Adesmia pinifolia, a Native High-Andean Species, as a Potential Candidate for Phytoremediation of Cd and Hg
Abstract
:1. Introduction
2. Results
2.1. Survival and Growth
2.2. Heavy Metal Uptake and Accumulation
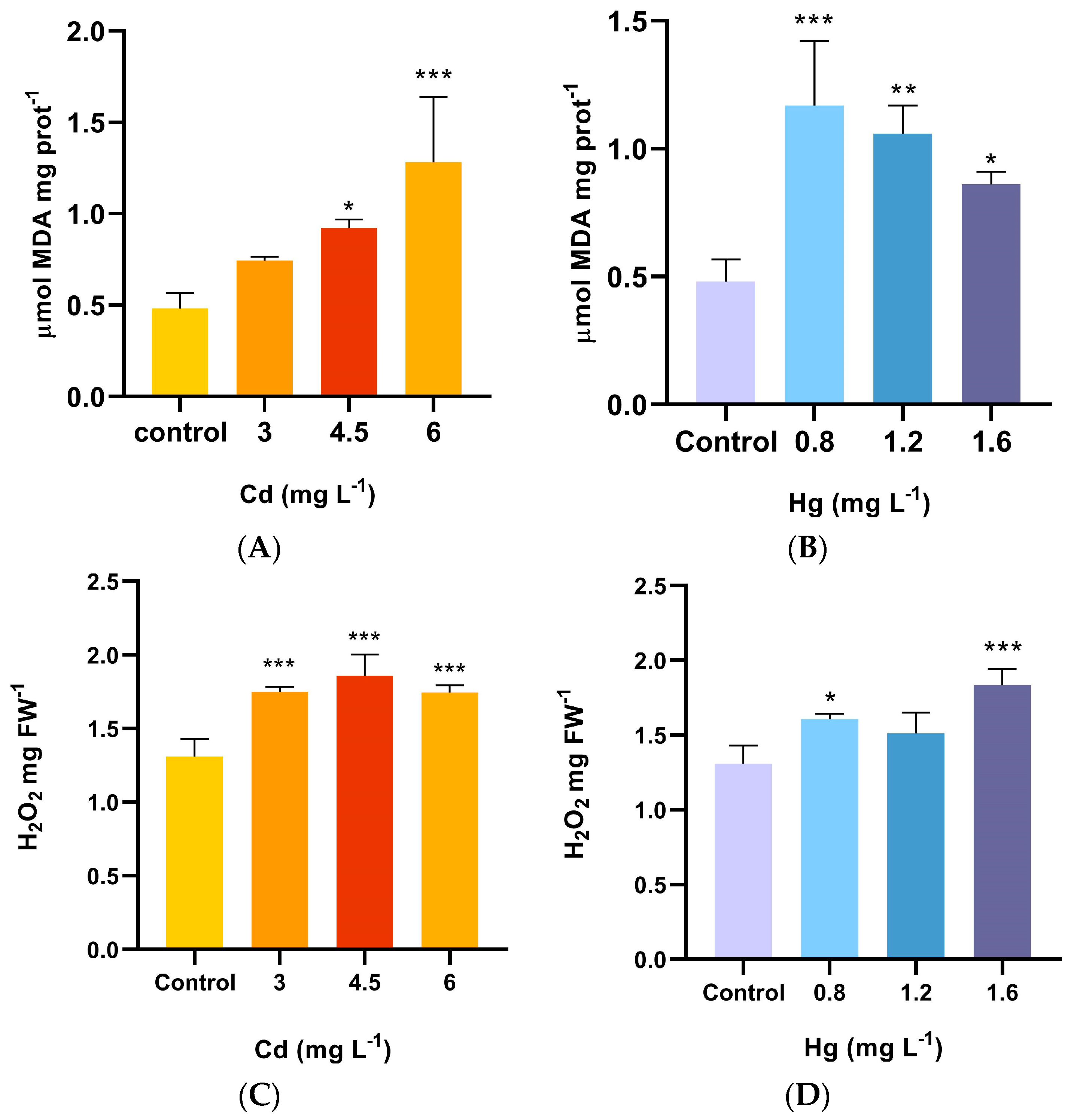
2.3. Lipid Peroxidation and H2O2
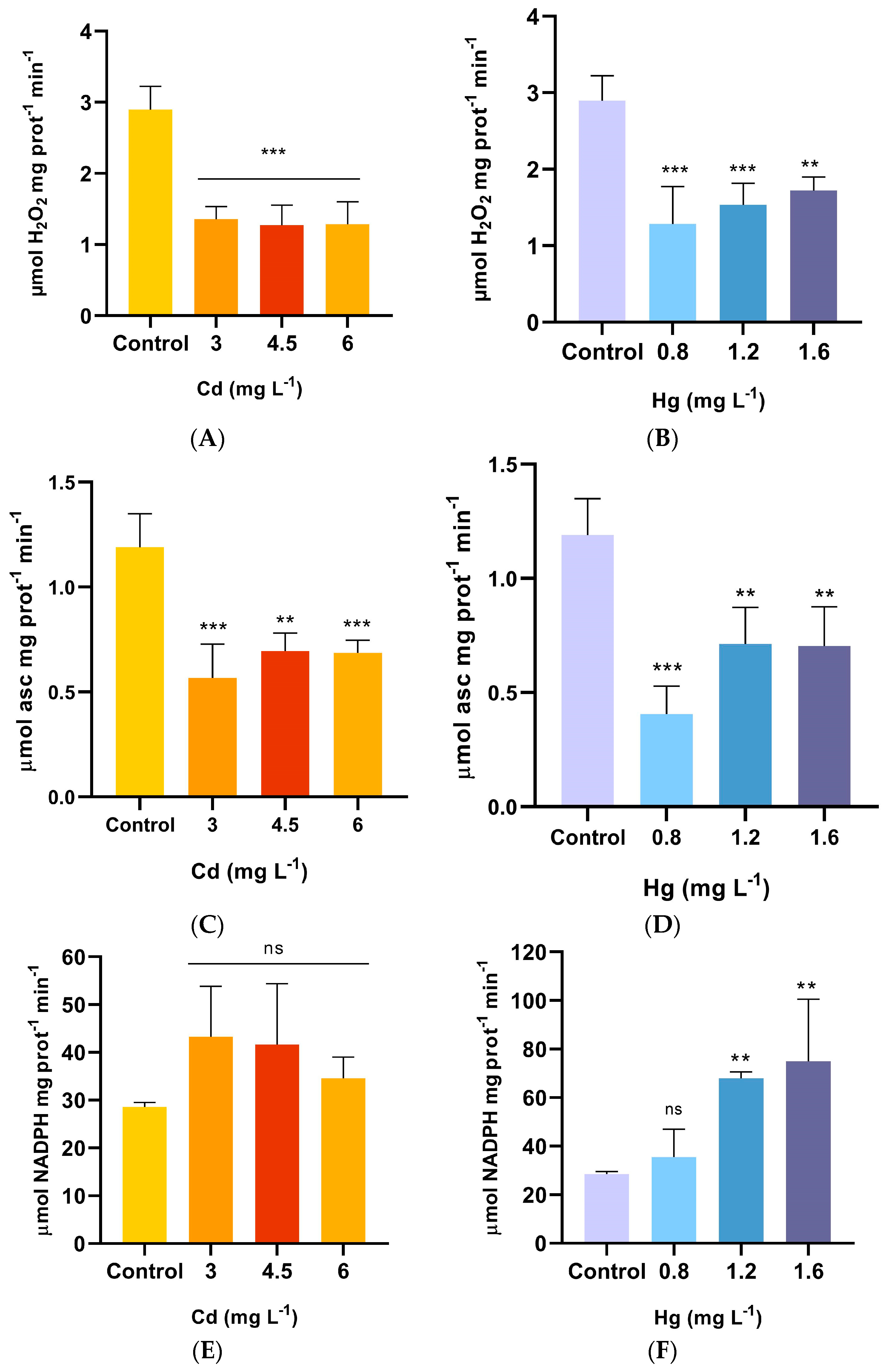
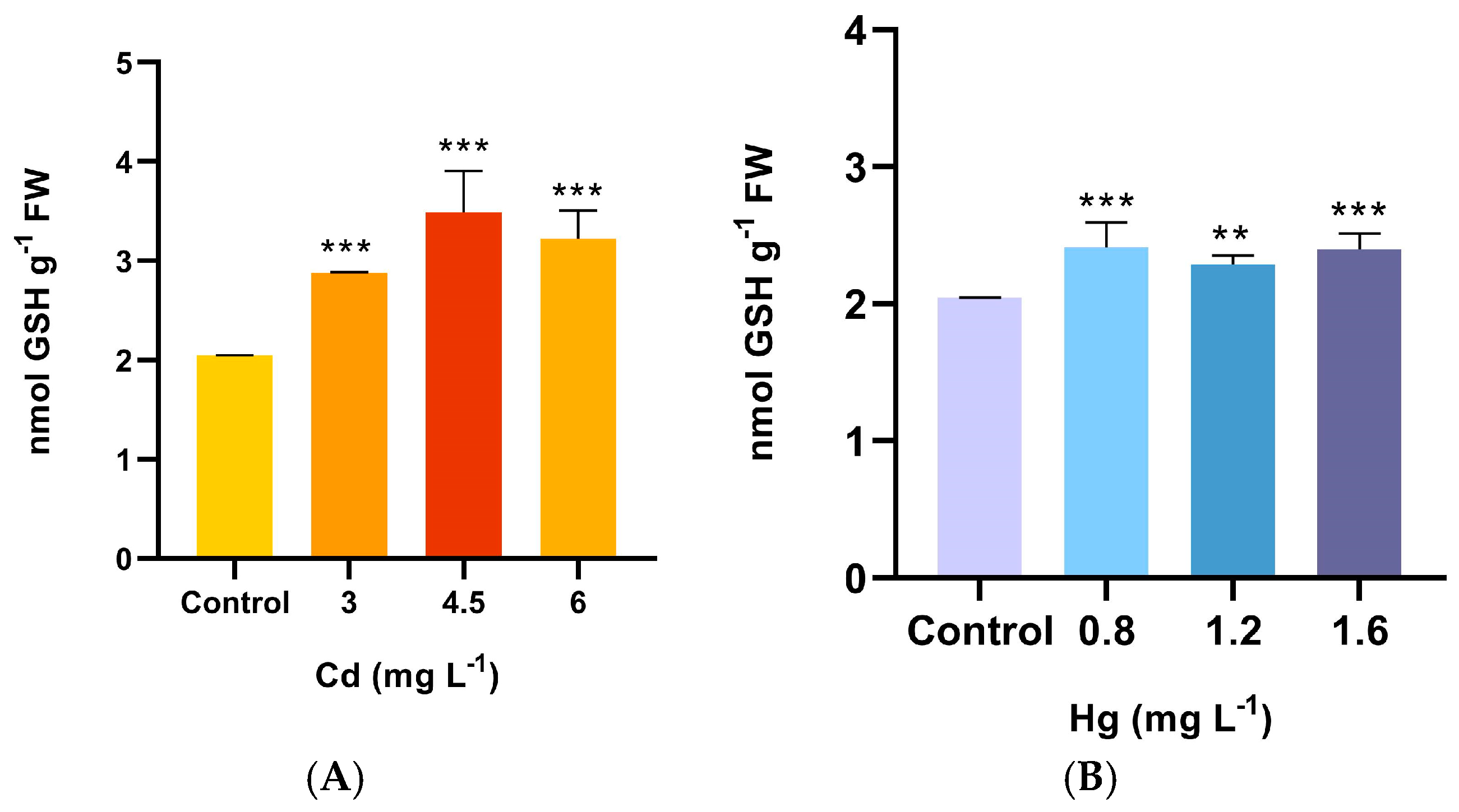
2.4. Antioxidant Enzyme Activities and GSH
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.1.1. Plant Material and Seed Collection
4.1.2. Growing Conditions
4.2. Heavy Metal Treatments
4.3. Harvesting of Plant Materials
4.4. Hydrogen Peroxide (H2O2)
4.5. Determination of MDA Concentration
4.6. Enzymatic Activities
4.7. Non-Enzymatic Antioxidants
4.8. Quantification of Cd and Hg in Plant Tissues
4.8.1. Total Cd and Hg Concentration
4.8.2. Calculation of Bioconcentration Factor and Translocation Factor
4.9. Data and Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- [SSPE] Subsecretaría de Planificación Económica. Secretaría de Política Económica y Planificación del desarrollo. Ministerio de Hacienda y Finanzas Públicas. Informes de Cadenas de Valor: Minería Metalífera y Rocas de Aplicación. ISSN 2525-2221. 2016. Available online: https://www.argentina.gob.ar/sites/default/files/sspe_cadena_de_valor_mineria.pdf (accessed on 6 May 2023).
- Sistema de Información Abierta a la Comunidad sobre la Actividad Minera en Argentina (SIACAM). Ministerio de Economía. Available online: https://www.argentina.gob.ar/economia/mineria/siacam (accessed on 16 May 2023).
- Zhao, X.; Sun, Y.; Huang, J.; Wang, H.; Tang, D. Effects of soil heavy metal pollution on microbial activities and community diversity in different land use types in mining areas. Environ. Sci. Pollut. 2020, 27, 20215–20226. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.; Maier, R.M. Phytostabilization of Mine Tailings in Arid and Semiarid Environments-An Emerging Remediation Technology. Environ. Health Perspect. 2008, 3, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, H.J.P.; Doronila, A.I.; Nicolas, M.; Ebbs, S.D.; Kolev, S.D. Growth of selected plant species in biosolids-amended mine tailings. Min. Eng. 2015, 80, 25–32. [Google Scholar] [CrossRef]
- Yan, B.; Xu, D.M.; Chen, T.; Yan, Z.A.; Li, L.L.; Wang, M.H. Leachability characteristic of heavy metals and associated health risk study in typical copper mining-impacted sediments. Chemosphere 2020, 239, 124748. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Bharti, R.; Sharma, R. Effect of heavy metals: An overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar] [CrossRef]
- Liu, S.; Yang, B.; Liang, Y.; Xiao, Y.; Fang, J. Prospect of phytoremediation combined with other approaches for remediation of heavy metal-polluted soils. Environ. Sci. Pollut. Res. 2020, 27, 16069–16085. [Google Scholar] [CrossRef]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Imran, A.; Naz, I. A critical review on the phytoremediation of heavy metals from environment: Performance and challenges. Chemosphere 2021, 291, 132979. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.S. Effects of nickel chloride on germination and seedling growth of different wheat (Triticum aestivum L. em Thell.) cultivars. J. Pharm. Phytochem. 2018, 7, 2227–2234. [Google Scholar]
