Genome-Wide Identification and Characterization of the Salvia miltiorrhiza Histone Deacetylase (HDAC) Family in Response to Multiple Abiotic Stresses
Abstract
:1. Introduction
2. Results
2.1. Identification of SmHDACs
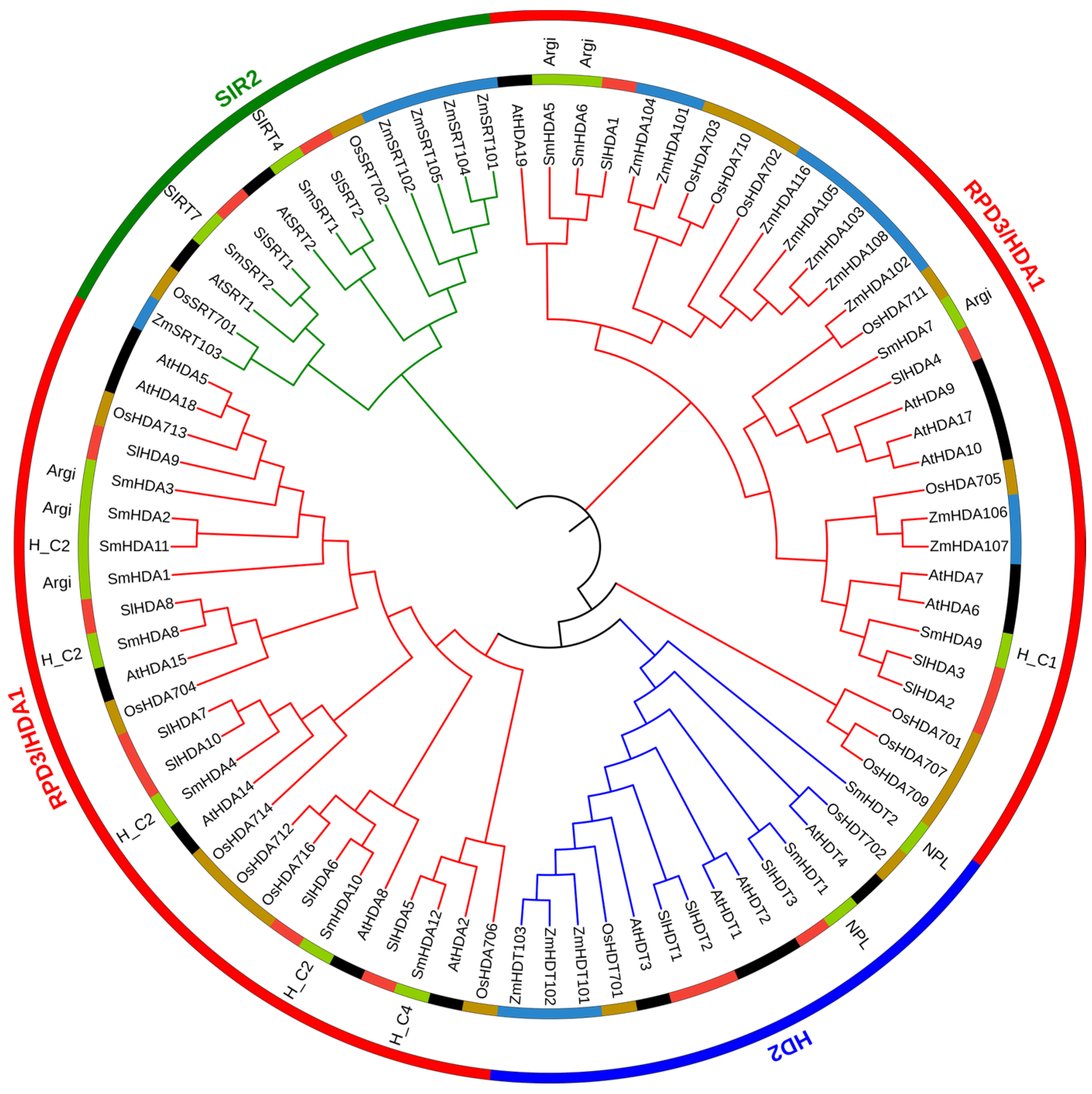
2.2. Phylogenetic Tree of SmHDACs
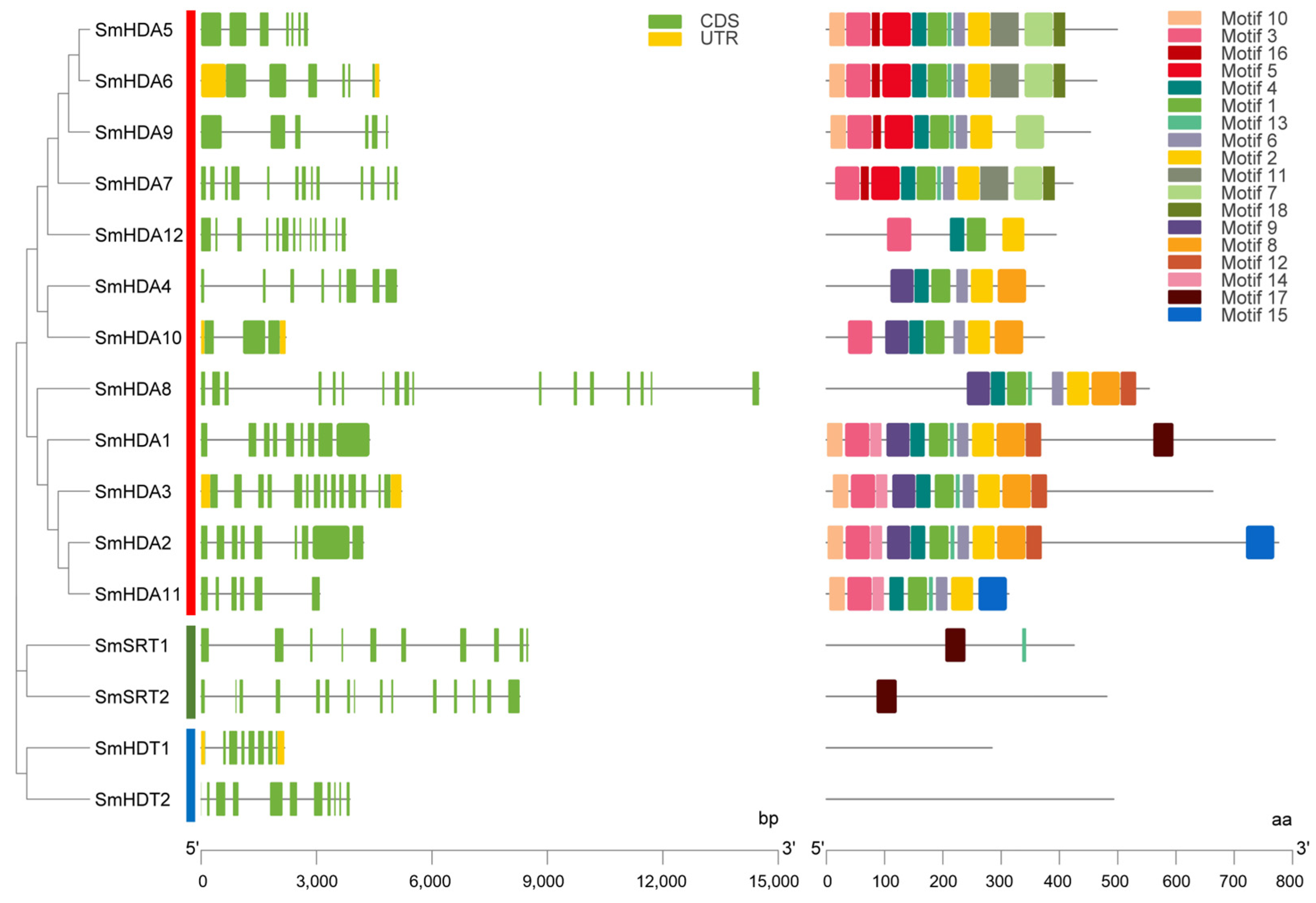
2.3. Gene Structure and Motif Analysis of SmHDACs
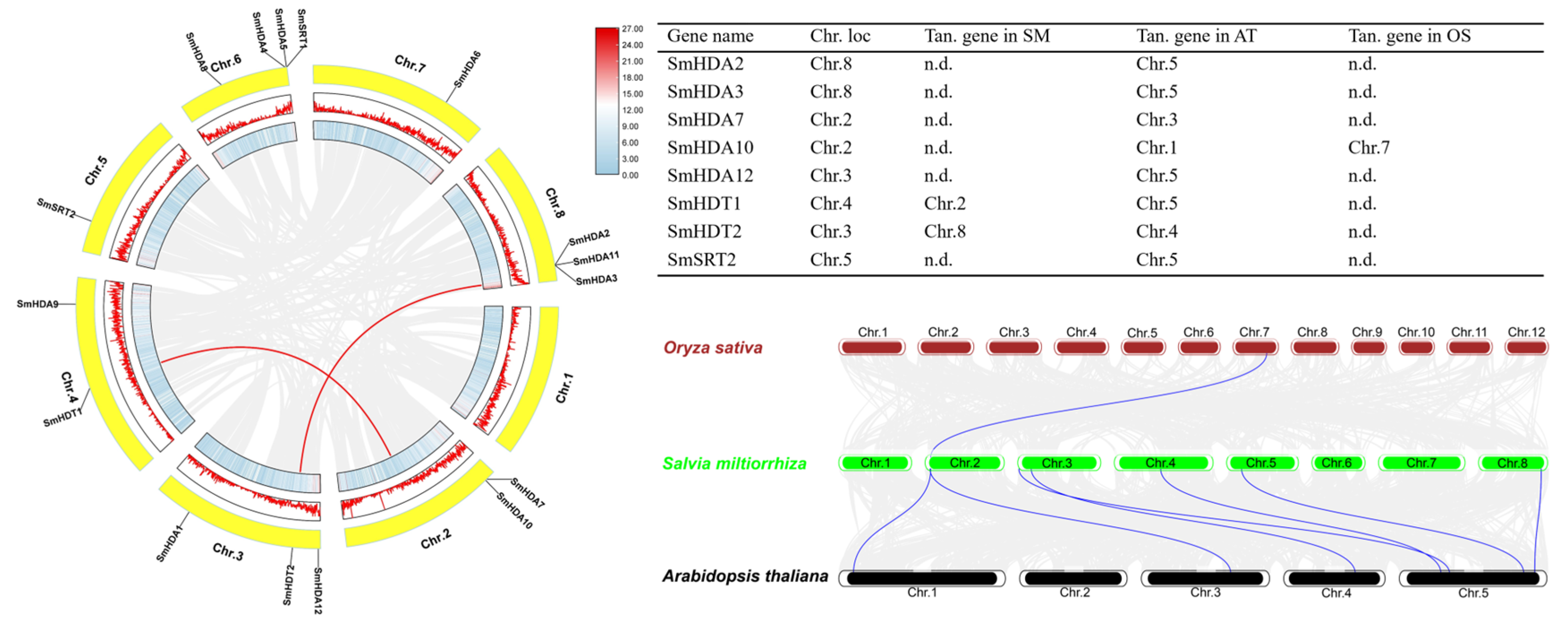
2.4. Chromosome Localization and Collinearity Analysis of SmHDACs
2.5. Cis-Acting Elements Analysis of SmHDACs
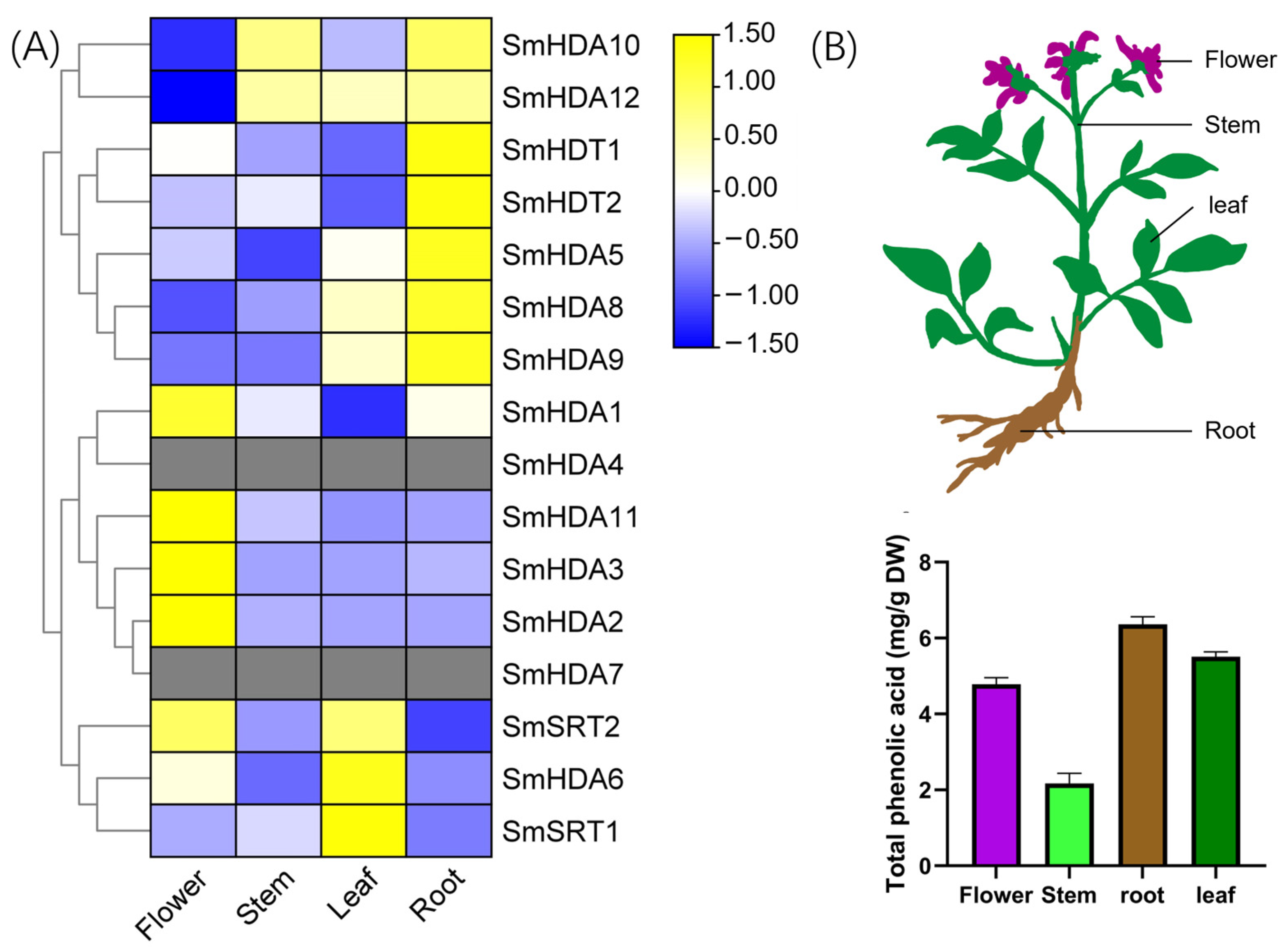
2.6. Tissue-Specific Analysis of SmHDACs
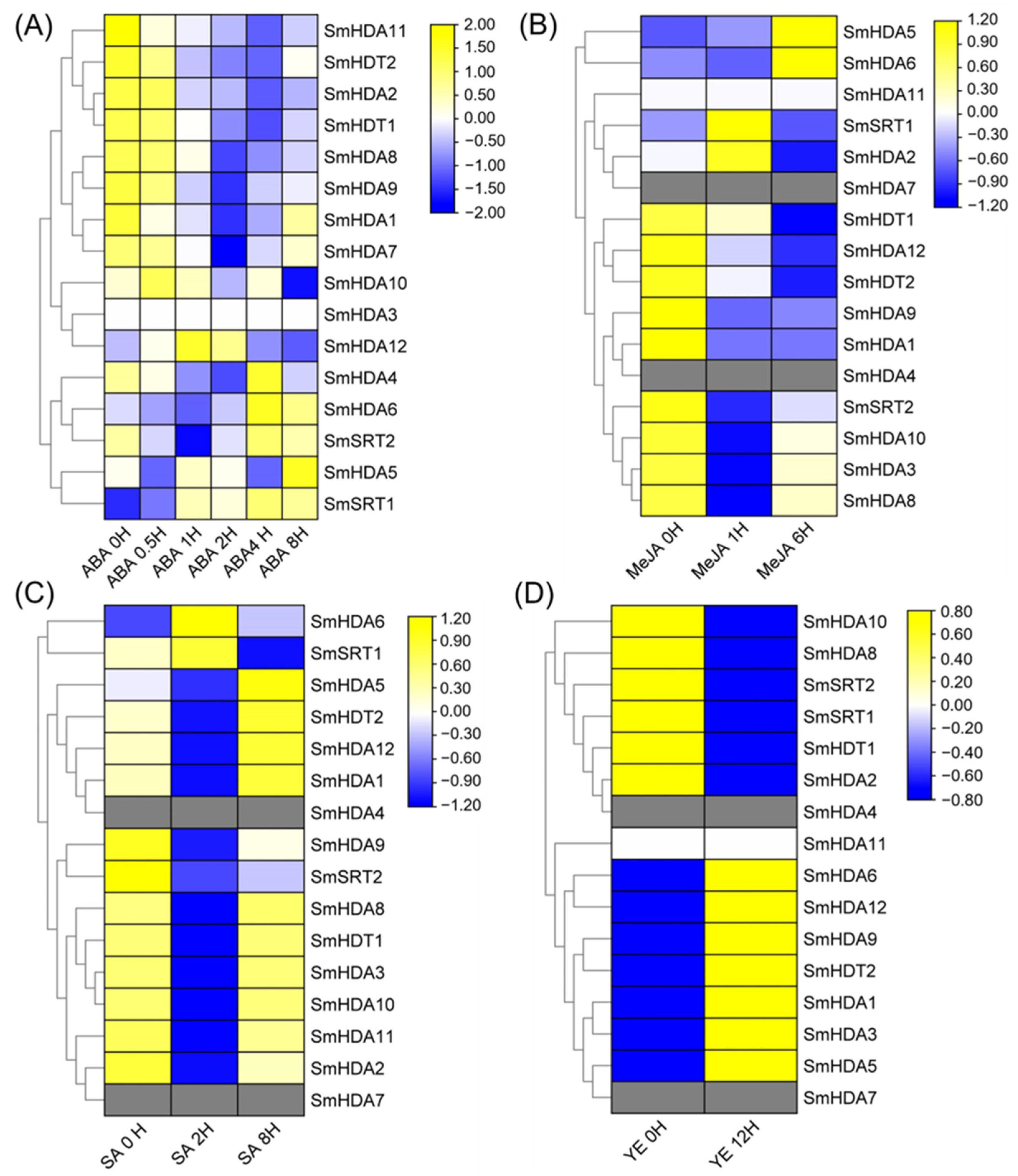
2.7. Induction of SmHDACs Expression Patterns by Each Inducer
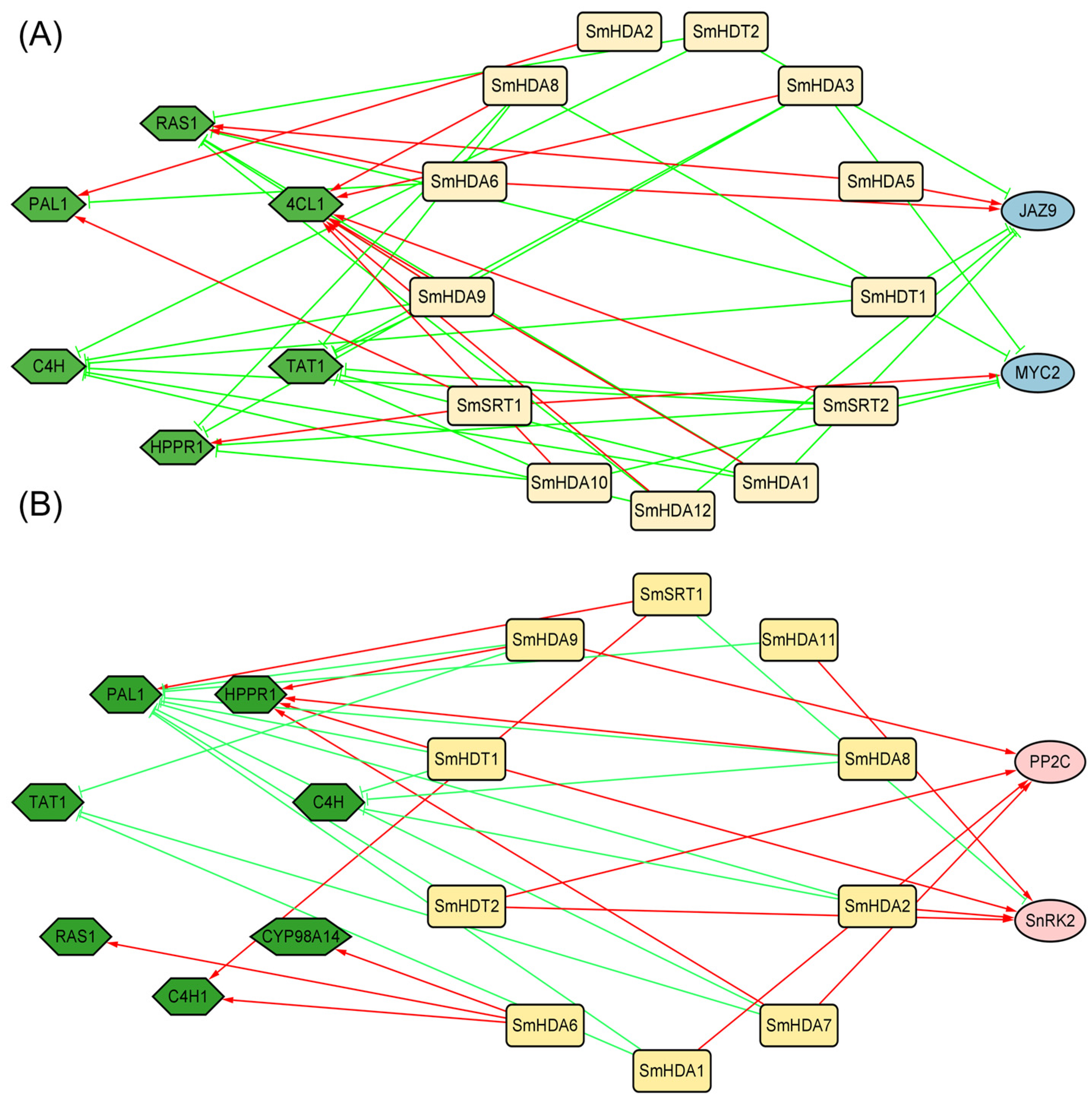
2.8. Co-Expression Network Analysis by MeJA and ABA Treatment
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Hormonal/Elicitor Treatment
4.2. Identification of the HDACs Family in S. miltiorrhiza
4.3. Phylogenetic Tree, Motif and Gene Structure Analysis of SmHDACs
4.4. Chromosome Localization and Collinearity Analysis of SmHDACs
4.5. Cis-Acting Elements Analysis of SmHDACs
4.6. Organ-Specific and Each Inducer Treated Expression Pattern of SmHDACs
4.7. Determination of Total Phenolic Acid
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, W.; Li, S.; Maoz, I.; Wang, Q.; Xu, M.; Feng, Y.; Hao, X.L.; Du, Z.Y.; Kai, G.Y. SmJRB1 positively regulates the accumulation of phenolic acid in Salvia miltiorrhiza. Ind. Crop. Prod. 2021, 164, 113417. [Google Scholar] [CrossRef]
- Liu, S.C.; Gao, X.K.; Shi, M.; Sun, M.H.; Li, K.L.; Cai, Y.; Chen, C.A.; Wang, C.; Maoz, I.; Guo, X.H.; et al. Jasmonic acid regulates the biosynthesis of medicinal metabolites via the JAZ9-MYB76 complex in Salvia miltiorrhiza. Hortic. Res. 2023, 10, uhad004. [Google Scholar] [CrossRef]
