Modulation of Drought-Induced Stress in Cowpea Genotypes Using Exogenous Salicylic Acid
Abstract
:1. Introduction
2. Results
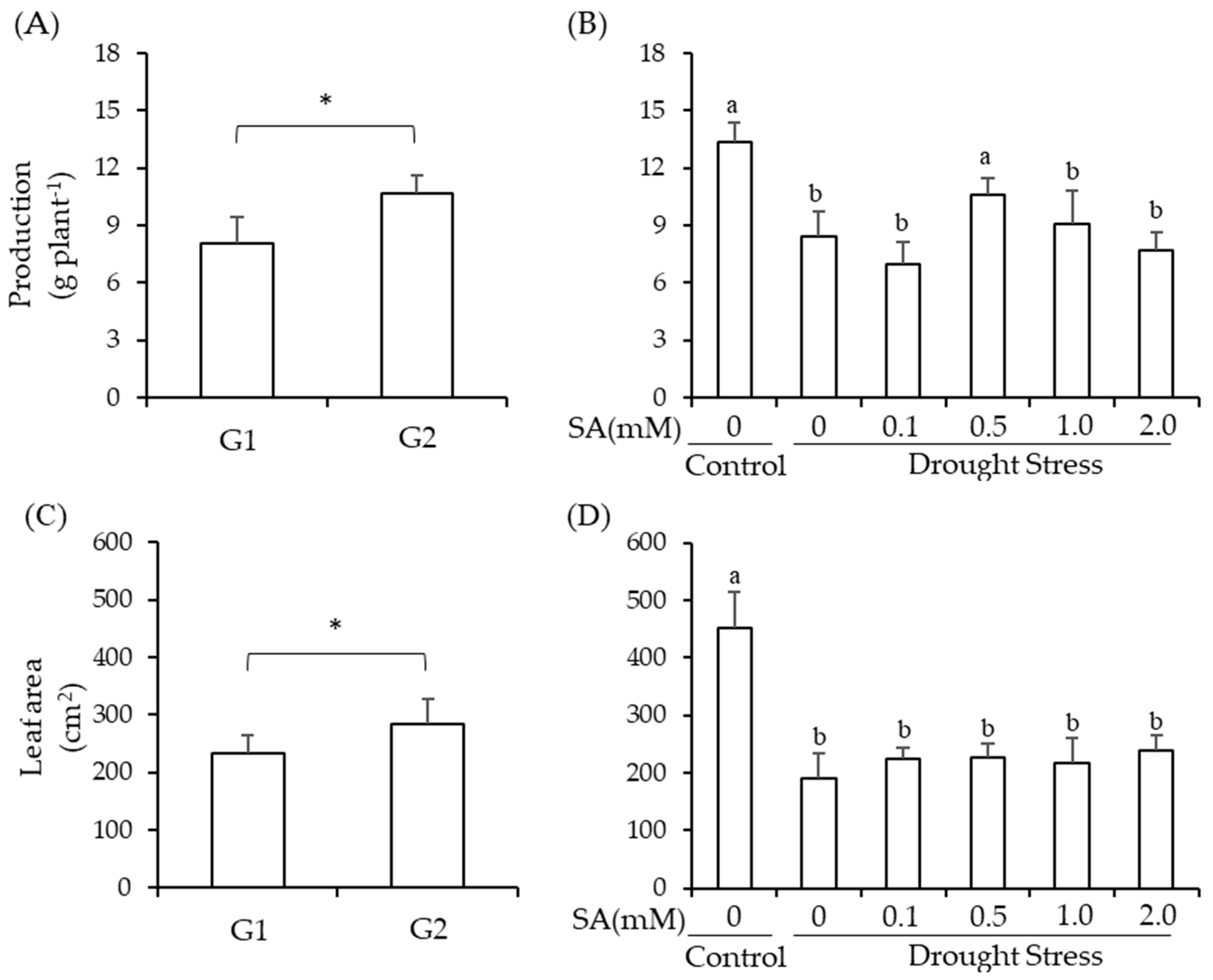
2.1. Production and Leaf Area
2.2. Stomatal Conductance and Net Photosynthesis
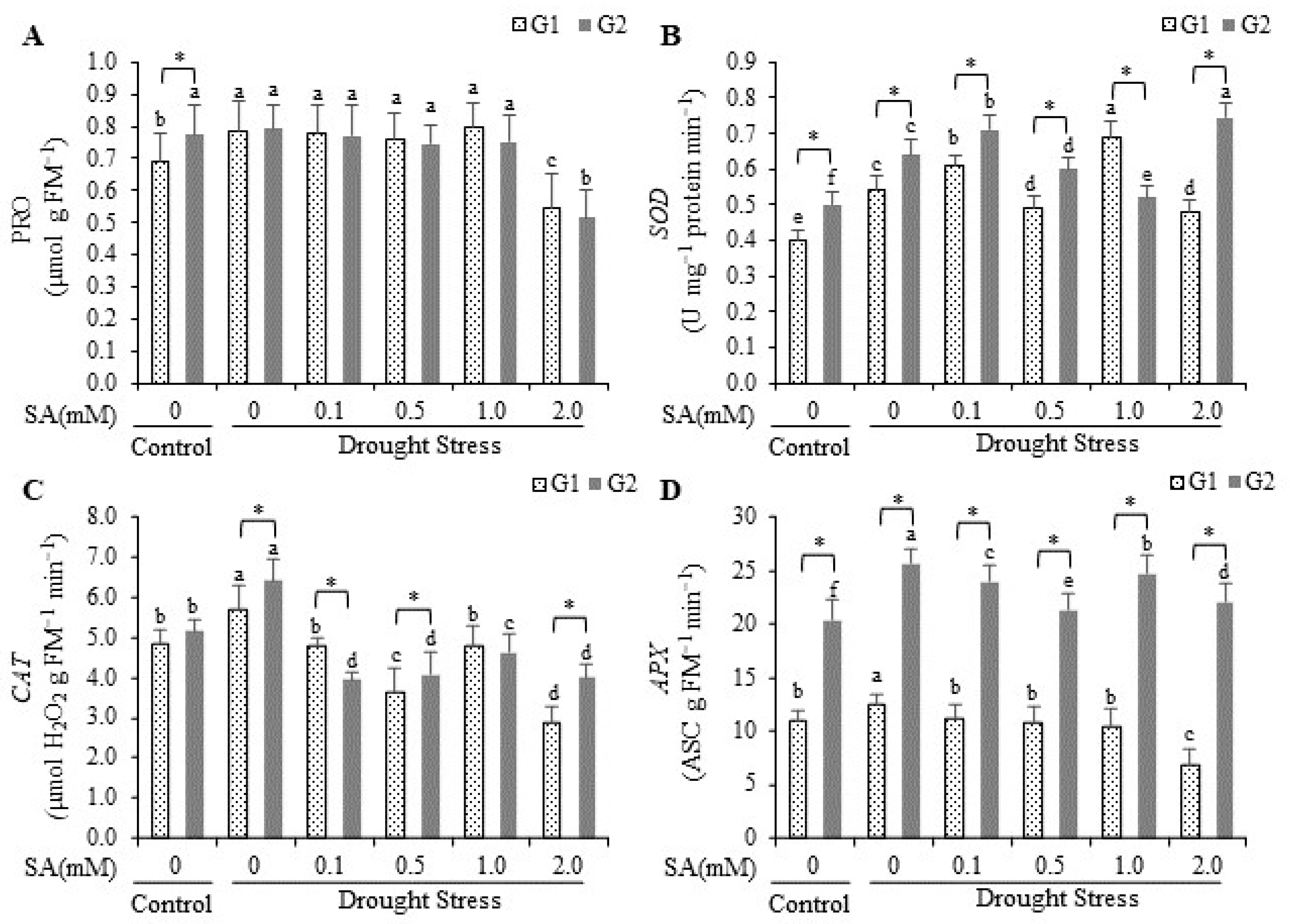
2.3. Proline and Superoxide Dismutase, Catalase, and Ascorbate Peroxidase Activity
3. Discussion
4. Materials and Methods
4.1. Location, Statistical Design, Treatments, and Plant Material
4.2. Soil, Water, and Plant Management
4.3. Experimental Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larcher, W. Ecofisiologia Vegetal; Prado, C.H.B.A., Ed.; Prado, C.H.B.A., Translator; Rima: São Carlos, Brazil, 2006; p. 531. (In Portuguese) [Google Scholar]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Morais, H.; Marur, C.J.; Caramori, P.H.; Ribeiro, A.M.A.; Gomes, J.C. Características fisiológicas e de crescimento de cafeeiro sombreado com guandu e cultivado a pleno sol. Pesqui. Agropecuária Bras. 2003, 38, 35–40. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Plant Physiol. 2013, 4, 35. [Google Scholar] [CrossRef]
- Belko, N.; Zaman-Allah, M.; Diop, N.N.; Cisse, N.; Zombre, G.; Ehlers, J.D.; Vadez, V. Restriction of transpiration rate under high vapor pressure deficit and non-limiting water conditions is important for terminal drought tolerance in cowpea. Plant Biol. 2013, 15, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Dutra, W.F.; Melo, A.S.; Suassuna, J.F.; Maia, J.M.; Dutra, A.F.; Silva, D.C. Antioxidative responses of cowpea cultivars to water deficit and salicylic acid treatment. Agron. J. 2017, 109, 895–905. [Google Scholar] [CrossRef]
- Andrade, W.L.; Melo, A.S.; Melo, Y.L.; Sá, F.V.S.; Rocha, M.M.; Oliveira, A.P.S.; Fernandes-Júnior, P.I. Bradyrhizobium inoculation plus foliar application of salicylic acid mitigates water deficit effects on cowpea. J. Plant Growth Regul. 2021, 40, 656–667. [Google Scholar] [CrossRef]