- Radziemska, M.; Koda, E.; Bilgin, A.; Vaverková, M.D. Concept of aided phytostabilization of contaminated soils in postindustrial areas. Int. J. Environ. Res. Public Health 2018, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, K.E.; Gerwing, P.D.; Greenberg, B.M. Opinion: Taking phytoremediation from proven technology to accepted practice. Plant Sci. 2017, 256, 170–185. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of heavy metals: Mechanisms, methods and enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Yang, H.; Li, X.; Cui, Z. Physiological responses of Suaeda glauca and Arabidopsis thaliana in phytoremediation of heavy metals. J. Environ. Manag. 2018, 223, 132–139. [Google Scholar] [CrossRef]
- Hou, D.; O’Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.; Sparks, D.L.; Yusuke, Y.; Jörg, R.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, D.; Strzałka, P.; Prasad, M.N.V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Tamás, L.; Mistrík, I.; Zelinová, V. Heavy metal-induced reactive oxygen species and cell death in barley root tip. Environ. Exp. Bot. 2017, 140, 34–40. [Google Scholar] [CrossRef]
- Bhuyan, M.B.; Hasanuzzaman, M.; Parvin, K.; Mohsin, S.M.; Al Mahmud, J.; Nahar, K.; Fujita, M. Nitric oxide and hydrogen sulfide: Two intimate collaborators regulating plant defense against abiotic stress. Plant Growth Regul. 2020, 90, 409–424. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Terron-Camero, L.C.; Pelaez-Vico, M.A.; Olmedilla, A.; Sandalio, L. Reactive oxygen and nitrogen species as key indicators of plant responses to Cd stress. Environ. Exp. Bot. 2019, 161, 107–119. [Google Scholar] [CrossRef]
- Thakur, M.; Praveen, S.; Divte, P.R.; Mitra, R.; Kumar, M.; Gupta, C.K.; Kalidindi, U.; Bansal, R.; Roy, S.; Anand, A.; et al. Metal tolerance in plants: Molecular and physicochemical interface determines the “not so heavy effect” of heavy metals. Chemosphere 2022, 287, 131957. [Google Scholar] [CrossRef] [PubMed]
- Sandalio, L.M.; Rodríguez-Serrano, M.; Gupta, D.K.; Archilla, A.; Romero-Puertas, M.C.; del Río, L.A. Reactive Oxygen Species and Nitric Oxide in Plants Under Cadmium Stress: From Toxicity to Signaling. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012; pp. 154–196. [Google Scholar]
- Pérez-Chaca, M.V.; Rodríguez-Serrano, M.; Molina, A.S.; Pedranzani, H.E.; Zirulnik, F.; Sandalio, L.M.; Romero-Puertas, M.C. Cadmium induces two waves of reactive oxygen species in Glycine max (L.) roots. Plant Cell Environ. 2014, 37, 1672–1687. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, M.N.V. Plant-lead interactions: Transport, toxicity, tolerance, and detoxification mechanisms. Ecotoxicol. Environ. Saf. 2018, 166, 401–418. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, L.; Wahid, Z.A.; Siddiqui, M.F.; Atnaw, S.M.; Din, M.F.M. Plant-driven removal of heavy metals from soil: Uptake, translocation, tolerance mechanism, challenges, and future perspectives. Environ. Monit. Assess. 2016, 188, 204. [Google Scholar] [CrossRef]