- Sun, C.T.; Han, B.; Zhai, Y.F.; Zhao, H.; Li, X.; Qian, J.; Hao, X.L.; Liu, Q.; Shen, J.Y.; Kai, G.Y. Dihydrotanshinone I inhibits ovarian tumor growth by activating oxidative stress through Keap1-mediated Nrf2 ubiquitination degradation. Free Radic. Biol. Med. 2022, 180, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.Q.; Zhu, R.Y.; Tian, Y.M.; Chen, B.B.; Li, R.; Li, L.; Wang, L.L.; Chen, Y.W.; Zhao, D.D.; Mo, F.F.; et al. Salvia miltiorrhiza in diabetes: A review of its pharmacology, phytochemistry, and safety. Phytomedicine 2019, 58, 152871. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Huang, F.F.; Deng, C.P.; Wang, Y.; Kai, G.Y. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 2019, 59, 953–964. [Google Scholar] [CrossRef]
- Fu, R.; Shi, M.; Deng, C.P.; Zhang, Y.; Zhang, X.C.; Wang, Y.; Kai, G.Y. Improved phenolic acid content and bioactivities of Salvia miltiorrhiza hairy roots by genetic manipulation of RAS and CYP98A14. Food Chem. 2020, 331, 127365. [Google Scholar] [CrossRef]
- Lu, S. Biosynthesis and regulatory mechanisms of bioactive compounds in Salvia miltiorrhiza, a model system for medicinal plant biology. Crit. Rev. Food Sci. Nutr. 2021, 40, 243–283. [Google Scholar] [CrossRef]
- Xu, Y.P.; Geng, L.J.; Zhang, Y.W.; Jones, J.A.; Zhang, M.H.; Chen, Y.; Tan, R.H.; Koffas, M.A.G.; Wang, Z.T.; Zhao, S.J. De novo biosynthesis of salvianolic acid B in Saccharomyces cerevisiae engineered with the rosmarinic acid biosynthetic pathway. J. Agric. Food Chem. 2022, 70, 2290–2302. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, Q.; Wu, X.; Zhou, Z.W.; Ding, M.Q.; Shi, M.; Huang, F.F.; Li, S.; Wang, Y.; Kai, G.Y. Comprehensive transcriptome profiling of Salvia miltiorrhiza for discovery of genes associated with the biosynthesis of tanshinones and phenolic acids. Sci. Rep. 2017, 7, 10554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shi, M.; Deng, C.P.; Lu, S.C.; Huang, F.F.; Wang, Y.; Kai, G.Y. The methyl jasmonate-responsive transcription factor SmMYB1 promotes phenolic acid biosynthesis in Salvia miltiorrhiza. Hortic. Res. 2021, 8, 10. [Google Scholar] [CrossRef]
- Deng, C.P.; Wang, Y.; Huang, F.F.; Lu, S.J.; Zhao, L.M.; Ma, X.Y.; Kai, G.Y. SmMYB2 promotes salvianolic acid biosynthesis in the medicinal herb Salvia miltiorrhiza. J. Integr. Plant Biol. 2020, 62, 1688–1702. [Google Scholar] [CrossRef]
- Sun, M.H.; Shi, M.; Wang, Y.; Huang, Q.; Yuan, T.P.; Wang, Q.; Wang, C.; Zhou, W.; Kai, G.Y. The biosynthesis of phenolic acids is positively regulated by the JA-responsive transcription factor ERF115 in Salvia miltiorrhiza. J. Exp. Bot. 2019, 70, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, X.; Gao, Y.; Zhou, C.G.; Chiang, V.L.; Li, W. Histone acetylation changes in plant response to drought stress. Genes 2021, 12, 1409. [Google Scholar] [CrossRef] [PubMed]
- Garske, A.L.; Oliver, S.S.; Wagner, E.K.; Musselman, C.A.; LeRoy, G.; Garcia, B.A.; Kutateladze, T.G.; Denu, J.M. Combinatorial profiling of chromatin binding modules reveals multisite discrimination. Nat. Chem. Biol. 2010, 6, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Lusser, A.; Kölle, D.; Loidl, P. Histone acetylation: Lessons from the plant kingdom. Trends Plant Sci. 2001, 6, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.L.; Ingle, R.A.; Petersen, L.N.; Denby, K.J. Basal resistance against Pseudomonas syringae in Arabidopsis involves WRKY53 and a protein with homology to a nematode resistance protein. Mol. Plant Microbe Interact. 