Phytochemicals and Their Usefulness in the Maintenance of Health
Abstract
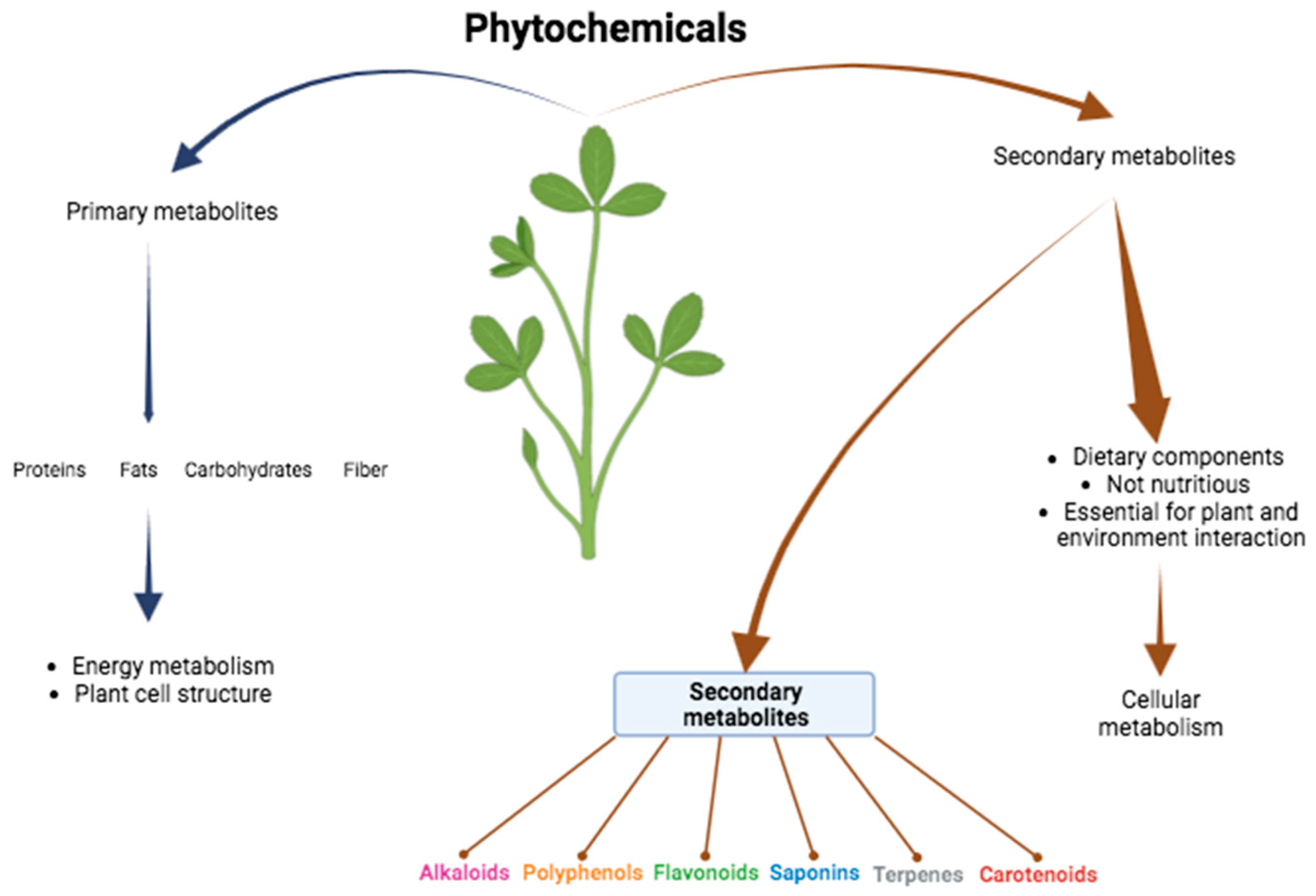
:1. Introduction
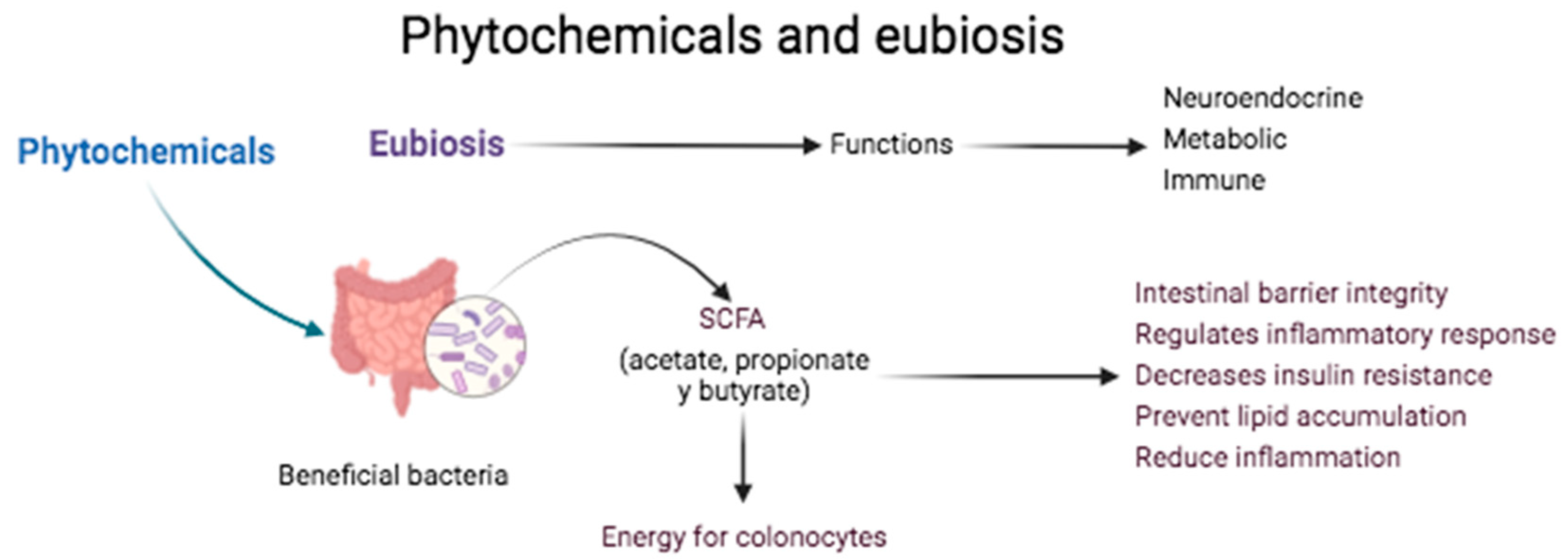
2. Function of the Phytochemicals in the Organism
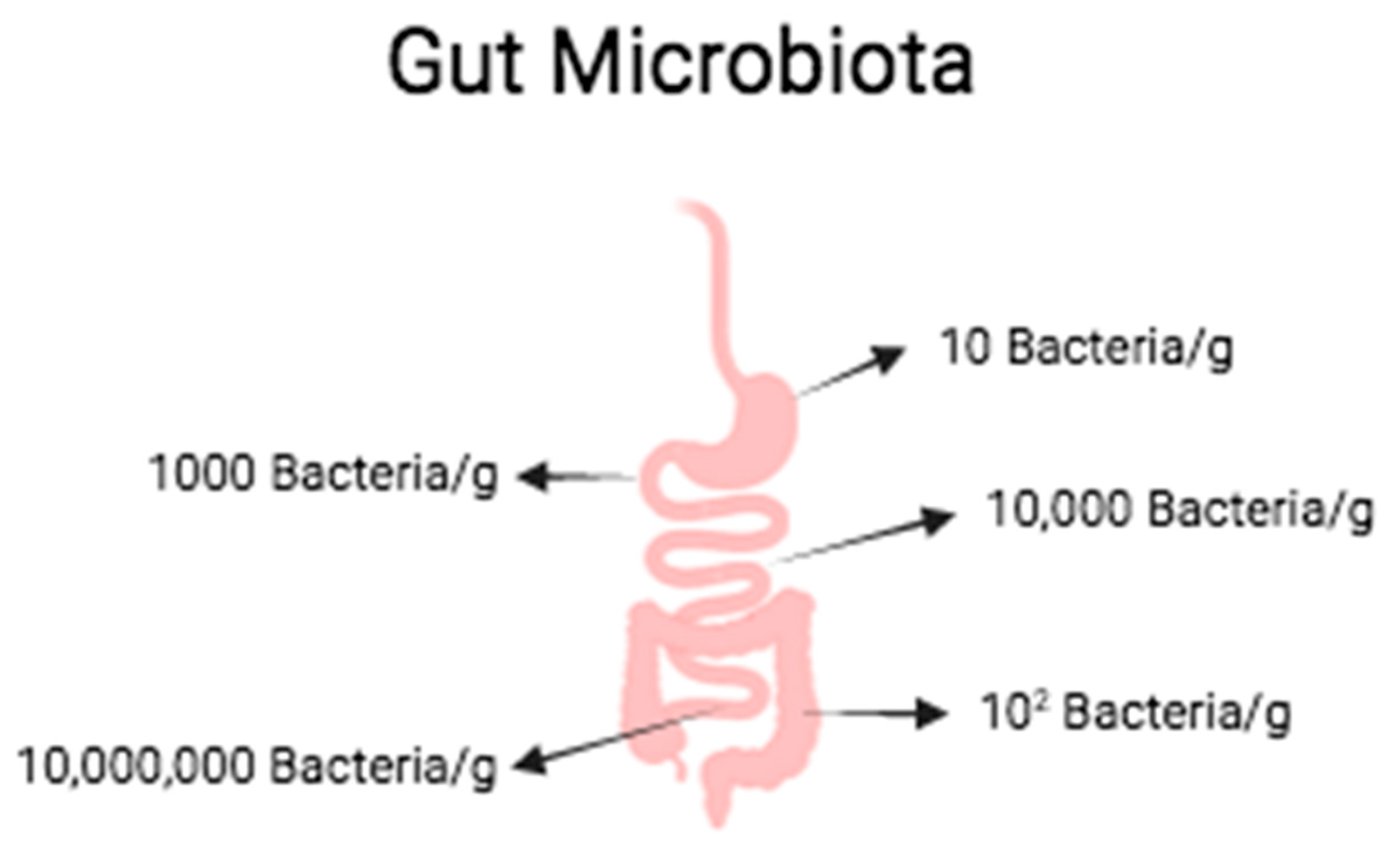
2.1. Intestinal Diseases
2.2. Inflammatory Bowel Disease (IBD)
2.3. Liver Diseases
2.4. Metabolic Syndrome
2.5. Cancer
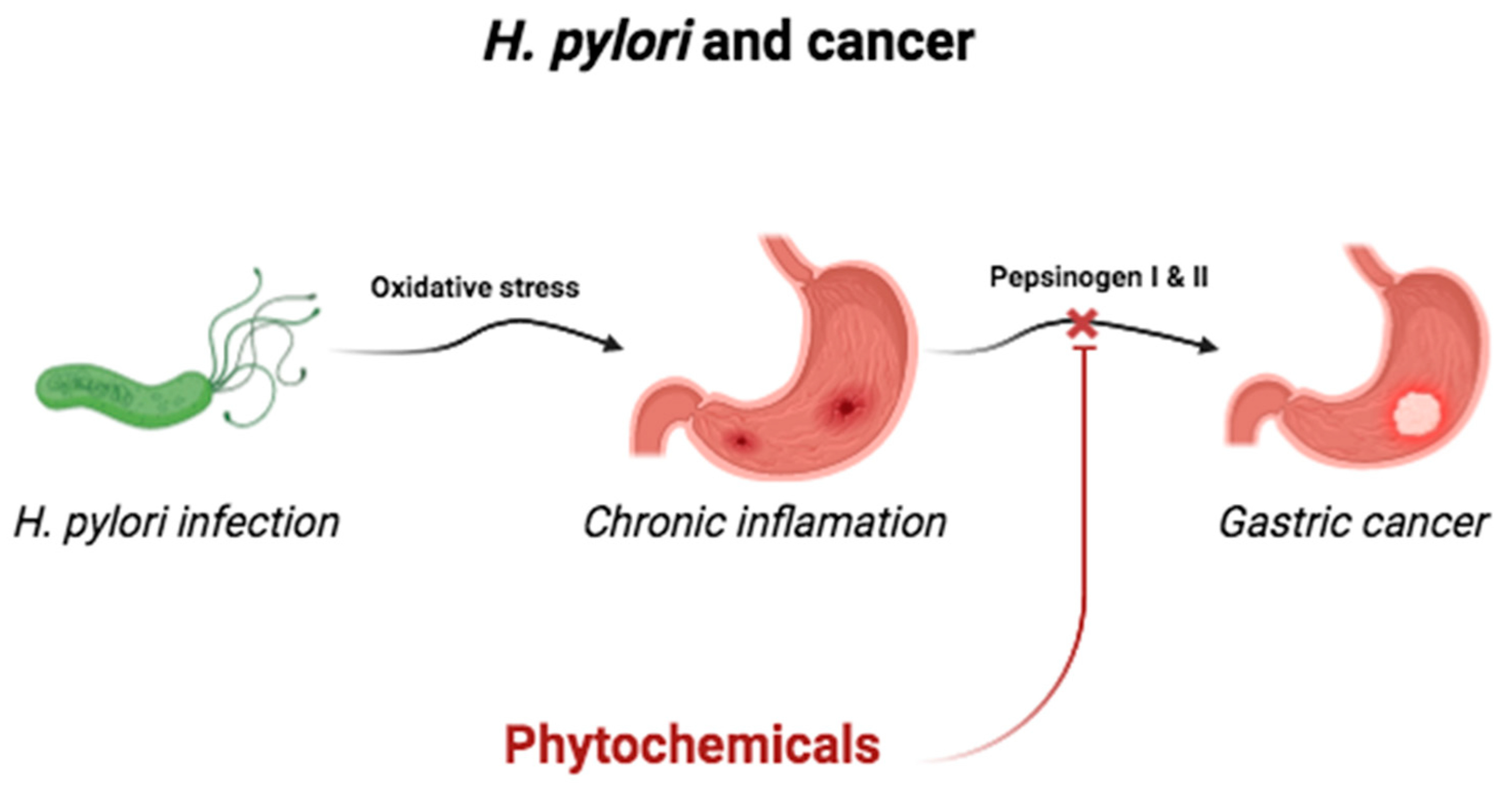
2.6. Infection by Helicobacter pylori
2.7. Diabetes
2.8. Bone Diseases
2.9. Kidney Disease
2.10. Cardiovascular Disease (CVD)
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. BioMed Res. Int. 2019, 2019, 516–536. [Google Scholar] [CrossRef]
- Dwivedi, S.; Kushalan, S.; Paithankar, J.G.; D’souza, L.C.; Hegde, S.; Sharma, A. Environmental toxicants, oxidative stress and health adversities: Interventions of phytochemicals. J. Pharm. Pharmacol. 2022, 74, 516–536. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, C. Characteristics and Health Benefits of Phytochemicals. Forsh Komplementmed 2016, 23, 69–74. [Google Scholar] [CrossRef]
- Wallace, T.C.; Blumberg, J.B.; Johnson, E.J.; Shao, A. Dietary Bioactives: Establishing a Scientific Framework for Recommended Intakes. Adv. Nutr. 2015, 6, 1–4. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, K.; Nam, H.; Lee, D. Discovering Health Benefits of Phytochemicals with Integrated Analysis of the Molecular Network, Chemical Properties and Ethnopharmacological Evidence. Nutrients 2018, 10, 1042. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Ko, Y.-F.; Young, J.D. Phytochemicals as Prebiotics and Biological Stress Inducers. Trends Biochem. Sci. 2020, 45, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Shan, J.; Zhong, L.; Liang, B.; Zhang, D.; Li, M.; Tang, H. Dietary Phytochemicals that Can Extend Longevity by Regulation of Metabolism. Plant Foods Hum. Nutr. 2022, 77, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Watzl, B.; Leitzmann, C. Other Biologically Active Substances in Plant Foods: Phytochemicals; Oxford University Press: Oxford, UK, 2012; pp. 254–264. [Google Scholar]
- Phillips, K.M.; Ruggio, D.M.; Ashraf-Khorassani, M. Phytosterol composition of nuts and seeds commonly consumed in the United States. J. Agri. Food Chem. 2005, 53, 9436–9445. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Van Hung, P. Phemolic compounds of cereals and their antioxidant capacity. Crit. Rev. Food Sci. Nutr. 2014, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-S.; Huang, G.-J.; Lu, Y.-H.; Chang, L.-W. Anti-inflammatory effects of an aqueous extract of Welsh onion green leaves in mice. Food Chem. 2019, 138, 751–756. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Guang, C.; Chen, J.; Sang, S.; Cheng, S. Biological Functionality of Soyasaponins and Soyasapogenols. J. Agric. Food Chem. 2014, 62, 8247–8255. [Google Scholar] [CrossRef]
- Bubonja-Šonje, M.; Knežević, S.; Abram, M. Challenges to antimicrobial susceptibility testing of plant-derived polyphenolic compounds. Arh. Hig. Rada Toksikol. 2020, 71, 300–311. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Wang, S.; Gao, X.; Sun, Q.; Zhu, Y.; Qin, L.; Zhu, B. The phytochemical properties, pharmacological effects and traditional uses of Actinidia eriantha Benth.: A review. Front. Pharmacol. 2022, 13, 959900. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, K.P.; Deans, S.G. Biological Activities of Essential Oils from Selected Aromatic Plants. Acta Hortic. 1995, 390, 203–209. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Focus: Plant-based medicine and pharmacology: Essential oils and health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar]