- De la Peña, T.C.; Fedorova, E.; Pueyo, J.J.; Lucas, M.M. The symbiosome: Legume and rhizobia co-evolution toward a nitrogenfixing organelle? Front. Plant Sci. 2018, 8, 2229. [Google Scholar] [CrossRef]
- Mahmud, J.A.; Borhannuddin Bhuyan, M.H.M.; Nahar, K.; Parvin, K.; Hasanuzzaman, M. Response and tolerance of Fabaceae plants to metal/metalloid toxicity. In The Plant Family Fabaceae: Biology and Physiological Responses to Environmental Stresses; Hasanuzzaman, M., Araújo, S., Gill, S., Eds.; Springer: Singapore, 2020; pp. 435–482. [Google Scholar] [CrossRef]
- Zaidi, A.; Wani, P.A.; Khan, M.S. Bioremediation: A Natural Method for the Management of Polluted Environment. In Toxicity of Heavy Metals to Legumes and Bioremediation; Zaidi, A., Wani, P., Khan, M., Eds.; Springer: Vienna, Austria, 2012; pp. 101–114. [Google Scholar] [CrossRef]
- Hao, X.; Taghavi, S.; Xie, P.; Orbach, M.J.; Alwathnani, H.A.; Rensing, C.; Wei, G. Phytoremediation of Heavy and Transition Metals Aided by Legume-Rhizobia Symbiosis. Int. J. Phytoremediat. 2014, 16, 179–202. [Google Scholar] [CrossRef] [PubMed]
- Arreghini, S.; de Cabo, L.; Serafini, R.; de Iorio, A.F. Effect of the combined addition of Zn and Pb on partitioning in sediments and their accumulation by the emergent macrophyte Schoenoplectus californicus. Environ. Sci. Pollut. Res. 2017, 24, 8098–8107. [Google Scholar] [CrossRef] [PubMed]
- Nedjimi, B. Germination characteristics of Peganum harmala L. (Nitrariaceae) subjected to heavy metals: Implications for the use in polluted dryland restoration. Int. J. Environ. Sci. Technol. 2019, 17, 2113–2122. [Google Scholar] [CrossRef]
- Ulibarri, E.A.; Burkart, A. Sinopsis de las especies de Adesmia (Leguminosae-Papilionoideae) de la Argentina. Darwiniana 2000, 38, 59–126. [Google Scholar]
- Plaza Cazón, J.; Benítez, L.; Murray, J.; Kirschbaum, A.; Kirschbaum, P.; Donati, E. Environmental impact on soil, water and plants from the abandoned Pan de Azúcar Mine. Adv. Mater. Res. 2013, 825, 88–91. [Google Scholar] [CrossRef]
- Lam, E.J.; Keith, B.F.; Montofré, L.; E Gálvez, M. Copper Uptake by Adesmia atacamensis in a Mine Tailing in an Arid Environment. Air Soil. Water Res. 2018, 11, 1178622118812462. [Google Scholar] [CrossRef]
- Parera, V.; Parera, C.A.; Feresin, G.E. Germination and Early Seedling Growth of High Andean Native Plants under Heavy Metal Stress. Diversity 2023, 15, 824. [Google Scholar] [CrossRef]
- Muszyńska, E.; Hanus-Fajerska, E.; Ciarkowska, K. Studies on lead and cadmium toxicity in Dianthus carthusianorum calamine ecotype cultivated in vitro. Plant Biol. 2018, 20, 474–482. [Google Scholar] [CrossRef]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int. J. Plant Prod. 2009, 3, 65–76. [Google Scholar]
- Amna, A.N.; Masood, S.; Mukhtar, T.; Kamran, M.A.; Rafique, M.; Munis, M.F.H.; Chaudhary, H.J. Differential effects of cadmium and chromium on growth, photosynthetic activity, and metal uptake of Linum usitatissimum in association with Glomus intraradices. Environ. Monit. Assess 2015, 187, 311. [Google Scholar] [CrossRef]