2007, 20, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Tian, L. Roles of dynamic and reversible histone acetylation in plant development and polyploidy. Biochim. Biophys. Acta 2007, 1769, 295–307. [Google Scholar] [CrossRef]
- Pandey, R.; Müller, A.; Napoli, C.A.; Selinger, D.A.; Pikaard, C.S.; Richards, E.J.; Bender, J.; Mount, D.W.; Jorgensen, R.A. Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res. 2002, 30, 5036–5055. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jing, Y.J.; Gao, Y.; Liu, Y.T.; Fang, X.F.; Lin, R.C. The PIF1/PIF3-MED25-HDA19 transcriptional repression complex regulates phytochrome signaling in Arabidopsis. New Phytol. 2023, 240, 1097–1115. [Google Scholar] [CrossRef]
- Tilak, P.; Kotnik, F.; Née, G.; Seidel, J.; Sindlinger, J.; Heinkow, P.; Eirich, J.; Schwarzer, D.; Finkemeier, I. Proteome-wide lysine acetylation profiling to investigate the involvement of histone deacetylase HDA5 in the salt stress response of Arabidopsis leaves. Plant J. 2023, 115, 275–292. [Google Scholar] [CrossRef]
- Ullah, F.; Xu, Q.; Zhao, Y.; Zhou, D.X. Histone deacetylase HDA710 controls salt tolerance by regulating ABA signaling in rice. J. Integr. Plant Biol. 2020, 63, 451–467. [Google Scholar] [CrossRef]
- An, C.P.; Deng, L.; Zhai, H.W.; You, Y.R.; Wu, F.M.; Zhai, Q.Z.; Goossens, A.; Li, C.Y. Regulation of jasmonate signaling by reversible acetylation of TOPLESS in Arabidopsis. Mol. Plant 2022, 15, 1329–1346. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Chen, Y.W.; Xia, Y.T.; Hong, X.Y.; You, H.Q.; Zhang, R.; Liang, Z.S.; Cui, Q.; Zhang, S.C.; Zhou, M.; et al. DNA methylation regulates biosynthesis of tanshinones and phenolic acids during growth of Salvia miltiorrhiza. Plant Physiol. 2023, kiad573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Teixeira, S.J.A.; Yu, Z.M.; Wang, H.B.; Si, C.; Zhao, C.H.; He, C.M.; Duan, J. Identification of histone deacetylase genes in Dendrobium officinale and their expression profiles under phytohormone and abiotic stress treatments. PeerJ 2020, 8, e10482. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Verslues, P.E.; Zhu, J.K. Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Nakashima, K.; Yoshida, T.; Katagiri, T.; Kidokoro, S.; Kanamori, N.; Umezawa, T.; Fujita, M.; Maruyama, K.; Ishiyama, K.; et al. Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol. 2009, 50, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cui, G.H.; Chen, T.; Ma, X.H.; Wang, R.S.; Jin, B.L.; Yang, J.; Kang, L.P.; Tang, J.F.; Lai, C.J.S.; et al. Expansion within the CYP71D subfamily drives the heterocyclization of tanshinones synthesis in Salvia miltiorrhiza. Nat. Commun. 2021, 12, 685. [Google Scholar] [CrossRef]
- Jin, P.; Gao, S.Q.; He, L.; Xu, M.Z.; Zhang, T.Y.; Zhang, F.; Jiang, Y.Y.; Liu, T.T.; Yang, J.; Yang, J.; et al. Genome-wide identification and expression analysis of the histone deacetylase gene family in wheat (Triticum aestivum L.). Plants 2020, 10, 19. [Google Scholar] [CrossRef]
- Zhao, L.M.; Lu, J.X.; Zhang, J.X.; Wu, P.Y.; Yang, S.G.; Wu, K.Q. Identification and characterization of histone deacetylases in tomato (Solanum lycopersicum). Front. Plant Sci. 2015, 5, 760. [Google Scholar] [CrossRef]