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as Modifiers of Gut Microbial Communities. Food Funct. 2020, 11, 8444–8471. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Role of te small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Ji, Y.; Yin, Y.; Sun, L.; Zhang, W. The Molecular and Mechanistic Insights Based on Gut–Liver Axis: Nutritional Target for Non-Alcoholic Fatty Liver Disease (NAFLD) Improvement. Int. J. Mol. Sci. 2020, 21, 3066. [Google Scholar] [CrossRef]
- Riccio, P.; Rossano, R. Diet, Gut Microbiota, and Vitamin D + A in Multiple Sclerosis. Neurotherapeutics 2018, 15, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.; Ediriweera, M.K.; Cho, S.K. Interplay between Phytochemicals and the Colonic Microbiota. Nutrients 2023, 15, 1989. [Google Scholar] [CrossRef]
- Wang, Q.; Jia, M.; Zhao, Y.; Hui, Y.; Pan, J.; Yu, H.; Yan, S.; Dai, X.; Liu, X.; Liu, Z. Supplementation of Sesamin Alleviates Stress-Induced Behavioral and Psychological Disorders via Reshaping the Gut Microbiota Structure. J. Agric. Food Chem. 2019, 67, 12441–12451. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, M.; Fu, X.; Zhang, Z.; Zhu, L.; Zheng, X.; Liu, J. Astaxanthin Prevents Alcoholic Fatty Liver Disease by Modulating Mouse Gut Microbiota. Nutrients 2018, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech. Dis. 2017, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Morales-González, Á.; Madrigal-Santillán, E.O.; Angeles-Valencia, M.; Anguiano-Robledo, L.; González-López, L.L.; Sosa-Gómez, A.; Fregoso-Aguilar, T.; Esquivel-Chirino, C.; Ruiz-Velazco-Benítez, Y.A.; et al. Phytochemicals and modulation of excercise-induced oxidative stress: A novel overview of antioxidants. Am. J. Transl. Res 2022, 14, 8292–8324. [Google Scholar] [PubMed]
- Ostrom, E.L.; Traustadóttir, T. Aerobic exer- cise training partially reverses the impairment of Nrf2 activation in older humans. Free. Radic. Biol. Med. 2020, 160, 418–432. [Google Scholar] [CrossRef]
- Haq, I.U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperina: A review of its biological effects. Phytother. Res. 2020, 35, 680–700. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Yoo, W.; Zieba, J.K.; Foegeding, N.J.; Torres, T.P.; Shelton, C.D.; Shealy, N.G.; Byndloss, A.J.; Cevallos, S.A.; Gertz, E.; Tiffany, C.R.; et al. High-fat diet–induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science 2021, 373, 813–818. [Google Scholar] [CrossRef]
- Hossen, I.; Hua, W.; Ting, L.; Mehmood, A.; Jingyi, S.; Duoxia, X.; Yanping, C.; Hongqing, W.; Zhipeng, G.; Kaiqi, Z.; et al. Phytochemicals and inflammatory bowel disease: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1321–1345. [Google Scholar] [CrossRef]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L. Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. CMAJ 2013, 185, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Danesi, F.; Del Rio, D.; Silva, P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr. Res. Rev. 2018, 31, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Mizgier, M.L.; Speisky, H.; Gotteland, M. Differential protective effects of quercetin, resveratrol, rutin and epigallocatechin gallate against mitochondrial dysfunction induced by indomethacin in Caco-2 cells. Chem. Biol. Interact. 2012, 195, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Schottelius, A.; Dinter, H. Cytokines, NF-κB, microenvironment, intestinal inflammation and cancer. Cancer Treat Res. 2006, 130, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Feng, R.; Wu, Q.-S.; Wan, J.-B.; Zhang, Q.-W. Total saponins from Panax japonicus attenuate acute alcoholic liver oxidative stress and hepatosteatosis by p62-related Nrf2 pathway and AMPK-ACC/PPARα axis in vivo and in vitro. J. Ethnopharmacol. 2023, 317, 116785. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The Potential and Action Mechanism of Polyphenoles in the Treatment of Liver Disease. Oxidative Med. Cell. Longev. 2018, 2018, 8394818. [Google Scholar] [CrossRef]
