Synergistic Influence of Arbuscular mycorrhizal Fungi Inoculation with Nanoparticle Foliar Application Enhances Chili (Capsicum annuum L.) Antioxidant Enzymes, Anatomical Characteristics, and Productivity under Cold-Stress Conditions
Abstract
:1. Introduction
2. Results
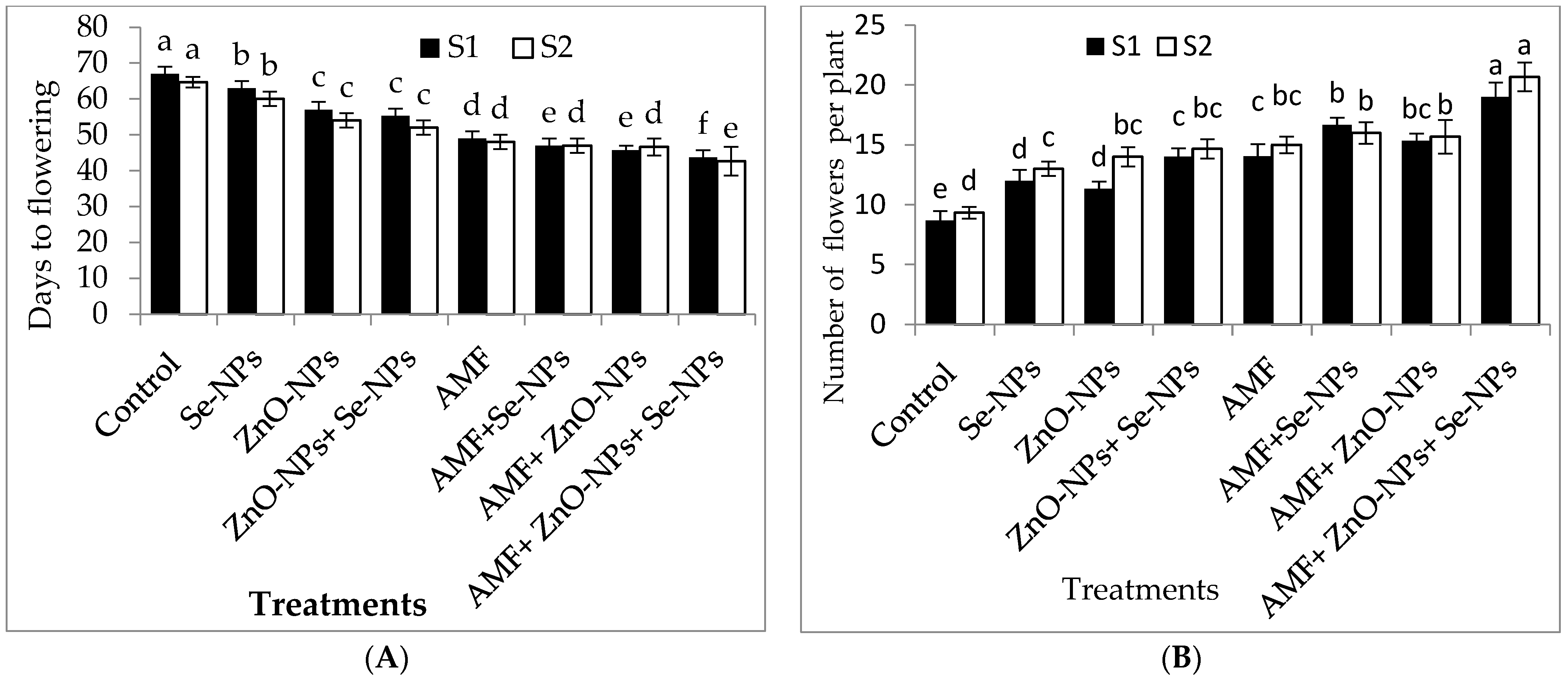
2.1. Plant Growth and Flowering Traits of Chili Plants
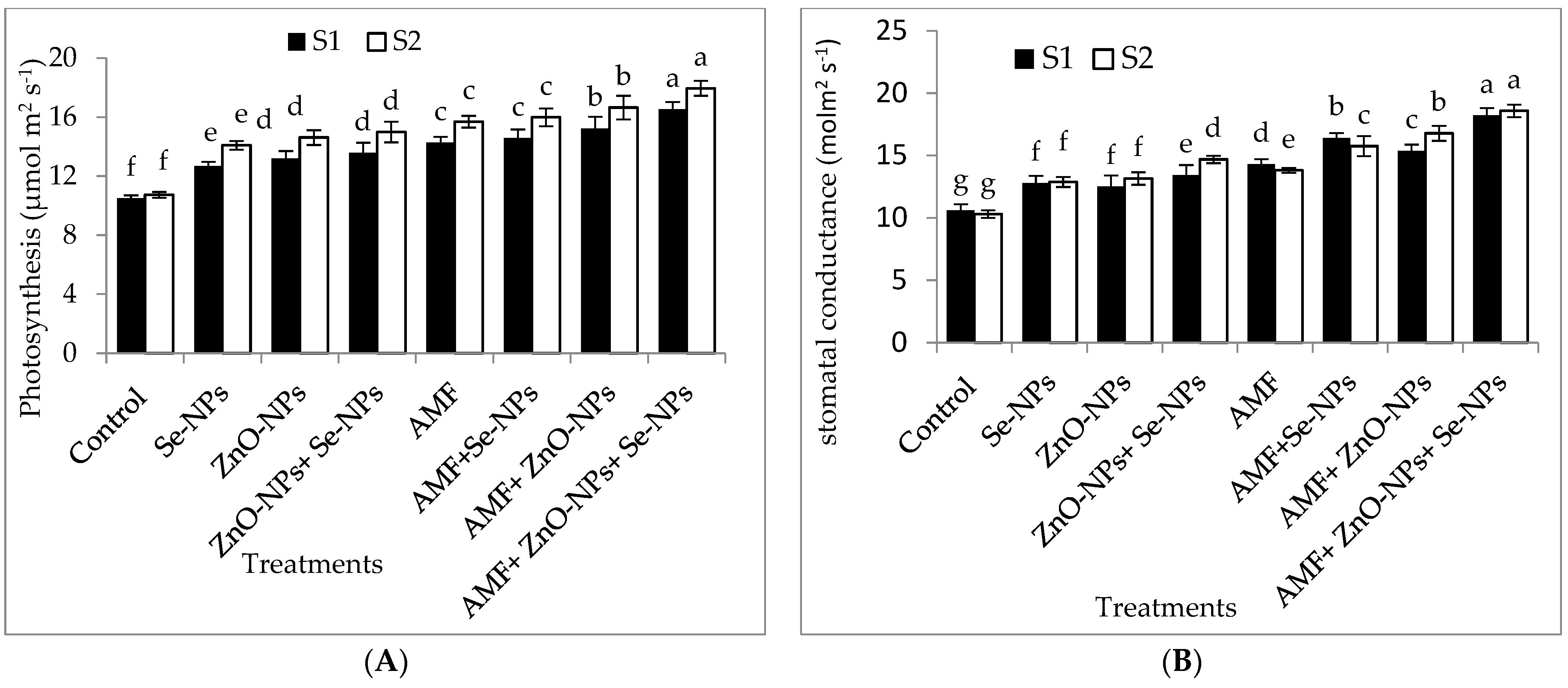
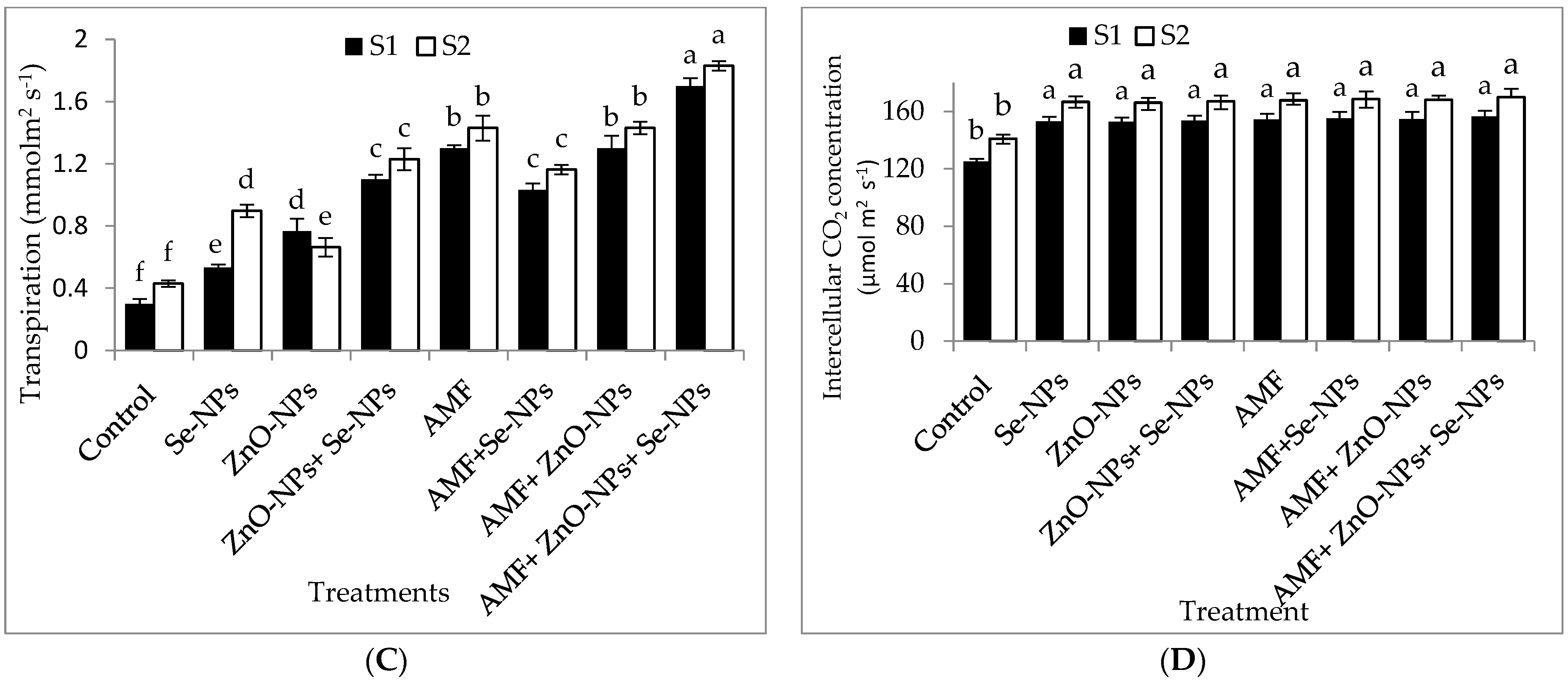
2.2. Photosynthetic Pigments of Chili Plants
2.3. Marketable Yield and Its Components
2.4. Quality Parameters of Chili Fruits
2.5. Mineral Content in Chili Leaves
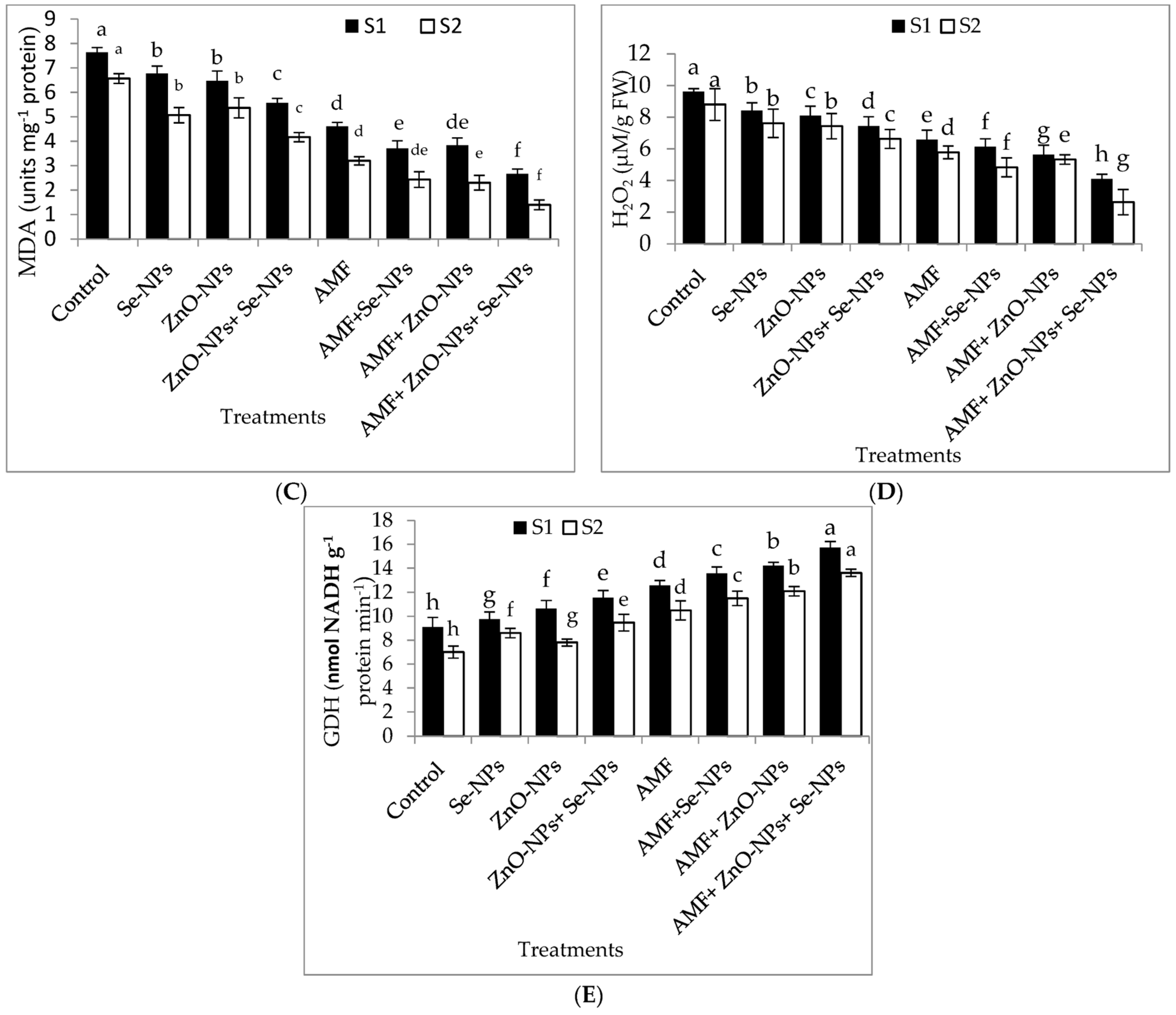
2.6. Plant Hormones, Nitrogen Metabolism Enzyme, Antioxidant Enzymes, Hydrogen Peroxide, and Lipid Peroxidation Content in Chili Leaves
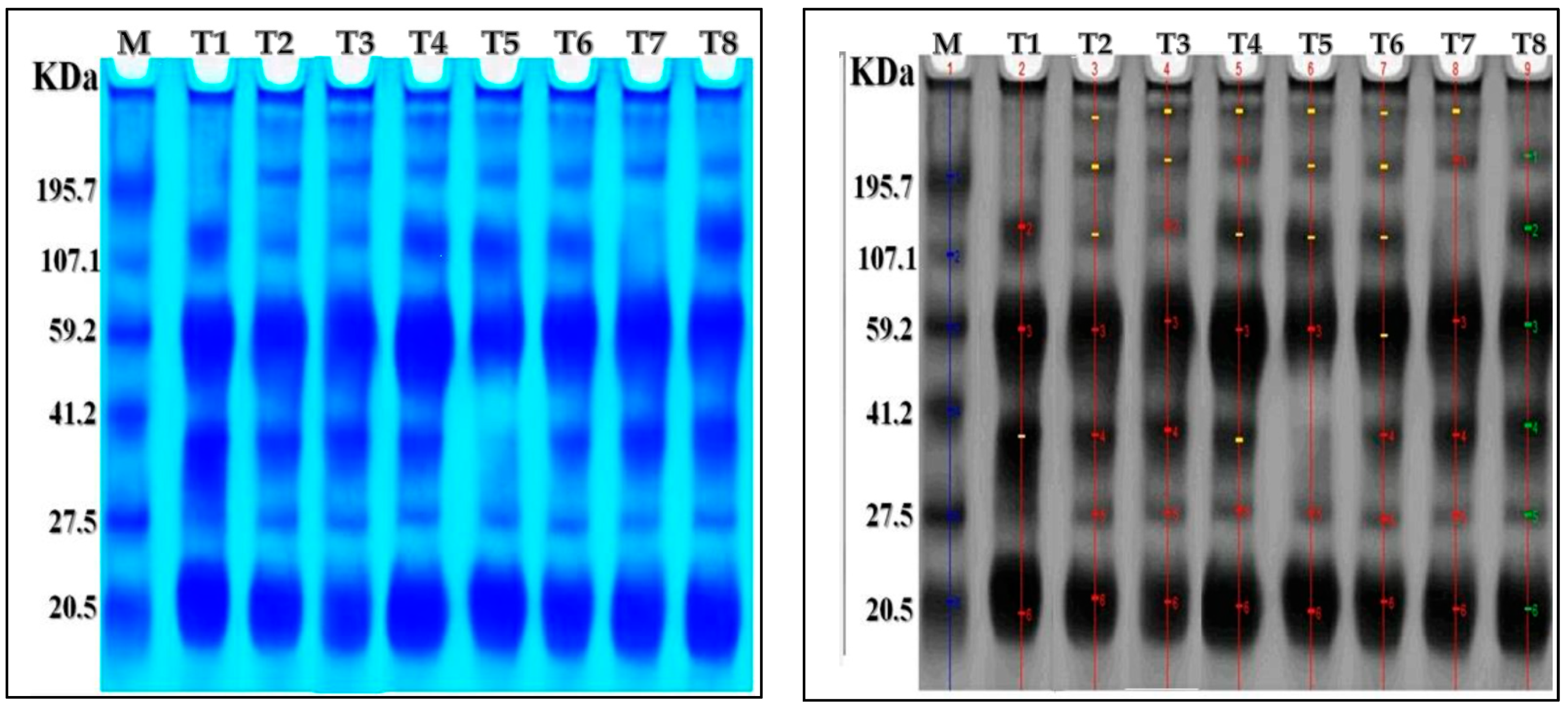
2.7. Protein Electrophoresis SDS-PAGE
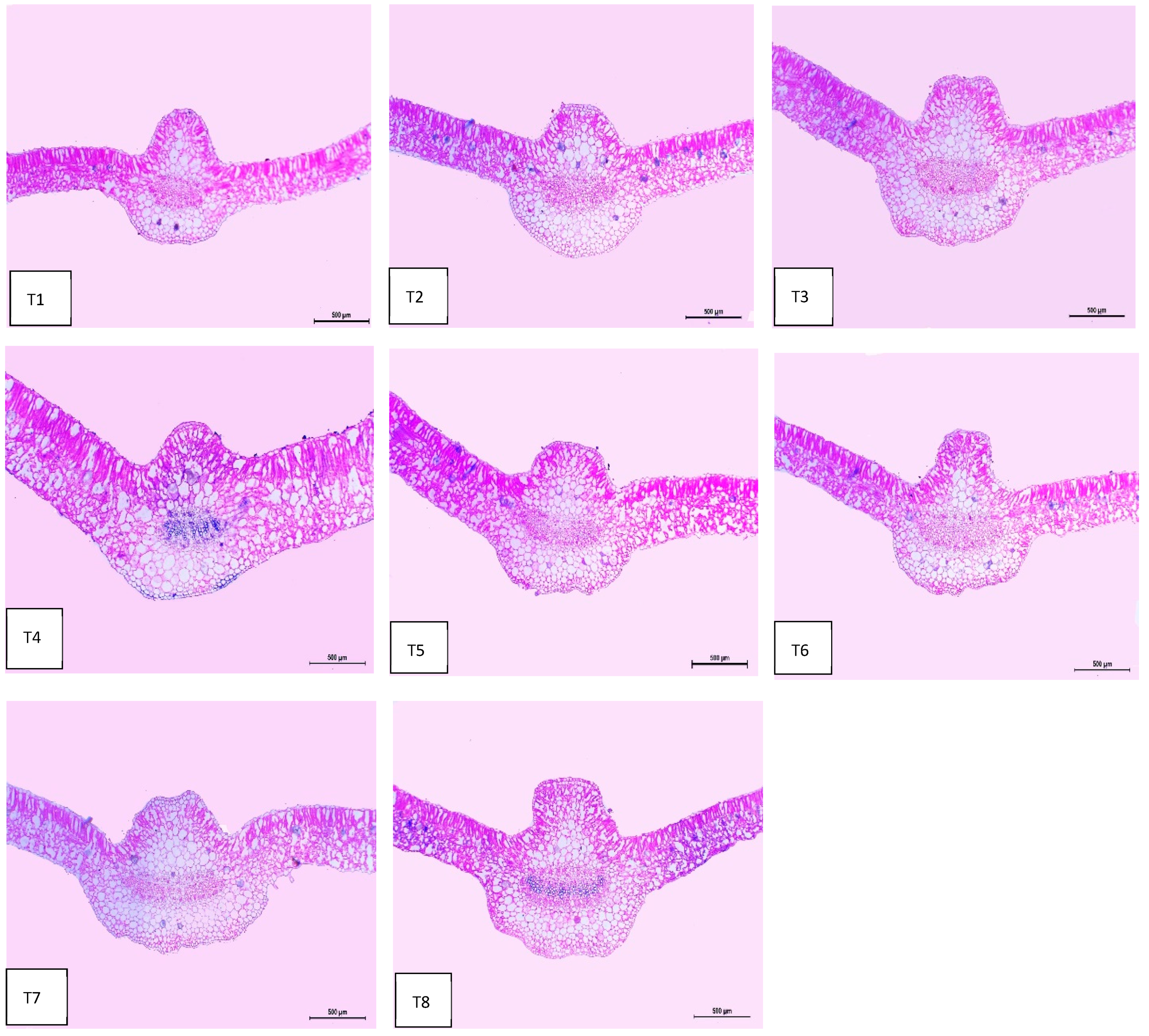
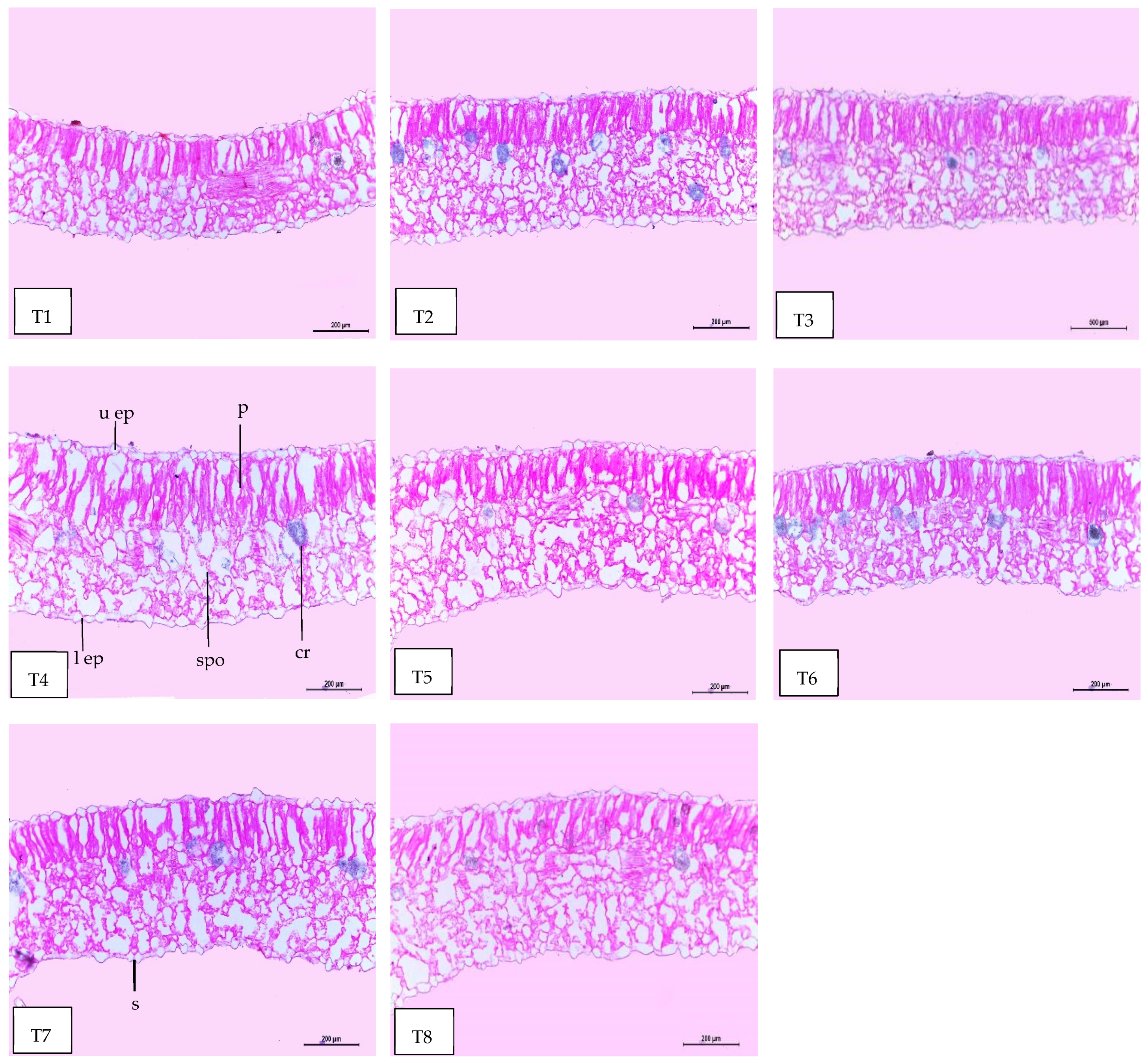
2.8. Leaf Anatomy
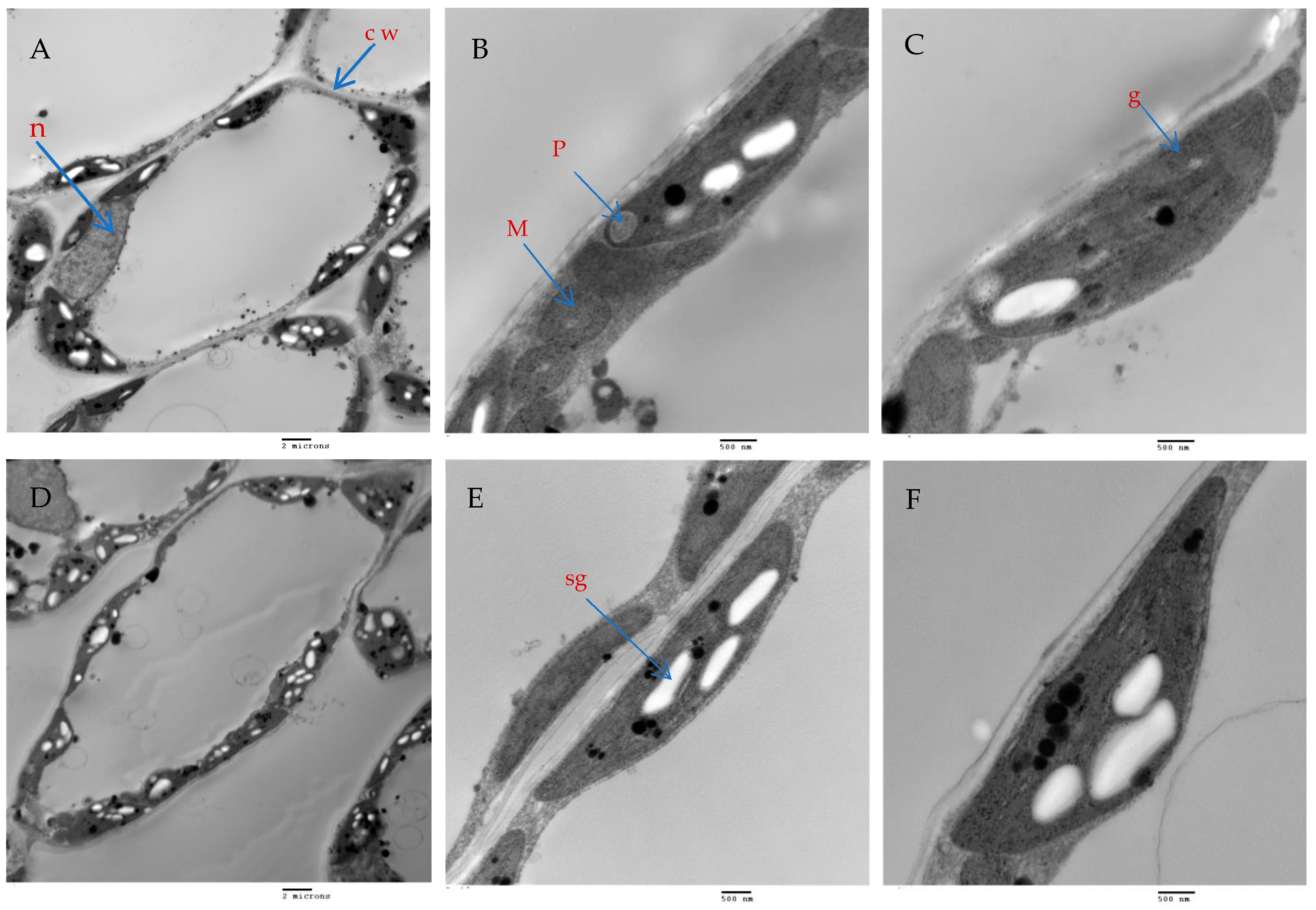
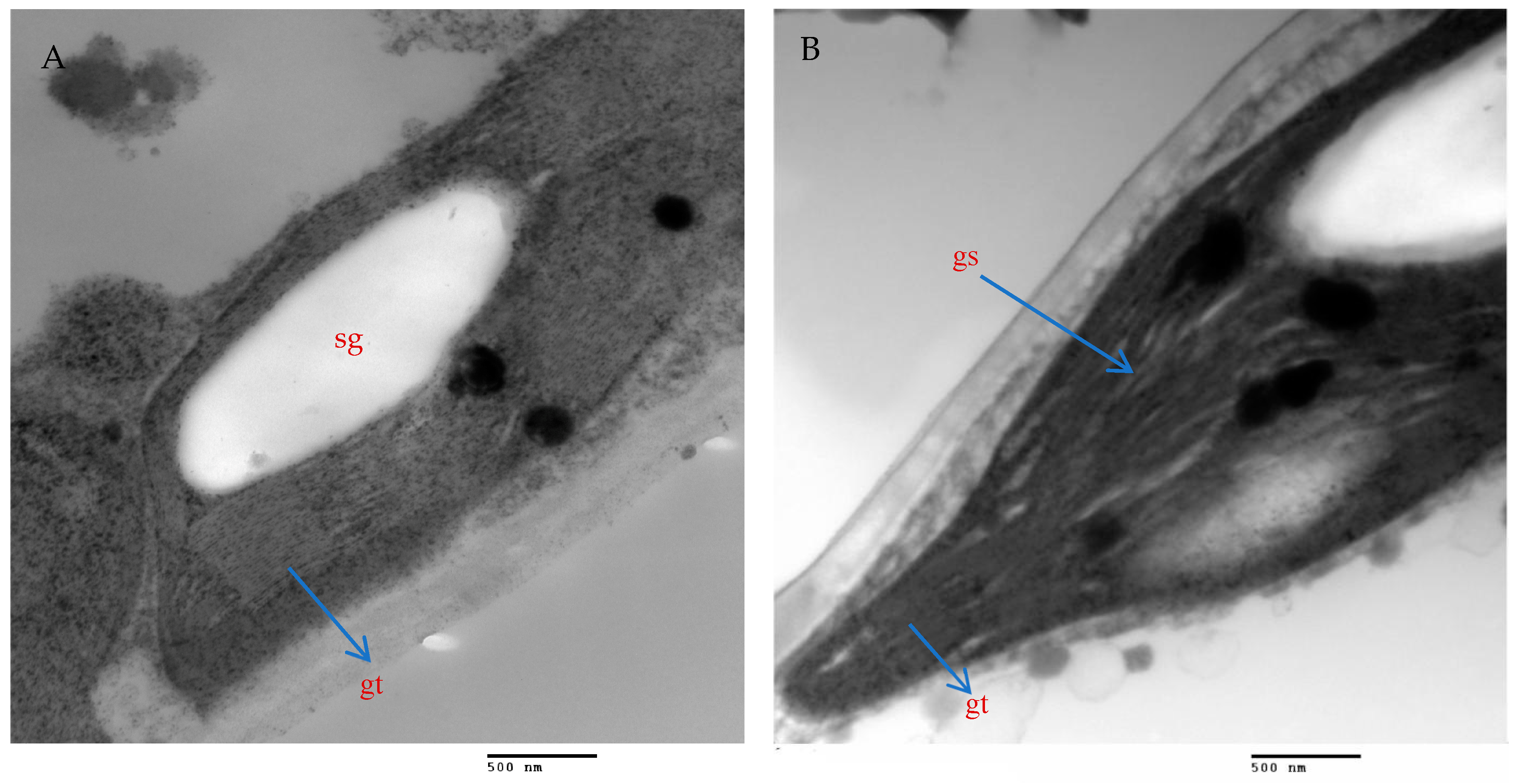
2.9. Mesophyll Parenchyma and Chloroplast Ultrastructure of Chili Pepper Leaves
3. Discussion
4. Materials and Methods
4.1. The Experimental Location and Plant Materials
4.2. Mycorrhizal Inoculums and Growth Conditions
4.3. Field Experiments
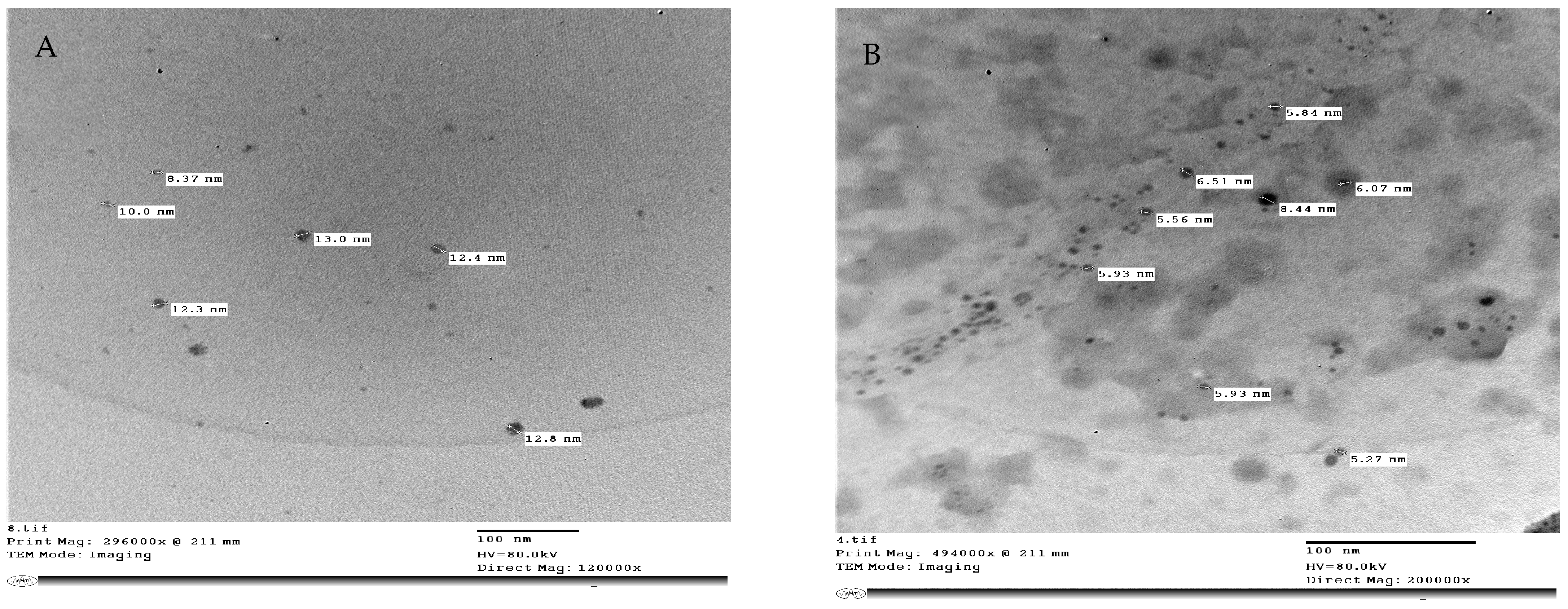
4.4. Preparation of Nanoparticles
4.5. Data Recorded
4.5.1. Plant Growth, Flowering, and Yield
4.5.2. Photosynthetic Parameters and Chlorophyll Content of Chili Plants
4.5.3. Fruit Quality Parameters
4.5.4. Mineral Content in Chili Leaves
4.5.5. Antioxidants Enzyme, Hormone, Lipid Peroxidation (MDA), Hydrogen Peroxide, and Nitrogen Metabolism Enzyme
4.5.6. Protein Electrophoresis SDS-PAGE
4.5.7. Anatomical of Chili Leaves
4.5.8. Ultrastructure of Chili Leaves
4.5.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mercado, A.J.; Mar, T.M.; Reid, M.S.; Valpuesta, V.; Quesada, M.A. Effects of low temperature on pepper pollen morphology and fertility: Evidence of cold induced exine alterations. J. Hortic. Sci. 1997, 72, 317–326. [Google Scholar] [CrossRef]
- Ou, L.J.; Wei, G.; Zhang, Z.Q.; Dai, X.Z.; Zou, X.X. Effects of low temperature and low irradiance on the physiological characteristics and related gene expression of different pepper species. Photosynthetica 2015, 53, 85–94. [Google Scholar] [CrossRef]
- Airaki, M.; Leterrier, M.; Mateos, R.M.; Valderrama, R.; Chaki, M.; Barroso, J.B.; del Río, L.A.; Palma, J.M.; Corpas, F.J. Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low-temperature stress. Plant Cell Environ. 2012, 35, 281–295. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Z.; Zhang, J.; Hemat, M.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Osmolyte accumulation plays important roles in the drought priming induced tolerance to post-anthesis drought stress in winter wheat (Triticum aestivum L.). Environ. Exp. Bot. 2019, 166, 103804. [Google Scholar] [CrossRef]
- Jan, N.; Majeed, U.; Andrabi, K.I.; John, R. Cold stress modulates osmolytes and antioxidant system in Calendula officinalis. Acta Physiol. Plant. 2018, 40, 73. [Google Scholar] [CrossRef]
- Megha, S.; Basu, U.N.K. Regulation of low-temperature stress in plants by microRNAs. Plant Cell Environ. 2017, 41, 12956. [Google Scholar] [CrossRef] [PubMed]
