Effects of Root–Root Interactions on the Physiological Characteristics of Haloxylon ammodendron Seedlings
Abstract
1. Introduction
2. Results
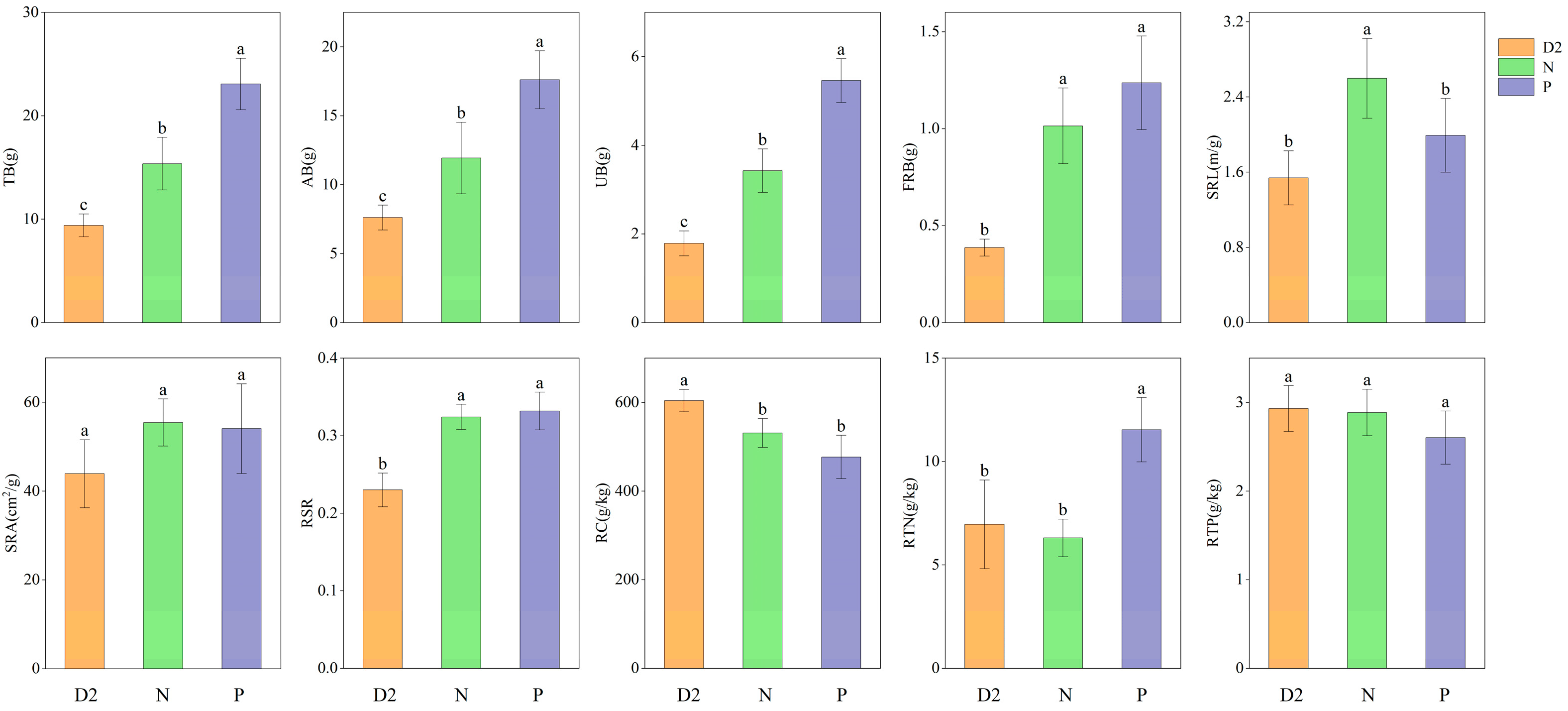
2.1. Effect of Root Separation on the Physiological Characteristics of H. ammodendron Seedlings
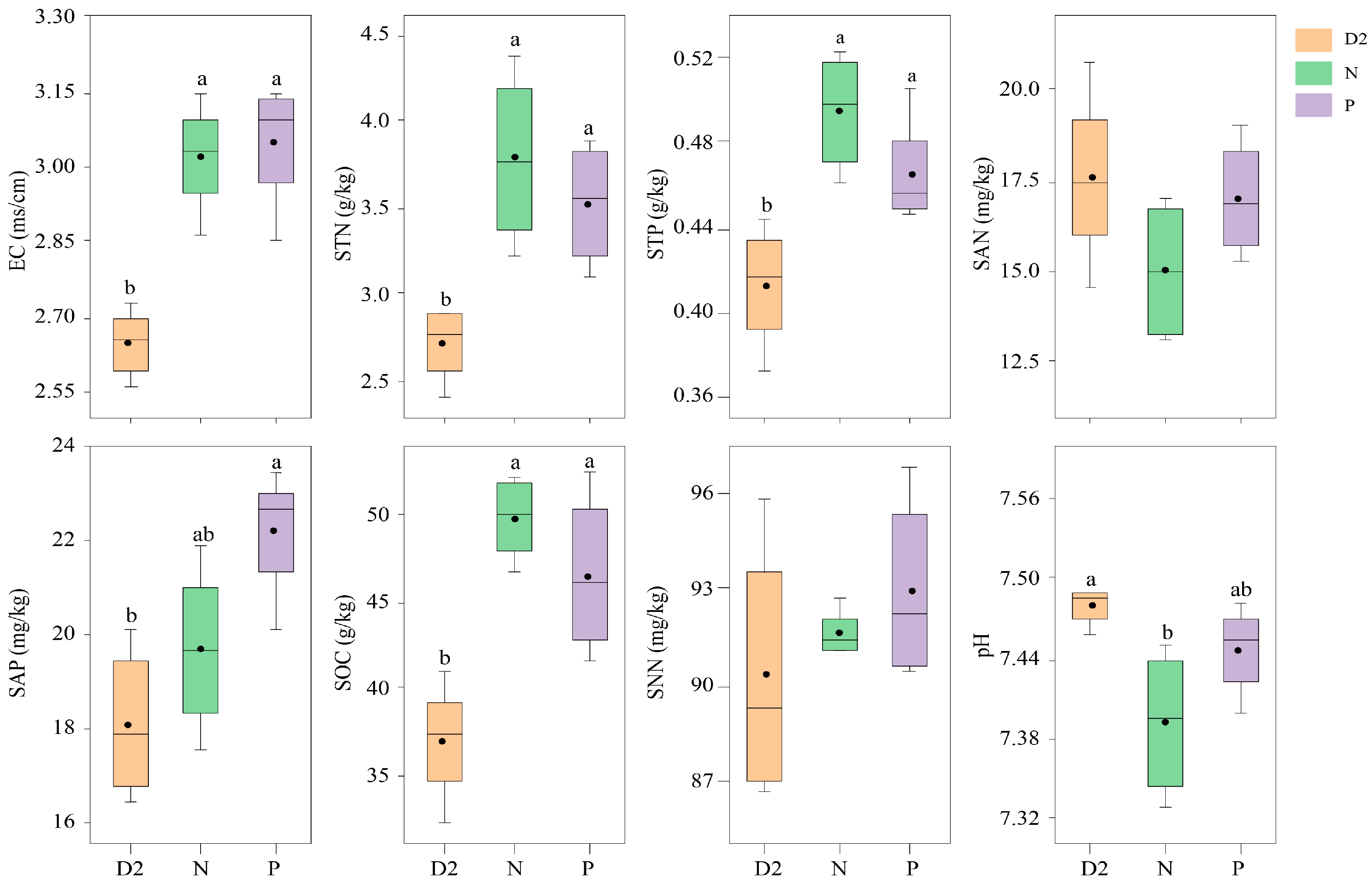
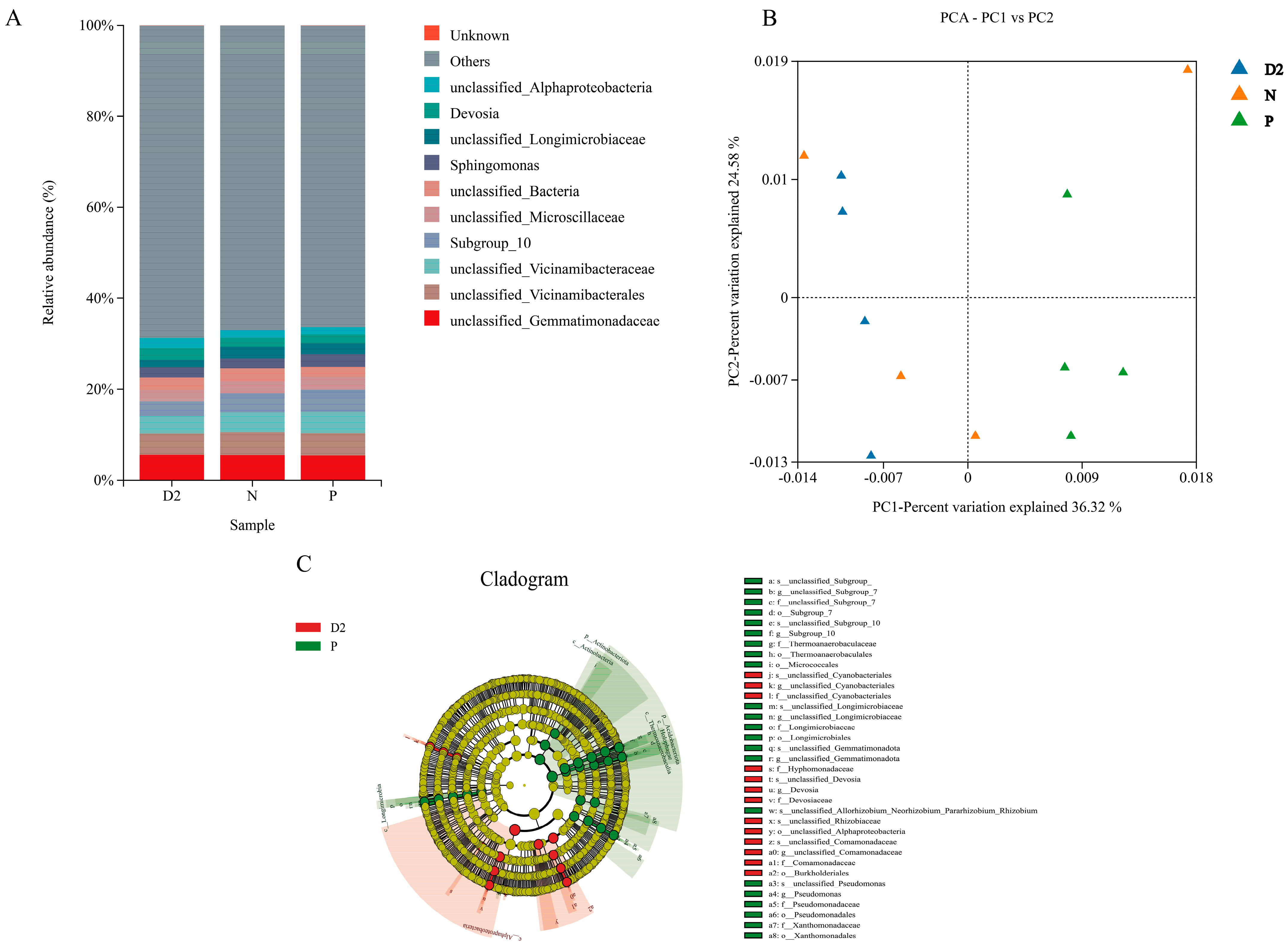
2.2. Soil Physicochemical Properties and Bacterial Community Structure Characteristics of H. ammodendron Seedlings under Different Separation Methods
2.3. Metabolite Composition and Quantitative Characteristics of H. ammodendron Seedling Roots under Different Isolation Methods
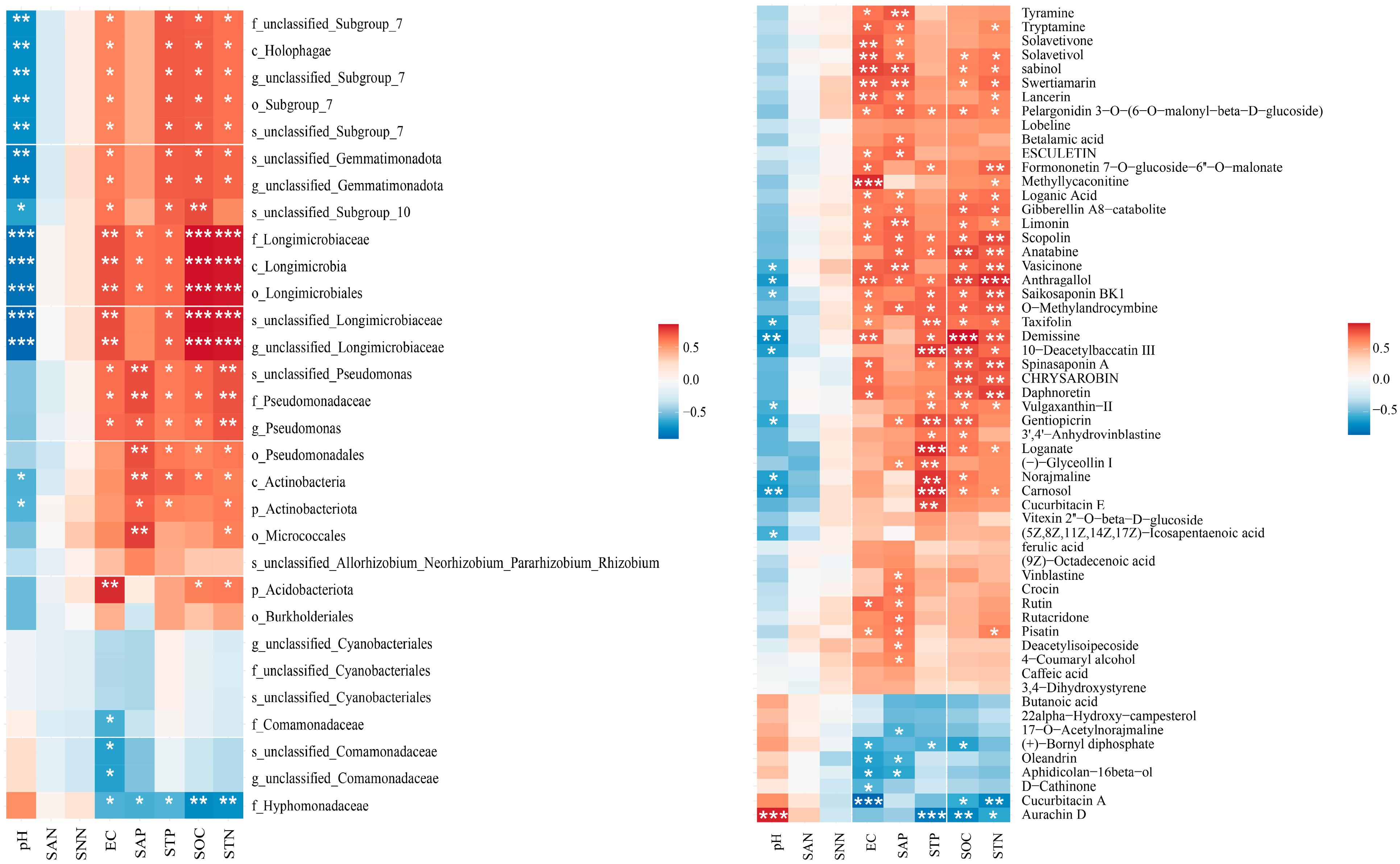
2.4. Relationships between Root Metabolites and Rhizosphere Bacteria and between Root Metabolites and Soil Physicochemical Properties