- Zhong, W.; Qian, K.; Xiong, J.; Ma, K.; Wang, A.; Zou, Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed. Pharmacother. 2016, 83, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Mathurin, P. Alcoholic liver disease:mechanism of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Solano-Urrusquieta, A.; Morales-González, J.A.; Castro-Narro, G.E.; Cerda-Reyes, E.; Flores-Rangel, P.D.; Fierros-Oceguera, R. NRF-2 and nonalcoholic fatty liver disease. Ann. Hepatol. 2020, 19, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Eilam, Y.; Pintel, N.; Khattib, H.; Shagug, N.; Taha, R.; Avni, D. Regulation of Cholesterol Metabolism by Phytochemicals Derived from Algae and Edible Mushrooms in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 13667. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Miller, M.L.; Shen, D.; Shertzer, H.G.; Stringer, K.F.; Wang, B.; Schneider, S.N.; Nebert, D.W.; Dalton, T.P. Hepatocyte-specific Gclc Deletion Leads to Rapid Onset of Steatosis with Mitochondrial Injury and Liver failure. Hepatology 2007, 45, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Osorio, A.S.; González-Reyes, S.; Pedraza-Chaverri, J. Natural Nrf2 activactors in diabetes. Clin. Chim. Acta 2015, 448, 182–192. [Google Scholar] [CrossRef] [PubMed]
- González, S. Dietary Bioactive Compounds and Human Health and Disease. Nutrients 2022, 12, 348. [Google Scholar] [CrossRef]
- Afrin, R.; Arumugam, S.; Rahman, A.; Wahed, M.I.I.; Karuppagounder, V.; Harima, M.; Suzuki, H.; Miyashita, S.; Suzuki, K.; Yoneyama, H.; et al. Curcumin ameliorates liver damage and progession of NASH in NASH-HCC mouse model possibly by modulating HMGB1-NF-kB translocation. Int. Immunopharmacol. 2017, 44, 174–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Zhong, L.; Wang, L.; Gong, D. Echinacoside promotes the proliferation of human renal tubular epithelial cells by blocking the HBX/TREM2-mediated NF-κB signalling pathway. Mol. Med. Rep. 2020, 22, 1137–1144. [Google Scholar] [CrossRef]
- Ren, Y.; Li, S.; Song, Z.; Luo, Q.; Zhang, Y.; Wang, H. The Regulatory Roles of Polysaccharides and Ferroptosis_related Phytochemicals in Liver Diseases. Nutrients 2022, 14, 2303. [Google Scholar] [CrossRef]
- Heeba, G.; Mahmoud, M.E. Therapeutic potential of morin against liver fibrosis in rats: Modulation of oxidative stress, cytokine production and nuclear factor kappa B. Environ. Toxicol. Pharmacol. 2014, 37, 662–671. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef]
- Cheng, C.; Li, Z.; Zhao, X.; Liao, C.; Quan, J.; Bode, A.M.; Cao, Y.; Luo, X. Natural alkaloid and polyphenol compounds targeting lipid metabolism: Treatment implications in metabolic diseases. Eur. J. Pharmacol. 2020, 870, 172922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Xiao, L.; Lai, B.; Yao, Q.; Liu, J.; Yang, H.; Wang, N. Nuciferin ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through a PPARα/PPARγ coactivator-1α pathway. Br. J. Pharmacol. 2018, 175, 4218–4228. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, L.; Giansanti, S.; Gallozzi, A.; Chiesa, C. Long chain omega-3 polyunsaturated fatty acids in pediatric metabolic syndrome. Mini Rev. Med. Chem. 2014, 14, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Chopra, H.; Baig, A.A.; Avula, S.K.; Kumari, S.; Mohanta, T.K.; Saravanan, M.; Mishra, A.K.; Sharma, N.; Mohanta, Y.K. Edible Mushrooms as Novel Myco-Therapeutics: Effects on Lipid Level, Obesity and BMI. J. Fungi 2022, 8, 211. [Google Scholar] [CrossRef]
- Lai, X.; Xia, W.; Wei, J.; Ding, X. Therapeutic Effect of Astragalus Polysaccharides on Hepatocellular Carcinoma H22-Bearing Mice. Dose-Response Int. J. 2017, 15, 1559325816685182. [Google Scholar] [CrossRef] [PubMed]