- Tang, W.S.; Zhong, L.; Ding, Q.Q.; Dou, Y.N.; Li, W.W.; Xu, Z.S.; Zhou, Y.B.; Chen, J.; Chen, M.; Ma, Y.Z. Histone deacetylase AtSRT2 regulates salt tolerance during seed germination via repression of vesicle-associated membrane protein 714 (VAMP714) in Arabidopsis. New Phytol. 2022, 234, 1278–1293. [Google Scholar] [CrossRef]
- Waadt, R. Phytohormone signaling mechanisms and genetic methods for their modulation and detection. Curr. Opin. Plant Biol. 2020, 57, 31–40. [Google Scholar] [CrossRef]
- Deng, C.P.; Shi, M.; Fu, R.; Zhang, Y.; Wang, Q.; Zhou, Y.; Wang, Y.; Ma, X.Y.; Kai, G.Y. ABA-responsive transcription factor bZIP1 is involved in modulating biosynthesis of phenolic acids and tanshinones in Salvia miltiorrhiza. J. Exp. Bot. 2020, 71, 5948–5962. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Yang, Y.H.; Chen, J.M.; Li, J.L.; Jian, G.T.; Yang, J.; Mao, K.Q.; Zeng, L.T.; Gu, D.C. Histone deacetylase CsHDA6 mediates the regulated formation of the anti-insect metabolite α-farnesene in tea (Camellia sinensis). Plant Sci. 2023, 326, 111501. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.C.; Wu, S.H.; Yu, Z.M.; Zeng, L.T.; Qian, J.J.; Zhou, X.J.; Yang, Z.Y. Involvement of histone deacetylase CsHDA2 in regulating (E)-nerolidol formation in tea (Camellia sinensis) exposed to tea green leafhopper infestation. Hortic. Res. 2022, 9, uhac158. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Guo, Q.; Lin, R. The SNL-HDA19 histone deacetylase complex antagonizes HY5 activity to repress photomorphogenesis in Arabidopsis. New Phytol. 2021, 229, 3221–3236. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, P.; Zhang, F.; Luo, X.; Xie, J. Histone deacetylase HDA19 interacts with histone methyltransferase SUVH5 to regulate seed dormancy in Arabidopsis. Plant Biol. 2020, 22, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.T.; Wu, K. Role of histone deacetylases HDA6 and HDA19 in ABA and abiotic stress response. Plant Signal. Behav. 2010, 5, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.H.; Zhang, L.; Duan, J.; Miki, B.; Wu, K.Q. HISTONE DEACETYLASE19 is involved in jasmonic acid and ethylene signaling of pathogen response in Arabidopsis. Plant Cell 2005, 17, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wei, W.; Zhu, W.J.; Su, L.F.; Xiong, Z.Y.; Zhou, M.; Zhen, G.Y.; Zhou, D.X. Histone deacetylase AtSRT1 links metabolic flux and stress response in Arabidopsis. Mol. Plant 2017, 10, 1510–1522. [Google Scholar] [CrossRef]
- Chen, C.A.; Wang, C.; Li, J.B.; Gao, X.K.; Huang, Q.K.; Gong, Y.F.; Hao, X.L.; Maoz, I.; Kai, G.Y. Genome-wide analysis of U-box E3 ubiquitin ligase family in response to ABA treatment in Salvia miltiorrhiza. Front. Plant Sci. 2022, 13, 829447. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Tang, H.B.; Debarry, J.D.; Tan, X.; Li, J.P.; Wang, X.Y.; Lee, T.H.; Jin, H.Z.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; de Peer, V.V.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]

| Name | Size/aa | Molecular Weight/kDa | PI | GRAVY | Aliphatic Index | Instability Index (II) | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| SmHDA1 | 770 | 83.873 | 5.81 | −0.249 | 83.14 | 40.40 | Nucleus |
| SmHDA2 | 776 | 85.280 | 6.39 | −0.385 | 84.36 | 53.08 | Nucleus |
| SmHDA3 | 663 | 73.814 | 5.27 | −0.248 | 85.78 | 49.16 | Chloroplast and Nucleus |
| SmHDA4 | 374 | 40.619 | 5.69 | −0.097 | 87.19 | 37.65 | Nucleus |
| SmHDA5 | 499 | 56.276 | 5.27 | −0.489 | 75.99 | 38.73 | Cytoplasm and Nucleus |
