Scientific RevertAid Master Mix Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA). UBQ2 and CAC were used as the internal reference genes [67]. Amplification was performed using a Roche LightCycler 480 fluorescent quantitative PCR instrument, and three replicates of each sample were averaged. The relative expression of target genes was calculated using 2−∆∆CT, Microsoft Excel 2016 and GraphPad Prism (version 8.0.2 for Windows, GraphPad Software, San Diego, CA, USA, www.graphpad.com, accessed on 19 November 2023) software programs were used for calculation and graphing, and IBM SPSS Statistics for Windows, version 26.0 (IBMCorp., Armonk, NY, USA) was used for significance analysis.

Table 4.
Fluorescent quantitative PCR primer information of MaD53 gene.
5. Conclusions
In this study, 3 MaD53, 3 MbD53 and 2 MiD53 genes were identified based on the genomic data of Musa acuminata, M. balbisiana and M. itinerans. The banana D53 gene promoter has a large number of light-responsive, hormone-responsive and adversity stress-responsive elements, indicating that this gene may have important roles in plant growth and development and adversity stress. The evolutionary tree and collinearity analysis hypothesised that the banana D53 genes may have undergone gene doubling events and loss events during the evolutionary process. Tissue-specific expression analysis revealed that the three MaD53 genes were expressed in various tissues, but the site of dominant expression was different for each gene, and it was hypothesised that each MaD53 gene had a different major site of function. GR24 and GA treatments reduced the expression of the MaD53 gene but did not significantly affect plant height in the “Yinniaijiao” dwarf banana, so it was hypothesised that “Yinniaijiao” dwarf banana was not sensitive to GA and SL and that MaD53 might not be able to regulate its plant height.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13030458/s1, Table S1: Similarity of D53 protein sequences.
Author Contributions
Conceptualisation, Z.L. (Zhongxiong Lai) and N.T.; methodology, N.T. and C.Z.; validation, Z.Z. (Zhilin Zhang) and X.X.; formal analysis, N.T. and C.Z.; investigation, N.T.; resources, J.L. and Z.L. (Zhaoyang Liu); data curation, N.T.; writing—original draft preparation, N.T.; writing—review and editing, N.T., Z.L. (Zhongxiong Lai) and Y.C.; supervision, Y.H. and Y.L.; project administration, Z.Z. (Zihao Zhang); funding acquisition, Z.L. (Zhongxiong Lai). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Project (2019YFD1000900), National Modern Agricultural Industrial Technology System (Banana) Special Fund (GARS-31-15), Fujian Province Plateau Science Section construction funding (102/71201801101), Fujian Agriculture and Forestry University Innovation Fund (KFb22021XA).
Data Availability Statement
Data are contained within the article.
Acknowledgments
Thanks to all the research workers of the Institute of Horticultural Biotechnology of Fujian Agriculture and Forestry University who contributed to our study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jiang, L.; Liu, X.; Xiong, G.; Liu, H.; Chen, F.; Wang, L.; Meng, X.; Liu, G.; Yu, H.; Yuan, Y.; et al. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 2013, 504, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Lin, Q.; Zhu, L.; Ren, Y.; Zhou, K.; Shabek, N.; Wu, F.; Mao, H.; Dong, W.; Gan, L.; et al. D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature 2013, 504, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, B.; Yu, H.; Guo, H.; Lin, T.; Kou, L.; Wang, A.; Shao, N.; Ma, H.; Xiong, G.; et al. Transcriptional regulation of strigolactone signalling in Arabidopsis. Nature 2020, 583, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, G.; Zhao, Y.; Wang, H.H.; Dai, Z.; Xue, W.; Yang, J.; Wei, H.; Shen, R.; Wang, H. DWARF53 interacts with transcription factors UB2/UB3/TSH4 to regulate maize tillering and tassel branching. Plant Physiol. 2021, 187, 947–962. [Google Scholar] [CrossRef]