- Karami, M.S.; Maali, A.R.; Kazemi, S.S. Effect of cold stress on oxidative damage and mitochondrial respiratory properties in chickpea. Plant Physiol. Biochem. 2018, 122, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, S.; Mishra, S.; Dames, J.F. Arbuscular mycorrhizal inoculation improves growth and antioxidative response of Jatropha curcas (L.) under Na2SO4 salt stress. Plant Biosyst. 2013, 49, 260–269. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Mishra, S. Evaluating effect of arbuscular mycorrhizal fungal consortia and Azotobacter chroococcum in improving biomass yield of Jatropha curcas. Plant Biosyst. 2016, 150, 1056–1064. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Hashem, A.; AbdAllah, E.F.; Ahmad, P. Arbuscular mycorrhiza in crop improvement under environmental stress. In Emerging Technologies and Management of Crop Stress Tolerance; Academic Press: Cambridge, MA, USA, 2014; pp. 69–95. [Google Scholar] [CrossRef]
- Hameed, A.; Wu, Q.S.; AbdAllah, E.F.; Hashem, A.; Kumar, A.; Lone, H.A.; Ahmad, P. Role of AM fungi in alleviating drought stress in plants. In Use of Microbes for the Alleviation of Soil Stresses; Miransari, M., Ed.; Springer Science Business Media: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Wahid, F.; Sharif, M.; Steinkellner, S.; Khan, M.A.; Marwat, K.B.; Khan, S.A. Inoculation of arbuscular mycorrhizal fungi and phosphate solubilizing bacteria in the presence of rock phosphate improves phosphorus uptake and growth of maize. Pak. J. Bot. 2016, 48, 739–747. [Google Scholar]
- Piotrowski, J.S.; Denich, T.; Klironomos, J.N.; Graham, J.M.; Rillig, M.C. The effects of arbuscular mycorrhizas on soil aggregation depend on the interaction between plant and fungal species. New Phytol. 2004, 164, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, L.; Wei, S.; Xiao, X.; Su, C.; Jiang, P. Effects of arbuscular mycorrhizal fungi on the growth, nutrient uptake and glycyrrhizic acid production of licorice (Glycyrrhiza uralensis Fisch.). Plant Growth Regul. 2007, 52, 29–39. [Google Scholar] [CrossRef]
- Ortas, I. The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. Field Crop. Res. 2012, 125, 35–48. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Greensynthesized nanoparticles enhanced seedling growth, yield, and quality of onion (Allium cepa L.). ACS Sustain. Chem. Eng. 2019, 7, 14580–14590. [Google Scholar] [CrossRef]
- Ikram, M.; Raja, N.I.; Javed, B.; Mashwani, Z.R.; Hussain, M.; Hussain, M.; Ehsan, M.; Rafique, N.; Malik, K.; Sultana, T.; et al. Foliar applications of biofabricated selenium nanoparticles to improve the growth of wheat plants under drought stress. Green. Process. Synth. 2020, 9, 706–714. [Google Scholar] [CrossRef]
- Hasanuzzaman, M. Silicon and selenium: Two vital trace elements that confer abiotic stress tolerance to plants. In Emerging Technologies and Management of Crop Stress Tolerance; Academic Press: San Diego, CA, USA, 2014; pp. 377–422. [Google Scholar]
- Singh, R.; Upadhyay, A.K.; Singh, D.P. Regulation of oxidative stress and mineral nutrient status by selenium in arsenic treated crop plant Oryza sativa. Ecotoxicol. Environ. Saf. 2017, 148, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qin, N.; Sun, L.; Yu, M.; Hu, W.; Qi, Z. Selenium Improves Physiological Parameters and Alleviates Oxidative Stress in Strawberry Seedlings under Low-Temperature Stress. Int. J. Mol. Sci. 2018, 19, 1913. [Google Scholar] [CrossRef]