- Patial, V.; Sharma, S.; Pratap, K.; Singh, D.; Padwad, Y.S. Synergistic effect of curcumin and piperine in suppression of DENA-induced hepatocellular carcinoma in rats. Environ. Toxicol. Pharmacol. 2015, 40, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Feng, Y. Gut-liver axis modulation of Panax notoginseng saponins in nonalcoholic fatty liver disease. Hepatol. Int. 2021, 15, 350–365. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, H.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Echinacea in hepatopathy: A review of its phytochemistry, pharmacology, and safety. Phytomedicine 2021, 87, 153572. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Ge, C.; Min, J.; Wang, F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022, 29, 467–480. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; García-Machorro, J.; Angeles-Valencia, M.; Martínez-Archundia, M.; Madrigal-Santillán, E.O.; Morales-González, Á.; Anguiano-Robledo, L.; Morales-González, J.A. Liver disorder in COVID-19, nutritional approaches and the use of phytochemicals. World J. Gastroenterol. 2021, 27, 5630–5665. [Google Scholar] [CrossRef]
- Houghton, C.; Fassett, R.G.; Coombes, J.S. Sulforaphane: Translational research from laboratory bench to clinic. Nutr. Rev. 2013, 71, 709–726. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Morales-González, A.; Morales-Martínez, M.; Soriano-Ursúa, M.A.; Delgado-Olivares, L.; Sandoval-Gallegos, E.M.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Madrigal-Santillán, E.; Morales-Gonzalez, J.A. Flavolignans from Sylimarin as Nrf2 Bioactivators and Their Therapeutic Applications. Biomedicines 2020, 8, 122. [Google Scholar] [CrossRef]
- Meshkibaf, M.; Maleknia, M.; Noroozi, S. Effect of curcumin on gene expression and protein level of methionine sulfoxide reductase A (MSRA), SOD, CAT and GPx in Freund’s adjuvant inflammation-induced male rats. J. Inflamm. Res. 2019, 12, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Halle, M.; Berg, A.; Northoff, H.; Keul, J. Importance of TNF-alpha and leptin in obesity and insulin resistance: A hypothesis on the impact of physical excercise. Exerc. Immunol. Rev. 1998, 4, 77–94. [Google Scholar] [PubMed]
- Santa, K.; Kumazawa, Y.; Nagaoka, I. Prevention of Metabolic Syndrome by Phytochemicals and Vitamin D. Int. J. Mol. Sci. 2023, 24, 2627. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Giau, V.V. Type 3 Diabetes and Its Role Implications in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Angelino, D. Plant Food, Nutrition, and Human Health. Nutrients 2020, 12, 2157. [Google Scholar] [CrossRef]
- Nurk, E.; Refsum, H.; Drevon, C.A.; Tell, G.S.; Nygaard, H.A.; Engedal, K.; Smith, A.D. Intake of Flavonoid-Rich Wine, Tea, and Chocolate by Elderly Men and Women Is Associated with Better Cognitive Test Performance. J. Nutr. 2009, 139, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Shanmugam, H.; Abdallah, H.; John Britto, J.S.; Galerati, I.; Gómez-Ambrosi, J.; Frühbeck, G.; Portincasa, P. The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome. Nutrients 2022, 14, 3112. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xu, W.; Zhang, Y.; Di, L.; Shan, J. A review of saponin intervention in metabolic syndrome suggests further study on intestinal microbiota. Pharmacol. Res. 2020, 160, 105088. [Google Scholar] [CrossRef]
- Atal, C.K.; Dubey, R.K.; Singh, J. Biochemical basis of enhanced drug bioavailability by piperine: Evidence that piperine is a potent inhibitor of drug metabolism. J. Pharmacol. Exp. Ther. 1985, 232, 258–262. [Google Scholar]
- Yaffe, P.B.; Power Coombs, M.R.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol. Carcinog. 2015, 54, 1070–1085. [Google Scholar] [CrossRef]