- Pandey, A.K.; Zori’c, L.; Sun, T.; Karanovi’c, D.; Fang, P.; Borišev, M.; Wu, X.; Luković, J.; Xu, P. The Anatomical Basis of Heavy Metal Responses in Legumes and Their Impact on Plant–Rhizosphere Interactions. Plants 2022, 11, 2554. [Google Scholar] [CrossRef]
- Yang, L.P.; Zhu, J.; Wang, P.; Zeng, J.; Tan, R.; Yang, Y.Z.; Liu, Z.M. Effect of Cd on growth, physiological response, Cd subcellular distribution and chemical forms of Koelreuteria paniculata. Ecotoxicol. Environ. Saf. 2018, 160, 10–18. [Google Scholar] [CrossRef]
- Xu, X.; Yang, B.; Qin, G.; Wang, H.; Zhu, Y.; Zhang, K.; Yang, H. Growth, accumulation, and antioxidative responses of two Salix genotypes exposed to cadmium and lead in hydroponic culture. Environ. Sci. Pollut. Res. 2019, 26, 19770–19784. [Google Scholar] [CrossRef]
- Abdal, N.; Abbas, G.; Asad, S.A.; Ghfar, A.A.; Shah, G.M.; Rizwan, M.; Ali, S.; Shahbaz, M. Salinity mitigates cadmium-induced phytotoxicity in quinoa (Chenopodium quinoa Willd.) by limiting the Cd uptake and improved responses to oxidative stress: Implications for phytoremediation. Environ. Geochem. Health 2023, 45, 171–185. [Google Scholar] [CrossRef]
- Żabka, A.; Winnicki, K.; Polit, J.T.; Wróblewski, M.; Maszewski, J. Cadmium (II)-Induced Oxidative Stress Results in Replication Stress and Epigenetic Modifications in Root Meristem Cell Nuclei of Vicia faba. Cells 2021, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Kintlová, M.; Vrána, J.; Hobza, R.; Blavet, N.; Hudzieczek, V. Transcriptome response to cadmium exposure in barley (Hordeum vulgare L.). Front. Plant Sci. 2021, 12, 629089. [Google Scholar] [CrossRef] [PubMed]
- Finger-Teixeira, A.; Ferrarese, M.D.L.L.; Soares, A.R.; da Silva, D.; Ferrarese-Filho, O. Cadmium-induced lignification restricts soybean root growth. Ecotoxicol. Environ. Saf. 2010, 73, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Hayat, M.T.; Zeb, B.S.; Abbas, Z.; Ahmed, T. Phytoremediation of Cd-contaminated soil and water. In Cadmium Toxicity and Tolerance in Plants; Hasanuzzaman, M., Prasad, M., Fujita, M., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; pp. 531–543. [Google Scholar] [CrossRef]
- Jawad Hassan, M.; Ali Raza, M.; Ur Rehman, S.; Ansar, M.; Gitari, H.; Khan, I.; Wajid, M.; Ahmed, M.; Abbas Shah, G.; Peng, Y.; et al. Effect of Cadmium Toxicity on Growth, Oxidative Damage, Antioxidant Defense System and Cadmium Accumulation in Two Sorghum Cultivars. Plants 2020, 9, 1575. [Google Scholar] [CrossRef]
- Uddin, M.M.; Chen, Z.; Xu, F.; Huang, L. Physiological and Cellular Ultrastructural Responses of Sesuvium portulacastrum under Cd Stress Grown Hydroponically. Plants 2023, 12, 3381. [Google Scholar] [CrossRef] [PubMed]
- Azizollahi, Z.; Ghaderian, S.M.; Ghotbi-Ravandi, A.A. Cadmium accumulation and its effects on physiological and biochemical characters of summer savory (Satureja hortensis L.). Int. J. Phytoremediat. 2019, 21, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Dinu, C.; Vasile, G.G.; Buleandra, M.; Popa, D.E.; Gheorghe, S.; Ungureanu, E.M. Translocation and accumulation of heavy metals in Ocimum basilicum L. plants grown in a mining-contaminated soil. J. Soils Sediments 2020, 20, 2141–2154. [Google Scholar] [CrossRef]