- Feng, J.N.; Guo, X.P.; Chen, Y.R.; Lu, D.P.; Niu, Z.S.; Tou, F.Y.; Hou, L.J.; Xu, J.; Liu, M.; Yang, Y. Time-dependent effects of ZnO nanoparticles on bacteria in an estuarine aquatic environment. Sci. Total Environ. 2020, 698, 134298–134309. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Jajoo, A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar] [CrossRef]
- Alharby, H.F.; Metwali, E.M.R.; Fuller, M.P.; Aldhebiani, A.Y. Impact of application of zinc oxide nanoparticles on callus induction, plant regeneration, element content and antioxidant enzyme activity in tomato (Solanum lycopersicum Mill.) under salt stress. Arch. Biol. Sci. 2016, 68, 723–735. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles-An eco-friendly approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Hussain, I.; Singh, H.; Yadav, V.; Singh, S.C. Green synthesis of nano zinc oxide and evaluation of its impact on germination and metabolic activity of Solanum lycopersicum. J. Biotechnol. 2016, 233, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Sharaf-Eldin, M.A.; Alshallash, K.S.; Alharbi, K.R.; Alqahtani, M.M.; Etman, A.A.; Yassin, A.M.; Azab, E.S.; El-Okkiah, S.A.F. Influence of Seed Soaking and Foliar Application Using Ozonated Water on Two Sweet Pepper Hybrids under Cold Stress. Sustainability 2022, 14, 13453. [Google Scholar] [CrossRef]
- Hafez, O.M.; Saleh, M.A.; El-Lethy, S.R. Response of some seedlings olive cultivars to foliar spray of yeast and garlic extracts with or without vascular arbuscular mycorrhizal fungi. J. World Appl. Sci. 2013, 24, 1119–1129. [Google Scholar]
- Latef, A.A.H.A.; Chaoxing, H. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomatoes grown under salinity stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Wang, C.; Hashem, A.M.; Tabl, K.M.; Nishawy, E.; Kuai, J.; Zhou, G.; Wang, B. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol. Environ. Saf. 2021, 225, 112695. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.A.; Darwesh, O.M.; Mekki, B.B. Environmentally friendly nano-selenium to improve the antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocatal. Agric. Biotechnol. 2019, 18, 101080. [Google Scholar] [CrossRef]
- Nandagopal, J.G.T.; Harinarayanan, U.N.D.; Raghavan, S.; Girija, S. Foliar selenium application mitigates low-temperature stress in chilli (Capsicum annuum L.) seedlings. Energy Nexus 2022, 6, 100079. [Google Scholar] [CrossRef]
- Sayed, E.G.; Mahmoud, A.W.M.; Abdel-Wahab, A.; El-bahbohy, R.M.; Azoz, S.N. Rootstock Priming with Shikimic Acid and Streptomyces griseus for Growth, Productivity, Physio-Biochemical, and Anatomical Characterisation of Tomato Grown under Cold Stress. Plants 2022, 11, 2822. [Google Scholar] [CrossRef]
- Garcia, C.L.; Dattamudi, S.; Chanda, S.; Jayachandran, K. Effect of Salinity Stress and Microbial Inoculations on Glomalin Production and Plant Growth Parameters of Snap Bean (Phaseolus vulgaris). Agronomy 2019, 9, 545. [Google Scholar] [CrossRef]
- El-Bassiouny, H.M.S.; Bakry, A.B.; Taha, M.H.; Younis, A.S.M. Alleviation of salt stress and improve crop productivity by using arbuscular mycorrhiza and ZnO-Nano or bulk particles in wheat. Plant Arch. 2019, 19, 205–214. [Google Scholar]
- Ali, E.A.; Mahmoud, A.M. Effect of foliar spray by different salicylic acid and zinc concentrations on seed yield and yield components of mungbean in sandy soil. Asian J. Crop Sci. 2012, 5, 33–40. [Google Scholar] [CrossRef]