- Boreddy, S.R.; Srivastava, S.K. Pancreatic Cancer Chemoprevention by Phytochemicals. Cancer Lett. 2013, 334, 86–94. [Google Scholar] [CrossRef]
- Feng, X.L.; Ho, S.C.; Mo, X.F.; Lin, F.Y.; Zhang, N.Q.; Luo, H.; Zhang, X.; Zhang, C.X. Association between flavonoids, flavonoid subclasses intake and breast cancer risk: A case-control study in China. Eur. J. Cancer Prev. 2020, 29, 493–500. [Google Scholar] [CrossRef]
- Singh, V.K.; Arora, D.; Ansari, M.I.; Sharma, P.K. Phytochemicals based chemopreventive and chemotherapeutic strategies and modern technologies to overcome limitations for better clinical applications. Phytother. Res. 2019, 33, 3064–3089. [Google Scholar] [CrossRef] [PubMed]
- Davoodvandi, A.; Sadeghi, S.; Alavi, S.M.A.; Alavi, S.S.; Jafari, A.; Khan, H.; Aschner, M.; Mirzaei, H.; Sharifi, M.; Asemi, Z. The therapeutic effects of berberine for gastrointestinal cancers. Asia-Pacific J. Clin. Oncol. 2023, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Elekofehinti, O.O.; Iwaloye, O.; Olawale, F.; Ariyo, E.O. Saponins in Cancer Treatment: Current Progress and Future Prospects. Pathophysiology 2021, 28, 250–272. [Google Scholar] [CrossRef]
- Zarifi, S.H.; Bagherniya, M.; Banach, M.; Johnston, T.P.; Sahebkar, A. Phytochemicals: A potential therapeutic intervention for the prevention and treatment of cachexia. Clin. Nutr. 2022, 41, 2843–2857. [Google Scholar] [CrossRef]
- Akash, S.; Bayıl, I.; Mahmood, S.; Mukerjee, N.; Mili, T.A.; Dhama, K.; Rahman, M.A.; Maitra, S.; Mohany, M.; Al-Rejaie, S.S.; et al. Mechanistic inhibition of gastric cancer-associated bacteria Helicobacter pylori by selected phytocompounds: A new cutting-edge computational approach. Heliyon 2023, 9, e20670. [Google Scholar] [CrossRef]
- Yanaka, A. Role of Sulforaphane in Protection of Gastrointestinal Tract Against H. pylori and NSAID-Induced Oxidative Stress. Curr. Pharm. Des. 2017, 23, 4066–4075. [Google Scholar] [CrossRef]
- Raza, H.; John, A.; Benedict, S. Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur. J. Pharmacol. 2011, 668, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Haristoy, X.; Angioi-Duprez, K.; Duprez, A.; Lozniewski, A. Efficacy of Sulforaphane in Eradicating Helicobacter pylori in Human Gastric Xenografts Implanted in Nude Mice. Antimicrob. Agents Chemother. 2003, 47, 3982–3984. [Google Scholar] [CrossRef] [PubMed]
- Vale, F.F.; Oleastro, M. Overview of the phytomedicine approaches against Helicobacter pylori. World J. Gastroenterol. 2014, 20, 5594–5609. [Google Scholar] [CrossRef] [PubMed]
- Paulo, L.; Oleastro, M.; Gallardo, E.; Queiroz, J. Anti-Helicobacter pylori and urease inhibitory activities of resveratrol and red wine. Food Res. Int. 2011, 44, 964–969. [Google Scholar] [CrossRef]
- Kundu, P.; De, R.; Pal, I.; Mukhopadhyay, A.K.; Saha, D.R.; Swarnakar, S. Curcumin Alleviates Matrix Metalloproteinase-3 and -9 Activities during Eradication of Helicobacter pylori Infection in Cultured Cells and Mice. PLoS ONE 2011, 6, e16306. [Google Scholar] [CrossRef] [PubMed]
- Simmler, C.; Pauli, G.F.; Chen, S.N. Phytochemistry and biological properties of glabridin. Fitoterapia 2013, 90, 160–184. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Kwon, Y.I.; Labbe, R.G.; Shetty, K. Inhibition of Helicobacter pylori and associated urease by oregano and cranberry phytochemical synergies. Appl. Environ. Microbiol. 2005, 71, 8558–8564. [Google Scholar] [CrossRef]
- Lima, Z.P.; Calvo, T.R.; Silva, E.F.; Pellizzon, C.H.; Vilegas, W.; Brito, A.R.; Bauab, T.M.; Hiruma-Lima, C.A. Brazilian medicinal plant acts on prostaglandin level and Helicobacter pylori. J. Med. Food 2008, 11, 701–708. [Google Scholar] [CrossRef]
