Interaction of Zinc Mineral Nutrition and Plant Growth-Promoting Bacteria in Tropical Agricultural Systems: A Review
Abstract
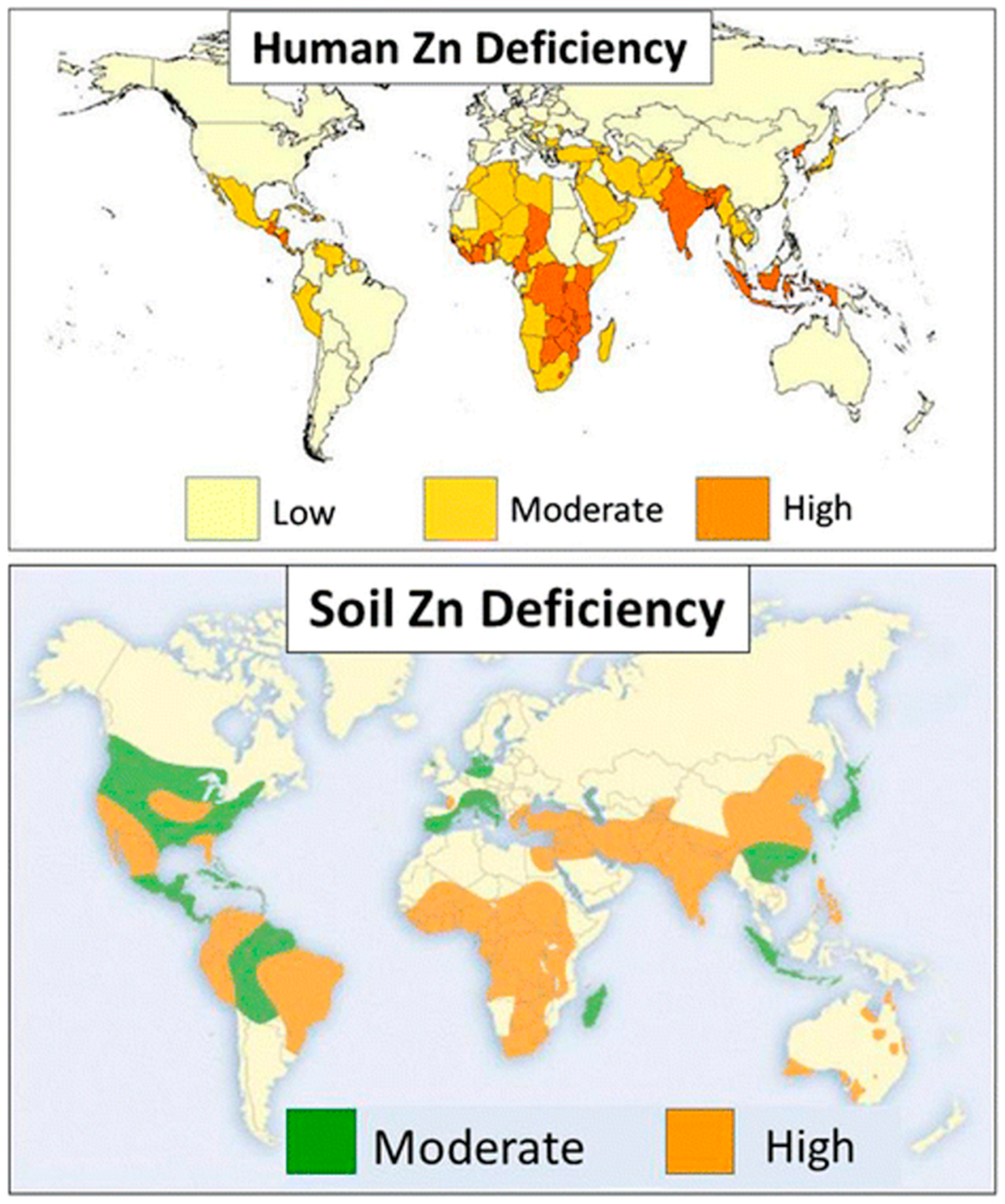
1. Introduction
2. Dynamics and Management of Zinc
2.1. Zinc Presence and Functions in Soil
2.2. Zinc Fertilization and Sources
2.3. Zinc Application Methods and Timings
3. Biofortification
3.1. Agronomic Biofortification
3.2. Mineral Biofortification
3.3. Microbe-Mediated Biofortification
4. Zinc Interaction with Root Mechanisms of Plant Growth-Promoting Bacteria (PGPBs)
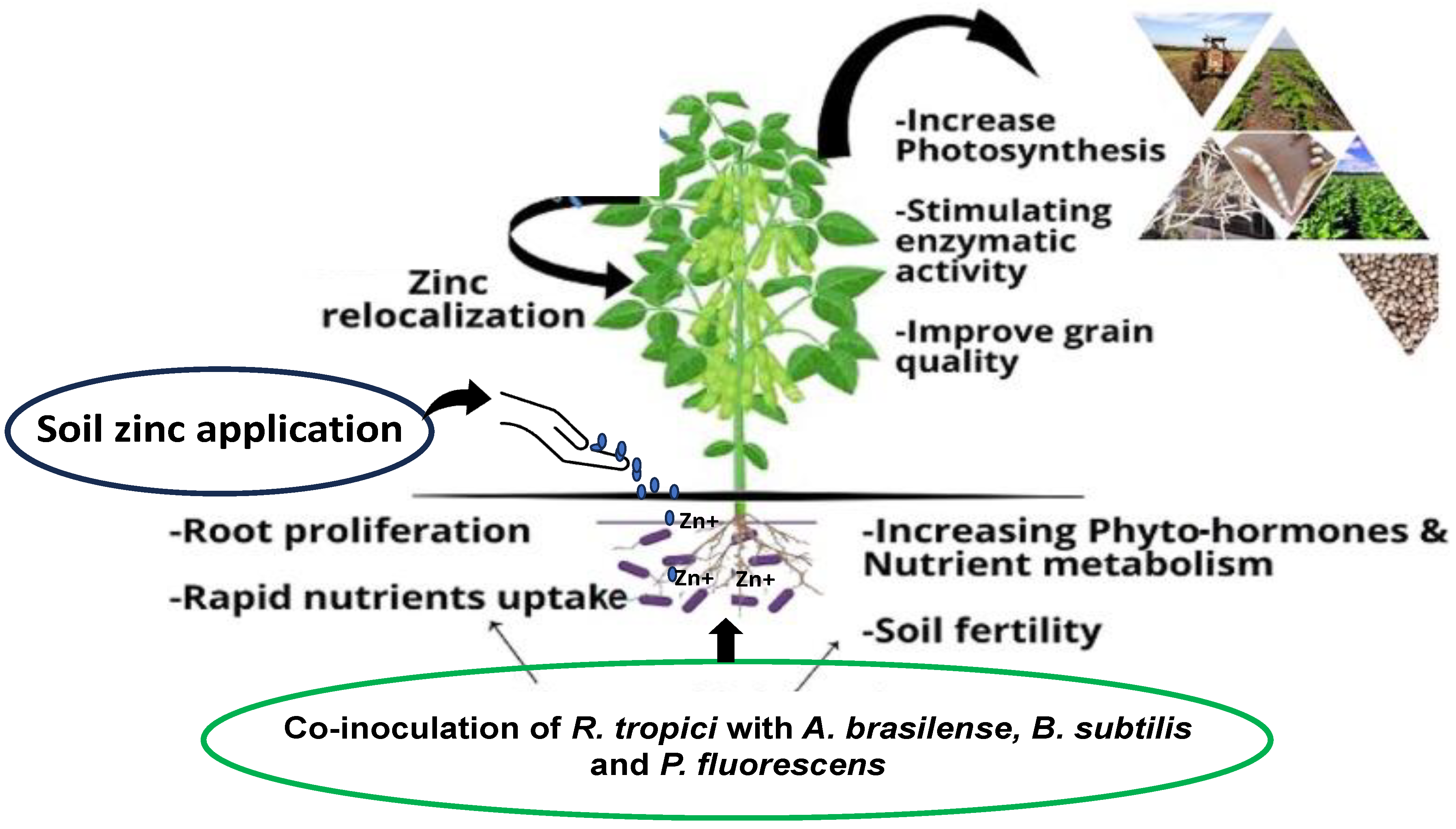
4.1. Zinc and Inoculation with PGPBs
4.2. Zinc and Co-Inoculation with PGPBs
5. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prosekov, A.Y.; Ivanova, S.A. Food security: The challenge of the present. Geoforum 2018, 91, 73–77. [Google Scholar] [CrossRef]
- Roriz, M.; Carvalho, S.M.P.; Castro, P.M.L.; Vasconcelos, M.W. Legume Biofortification and the Role of plant growth-promoting bacteria in a sustainable agricultural era. Agronomy 2020, 10, 435. [Google Scholar] [CrossRef]
- Debnath, S.; Mandal, B.; Saha, S.; Sarkar, D.; Batabyal, K.; Murmu, S.; Patra, B.C.; Mukherjee, D.; Biswas, T. Are the modern-bred rice and wheat cultivars in India inefficient in zinc and iron sequestration? Environ. Exp. Bot. 2021, 189, 104535. [Google Scholar] [CrossRef]
- Harding, K.L.; Aguayo, V.M.; Webb, P. Hidden hunger in South Asia: A review of recent trends and persistent challenges. Public Health Nutr. 2018, 21, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M. The global challenge of hidden hunger: Perspectives from the field. Proc. Nutr. Soc. 2021, 80, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, L.; Crane, J.S. Zinc Deficiency. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493231/ (accessed on 18 October 2023).