- Soundappan, I.; Bennett, T.; Morffy, N.; Liang, Y.; Stanga, J.P.; Abbas, A.; Leyser, O.; Nelson, D.C. SMAX1-LIKE/D53 Family Members Enable Distinct MAX2-Dependent Responses to Strigolactones and Karrikins in Arabidopsis. Plant Cell 2015, 27, 3143–3159. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhu, J.; Huang, X. Diversification of plant SUPPRESSOR OF MAX2 1 (SMAX1)-like genes and genome-wide identification and characterization of cotton SMXL gene family. BMC Plant Biol. 2023, 23, 419. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.P. Cloning and Expression of PagD53 Gene and Its Interaction with PagD14 Gene in Poplar. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2020. [Google Scholar]
- Gray, W.M. Hormonal regulation of plant growth and development. PLoS Biol. 2004, 2, E311. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tao, B.J.; Hua, X.; Lv, B.; Liu, L.J.; Chen, Y. Research progress on the interaction of chrysomolactone and hormone in regulating root growth. Biotechnol. Bull. 2022, 38, 24–31. [Google Scholar]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of Witchweed (Striga lutea Lour.): Isolation and Properties of a Potent Stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef]
- Kohlen, W.; Charnikhova, T.; Liu, Q.; Bours, R.; Domagalska, M.A.; Beguerie, S.; Verstappen, F.; Leyser, O.; Bouwmeester, H.; Ruyter-Spira, C. Strigolactones Are Transported through the Xylem and Play a Key Role in Shoot Architectural Response to Phosphate Deficiency in Nonarbuscular Mycorrhizal Host Arabidopsis. Plant Physiol. 2010, 155, 974–987. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Ha, C.V.; Leyva-González, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Dong, N.V.; et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Li, J.; Xie, D. Recent advances in molecular basis for strigolactone action. Sci. China. Life Sci. 2018, 61, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Sorefan, K.; Booker, J.; Haurogné, K.; Goussot, M.; Bainbridge, K.; Foo, E.; Chatfield, S.; Ward, S.; Beveridge, C.; Rameau, C.; et al. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev. 2003, 17, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Maekawa, M.; Arite, T.; Onishi, K.; Takamure, I.; Kyozuka, J. Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 2005, 46, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shang, L.; Yu, H.; Zeng, L.; Hu, J.; Ni, S.; Rao, Y.; Li, S.; Chu, J.; Meng, X.; et al. A Strigolactone Biosynthesis Gene Contributed to the Green Revolution in Rice. Mol. Plant 2020, 13, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Seto, Y.; Yasui, R.; Kameoka, H.; Tamiru, M.; Cao, M.; Terauchi, R.; Sakurada, A.; Hirano, R.; Kisugi, T.; Hanada, A.; et al. Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nat. Commun. 2019, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Stirnberg, P.; Furner, I.J.; Ottoline Leyser, H.M. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. Cell Mol. Biol. 2007, 50, 80–94. [Google Scholar] [CrossRef]
- Claus, S. Gibberellin—Mechanism of Action; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Davière, J.M.; Achard, P. Gibberellin signaling in plants. Devevelopment 2013, 140, 1147–1151. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; Martínez-Montiel, N.; García-Sánchez, J.; Pérez, Y.T.R.; Martínez-Contreras, R.D. Gibberellin biosynthesis and metabolism: A convergent route for plants, fungi and bacteria. Microbiol. Res. 2018, 208, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Bolle, C. The role of GRAS proteins in plant signal transduction and development. Planta 2004, 218, 683–692. [Google Scholar] [CrossRef]
- McGinnis, K.M.; Thomas, S.G.; Soule, J.D.; Strader, L.C.; Zale, J.M.; Sun, T.P.; Steber, C.M. The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 2003, 15, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Dill, A.; Thomas, S.G.; Hu, J.; Steber, C.M.; Sun, T.P. The Arabidopsis F-box protein SLEEPY1 targets gibberellin signaling repressors for gibberellin-induced degradation. Plant Cell 2004, 16, 1392–1405. [Google Scholar] [CrossRef] [PubMed]