- Pusz, A.; Wisniewska, M.; Rogalski, D. Assessment of the Accumulation Ability of Festuca rubra L. and Alyssum saxatile L. Tested on Soils Contaminated with Zn, Cd, Ni, Pb, Cr, and Cu. Resources 2021, 10, 46. [Google Scholar] [CrossRef]
- Hamim, H.; Miftahudin, M.; Setyaningsih, L. Cellular and ultrastructure alteration of plant roots in response to metal stress. In Plant Growth and Regulation-Alterations to Sustain Unfavorable Conditions; Ratnadewi, D., Ed.; Intech Open: London, UK, 2018; pp. 21–44. [Google Scholar]
- Gishini, M.F.S.; Azizian, A.; Alemzadeh, A.; Shabani, M.; Amin, S.; Hildebrand, D. Response of Prosopis farcta to copper and cadmium stress and potential for accumulation and translocation of these heavy metals. bioRxiv 2020, 11, 36519. [Google Scholar] [CrossRef]
- Ramana, S.; Tripathi, A.K.; Kumar, A.; Dey, P.; Saha, J.K.; Patra, A.K. Phytoremediation of soils contaminated with cadmium by Agave americana. J. Nat. Fibers 2022, 19, 4984–4992. [Google Scholar] [CrossRef]
- Zou, T.; Li, T.; Zhang, X.; Yu, H.; Huang, H. Lead accumulation and phytostabilization potential of dominant plant species growing in a lead–zinc mine tailing. Environ. Earth Sci. 2012, 65, 621–630. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Zayneb, C.; Bassem, K.; Zeineb, K.; Grubb, C.D.; Noureddine, D.; Hafedh, M.; Amine, E. Physiological responses of fenugreek seedlings and plants treated with cadmium. Environ. Sci. Pollut. R. 2015, 22, 10679–10689. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, Y.; Cao, X.; Jiang, W.; Liu, X.; Liu, Q.; Chen, Z.; Zhou, W.C.; Wang, Q. Phytoremediation of Cd and Pb interactive polluted soils by switchgrass (Panicum virgatum L.). Int. J. Phytoremediat. 2019, 21, 1486–1496. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. AComprehensive Review on the Heavy Metal Toxicity and Sequestration in Plants. Biomolecules 2022, 12, 43. [Google Scholar] [CrossRef]
- Çatav, Ş.S.; Genç, T.O.; Oktay, M.K.; Küçükakyüz, K. Cadmium toxicity in wheat: Impacts on element contents, antioxidant enzyme activities, oxidative stress, and genotoxicity. Bull. Environ. Contam. Toxicol. 2020, 104, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Pan, X.; Zhao, Q.; Zhao, T. Plant growth, antioxidative enzyme, and cadmium tolerance responses to cadmium stress in Canna orchioides. Hortic. Plant J. 2021, 7, 256–266. [Google Scholar] [CrossRef]
- Pál, M.; Horváth, E.; Janda, T.; Páldi, E.; Szalai, G. Cadmium stimulates the accumulation of salicylic acid and its putative precursors in maize (Zea mays) plants. Physiol. Plant 2005, 125, 356–364. [Google Scholar] [CrossRef]
- Ulusu, Y.; Öztürk, L.; Elmastas, M. Antioxidant capacity and cadmium accumulation in parsley seedlings exposed to cadmium stress. Russ. J. Plant Phys. 2017, 64, 883–888. [Google Scholar] [CrossRef]