- Smirnoff, N. Antioxidant systems and plant response to environment. In Environmentand Plant Metabolism: Flexibility and Acclimation; Smirnoff, N., Ed.; BIOS Scientific Publishers: Oxford, UK, 1995; pp. 217–243. [Google Scholar]
- Uresti-Porras, J.G.; Cabrera, D.; Fuente, M.; Benavidez, M.A.; Olivares, S.E.; Cabrera, R.I.; Juárez, M.A. Effect of Graft and Nano ZnO on Nutraceutical and Mineral Content in Bell Pepper. Plants 2021, 10, 2793. [Google Scholar] [CrossRef]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez, R.V.; Jauneau, A.; Roy, S. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, e226. [Google Scholar] [CrossRef] [PubMed]
- El Lateef Gharib, F.A.; Zeid, I.M.; Ghazi, S.M.; Ahmed, E.Z. The response of cowpea (Vigna unguiculata L.) plants to foliar application of sodium selenate and selenium nanoparticles (SeNPs). J. Nanomater. Mol. Nanotechnol. 2019, 8, 4. [Google Scholar]
- Zhu, X.; Song, F.; Liu, F. Arbuscular Mycorrhizal Fungi and Tolerance of Temperature Stress in Plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer Science and Business Media LLC: Berlin, Germany, 2017; Volume 33, pp. 163–194. [Google Scholar]
- Vankova, R.; Landa, P.; Podlipna, R.; Dobrev, P.I.; Prerostova, S.; Langhansova, L.; Gaudinova, A.; Motkova, K.; Knirsch, V.; Vanek, T. ZnO nanoparticle effects on hormonal pools in Arabidopsis thaliana. Sci. Total Environ. 2017, 593–594, 535–542. [Google Scholar] [CrossRef]
- Feng, R.; Wang, L.; Yang, J.; Zhao, P.; Zhu, Y.; Li, Y.; Yu, Y.; Liu, H.; Rensing, C.; Wu, Z. Underlying mechanisms responsible for restriction of uptake and translocation of heavy metals (metalloids) by selenium via root application in plants. J. Hazard. Mater. 2021, 402, 123570. [Google Scholar] [CrossRef] [PubMed]
- Diab, H.; Limami, A.M. Metabolism upon Hypoxia Stress and Recovery: Roles of Alanine Aminotransferase (AlaAT) and Glutamate Dehydrogenase (GDH) Alternative route for nitrogen assimilation in higher plants. Plants 2016, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Joudmand, A.; Aliasgharzad, N.; Tolrá, R.; Poschenrieder, C. Arbuscular mycorrhizal fungialleviate low-temperature stress and increase freezing resistance as a substitute for acclimation treatment in barley. Crop. Pasture Sci. 2019, 70, 218–233. [Google Scholar] [CrossRef]
- Ebrahimian, E.; Bybordi, A. Exogenous silicium and zinc increase antioxidant enzyme activity and alleviate salt stress in leaves of sunflower. J. Food Agric. Environ. 2011, 9, 422–427. [Google Scholar]
- Ouledali, S.; Ennajeh, M.; Ferrandino, A.; Khemira, H.; Schubert, A.; Secchi, F. Influence of arbuscular mycorrhizal fungi inoculation on the control of stomata functioning by abscisic acid (ABA) in drought-stressed olive plants. S. Afr. J. Bot. 2019, 121, 152–158. [Google Scholar] [CrossRef]
- Xue, T.L.; Hou, S.F.; Tan, J.A.; Liu, G.L. The antioxidative function of selenium in higher plants: II. Non-enzymatic mechanisms. Chin. Sci. Bull. 1993, 38, 356–358. [Google Scholar]
- Korkmazm, A.; Deger, O.; Szafranska, K.; Koklü, S.; Karaca, A.; Yakupoglu, G.; Kocacinar, F. Melatonin effects in enhancing chilling stress tolerance of pepper. Sci. Hortic. 2022, 289, 110434. [Google Scholar] [CrossRef]
- Khater, M.; El-Awadi, M.; Elashtokhy, M.; Abdel-Baky, Y.; Shalaby, M. Physiological and Molecular Changes in Fenugreek (Trigonella foenum-graecum L.) as a Response to Gamma Rays. Int. J. Pharm. Tech. Res. 2016, 9, 306–316. [Google Scholar]