- Gomi, K.; Sasaki, A.; Itoh, H.; Ueguchi-Tanaka, M.; Ashikari, M.; Kitano, H.; Matsuoka, M. GID2, an F-box subunit of the SCF E3 complex, specifically interacts with phosphorylated SLR1 protein and regulates the gibberellin-dependent degradation of SLR1 in rice. Plant J. Cell Mol. Biol. 2004, 37, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Hamiaux, C.; Drummond, R.S.; Janssen, B.J.; Ledger, S.E.; Cooney, J.M.; Newcomb, R.D.; Snowden, K.C. DAD2 Is an α/β Hydrolase Likely to Be Involved in the Perception of the Plant Branching Hormone, Strigolactone. Curr. Biol. 2012, 22, 2032–2036. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M. Strigolactones and Gibberellins: A New Couple in the Phytohormone World? Trends Plant Sci. 2017, 22, 813–815. [Google Scholar] [CrossRef]
- Nakamura, H.; Xue, Y.L.; Miyakawa, T.; Hou, F.; Qin, H.M.; Fukui, K.; Shi, X.; Ito, E.; Ito, S.; Park, S.H.; et al. Molecular mechanism of strigolactone perception by DWARF14. Nat. Commun. 2013, 4, 2613. [Google Scholar] [CrossRef]
- de Saint Germain, A.; Ligerot, Y.; Dun, E.A.; Pillot, J.-P.; Ross, J.J.; Beveridge, C.A.; Rameau, C. Strigolactones Stimulate Internode Elongation Independently of Gibberellins. Plant Physiol. 2013, 163, 1012–1025. [Google Scholar] [CrossRef]
- Shu, H.Y.; Sun, W.; Wang, Z.; Silver, M.; Han, Q.; Zhou, Z.X.; Dai, M.J.; Jin, Z.Q.; Li, J.Y.; Chang, S.H. Feasibility analysis of banana breeding for wind resistance. Mol. Plant Breed. 2016, 14, 3511–3515. [Google Scholar]
- Huang, J.L. Integrated technology of banana wind resistance and lodging resistance. Trop. Agric. China 2008, 5, 61. [Google Scholar]
- Chen, J.J.; Hu, Y.L.; Pang, Z.C.; Xie, J. A preliminary study on the causes of dwarfing in Williams banana mutant. J. Trop. Crops 2014, 35, 2144–2150. [Google Scholar]
- Wei, Y.R.; Kwong, R.B.; Yang, H. Breeding of a new dwarf banana variety ‘Zhongjiao 11’. J. Fruit Trees 2019, 36, 957–959. [Google Scholar]
- D’Hont, A.; Denoeud, F.; Aury, J.M.; Baurens, F.C.; Carreel, F.; Garsmeur, O.; Noel, B.; Bocs, S.; Droc, G.; Rouard, M.; et al. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 2012, 488, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Miao, H.; Liu, J.; Xu, B.; Yao, X.; Xu, C.; Zhao, S.; Fang, X.; Jia, C.; Wang, J.; et al. Musa balbisiana genome reveals subgenome evolution and functional divergence. Nat. Plants 2019, 5, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Chen, J. A reappraisal of the phylogenetic placement of the Aquilegia whole-genome duplication. Genome Biol. 2020, 21, 295. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, B.; Jiang, L.; Liu, X.; Li, X.; Lu, Z.; Meng, X.; Wang, Y.; Smith, S.M.; Li, J. Strigolactone Signaling in Arabidopsis Regulates Shoot Development by Targeting D53-Like SMXL Repressor Proteins for Ubiquitination and Degradation. Plant Cell 2015, 27, 3128–3142. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, V.K.; Negi, N.; Khurana, P. Genome-wide Identification and Structural, Functional and Evolutionary Analysis of WRKY Components of Mulberry. Sci. Rep. 2016, 6, 30794. [Google Scholar] [CrossRef]
- Zhang, R.M.; Li, C.; Chen, D.L. Breeding of vertical plant type material DW 871 in Xiangyang, Brassica napus. Seed 2019, 38, 116–120+123. [Google Scholar]
- Hou, C.; Liu, S.M.; Xue, Y.; Xu, Z.H.; Wang, P.Y. Research progress on hormone regulation of dwarfing in cucumber and vegetable plants. Chin. Melon 2020, 33, 1–7. [Google Scholar]
- Xing, M.; Su, H.; Liu, X.; Yang, L.; Zhang, Y.; Wang, Y.; Fang, Z.; Lv, H. Morphological, transcriptomics and phytohormone analysis shed light on the development of a novel dwarf mutant of cabbage (Brassica oleracea). Plant Sci. Int. J. Exp. Plant Biol. 2020, 290, 110283. [Google Scholar] [CrossRef]
- Tong, L.; Chen, S.H.; Gu, L.X.; Xu, Y.; Rei, X.; Ni, M.; Wang, Z.W.; Zhu, G.P.; Chen, Y.L. Effect of gibberellin treatment on yield and quality of main side moss of Chinese mustard. J. Trop. Biol. 2020, 11, 7–10+19. [Google Scholar]