- Leite, C.M.D.C.; Muraoka, T.; Colzato, M.; Alleoni, L.R.F. Soil-applied Zn effect on soil fractions. Scientia Agricola 2019, 77, e20180124. [Google Scholar] [CrossRef]
- Galindo, F.S.; Bellotte, J.L.; Santini, J.M.K. Zinc use efficiency of maize-wheat crop after inoculation with Azospirillum brasilense. Nutr. Cycl. Agroecosyst. 2021, 120, 205–221. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Rehman, R.; Asif, M.; Cakmak, I.; Ozturk, L. Differences in uptake and translocation of foliar-applied Zn in maize and wheat. Plant Soil 2021, 462, 235–244. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Verma, V.; Kaur, M.; Shivay, Y.S.; Nisar, S.; Gaber, A.; Brestic, M.; Barek, V.; et al. Biofortification—A Frontier Novel Approach to Enrich Micronutrients in Field Crops to Encounter the Nutritional Security. Molecules 2022, 27, 1340. [Google Scholar] [CrossRef]
- Li, J.; Martin, C.; Fernie, A. Biofortification’s contribution to mitigating micronutrient deficiencies. Nat. Food 2024, 5, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Farooq, M.; Rehman, A.; Hussain, M.; Siddique, K.H. Zinc nutrition in chickpea (Cicer arietinum): A review. Crop Pasture Sci. 2020, 71, 199–218. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations (FAO) Sustainable Food and Agriculture. 2022. Available online: https://www.fao.org/sustainability/background/en/ (accessed on 12 October 2023).
- Campanhola, C.; Pandey, S. (Eds.) Sustainable Food and Agriculture: An Integrated Approach; Academic Press: New York, NY, USA, 2018. [Google Scholar]
- Van Der Straeten, D.; Bhullar, N.K.; De Steur, H.; Gruissem, W.; MacKenzie, D.; Pfeiffer, W.; Qaim, M.; Slamet-Loedin, I.; Strobbe, S.; Tohme, J.; et al. Multiplying the efficiency and impact of biofortification through metabolic engineering. Nat. Commun. 2020, 11, 5203. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, R.K.; Zhou, Y.; Wang, J.; Jiang, Y.; Shen, N.; Wang, Y.; Yang, L.; Jiang, M. Unlocking the strength of plant growth promoting Pseudomonas in improving crop productivity in normal and challenging environments: A review. J. Plant Interact. 2022, 17, 220–238. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Kaur, T. Endophytic microbes: Biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Van Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef]
- Nitu, R.; Rajinder, K.; Sukhminderjit, K. Zinc solubilizing bacteria to augment soil fertility–a comprehensive review. Int. J. Agric. Sci. Vet. Med. 2020, 8, 38–44. [Google Scholar]
- Jalal, A.; Oliveira, C.E.d.S.; Rosa, P.A.L.; Galindo, F.S.; Teixeira Filho, M.C.M. Beneficial Microorganisms Improve Agricultural Sustainability under Climatic Extremes. Life 2023, 13, 1102. [Google Scholar] [CrossRef]
- Kumar, A.; Patel, J.S.; Meena, V.S.; Ramteke, P.W. Plant growth-promoting rhizobacteria: Strategies to improve abiotic stresses under sustainable agriculture. J. Plant Nutr. 2019, 42, 1402–1415. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.E.D.S.; Fernandes, G.C.; da Silva, E.C.; da Costa, K.N.; de Souza, J.S.; Leite, G.D.S.; Biagini, A.L.C.; Galindo, F.S.; Teixeira Filho, M.C.M. Integrated use of plant growth-promoting bacteria and nano-zinc foliar spray is a sustainable approach for wheat biofortification, yield, and zinc use efficiency. Front. Plant Sci. 2023, 14, 1146808. [Google Scholar] [CrossRef]
- Kushwaha, M.; Mishra, A.; Shankar, S.; Goel, D.; Joshi, S.; Ram, S. Plant growth-promoting rhizobacteria for sustainable agriculture: Recent progress and challenges. In Role of Green Chemistry in Ecosystem Restoration to Achieve Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2024; pp. 333–342. [Google Scholar]