- Abedi, T.; Alemzadeh, A.; Kazemeini, S.A. Wheat Yield and Grain Protein Response to Nitrogen Amount and Timing. Aust. J. Crop Sci. 2011, 5, 330–336. [Google Scholar]
- Bassuony, F.; Hashem, H.A.; Hassanein, R.; Baraka, D.; Khalil, R.R. The Impact of Stigmasterol on Growth, Productivity and Biochemical Response of Vicia faba L. Plants Grown under Salt Stress. Egypt. J. Bot. 2014, 54, 203–218. [Google Scholar]
- Liu, Y.; Lu, J.; Cui, L.; Tang, L.; Ci, D.; Zou, X.; Zhang, X.; Yu, X.; Wang, Y.; Si, Y. The multifaceted roles of Arbuscular Mycorrhizal Fungi in peanut responses to salt, drought, and cold stress. BMC Plant Biol. 2023, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Sarkar, A.; Dasgupta, D.; Acharya, K.; Keswani, C.; Popova, V.; Minkina, T.; Maksimov, A.; Chakraborty, N. Unravelling the Efficient Applications of Zinc and Selenium for Mitigation of Abiotic Stresses in Plants. Agriculture 2022, 12, 1551. [Google Scholar] [CrossRef]
- Mahdi, A.; Abd, A.; Awad, K. Effect of Foliar Application of Nano-selenium on the Anatomical Characteristics of Date Palm Phoenix dactylifera L. Barhi Cultivar under salt stress. Basrah J. Agric. Sci. 2022, 35, 313–325. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Z.; Wang, Q.; Guan, Y. Effects of Low Temperature on Leaf Anatomy and Photosynthetic Performance in Different Genotypes of Wheat Following a Rice Crop. Int. J. Agric. Biol. 2015, 17, 1165–1171. [Google Scholar] [CrossRef]
- Kang, P.; Wu, Y.; Zhang, J.; An, Q.; Zhou, C.; Li, D.; Pan, C. Nano-selenium enhances the antioxidant capacity, organic acids and cucurbitacin B in melon (Cucumis melo L.) plants. Ecotoxicol. Environ. Saf. 2022, 241, 113777. [Google Scholar] [CrossRef]
- Venzhik, Y.; Shchyogolev, S.; Dykman, L. Ultrastructural Reorganization of Chloroplasts during Plant Adaptation to Abiotic Stress Factors. Russ. J. Plant Physiol. 2019, 66, 850–863. [Google Scholar] [CrossRef]
- Ejsmont, A.; Goscianska, J. Hydrothermal Synthesis of ZnO Superstructures with Controlled Morphology via Temperature and pH Optimization. Materials 2023, 16, 1641. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Zendehcheshm, S.; Khademi, F. Selenium nanoparticles: Synthesis, in-vitro cytotoxicity, antioxidant activity and interaction studies with ct-DNA and HSA, HHb and Cyt c serum proteins. Biotechnol. Rep. 2021, 30, e00615. [Google Scholar] [CrossRef]
- Boyhan, G.E.; McGregor, C.; O’Connell, S.; Biang, J.; Berle, D.A. Comparison of 13 sweet pepper varieties under an organic farming system. HortTechnology 2020, 30, 135–143. [Google Scholar] [CrossRef]
- Thaib, N.; Katja, D.G.; Aritonang, H.F. Isolasi capsaicin dari oleoresin cabairawit (Capsicum frutescens L.). Chem. Prog. 2015, 8, 71–76. [Google Scholar]
- Kumar, O.A.; Tata, S.S. Ascorbic Acid Contents in Chili Peppers (Capsicum L.). Not. Sci. Biol. 2009, 1, 50–52. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis, 15th ed.; Association of Official Agricultural Chemist: Arlington, VA, USA, 1990; Volume 1, p. 673. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Alfosea, S.M.; Simón, G.S.; Zavala, G.E.A.; Cámara, Z.J.M.; Simón, I.; Martínez, N.J.J.; Lidón, V.; García, S.F. Physiological, nutritional and metabolomic responses of tomato plants after the foliar application of amino acids Aspartic acid, Glutamic Acid and Alanine. Front. Plant Sci. 2020, 11, 581234. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.L. Soil Chemical Analysis; Text Book; Printice-Hall of India Privat Limited: New Delhi, India, 1973; Volume 144–197, p. 381. [Google Scholar]