- Spielmeyer, W.; Ellis, M.H.; Chandler, P.M. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc. Natl. Acad. Sci. USA 2002, 99, 9043–9048. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef]
- Booker, J.; Auldridge, M.; Wills, S.; McCarty, D.; Klee, H.; Leyser, O. MAX3/CCD7 Is a Carotenoid Cleavage Dioxygenase Required for the Synthesis of a Novel Plant Signaling Molecule. Curr. Biol. 2004, 14, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, K.P.; Vishwakarma, C.; Sahoo, S.P.; Lima, J.M.; Nath, M.; Dokku, P.; Gacche, R.N.; Mohapatra, T.; Robin, S.; Sarla, N.; et al. A substitution mutation in OsCCD7 cosegregates with dwarf and increased tillering phenotype in rice. J. Genet. 2014, 93, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ma, R.; Han, Q.; Zhang, J.-m.; Shi, Z.; Qi, Z.; Huang, Y.; Sha, G.-l.; Ge, H.-j. Transcriptome analysis for deep understanding the dwarfing mechanism of Malus dwarf mutant rootstock A1d. Sci. Hortic. 2023, 319, 112163. [Google Scholar] [CrossRef]
- Santner, A.; Calderon-Villalobos, L.I.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef]
- Lopez-Obando, M.; Ligerot, Y.; Bonhomme, S.; Boyer, F.D.; Rameau, C. Strigolactone biosynthesis and signaling in plant development. Development 2015, 142, 3615–3619. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, M.L.; Chen, M.S.; Pan, B.Z.; Tao, Y.B.; Xu, Z.F. Comparative transcriptome analysis of axillary buds in response to the shoot branching regulators gibberellin A3 and 6-benzyladenine in Jatropha curcas. Sci. Rep. 2017, 7, 11417. [Google Scholar] [CrossRef]
- Ito, S.; Yamagami, D.; Umehara, M.; Hanada, A.; Yoshida, S.; Sasaki, Y.; Yajima, S.; Kyozuka, J.; Ueguchi-Tanaka, M.; Matsuoka, M.; et al. Regulation of Strigolactone Biosynthesis by Gibberellin Signaling. Plant Physiol. 2017, 174, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.N. Study on the Interaction of SL Transduction Genes CsDAD2 and GA_3 to Regulate Branch Development in Cucumber. Master’s Thesis, Harbin Normal University, Harbin, China, 2020. [Google Scholar]
- Martin, G.; Baurens, F.-C.; Droc, G.; Rouard, M.; Cenci, A.; Kilian, A.; Hastie, A.; Doležel, J.; Aury, J.-M.; Alberti, A.; et al. Improvement of the banana “Musa acuminata” reference sequence using NGS data and semi-automated bioinformatics methods. BMC Genom. 2016, 17, 243. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2019, 48, D265–D268. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2020, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Piffanelli, P.; Ciampi, A.Y.; Ruiz, M.; Blanc, G.; Leebens-Mack, J.; da Silva, F.R.; Santos, C.M.; d’Hont, A.; Garsmeur, O.; et al. Insights into the Musa genome: Syntenic relationships to rice and between Musa species. BMC Genomics 2008, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Wiederschain, G.Y. The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2006; Volume 71, p. 696. [Google Scholar]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Gribskov, M. Combining evidence using p-values: Application to sequence homology searches. Bioinformatics 1998, 14, 48–54. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016, 45, D1040–D1045. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, C.; Lin, Y.; XuHan, X.; Lai, Z. Genome-wide identification and characterization of mRNAs and lncRNAs involved in cold stress in the wild banana (Musa itinerans). PLoS ONE 2018, 13, e0200002. [Google Scholar] [CrossRef]
- Zorrilla-Fontanesi, Y.; Rouard, M.; Cenci, A.; Kissel, E.; Do, H.; Dubois, E.; Nidelet, S.; Roux, N.; Swennen, R.; Carpentier, S.C. Differential root transcriptomics in a polyploid non-model crop: The importance of respiration during osmotic stress. Sci. Rep. 2016, 6, 22583. [Google Scholar] [CrossRef]
- Chen, L.; Zhong, H.; Kuang, J.; Li, J.; Lu, W.; Chen, J. Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta 2011, 234, 377–390. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).