- Sudha, S.; Stalin, P. Zinc deficiency in soil and role of zinc in human and plant. Int. J. Farm Sci. 2017, 7, 30–38. [Google Scholar]
- Wessells, K.R.; Brown, K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef]
- Alloway, B. Zinc in Soils and Crop Nutrition, 2nd ed.; International Zinc Association: Brussels, Belgium; International Fertilizer Industry Association: Paris, France, 2008. [Google Scholar]
- Khatun, M.A.; Hossain, M.M.; Bari, M.A.; Abdullahil, K.M.; Parvez, M.S.; Alam, M.F.; Kabir, A.H. Zinc deficiency tolerance in maize is associated with the up-regulation of Zn transporter genes and antioxidant activities. Plant Biol. 2018, 20, 765–770. [Google Scholar] [CrossRef]
- Suganya, A.; Saravanan, A.; Manivannan, N. Role of zinc nutrition for increasing zinc availability, uptake, yield, and quality of maize (Zea mays L.) grains: An Overview. Commun. Soil Sci. Plant Anal. 2020, 51, 2001–2021. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.E.D.S.; Bastos, A.D.C.; Fernandes, G.C.; de Lima, B.H.; Furlani Junior, E.; de Carvalho, P.H.G.; Galindo, F.S.; Gato, I.M.B.; Teixeira Filho, M.C.M. Nanozinc and plant growth-promoting bacteria improve biochemical and metabolic attributes of maize in tropical Cerrado. Front. Plant Sci. 2023, 13, 1046642. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Bio/Technol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Sinclair, S.A.; Krämer, U. The zinc homeostasis network of land plants. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 1553–1567. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz MRasheed, A.; Afzal, A.; Liu, Y.; Guoqin, H. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Ullah, A.; Nadeem, F.; Im, S.Y.; Park, S.K.; Lee, D.J. Agronomic biofortification of zinc in Pakistan: Status, benefits, and constraints. Front. Sustain. Food Syst. 2020, 4, 591722. [Google Scholar] [CrossRef]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef] [PubMed]
- FAO (2015–2019) 2019. FAO Office for Corporate Communication. 2019. Rome. Available online: https://ourworldindata.org/grapher/global-prevalence-of-zinc-deficiency?tab=chart®ion=SouthAmerica (accessed on 1 November 2023).
- Knijnenburg, J.T.; Laohhasurayotin, K.; Khemthong, P.; Kangwansupamonkon, W. Structure, dissolution, and plant uptake of ferrous/zinc phosphates. Chemosphere 2019, 223, 310–318. [Google Scholar] [CrossRef]
- Jalal, A.; Galindo, F.S.; Freitas, L.A.; Oliveira, C.E.S.; de Lima, B.H.; Pereira, Í.T.; Ferraz, G.F.; de Souza, J.S.; da Costa, K.N.; Nogueira, T.A.R.; et al. Yield, zinc efficiencies and biofortification of wheat with zinc sulfate application in soil and foliar nanozinc fertilisation. Crop Pasture Sci. 2022, 73, 749–759. [Google Scholar] [CrossRef]
- Zou, C.Q.; Zhang, Y.Q.; Rashid, A.; Ram, H.; Savasli, E.; Arisoy, R.Z.; Ortiz-Monasterio, I.; Simunji, S.; Wang, Z.H.; Sohu, V.; et al. Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil 2012, 361, 119–130. [Google Scholar] [CrossRef]
- Weisany, W.; Mohammadi, M.; Tahir NA, R.; Aslanian, N.; Omer, D.A. Changes in growth and nutrient status of maize (Zea mays L.) in response to two zinc sources under drought stress. J. Soil Sci. Plant Nutr. 2021, 21, 3367–3377. [Google Scholar] [CrossRef]
- Barreto, M.S.C.; Elzinga, E.J.; Rouff, A.A.; Siebecker, M.G.; Sparks, D.L.; Alleoni, L.R.F. Zinc speciation in highly weathered tropical soils affected by large scale vegetable production. Sci. Total Environ. 2024, 916, 170223. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Beshir, A. Zinc biofortification of maize (Zea mays L.): Status and challenges. Plant Breed. 2019, 138, 1–28. [Google Scholar] [CrossRef]