- Polle, A.; Otter, T.; Mehne-Jakobs, B. Effect of magnesium deficiency on antioxidative systems in needles of Norway spruce [Picea abies (L.) Karst.] grown with different ratios of nitrate and ammonium as nitrogen souices. New Phytol. 1994, 128, 621–628. [Google Scholar] [CrossRef]
- Fales, T.M.; Jaouni, J.F.; Babashak, I. Simple device for preparing ethereal diazomethane without resorting to distillation. Ann. Chem. 1973, 45, 2302–2303. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Rasheed, R.; Hussain, I.; Iqbal, M.; Haider, M.Z.; Parveen, S.; Sajid, M.A. Hydrogen peroxide modulates antioxidant system and nutrient relation in maize (Zea mays L.) under water-deficit conditions. Arch. Agron. Soil Sci. 2015, 61, 507–523. [Google Scholar] [CrossRef]
- Majerowicz, N.; Kerbauy, G.; Nievola, C.; Suzuki, R. Growth and nitrogen metabolism of Catasetum fimbriatum (Orchidaceae) grown with different nitrogen sources. Environ. Exp. Bot. 2000, 44, 195–206. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hunter, E. Paractical Electron Microscopy: A Beginners Illustrated Guide, 2nd ed.; Cambridge University Press: Cambridge, NY, USA, 1993. [Google Scholar]
- Bricker, B. MSTATC: A Micro Computer Program from the Design Management and Analysis of Agronomic Research Experiments; Michigan State University: East Lansing, MI, USA, 1991. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980; p. 507. [Google Scholar]




| Treatment | Plant Height (cm) | Number of Leaves | Plant Fresh Weight (g) | Plant Dry Weight (g) | Leaf Area (cm2) |
|---|---|---|---|---|---|
| 2021 Season | |||||
| Control | 50.3 h | 40.3 h | 101.3 f | 18.6 f | 51.7 h |
| Se-NPs | 59.0 g | 52.0 g | 156.0 e | 29.7 e | 55.3 g |
| ZnO-NPs | 67.6 f | 63.0 f | 207.3 d | 38.3 d | 58.7 f |
| ZnO-NPs + Se-NPs | 77.0 e | 75.0 e | 255.7 c | 48.0 c | 61.0 e |
| AMF | 84.3 d | 88.7 d | 303.3 b | 52.3 bc | 65.0 d |
| AMF + Se-NPs | 92.3 c | 102.3 c | 318.7 b | 58.7 b | 67.7 c |
| AMF + ZnO-NPs | 102.3 b | 117.7 b | 312.0 b | 58.0 b | 71.0 b |
| AMF + ZnO-NPs + Se-NPs | 115.7 a | 135.7 a | 388.3 a | 74.7 a | 80.0 a |
| LSD value at 0.05: | 3.1 | 2.4 | 22.59 | 7.4 | 1.6 |
| 2022 Season | |||||
| Control | 49.03 g | 35.0 g | 93.3 h | 14.7 g | 54.7 h |
| Se-NPs | 58.1 f | 55.3 f | 142.7 g | 25.0 f | 58.3 g |
| ZnO-NPs | 66.4 e | 61.0 f | 166.4 f | 31.9 e | 61.7 f |
| ZnO-NPs + Se-NPs | 76.0 d | 73.0 e | 203.1 e | 40.7 d | 63.4 e |
| AMF | 88.1 c | 86.6 d | 244.1 d | 50.2 c | 67.3 d |
| AMF + Se-NPs | 90.8 c | 99.7 c | 282.5 c | 61.1 b | 69.9 c |
| AMF + ZnO-NPs | 100.2 b | 110.3 b | 299.5 b | 62.0 b | 73.3 b |
| AMF + ZnO-NPs + Se-NPs | 113.6 a | 129.3 a | 336.2 a | 72.7 a | 82.3 a |
| LSD value at 0.05: | 6.3 | 8.09 | 7.1 | 3.3 | 1.7 |
| Treatment | Single-Fruit Weight (g) | Fruit Length (cm) | Fruit Diameter (cm) | Capsaicin (µg/g) | Ascorbic Acid (mg/100 g) |
|---|---|---|---|---|---|
| 2021 Season | |||||
| Control | 13.0 e | 8.0 g | 0.67 e | 133.3 h | 85.5 f |
| Se-NPs | 15.3 c | 11.7 f | 1.4 d | 136.7 f | 89.2 de |
| ZnO-NPs | 14.3 d | 10.5 f | 1.03 e | 134.6 g | 88.4 ef |
| ZnO-NPs + Se-NPs | 16.7 b | 13.1 e | 1.0 d | 137.7 e | 92.0 d |
| AMF | 14.33 d | 14.6 d | 2.9 b | 139.2 d | 100.2 c |
| AMF + Se-NPs | 15.6 c | 17.8 b | 3.9 a | 140.5 b | 115.4 b |