- Hassani, A.R.; Ordouzadeh, N.; Ghaemi, A.; Amirmozafari, N.; Hamdi, K.; Nazari, R. In vitro inhibition of Helicobacter pylori urease with non and semi fermented Camellia sinensis. Indian J. Med. Microbiol. 2009, 27, 30–34. [Google Scholar] [CrossRef]
- Adeniyi, B.A.; Anyiam, F.M. In vitro anti-Helicobacter pylori potential of methanol extract of Allium ascalonicum Linn. (Liliaceae) leaf: Susceptibility and effect on urease activity. Phytother. Res. 2004, 18, 358–361. [Google Scholar] [CrossRef]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef]
- Sutariya, B.; Jhonsa, D.; Saraf, M.N. TGF-B: The connecting link between nephopaty and fibrosis. Immunopharmacol. Immunotoxicol. 2016, 38, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Rafieian-Kopaie, M.; Nasri, H. Silymarin and diabetic nephropathy. J. Ren. INJ Prev. 2012, 1, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, K.; Sharma, S.; Gupta, J. Metabolic memory and diabetic nephropathy: Beneficial effects of natural epigenetic modifiers. Biochimie 2020, 170, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Khanra, R.; Dewanjee, S.; K Dua, T.; Sahu, R.; Gangopadhyay, M.; De Feo, V.; Zia-Ul-Haq, M. Abroma augusta L.(Malvaceae) leaf extract attenuates dia- betes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J. Transl. Med. 2015, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.H.; Shao, Z.M.; Tang, D.Q.; Lu, Q.; Chen, X.; Yin, X.X.; Wu, J.; Chen, H. Preventive effects of rutin on the development of experimental diabetic nephropaty in rats. Life Sci. 2012, 91, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Sutariya, B.; Saraf, M. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-B pathway. J. Ethnopharmacol. 2017, 198, 432–443. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhan, J.; Liu, X.L.; Wang, Y.; Ji, J.; He, Q.Q. Dietary flavonoids intake and risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Clin. Nutr. 2014, 33, 59–63. [Google Scholar] [CrossRef]
- Sluijs, I.; Cadier, E.; Beulens, J.W.; van der A, D.L.; Spijkerman, A.M.; van der Schouw, Y.T. Dietary intake of carotenoids and risk of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 376–381. [Google Scholar] [CrossRef]
- Kaur, G.; Invally, M.; Chintamaneni, M. Influence of piperine and quercetin on antidiabetic potential of curcumin. J. Complement. Integr. Med. 2016, 13, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Watanabe, K.; Kimura, I. Gut Microbiota Dysbiosis Drives and Implies Novel Therapeutic Strategies for Diabetes mellitus and Related Metabolic Diseases. Front. Immunol. 2017, 8, 1882. [Google Scholar] [CrossRef]
- Tang, C.; Ahmed, K.; Gille, A.; Lu, S.; Gröne, H.J.; Tunaru, S.; Offermanns, S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat. Med. 2015, 21, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kim, C.S.; Han, I.S.; Kawada, T.; Yu, R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007, 581, 4389–4396. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Balan, P.; Popovich, D.G. Review of Ginseng Anti-Diabetic Studies. Molecules 2019, 24, 4501. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, W.; He, S.; Sun, Y.; Meng, X.; Sun, G.; Sun, X. Ginsenoside Rb1 as an Anti-Diabetic Agent and Its Underlying Mechanism Analysis. Cells 2019, 8, 204. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 14, 785–795. [Google Scholar] [CrossRef]