- Jozefczak, M.; Keunen, E.; Schat, H.; Bliek, M.; Hernández, L.E.; Carleer, R.; Remans, T.; Bohler, S.; Vangronsveld, J.; Cuypers, A. Differential response of Arabidopsis leaves and roots to cadmium: Glutathione-related chelating capacity vs antioxidant capacity. Plant Physiol. Biochem. 2014, 83, 1–9. [Google Scholar] [CrossRef]
- Labudda, M.; Dziurka, K.; Fidler, J.; Gietler, M.; Rybarczyk-Płonska, A.; Nykiel, M.; Prabucka, B.; Morkunas, I.; Muszynska, E. The Alleviation of Metal Stress Nuisance for Plants—A Review of Promising Solutions in the Face of Environmental Challenges. Plants 2022, 11, 2544. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Z.M. Mercury toxicity, molecular response and tolerance in higher plants. Biometals 2012, 25, 847–857. [Google Scholar] [CrossRef]
- Mei, L.; Zhu, Y.; Zhang, X.; Zhou, X.; Zhong, Z.; Li, H.; Li, Y.; Li, X.; Daud, M.K.; Chen, J.; et al. Mercury-Induced Phytotoxicity and Responses in Upland Cotton (Gossypium hirsutum L.) Seedlings. Plants 2021, 10, 1494. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Durango-Hernández, J.; Pinedo-Hernández, J.; Olivero-Verbel, J.; Díez, S. Phytoremediation of mercury-contaminated soils by Jatropha curcas. Chemosphere 2015, 127, 58–63. [Google Scholar] [CrossRef]
- Xun, Y.; Feng, L.; Li, Y.; Dong, H. Mercury accumulation plant Cyrtomium macrophyllum and its potential for phytoremediation of mercury polluted sites. Chemosphere 2017, 189, 161–170. [Google Scholar] [CrossRef]
- Pérez-Sanz, A.; Millán, R.; Sierra, M.J.; Alarcón, R.; García, P.; Gil-Díaz, M.; Vazquez, S.; Lobo, M.C. Mercury uptake by Silene vulgaris grown on contaminated spiked soils. J. Environ. Manag. 2012, 95, S233–S237. [Google Scholar] [CrossRef]
- Lomonte, C.; Doronila, A.I.; Gregory, D.; Baker, A.J.; Kolev, S.D. Phytotoxicity of biosolids and screening of selected plant species with potential for mercury phytoextraction. J. Hazard. Mater. 2010, 173, 494–501. [Google Scholar] [CrossRef]
- Kranner, I.; Colville, L. Metals and seeds: Biochemical and molecular implications and their significance for seed germination. Environ. Exp. Bot. 2011, 72, 93–105. [Google Scholar] [CrossRef]
- Agathokleous, E. Environmental hormesis, a fundamental non-monotonic biological phenomenon with implications in ecotoxicology and environmental safety. Ecotoxicol. Environ. Saf. 2018, 148, 1042–1053. [Google Scholar] [CrossRef]
- Alcantara, H.J.P.; Doronila, A.I.; Kolev, S.D. Phytoextraction potential of Manihot esculenta Crantz. (cassava) grown in mercury-and gold-containing biosolids and mine tailings. Miner. Eng. 2017, 114, 57–63. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Gamarra, R.; Carpena-Ruiz, R.O.; Millán, R.; Peñalosa, J.M.; Esteban, E. Mercury bioaccumulation and phytotoxicity in two wild plant species of Almadén area. Chemosphere 2006, 63, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Malar, S.; Sahi, S.V.; Favas, P.J.C.; Venkatachalam, P. Assessment of mercury heavy metal toxicity-induced physiochemical and molecular changes in Sesbania grandiflora L. Int. J. Environ. Sci. Technol. 2015, 12, 3273–3282. [Google Scholar] [CrossRef]