- Małecki, J.J.; Kadzikiewicz-Schoeneich, M.; Szostakiewicz-Hołownia, M. Concentration and mobility of copper and zinc in the hypergenic zone of a highly urbanized area. Environ. Earth Sci. 2016, 75, 24. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Campos, M.G.; Fugice, J.; Glass, K.; Ozcan, A.; Huang, Z.; Singh, U.; Santra, S. Synthesis and characterization of novel dual-capped Zn–urea nanofertilizers and application in nutrient delivery in wheat. Environ. Sci. Adv. 2022, 1, 47–58. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2022, 423, 127021. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Jajoo, A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Younas, N.; Fatima, I.; Ahmad, I.A.; Ayyaz, M.K. Alleviation of zinc deficiency in plants and humans through an effective technique; biofortification: A detailed review. Acta Ecol. Sin. 2023, 43, 419–425. [Google Scholar] [CrossRef]
- Ofori, K.F.; Antoniello, S.; English, M.M. Aryee AN. Improving nutrition through biofortification—A systematic review. Front. Nutr. 2022, 9, 1043655. [Google Scholar] [CrossRef]
- Obaid, H.; Shrestha, R.K.; Liu, D.; Elsayed, N.S.; Ni, J.; Ni, C. Biofortification of Maize with Zinc and Its Effect on Human Health. J. Soil Sci. Plant Nutr. 2022, 22, 1792–1804. [Google Scholar] [CrossRef]
- Bhatt, R.; Hossain, A.; Sharma, P. Zinc biofortification as an innovative technology to alleviate the zinc deficiency in human health: A review. Open Agric. 2020, 5, 176–187. [Google Scholar] [CrossRef]
- Jalal, A.; Shah, S.; Teixeira Filho, M.C.M.; Khan, A.; Shah, T.; Ilyas, M.; Rosa, P.A.L. Agro-biofortification of zinc and iron in wheat grains. Gesunde Pflanz. 2020, 72, 227–236. [Google Scholar] [CrossRef]
- Mabesa, R.Á.; Impa, S.Á.; Grewal, D.; Johnson-Beebout, S.E.E. Contrasting grain-Zn response of biofortification rice (Oryza sativa L.) breeding lines to foliar Zn application. Field Crops Res. 2013, 149, 223–233. [Google Scholar] [CrossRef]
- Kandil, E.E.; El-Banna, A.A.; Tabl, D.M.; Mackled, M.I.; Ghareeb, R.Y.; Al-Huqail, A.A.; Ali, H.M.; Jebril, J.; Abdelsalam, N.R. Zinc nutrition responses to agronomic and yield traits, kernel quality, and pollen viability in rice (Oryza sativa L.). Front. Plant Sci. 2022, 13, 791066. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Liu, D.Y.; Zhao, Q.Y.; Zhang, W.; Chen, X.X.; Xu, S.J.; Zou, C.Q. Zinc fractions in soils and uptake in winter wheat as affected by repeated applications of zinc fertilizer. Soil Tillage Res. 2020, 200, 104612. [Google Scholar] [CrossRef]
- Kumar, B.; Ram, H. Biofortification of maize fodder with zinc improves forage productivity and nutritive value for livestock. J. Anim. Feed Sci. 2021, 30, 149–158. [Google Scholar] [CrossRef]
- Jalal, A.; Shah, S.; Teixeira Filho, M.; Carvalho, M.; Khan, A.; Shah, T.; Hussain, Z.; Younis, M.; Ilyas, M. Yield and phenological indices of wheat as affected by exogenous fertilization of zinc and iron. Braz. J. Agric. Sci./Rev. Bras. Ciênc. Agrár. 2020, 15, e7730. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Ahmad, M.; Zafar-ul-Hye, M.; Saqib, M.; Akhtar, M.F.U.Z.; Zaheer, M.S. Seed-applied zinc-solubilising Bacillus biofertilisers improve antioxidant enzyme activities, crop productivity, and biofortification of maize. Crop Pasture Sci. 2022, 73, 503–514. [Google Scholar] [CrossRef]
- Adele, N.C.; Ngwenya, B.T.; Heal, K.V.; Mosselmans, J.F.W. Role of plant growth promoting bacteria in driving speciation gradients across soil-rhizosphere-plant interfaces in zinc-contaminated soils. Environ. Pollut. 2021, 279, 116909. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, R.; Srinivasan, V.; Hamza, S.; Sarathambal, C.; Anke Gowda, S.J.; Ganeshamurthy, A.N.; Gupta, S.B.; Aparna Nair, V.; Subila, K.P.; Lijina, A.; et al. Isolation and characterization of potential Zn solubilizing bacteria from soil and its effects on soil Zn release rates, soil available Zn and plant Zn content. Geoderma 2018, 321, 173–186. [Google Scholar] [CrossRef]