- Merwad, A.R.M.; Desoky, E.S.M.; Rady, M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Carvalho, M.; Castro, I.; Moutinho-Pereira, J.; Correia, C.; Egea-Cortines, M.; Matos, M.; Rosa, E.; Carnide, V.; Lino-Neto, T. Evaluating stress responses in cowpea under drought stress. J. Plant Physiol. 2019, 241, 153001. [Google Scholar] [CrossRef] [PubMed]
- CONAB—Companhia Nacional de Abastecimento. Monitoring the Brazilian Grain Harvest; CONAB: Russas, Brazil, 2021. (In Portuguese)
- Saboya, R.C.C.; Borges, P.R.S.; Saboya, L.M.F.; Monteiro, F.P.R.; Souza, S.E.A.; Santos, A.F.; Santos, E.R. Response of cowpea to inoculation with nitrogen-fixing strains in Gurupi-TO. J. Biotechnol. Biodivers. 2013, 4, 40–48. [Google Scholar] [CrossRef]
- Goufo, P.; Moutinho-Pereira, J.M.; Jorge, T.F.; Correia, C.M.; Oliveira, M.R.; Rosa, E.A.S. Cowpea (Vigna unguiculata L. Walp.) metabolomics: Osmoprotection as a physiological strategy for drought stress resistance and improved yield. Front. Plant Sci. 2017, 8, e586. [Google Scholar] [CrossRef]
- Zegaoui, Z.; Planchais, S.; Cabassa, C.; Djebbar, R.; Belbachir, O.A.; Carol, P. Variation in relative water content, proline accumulation and stress gene expression in two cowpea landraces under drought. J. Plant Physiol. 2017, 218, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.C.; Melo, A.S.; Melo, Y.L.; Andrade, W.L.; Lima, L.M.; Santos, A.R. Silicon foliar application attenuates the effects of water suppression on cowpea cultivars. Cienc. Agrotec. 2019, 43, 023019. [Google Scholar] [CrossRef]
- Santos, A.R.; Melo, Y.L.; Oliveira, L.F.; Cavalcante, I.E.; Ferraz, R.L.S.; Sá, F.V.S.; Lacerda, C.F.; Melo, A.S. Exogenous silicon and proline modulate osmoprotection and antioxidant activity in cowpea under drought stress. J. Soil. Sci. Plant Nutr. 2022, 22, 1692–1699. [Google Scholar] [CrossRef]
- Srivastav, M.; Kishor, A.; Dahuja, A.; Sharma, R.R. Effect of paclobutrazol and salinity on ion leakage, proline content and activities of antioxidant enzymes in mango (Mangifera indica L.). Sci. Hortic. 2010, 125, 785–788. [Google Scholar] [CrossRef]
- Nassef, D.M. Impact of irrigation water deficit and foliar application with salicylic acid on the productivity of two cowpea cultivars. Egypt. J. Hortic. 2017, 44, 75–90. [Google Scholar] [CrossRef]