- Xiao, P.L.; Cui, A.Y.; Hsu, C.J.; Peng, R.; Jiang, N.; Xu, X.H.; Ma, Y.G.; Liu, D.; Lu, H.D. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: A systematic review and meta-analysis. Osteoporos. Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 17, 321–333. [Google Scholar] [CrossRef]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Liu, M.; Chen, Y.; Chen, D.C. Systematic review of soy isoflavone supplements on osteoporosis in women. Asian Pac. J. Trop. Med. 2012, 5, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Yamamoto, H.; Sato, T.; Mizuha, Y.; Kawai, Y.; Taketani, Y.; Kato, S.; Terao, J.; Inakuma, T.; Takeda, E. Quercetin Inhibits Bone Loss without Effect on the Uterus in Ovariectomized Mice. J. Bone Miner. Metab. 2009, 27, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Babikova, M.; Mondockova, V.; Blahova, J.; Kovacova, V.; Omelka, R. The Role of Macronutrients, Micronutrients and Flavonoid Polyphenols in the Prevention and Treatment of Osteoporosis. Nutrients 2022, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Min, J.; Zhao, Y.; Cheng, Q.; Wang, K.; Lin, S.; Luo, J.; Liu, H. Quercetin Rescued TNF-Alpha-Induced Impairments in Bone Marrow-Derived Mesenchymal Stem Cell Osteogenesis and Improved Osteoporosis in Rats. Am. J. Transl. Res. 2018, 10, 4313–4321. [Google Scholar]
- Zhang, S.; Shen, S.; Ma, P.; Fan, D. Biochemical Targets and Molecular Mechanism of Ginsenoside Compound K in Treating Osteoporosis Based on Network Pharmacology. Int. J. Mol. Sci. 2022, 23, 13921. [Google Scholar] [CrossRef]
- Gao, S.S.; Zhao, Y. The effects of β-carotene on osteoporosis: A systematic review and meta-analysis of observational studies. Osteoporos. Int. 2023, 34, 627–639. [Google Scholar] [CrossRef]
- Sukhikh, S.; Noskova, S.; Ivanova, S.; Ulrikh, E.; Izgaryshev, A.; Babich, O. Chondroprotection and Molecular Mechanism of Action of Phytonutraceuticals on Osteoarthritis. Molecules 2021, 26, 2391. [Google Scholar] [CrossRef]
- Marotte, H.; Ruth, J.H.; Campbell, P.L.; Koch, A.E.; Ahmed, S. Green tea extract inhibits chemokine production, but up-regulates chemokine receptor expression, in rheumatoid arthritis synovial fibroblasts and rat adjuvant-induced arthritis. Rheumatology 2010, 49, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Xu, P.; Zheng, J.; Deng, X.; Ye, Q.; Huang, Z.; Wang, N. Effects and mechanisms of natural alkaloids for prevention and treatment of osteoporosis. Front. Pharmacol. 2022, 13, 1014173. [Google Scholar] [CrossRef] [PubMed]
- Tiselius, H.G. Epidemiology and medical managment of stone disease. BJU Int. 2003, 91, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Baumann, J.M. Stone prevention: Why so little progress? Urol. Res. 1998, 26, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G.C.; Willett, W.C.; Knight, E.L.; Stampfer, M.J. Dietary Factors and the Risk of Incident Kidney Stones in Younger Women. Arch. Intern. Med. 2004, 164, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Nirumand, M.C.; Hajialyani, M.; Rahimi, R.; Farzaei, M.H.; Zingue, S.; Nabavi, S.M.; Bishayee, A. Dietary Plants for the Prevention and Managment of Kidney Stones: Preclinical and Clinical Evidence and Molecular Mechanisms. Int. J. Mol. Sci. 2018, 19, 765. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.C.; Kim, B.S.; Kim, J.I.; Kim, H.H. Effects of Green Tea on Urinary Stoen Formation: An in Vivo and in Vitro Study. J. Endourol. 2006, 20, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Yasui, T.; Okada, A.; Tozawa, K.; Hayashi, Y.; Kohri, K. Preventive effects of green tea on renal stone formation and the role of oxidative stress in nepholitiasis. J. Urol. 2005, 173, 271–275. [Google Scholar] [CrossRef]
- Ghalayini, I.F.; Al-Ghazo, M.A.; Harfeil, M.N. Prophylaxis and Therapeutic Effects of Raspberry (Rubus idasus) on Renal Srone Formation in Balb/c mice. Int. Braz. J. Urol. 2011, 37, 259–267. [Google Scholar] [CrossRef]
- Divakar, K.; Pawar, A.T.; Harfeil, M.N. Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem. Toxicol. 2010, 48, 1013–1018. [Google Scholar] [CrossRef]