- Gontia-Mishra, I.; Sapre, S.; Tiwari, S. Zinc solubilizing bacteria from the rhizosphere of rice as prospective modulator of zinc biofortification in rice. Rhizosphere 2017, 3, 185–190. [Google Scholar] [CrossRef]
- Aiqing, Z.; Zhang, L.; Ning, P.; Chen, Q.; Wang, B.; Zhang, F.; Zhang, Y. Zinc in cereal grains: Concentration, distribution, speciation, bioavailability, and barriers to transport from roots to grains in wheat. Crit. Rev. Food Sci. Nutr. 2022, 62, 7917–7928. [Google Scholar] [CrossRef]
- Helliwell, J.R.; Sturrock, C.J.; Mairhofer, S.; Craigon, J.; Ashton, R.W.; Miller, A.J.; Whalley, W.R.; Mooney, S.J. The emergent rhizosphere: Imaging the development of the porous architecture at the root-soil interface. Sci. Rep. 2017, 7, 14875. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Z.; Xie, J.; Hesselberg-Thomsen, V.; Tan, T.; Zheng, D.; Strube, M.L.; Dragoš, A.; Shen, Q.; Zhang, R.; et al. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2022, 16, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Havlin, J.L. Soil: Fertility and nutrient management. In Landscape and Land Capacity; CRC Press: Boca Raton, FL, USA, 2020; pp. 251–265. [Google Scholar]
- Singh, D.; Geat, N.; Rajawat, M.V.S.; Mahajan, M.M.; Prasanna, R.; Singh, S.; Kaushik RSingh RNKumar, K.; Saxena, A.K. Deciphering the mechanisms of endophyte-mediated biofortification of Fe and Zn in wheat. J. Plant Growth Regul. 2018, 37, 174–182. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-solubilizing microorganisms: Mechanism and their role in phosphate solubilization and uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Karnwal, A. Zinc solubilizing Pseudomonas spp. from vermicompost bestowed with multifaceted plant growth promoting properties and having prospective modulation of zinc biofortification in Abelmoschus esculentus L. J. Plant Nutr. 2021, 44, 1023–1038. [Google Scholar] [CrossRef]
- Yasmin, H.; Naeem, S.; Bakhtawar, M.; Jabeen, Z.; Nosheen, A.; Naz, R.; Keyani, R.; Mumtaz, S.; Hassan, M.N. Halotolerant rhizobacteria Pseudomonas pseudoalcaligenes and Bacillus subtilis mediate systemic tolerance in hydroponically grown soybean (Glycine max L.) against salinity stress. PLoS ONE 2020, 15, e0231348. [Google Scholar] [CrossRef]
- Singh, D.; Rajawat, M.V.S.; Kaushik, R.; Prasanna, R.; Saxena, A.K. Beneficial role of endophytes in biofortification of Zn in wheat genotypes varying in nutrient use efficiency grown in soils sufficient and deficient in Zn. Plant Soil 2017, 416, 107–116. [Google Scholar] [CrossRef]
- Joshi, D.; Negi, G.; Vaid, S.; Sharma, A. Enhancement of wheat growth and Zn content in grains by zinc solubilizing bacteria. Int. J. Agric. Environ. Biotechnol. 2013, 6, 363–370. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Plant growth-promoting traits in Enterobacter cloacae subsp. dissolvens MDSR9 isolated from soybean rhizosphere and its impact on growth and nutrition of soybean and wheat upon inoculation. Agric. Res. 2014, 3, 53–66. [Google Scholar] [CrossRef]
- Kamran, S.; Shahid, I.; Baig, D.N.; Rizwan, M.; Malik, K.A.; Mehnaz, S. Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front. Microbiol. 2017, 8, 2593. [Google Scholar] [CrossRef]
- Shaikh, S.; Saraf, M. Biofortification of Triticum aestivum through the inoculation of zinc solubilizing plant growth promoting rhizobacteria in field experiment. Biocatal. Agric. Biotechnol. 2017, 9, 120–126. [Google Scholar] [CrossRef]