- Ding, W.; Zhang, J.; Wu, S.C.; Zhang, S.; Christie, P.; Liang, P. Responses of the grass Paspalum distichum L. to Hg stress: A proteomic study. Ecotoxicol. Environ. Saf. 2019, 183, 109549. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, L.A.; Xu, J.; Ding, S.; Feng, X.; Xiao, H. Effects of different concentrations of mercury on accumulation of mercury by five plant species. Ecol. Eng. 2017, 106, 273–278. [Google Scholar] [CrossRef]
- Anjum, N.A.; Duarte, A.C.; Pereira, E.; Ahmad, I. Oxidative stress status, antioxidant metabolism and polypeptide patterns in Juncus maritimus shoots exhibiting differential mercury burdens in Ria de Aveiro coastal lagoon (Portugal). Environ. Sci. Pollut. Res. 2014, 21, 6652–6661. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, Q.; Su, C.; Yang, Y.; Hu, D.; Xu, Q. Mercury induced oxidative stress, DNA damage, and activation of antioxidative system and Hsp70 induction in duckweed (Lemna minor). Ecotoxicol. Environ. Saf. 2017, 143, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Malar, S.; Sahi, S.V.; Favas, P.J.; Venkatachalam, P. Mercury heavy-metal-induced physiochemical changes and genotoxic alterations in water hyacinths [Eichhornia crassipes (Mart)]. Environ. Sci. Pollut. Res. 2015, 22, 4597–4608. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, A.; Wei, Y.Y.; Meng, Q.; Zheng, Q.; Yang, Z.M. Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicology 2010, 19, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Pirzadah, T.B.; Malik, B.; Tahir, I.; Irfan, Q.M.; Rehman, R.U. Characterization of mercury-induced stress biomarkers in Fagopyrum tataricum plants. Int. J. Phytoremediat. 2018, 20, 225–236. [Google Scholar] [CrossRef]
- Feng, Z.; Ji, S.; Ping, J.; Cui, D. Recent advances in metabolomics for studying heavy metal stress in plants. TrAC Trends Analyt Chem. 2021, 143, 116402. [Google Scholar] [CrossRef]
- Kiesling, R.; de San Juan, F. República Argentina: Vol.1, Pteridófitas Gimnospermas, Dicotiledóneas, Dialipétalas (Salicáceas a leguminosas); Vazquez Mazzini: Buenos Aires, Argentina, 1994; pp. 291–301. [Google Scholar]
- Parera, C.A.; Ruiz, M. Adesmia subterranea Clos Germination Physiology and Presowing Treatments. J. Range Manag. 2003, 56, 273. [Google Scholar] [CrossRef]
- Župunski, M.; Borišev, M.; Orlović, S.; Arsenov, D.; Nikolić, N.; Pilipović, A.; Pajević, S. Hydroponic screening of black locust families for heavy metal tolerance and accumulation. Int. J. Phytoremediat. 2016, 18, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Chen, G.X.; Asada, K. Ascorbate peroxidase in tea leaves: Occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1989, 30, 987–998. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.A.; Rawsthone, S.; Mullineaux, P.M. Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L). Planta 1990, 180, 278–284. [Google Scholar] [CrossRef]
- Akerboon, T.; Sies, H. Assay of glutathione disulfide and glutathion mixed disulfides in biological samples. Methods Enzymol. 1981, 77, 373–382. [Google Scholar]