- Tracy, C.R.; Henning, J.R.; Newton, M.R.; Aviram, M.; Bridget Zimmerman, M. Oxidative stress and nephrolithiasis: A comparative pilot study evaluating the effect of pomegranate extract on stone risk factors and elevated oxidative stress levels of recurrent stone formers and controls. Urolithiasis 2014, 42, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Ogborn, M.; Nitschmann, E.; Bankovic-Calic, N.; Weiler, H.A.; Aukema, H.M. Dietary soy protein benefit in experimental kidney disease is preserved after isoflavone depletion of diet. Exp. Biol. Med. 2010, 235, 1315–1320. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, H.; Lei, T.; Zhou, L.; Li, W.; Li, X.; Yang, B. Curcumin inhibits renal cyst formation and enlargement in vitro by regulating in intracellular signaling pathways. Eur. J. Pharmacol. 2011, 654, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Leonhard, W.; van der Wal, A.; Novalic, Z.; Kunnen, S.J.; Gansevoort, R.T.; Breuning, M.H.; de Heer, E.; Peters, D.J. Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: In vivo evidence from a Pkdl-deletion model. Am. J. Phisiology 2010, 300, 1193–11202. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2013, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Gu, K.; Zhou, Y. Flavonoid intake is associated with lower all-cause and disease-specific mortality: The National Health and Nutrition Examination Survey 2007–2010 and 2017–2018. Front. Nutr. 2023, 10, 1046998. [Google Scholar] [CrossRef]
- Li, X.W.; Guo, B.; Shen, Y.Y.; Yang, J.R. Effect of chrysin on expression of NOX4 and NF-κB in right ventricle of monocrotaline- induced pulmonary arterial hypertension of rats. Acta Pharm. Sin. 2015, 50, 1128–1134. [Google Scholar]
- Wang, D.; Lv, L.; Xu, Y.; Jiang, K.; Chen, F.; Qian, J.; Chen, M.; Liu, G.; Xiang, Y. Cardioprotection of Panax Notoginseng saponins against acute myocardial infarction and heart failure through inducing autophagy. Biomed. Pharmacother. 2021, 136, 111287. [Google Scholar] [CrossRef]
- Cicero, A.F.; Baggioni, A. Berberine and Its Role in Chronic Disease. Adv. Exp. Med. Biol. 2016, 928, 27–45. [Google Scholar] [CrossRef]
- Ito, F. Polyphenols can Potentially Prevent Atherosclerosis and Cardiovascular Disease by Modulating Macrophage Cholesterol Metabolism. Curr. Mol. Pharmacol. 2021, 14, 175–190. [Google Scholar] [CrossRef]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Negrete, E.V.; Morales-González, Á.; Madrigal-Santillán, E.O.; Sánchez-Reyes, K.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Valadez-Vega, C.; Chamorro-Cevallos, G.; Garcia-Melo, L.F.; Morales-González, J.A. Phytochemicals and Their Usefulness in the Maintenance of Health. Plants 2024, 13, 523. https://doi.org/10.3390/plants13040523
Rodríguez-Negrete EV, Morales-González Á, Madrigal-Santillán EO, Sánchez-Reyes K, Álvarez-González I, Madrigal-Bujaidar E, Valadez-Vega C, Chamorro-Cevallos G, Garcia-Melo LF, Morales-González JA. Phytochemicals and Their Usefulness in the Maintenance of Health. Plants. 2024; 13(4):523. https://doi.org/10.3390/plants13040523
Chicago/Turabian StyleRodríguez-Negrete, Elda Victoria, Ángel Morales-González, Eduardo Osiris Madrigal-Santillán, Karina Sánchez-Reyes, Isela Álvarez-González, Eduardo Madrigal-Bujaidar, Carmen Valadez-Vega, German Chamorro-Cevallos, Luis Fernando Garcia-Melo, and José A. Morales-González. 2024. "Phytochemicals and Their Usefulness in the Maintenance of Health" Plants 13, no. 4: 523. https://doi.org/10.3390/plants13040523
APA StyleRodríguez-Negrete, E. V., Morales-González, Á., Madrigal-Santillán, E. O., Sánchez-Reyes, K., Álvarez-González, I., Madrigal-Bujaidar, E., Valadez-Vega, C., Chamorro-Cevallos, G., Garcia-Melo, L. F., & Morales-González, J. A. (2024). Phytochemicals and Their Usefulness in the Maintenance of Health. Plants, 13(4), 523. https://doi.org/10.3390/plants13040523