- Sirohi, G.; Upadhyay, A.; Srivastava, P.S.; Srivastava, S. PGPR mediated Zinc biofertilization of soil and its impact on growth and productivity of wheat. J. Soil Sci. Plant Nutr. 2015, 15, 202–216. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl. Soil Ecol. 2014, 73, 87–96. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.E.d.S.; Fernandes, H.B.; Galindo, F.S.; Silva, E.C.d.; Fernandes, G.C.; Nogueira, T.A.R.; De Carvalho, P.H.G.; Balbino, V.R.; Lima, B.H.d.; et al. Diazotrophic bacteria is an alternative strategy for increasing grain biofortification, yield and zinc use efficiency of maize. Plants 2022, 11, 1125. [Google Scholar] [CrossRef]
- Shakeel, M.; Rais, A.; Hassan, M.N.; Hafeez, F.Y. Root associated Bacillus sp. improves growth, yield and zinc translocation for basmati rice (Oryza sativa) varieties. Front. Microbiol. 2015, 6, 1286. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Zhang, X.; Dong, L.; Zhang, J.; Wei, Y.; Feng, Y.; Lu, L. Improved plant growth and Zn accumulation in grains of rice (Oryza sativa L.) by inoculation of endophytic microbes isolated from a Zn Hyperaccumulator, Sedum alfredii H. J. Agric. Food Chem. 2014, 62, 1783–1791. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Vadlamudi, S.; Samineni, S.; Sameer Kumar, C.V. Plant growth-promotion and biofortification of chickpea and pigeonpea through inoculation of biocontrol potential bacteria, isolated from organic soils. Springerplus 2016, 5, 1882. [Google Scholar] [CrossRef]
- Jalal, A.; Mortinho, E.S.; da Silva Oliveira, C.E.; Fernandes, G.C.; Junior, E.F.; de Lima, B.H.; Moreira, A.; Nogueira, T.A.; Galindo, F.S.; Filho, M.C. Nano-zinc and plant growth-promoting bacteria is a sustainable alternative for improving productivity and agronomic biofortification of common bean. Chem. Biol. Technol. Agric. 2023, 10, 77. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.E.D.S.; Freitas, L.A.; Galindo, F.S.; Lima, B.H.; Boleta, E.H.M.; Da Silva, E.C.; do Nascimento, V.; Nogueira, T.A.R.; Buzetti, S.; et al. Agronomic biofortification and productivity of wheat with soil zinc and diazotrophic bacteria in tropical savannah. Crop Pasture Sci. 2022, 73, 817–830. [Google Scholar] [CrossRef]
- Santos, F.L.; da Silva, F.B.; de Sá, E.L.; Vian, A.L.; Muniz, A.W.; dos Santos, R.N. Inoculation and co-inoculation of growth promoting rhizobacteria in irrigated rice plants. Rev. Bras. Ciênc. Agrár. 2019, 14, 1–5. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Gomes, E.A.; de Sousa, S.M.; de Paula Lana, U.G.; Coelho, A.M.; Marriel, I.E.; de Oliveira-Paiva, C.A. Co-inoculation with tropical strains of Azospirillum and Bacillus is more efficient than single inoculation for improving plant growth and nutrient uptake in maize. Arch. Microbiol. 2022, 204, 143. [Google Scholar] [CrossRef] [PubMed]
- Tahami, M.K.; Jahan, M.; Khalilzadeh, H.; Mehdizadeh, M. Plant growth growing rhizobacteria in an ecological cropping system: A study on basil (Ocimum basilicum L.) essential oil production. Ind. Crop Prod. 2017, 107, 97–104. [Google Scholar] [CrossRef]
- Khan, K.; Pankaj, U.; Verma, S.K.; Gupta, A.K.; Singh, R.P.; Verma, R.K. Bio-inoculants and vermicompost influence on yield, quality of Andrographis paniculata, and soil properties. Ind. Crop Prod. 2015, 70, 404–409. [Google Scholar] [CrossRef]
- Korir, H.; Mungai, N.W.; Thuita, M.; Hamba, Y.; Masso, C. Co-inoculation effect of rhizobia and plant growth promoting rhizobacteria on common bean growth in a low phosphorus soil. Front. Plant Sci. 2017, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Stajkovic, O.; Delic, D.; Josic, D.; Kuzmanovic, D.; Rasulic, N.; Knezevic-Vukcevic, J. Improvement of common bean growth by co-inoculation with Rhizobium and plant growth-promoting bacteria. Rom. Biotechnol. Lett. 2011, 16, 5919–5926. [Google Scholar]