| AMF + ZnO-NPs | 17.0 b | 16.2 c | 2.5 c | 139.6 c | 113.1 b |
| AMF + ZnO-NPs + Se-NPs | 18.6 a | 21.70 a | 3.5 a | 142.9 a | 125.0 a |
| LSD value at 0.05: | 0.93 | 1.38 | 0.39 | 0.34 | 3.3 |
| 2022 Season | |||||
| Control | 15.3 d | 8.7 f | 0.63 e | 134.0 h | 88.0 f |
| Se-NPs | 17.0 bc | 14.4 d | 1.30 d | 137.5 f | 91.7 de |
| ZnO-NPs | 16.7 c | 13.0 e | 1.20 d | 135.3 g | 90.9 de |
| ZnO-NPs + Se-NPs | 16.7 c | 15.0 d | 1.7 c | 138.4 e | 94.5 d |
| AMF | 17.3 bc | 15.5 cd | 1.60 c | 139.7 d | 103.7 c |
| AMF + Se-NPs | 17.6 bc | 17.9 b | 2.6 a | 141.0 b | 119.4 b |
| AMF + ZnO-NPs | 18.0 b | 16.2 c | 2.1 b | 140.1 c | 117.1 b |
| AMF + ZnO-NPs + Se-NPs | 19.6 a | 21.0 a | 2.7 a | 143.3 a | 129.0 a |
| LSD value at 0.05: | 1.26 | 1.1 | 0.26 | 0.27 | 3.41 |
| Treatment | N% | P% | K% | Ca% |
|---|---|---|---|---|
| 2021 Season | ||||
| Control | 2.5 f | 0.08 d | 2.1 d | 0.6 d |
| Se-NPs | 3.0 e | 0.26 c | 3.2 c | 1.1 c |
| ZnO-NPs | 2.9 e | 0.27 bc | 3.1 c | 1.2 bc |
| ZnO-NPs + Se-NPs | 3.2 d | 0.31 bc | 3.4 bc | 1.3 bc |
| AMF | 3.3 c | 0.35 bc | 3.6 bc | 1.5 bc |
| AMF + Se-NPs | 3.4 c | 0.38 b | 3.7 b | 1.4 bc |
| AMF + ZnO-NPs | 3.7 b | 0.37 b | 3.8 b | 1.6 b |
| AMF + ZnO-NPs + Se-NPs | 3.9 a | 0.70 a | 4.4 a | 1.9 a |
| LSD value at 0.05: | 0.14 | 0.11 | 0.46 | 0.35 |
| 2022 Season | ||||
| Control | 2.3 f | 0.12 h | 1.9 | 0.5 h |
| Se-NPs | 3.2 e | 0.35 f | 2.9 d | 0.9 g |
| ZnO-NPs | 3.3 e | 0.28 g | 2.9 cd | 1.2 f |
| ZnO-NPs + Se-NPs | 3.4 d | 0.43 e | 3.5 bc | 1.53 e |
| AMF | 3.5 cd | 0.50 d | 3.8 b | 1.8 d |
| AMF + Se-NPs | 3.6 c | 0.64 b | 3.9 b | 2.15 c |
| AMF + ZnO-NPs | 3.9 b | 0.58 c | 4.0 b | 2.4 b |
| AMF + ZnO-NPs + Se-NPs | 4.2 a | 0.84 a | 4.7 a | 2.7 a |
| LSD value at 0.05: | 0.44 | 0.055 | 0.61 | 0.11 |
| Treatment | Fe (ppm) | Mn (ppm) | Cu (ppm) | Zn (ppm) | Se (ppm) |
|---|---|---|---|---|---|
| 2021 Season | |||||
| Control | 56.2 h | 12.7 h | 2.1 g | 32.9 f | 0.5 g |
| Se-NPs | 60.1 g | 20.9 f | 2.5 f | 30.1 g | 1.4 cd |
| ZnO-NPs | 62.9 f | 18.1 g | 2.5 f | 37.6 d | 0.9 f |
| ZnO-NPs + Se-NPs | 65.1 e | 23.1 e | 2.7 e | 40.5 c | 1.6 bc |
| AMF | 67.6 d | 25.6 d | 2.9 d | 23.7 h | 1.3 de |
| AMF + Se-NPs | 70.5 c | 30.8 b | 3.04 c | 35.1 e | 1.7 b |
| AMF + ZnO-NPs | 72.8 b | 28.5 c | 3.3 b | 42.8 b | 1.1 ef |
| AMF + ZnO-NPs + Se-NPs | 78.2 a | 35.9 a | 3.5 a | 48.2 a | 2.2 a |
| LSD value at 0.05: | 1.14 | 1.41 | 0.078 | 0.99 | 0.22 |
| 2022 Season | |||||
| Control | 55.0 h | 14.3 h | 2.13 h | 22.1 g | 0.75 f |
| Se-NPs | 60.50 g | 23.3 f | 2.8 f | 30.5 f | 1.18 cd |
| ZnO-NPs | 63.33 f | 20.2 g | 2.5 g | 35.3 de | 0.94 e |
| ZnO-NPs + Se-NPs | 66.0 e | 25.5 e | 3.16 e | 40.7 c | 1.3 bc |
| AMF | 68.0 d | 27.9 d | 3.5 b | 37.7 d | 1.02 de |
| AMF + Se-NPs | 71.0 c | 33.2 b | 3.4 c | 33.3 e | 1.4 b |
| AMF + ZnO-NPs | 73.3 b | 30.8 c | 3.3 d | 43.8 b | 1.11 c–e |
| AMF + ZnO-NPs + Se-NPs | 78.9 a | 39.8 a | 3.9 a | 46.9 a | 1.7 a |
| LSD value at 0.05: | 1.113 | 1.66 | 0.055 | 2.5 | 0.17 |
| Mwt (kD) | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 |
|---|---|---|---|---|---|---|---|---|
| 320.23 | - | + | + | + | + | + | + | - |
| 228.02 | - | + | + | + | + | + | + | + |
| 133.56 | + | + | + | + | + | + | - | + |
| 61.96 | + | + | + | + | + | + | + | + |
| 38.82 | + | + | + | + | - | + | + | + |
| 28.09 | - | + | + | + | + | + | + | + |
| 20.74 | + | + | + | + | + | + | + | + |
| Number of bands | 7 | 7 | 4 | 7 | 6 | 7 | 6 | 7 |