- Niazi, N.K.; Bibi, I.; Fatimah, A.; Shahid, M.; Javed, M.T.; Wang, H.; Ok, Y.S.; Bashir, S.; Murtaza, B.; Saqib, Z.A.; et al. Phosphate-assisted phytoremediation of arsenic by Brassica napus and Brassica juncea: Morphological and physiological response. Int. J. Phytoremediat. 2017, 19, 670–678. [Google Scholar] [CrossRef]
| Heavy Metal Treatment (mg L−1) | Survival (%) | Shoot Biomass (mg) | Root Biomass (mg) | |
|---|---|---|---|---|
| Control | 92 ± 8 a | 29.69 ± 1.11 a | 15.87 ± 1.41 a | |
| Cd | 3 | 85.3 ± 4.6 a | 11.2 ± 1.41 b | 1.51 ± 0.38 b |
| 4.5 | 81.3 ± 8.3 a | 8.67 ± 1.28 c | 1.45 ± 0.36 b | |
| 6 | 84 ± 8 a | 6.93 ± 0.93 d | 1.45 ± 0.35 b | |
| Heavy Metal Treatment (mg L−1) | Survival (%) | Shoot Biomass (mg) | Root Biomass (mg) | |
|---|---|---|---|---|
| Control | 92 ± 8 a | 29.69 ± 1.11 a | 15.87 ± 1.41 a | |
| Hg | 0.8 | 88 ± 8 a | 25.93 ± 3.56 ab | 7.94 ± 2.67 b |
| 1.2 | 90.6 ± 2.3 a | 23.99 ± 3.28 b | 6.78 ± 2.42 b | |
| 1.6 | 85.3 ± 12.8 a | 27.09 ± 5.3 ab | 18.2 ± 4.73 a | |
| Heavy Metal Treatment (mg L−1) | Metal Concentration (µg g−1) | BCF | TF | |||
|---|---|---|---|---|---|---|
| Shoots | Roots | Shoots | Roots | |||
| Control | ND | ND | - | - | - | |
| Cd | 3 | 302.97 ± 18.04 a | 2416.76 ± 30.47 b | 125.57 ± 48.06 a | 805.59 ± 54.38 a | 0.12 ± 0.03 a |
| 4.5 | 346.56 ± 14.18 a | 2605.24 ± 29.47 b | 77.01 ± 23.85 a | 578.94 ± 94.96 b | 0.13 ± 0.04 a | |
| 6 | 546.06 ± 39.96 a | 2580.10 ± 6.72 b | 91.01 ± 14.74 a | 475.34 ± 85.82 c | 0.19 ± 0.04 a | |
| Heavy Metal Treatment (mg L−1) | Metal Concentration (µg g−1) | BCF | TF | |||
|---|---|---|---|---|---|---|
| Shoots | Roots | Shoots | Roots | |||
| Control | ND | ND | - | - | - | |
| Hg | 0.8 | 138.74 ± 16.25 a | 643.63 ± 30.47 a | 173.43 ±20.30 a | 804.54 ± 38.09 a | 0.22 ± 0.02 a |
| 1.2 | 118.75 ± 14.18 a | 506.11 ± 52.34 b | 98.96 ± 11.82 b | 421.76 ± 43.62 b | 0.24 ± 0.04 a | |
| 1.6 | 164.91 ± 39.96 a | 639.53 ± 6.72 a | 103.07 ± 24.98 b | 399.71 ± 4.20 b | 0.26 ± 0.06 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parera, V.; Pérez-Chaca, M.V.; Gallardo, L.V.; Gatica-Aguilar, C.V.; Parera, C.A.; Feresin, G.E. Adesmia pinifolia, a Native High-Andean Species, as a Potential Candidate for Phytoremediation of Cd and Hg. Plants 2024, 13, 464. https://doi.org/10.3390/plants13040464
Parera V, Pérez-Chaca MV, Gallardo LV, Gatica-Aguilar CV, Parera CA, Feresin GE. Adesmia pinifolia, a Native High-Andean Species, as a Potential Candidate for Phytoremediation of Cd and Hg. Plants. 2024; 13(4):464. https://doi.org/10.3390/plants13040464
Chicago/Turabian StyleParera, Victoria, M. Verónica Pérez-Chaca, Laura V. Gallardo, Camila V. Gatica-Aguilar, Carlos A. Parera, and Gabriela E. Feresin. 2024. "Adesmia pinifolia, a Native High-Andean Species, as a Potential Candidate for Phytoremediation of Cd and Hg" Plants 13, no. 4: 464. https://doi.org/10.3390/plants13040464
APA StyleParera, V., Pérez-Chaca, M. V., Gallardo, L. V., Gatica-Aguilar, C. V., Parera, C. A., & Feresin, G. E. (2024). Adesmia pinifolia, a Native High-Andean Species, as a Potential Candidate for Phytoremediation of Cd and Hg. Plants, 13(4), 464. https://doi.org/10.3390/plants13040464