- Jalal, A.; Galindo, F.S.; Boleta, E.H.M.; Oliveira, C.E.D.S.; Reis, A.R.D.; Nogueira, T.A.R.; Moretti Neto, M.J.; Mortinho, E.S.; Fernandes, G.C.; Teixeira Filho, M.C.M. Common bean yield and zinc use efficiency in association with diazotrophic bacteria co-inoculations. Agronomy 2021, 11, 959. [Google Scholar] [CrossRef]
- Oliveira, T.J.; da Silva Oliveira, C.E.; Jalal, A.; Gato, I.M.; Rauf, K.; de Almeida Moreira, V.; de Lima, B.H.; Vitória, L.S.; Giolo, V.M.; Teixeira Filho, M.C. Inoculation reduces nitrate accumulation and increases growth and nutrient accumulation in hydroponic arugula. Sci. Hortic. 2023, 320, 112213. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Mohapatra, S.; Bhayana, S.; Varma, A. Revisiting plant–microbe interactions and microbial consortia application for enhancing sustainable agriculture: A review. Front. Microbiol. 2020, 11, 560406. [Google Scholar] [CrossRef]
- Silva, P.S.; Cassiolato, A.M.; Galindo, F.S.; Jalal, A.; Nogueira, T.A.; Oliveira, C.E.; Filho, M.C. Azospirillum brasilense and Zinc Rates Effect on Fungal Root Colonization and Yield of Wheat-Maize in Tropical Savannah Conditions. Plants 2022, 11, 3154. [Google Scholar] [CrossRef]
- Galindo, F.S.; Pagliari, P.H.; da Silva, E.C.; Silva, V.M.; Fernandes, G.C.; Rodrigues, W.L.; Céu, E.G.; de Lima, B.H.; Jalal, A.; Muraoka, T.; et al. Co-Inoculation with Azospirillum brasilense and Bradyrhizobium sp. enhances nitrogen uptake and yield in field-grown cowpea and did not change n-fertilizer recovery. Plants 2022, 11, 1847. [Google Scholar] [CrossRef]


| Crop | Types of Inoculants | Biofortification of Nutrients | References |
|---|---|---|---|
| Wheat | Pseudomonas sp. | 31% increase in Zn | [72] |
| E. cloacae subsp. dissolvens MDSR9 | 37% and 21% increase in Zn and Fe | [73] | |
| P. fragi EPS1 | Twofold increase in Zn | [74] | |
| Exiguobacterium aurantiacum MS-ZT10 | Sixfold increase in Fe and Zn | [75] | |
| P. fluorescens strain Psd | 85% increase Zn | [76] | |
| Bacillus aryabhattai MDSR 7 | 45% increase in Zn | [77] | |
| B. subtilis, Arthrobacter sp. | Twofold increase in Zn | [22] | |
| Maize | P. fluorescens | Increased Zn uptake by 33–35% in shoot and 37–42% in grain | [78] |
| B. subtilis ZM63 and B. aryabhattai ZM31 | 68% in Zn | [59] | |
| Rice | Bacillus sp. SH10 and B. cereus SH17 | 22–49% increase in Zn translocation to grain | [79] |
| Sphingomonas sp. SaMR12, Enterobacter sp. SaCS20 | 22% in Zn | [80] | |
| Chickpea & pigeon pea | P. plecoglossicida, B. antiquum, E. ludwigii, Acinetobacter tandoii, P. monteilii | 5–23% in Zn | [81] |
| Soybean | E. cloacae subsp. dissolvens MDSR9 | 33% and 25% increase in Zn and Fe | [73] |
| B. aryabhattai MDSR 14 | 36% in increase in Zn | ||
| Common beans | R. tropici + B. subtilis | Increased Zn partitioning to grain by 11–14% | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalal, A.; Júnior, E.F.; Teixeira Filho, M.C.M. Interaction of Zinc Mineral Nutrition and Plant Growth-Promoting Bacteria in Tropical Agricultural Systems: A Review. Plants 2024, 13, 571. https://doi.org/10.3390/plants13050571
Jalal A, Júnior EF, Teixeira Filho MCM. Interaction of Zinc Mineral Nutrition and Plant Growth-Promoting Bacteria in Tropical Agricultural Systems: A Review. Plants. 2024; 13(5):571. https://doi.org/10.3390/plants13050571
Chicago/Turabian StyleJalal, Arshad, Enes Furlani Júnior, and Marcelo Carvalho Minhoto Teixeira Filho. 2024. "Interaction of Zinc Mineral Nutrition and Plant Growth-Promoting Bacteria in Tropical Agricultural Systems: A Review" Plants 13, no. 5: 571. https://doi.org/10.3390/plants13050571
APA StyleJalal, A., Júnior, E. F., & Teixeira Filho, M. C. M. (2024). Interaction of Zinc Mineral Nutrition and Plant Growth-Promoting Bacteria in Tropical Agricultural Systems: A Review. Plants, 13(5), 571. https://doi.org/10.3390/plants13050571