- Bastos, E.A.; Nascimento, S.P.; Silva, E.M.; Freire-Filho, F.R.; Gomide, R.L. Identification of cowpea genotypes for drought tolerance. Rev. Cienc. Agron. 2011, 42, 100–107. [Google Scholar] [CrossRef]
- Woo, S.Y.; Hinckley, T.M. The effects of ozone on growth and stomatal response in the F2 generation of hybrid poplar (Populus trichocarpa × Populus deltoides). Biol. Plant. 2005, 49, 395–404. [Google Scholar] [CrossRef]
- Rivas-San, V.M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Afshari, M.; Shekari, F.; Azimkhani, R.; Habibi, H.; Fotokian, M.H. Effects of foliar application of salicylic acid on growth and physiological attributes of cowpea under water stress conditions. Iran. Agric. Res. 2013, 32, 55–70. [Google Scholar] [CrossRef]
- Jini, D.; Joseph, B. Physiological Mechanism of Salicylic Acid for Alleviation of Salt Stress in Rice. Rice Sci. 2017, 24, 97–108. [Google Scholar] [CrossRef]
- Araújo, E.D.; Melo, A.S.; Rocha, M.S.; Silva, P.C.C.; Ferraz, R.L.S.; Melo, Y.L.; ·Alencar, R.S.; ·Sá, F.V.S.; Lacerda, C.F. Improvement of Silicon-Induced Tolerance to Water Stress Is Dependent on Genotype Sensitivity and Phenological Stage. J. Soil. Sci. Plant Nutr. 2023, 23, 1648–1659. [Google Scholar] [CrossRef]
- Anosheh, H.P.; Emam, Y.; Ashraf, M.; Fonland, M.R. Exogenous Application of Salicylic Acid and Chlormequat Chloride Alleviates Negative Effects of Drought Stress in Wheat. Adv. Stud. Biol. 2012, 4, 501–520. [Google Scholar]
- Yaish, M.W. Proline accumulation is a general response to abiotic stress in the date palm tree (Phoenix dactylifera L.). Genet. Mol. Res. 2015, 14, 9943–9950. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.K.; Hemantaranjan, A.; Sarma, B.K.; Singh, R. Growth and antioxidant system under drought stress in chickpea (Cicer arietinum L.) as sustained by salicylic acid. J. Stress. Physiol. Biochem. 2011, 7, 130–144. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Short. Comm. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bezerra Neto, E.; Barreto, L.P. Análises Químicas e Bioquímicas em Plantas; UFRPE: Recife, Brazil, 2011; p. 267. (In Portuguese) [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase—Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Sudhakar, C.; Lakshmi, A.; Giridarakumar, S. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 2001, 161, 613–619. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidases in spinach chloroplast. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]

| Physical Characteristics | ||||||||||
| Particle Size (%) | TC | BD | PD | TP | Water Content (dag kg−1) | |||||
| Sand | Silt | Clay | 10kPa | 1500kPa | AW | |||||
| 87.75 | 5.45 | 6.80 | Loamy sand | 1.53 | 2.52 | 39.26 | 13.73 | 3.51 | 10.22 | |
| Chemical Characteristics | ||||||||||
| Ca2+ | Mg2+ | Na+ | K+ | S | H+ | Al3+ | P | OM | pH | EC |
| (cmolc/dm−3) | mg kg−1 | |||||||||
| 2.23 | 0.66 | 0.26 | 0.40 | 3.55 | 0.00 | 0.00 | 24.4 | 0.50 | 7.01 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, A.S.d.; Costa, R.R.d.; Sá, F.V.d.S.; Dias, G.F.; Alencar, R.S.d.; Viana, P.M.d.O.; Peixoto, T.D.C.; Suassuna, J.F.; Brito, M.E.B.; Ferraz, R.L.d.S.; et al. Modulation of Drought-Induced Stress in Cowpea Genotypes Using Exogenous Salicylic Acid. Plants 2024, 13, 634. https://doi.org/10.3390/plants13050634
Melo ASd, Costa RRd, Sá FVdS, Dias GF, Alencar RSd, Viana PMdO, Peixoto TDC, Suassuna JF, Brito MEB, Ferraz RLdS, et al. Modulation of Drought-Induced Stress in Cowpea Genotypes Using Exogenous Salicylic Acid. Plants. 2024; 13(5):634. https://doi.org/10.3390/plants13050634
Chicago/Turabian StyleMelo, Alberto Soares de, Rayssa Ribeiro da Costa, Francisco Vanies da Silva Sá, Guilherme Felix Dias, Rayanne Silva de Alencar, Priscylla Marques de Oliveira Viana, Tayd Dayvison Custódio Peixoto, Janivan Fernandes Suassuna, Marcos Eric Barbosa Brito, Rener Luciano de Souza Ferraz, and et al. 2024. "Modulation of Drought-Induced Stress in Cowpea Genotypes Using Exogenous Salicylic Acid" Plants 13, no. 5: 634. https://doi.org/10.3390/plants13050634