| SmHDA6 | 464 | 52.436 | 5.15 | −0.544 | 72.87 | 38.75 | Cytoplasm and Nucleus |
| SmHDA7 | 423 | 47.742 | 5.01 | −0.365 | 81.82 | 31.80 | Cytoplasm and Nucleus |
| SmHDA8 | 554 | 60.571 | 5.68 | −0.229 | 79.82 | 41.16 | Nucleus |
| SmHDA9 | 453 | 51.454 | 5.82 | −0.513 | 76.16 | 47.61 | Nucleus |
| SmHDA10 | 374 | 40.411 | 5.38 | −0.124 | 86.55 | 34.18 | Nucleus |
| SmHDA11 | 313 | 35.305 | 6.74 | −0.416 | 86.36 | 42.16 | Nucleus |
| SmHDA12 | 394 | 43.173 | 7.21 | −0.112 | 94.85 | 36.19 | Nucleus |
| SmSRT1 | 425 | 47.983 | 9.40 | −0.450 | 77.13 | 45.82 | Chloroplast |
| SmSRT2 | 481 | 53.433 | 9.34 | −0.180 | 89.58 | 39.70 | Chloroplast |
| SmHDT1 | 284 | 30.699 | 5.07 | −1.059 | 50.28 | 46.80 | Nucleus |
| SmHDT2 | 493 | 53.822 | 5.09 | −0.992 | 61.08 | 47.81 | Vacuole and Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Ying, Y.; Yao, L.; Xu, Z.; Yu, Z.; Kai, G. Genome-Wide Identification and Characterization of the Salvia miltiorrhiza Histone Deacetylase (HDAC) Family in Response to Multiple Abiotic Stresses. Plants 2024, 13, 580. https://doi.org/10.3390/plants13050580
Chen J, Ying Y, Yao L, Xu Z, Yu Z, Kai G. Genome-Wide Identification and Characterization of the Salvia miltiorrhiza Histone Deacetylase (HDAC) Family in Response to Multiple Abiotic Stresses. Plants. 2024; 13(5):580. https://doi.org/10.3390/plants13050580
Chicago/Turabian StyleChen, Junyu, Yuxin Ying, Lingtiao Yao, Zhangting Xu, Zhenming Yu, and Guoyin Kai. 2024. "Genome-Wide Identification and Characterization of the Salvia miltiorrhiza Histone Deacetylase (HDAC) Family in Response to Multiple Abiotic Stresses" Plants 13, no. 5: 580. https://doi.org/10.3390/plants13050580