| Treatments | Histological Aspects | |||||||
|---|---|---|---|---|---|---|---|---|
| Thickness of Midvein | Thickness of Upper Epiderm | Thickness of Lower Epiderm | Thickness of Lamina | Thickness of Palisade Tissue | Thickness of Spongy Tissue | Dimensions of Main Midvein Bundle | The Mean Diameter of the Vessel | |
| Control | 939.651 | 14.854 | 19.954 | 279.527 | 119.885 | 160.205 | 280.090 | 19.285 |
| Se-NPs | 1110.333 | 14.920 | 20.639 | 309.849 | 125.253 | 184.596 | 350.562 | 20.861 |
| ZnO-NPs | 1234.779 | 15.560 | 20.354 | 334.560 | 126.276 | 208.284 | 394.060 | 20.901 |
| ZnO-NPs + Se-NPs | 1255.086 | 17.208 | 21.758 | 463.622 | 194.225 | 269.397 | 286.452 | 20.333 |
| AMF | 1200.086 | 16.450 | 21.070 | 341.252 | 126.057 | 215.195 | 382.586 | 20.900 |
| AMF + Se-NPs | 1230.381 | 15.845 | 21.465 | 320.655 | 120.221 | 200.434 | 422.038 | 21.347 |
| AMF + ZnO-NPs | 1245.915 | 17.107 | 22.164 | 397.821 | 140.370 | 257.451 | 466.477 | 21.445 |
| AMF + ZnO-NPs + Se-NPs | 1260.588 | 17.886 | 22.747 | 419.633 | 144.979 | 275.654 | 469.774 | 21.762 |
| Soil Parameters | 2021 | 2022 |
|---|---|---|
| Physical properties | ||
| pH | 7.45 ± 0.5 | 7.35 ± 0.3 |
| Clay (%) | 42.2 ± 1.3 | 40.5 ± 1.2 |
| Silt (%) | 27.2 ± 5.1 | 28.7 ± 2.2 |
| Sand (%) | 20.5 ± 3.1 | 22.8 ± 5.1 |
| Soil texture | Clay loam | Clay loam |
| Chemical properties | ||
| Soluble Cations (meq L−1) | ||
| Mg2+ | 2.45 ± 0.4 | 1.98 ± 0.2 |
| K+ | 0.30 ± 0.05 | 0.30 ± 0.03 |
| Ca2+ | 3.44 ± 0.4 | 3.50 ± 0.5 |
| Na+ | 1.61 ± 0.5 | 1.75 ± 0.4 |
| Available phosphorus (mg L−1) | 0.12 ± 0.03 | 0.1 ± 0.01 |
| Total nitrogen (TN) (%) | 0.19 ± 0.03 | 0.17 ± 0.01 |
| Soluble Anions (meqL−1) | ||
| HCO3− | 0.70 ± 0.2 | 0.78 ± 0.1 |
| Cl− | 1.20 ± 0.3 | 1.50 ± 0.2 |
| SO42− | 1.80 ± 0.5 | 1.35 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayed, E.G.; Desoukey, S.F.; Desouky, A.F.; Farag, M.F.; EL-kholy, R.I.; Azoz, S.N. Synergistic Influence of Arbuscular mycorrhizal Fungi Inoculation with Nanoparticle Foliar Application Enhances Chili (Capsicum annuum L.) Antioxidant Enzymes, Anatomical Characteristics, and Productivity under Cold-Stress Conditions. Plants 2024, 13, 517. https://doi.org/10.3390/plants13040517
Sayed EG, Desoukey SF, Desouky AF, Farag MF, EL-kholy RI, Azoz SN. Synergistic Influence of Arbuscular mycorrhizal Fungi Inoculation with Nanoparticle Foliar Application Enhances Chili (Capsicum annuum L.) Antioxidant Enzymes, Anatomical Characteristics, and Productivity under Cold-Stress Conditions. Plants. 2024; 13(4):517. https://doi.org/10.3390/plants13040517
Chicago/Turabian StyleSayed, Eman G., S. F. Desoukey, Abeer F. Desouky, Mervat F. Farag, Ragab I. EL-kholy, and Samah N. Azoz. 2024. "Synergistic Influence of Arbuscular mycorrhizal Fungi Inoculation with Nanoparticle Foliar Application Enhances Chili (Capsicum annuum L.) Antioxidant Enzymes, Anatomical Characteristics, and Productivity under Cold-Stress Conditions" Plants 13, no. 4: 517. https://doi.org/10.3390/plants13040517
APA StyleSayed, E. G., Desoukey, S. F., Desouky, A. F., Farag, M. F., EL-kholy, R. I., & Azoz, S. N. (2024). Synergistic Influence of Arbuscular mycorrhizal Fungi Inoculation with Nanoparticle Foliar Application Enhances Chili (Capsicum annuum L.) Antioxidant Enzymes, Anatomical Characteristics, and Productivity under Cold-Stress Conditions. Plants, 13(4), 517. https://doi.org/10.3390/plants13040517