3. Discussion
3.1. Effects of Root Contact and Secretions on the Physiological Traits of H. ammodendron Seedlings
3.2. Root Interactions Alter the Soil Physicochemical Properties and Bacterial Community Structure of H. ammodendron Seedlings
3.3. The Impact of Root Interactions on the Metabolite Composition of H. ammodendron Seedling Roots
3.4. Effects of Root Metabolites on Soil Nutrients, Plant Traits, and Soil Microbial Communities
4. Materials and Methods
4.1. Experimental Design
4.2. Sample Collection and Processing
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

| Group | Total | Diff | Up | Down |
|---|---|---|---|---|
| D2 vs. N | 4010 | 358 | 301 | 57 |
| D2 vs. P | 4010 | 1182 | 916 | 266 |
| N vs. P | 4010 | 824 | 678 | 146 |
References
- Brooker, R.W. Plant-plant interactions and environmental change. New Phytol. 2006, 171, 271–284. [Google Scholar] [CrossRef] [PubMed]
- de Kroon, H. How Do Roots Interact? Science 2007, 318, 1562–1563. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.Q.; Kong, C.H.; Wang, P.; Meiners, S.J. Root exudate signals in plant-plant interactions. Plant Cell Environ. 2021, 44, 1044–1058. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Ahkami, A.H.; White, R.A.; Handakumbura, P.P.; Jansson, C. Rhizosphere engineering: Enhancing sustainable plant ecosystem productivity. Rhizosphere 2017, 3, 233–243. [Google Scholar] [CrossRef]
- Cantó, C.D.; Simonin, M.; King, E.; Moulin, L.; Bennett, M.J.; Castrillo, G.; Laplaze, L. An extended root phenotype: The rhizosphere, its formation and impacts on plant fitness. Plant J. 2020, 103, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interations with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Front. Plant Sci. 2019, 10, 420. [Google Scholar] [CrossRef]
- Hartmann, A.; Schmid, M.; van Tuinen, D.; Berg, G. Plant-driven selection of microbes. Plant Soil 2009, 321, 235–257. [Google Scholar] [CrossRef]
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 2010, 329, 1–25. [Google Scholar] [CrossRef]
- Gunina, A.; Smith, A.R.; Kuzyakov, Y.; Jones, D.L. Microbial uptake and utilization of low molecular weight organic substrates in soil depend on carbon oxidation state. Biogeochemistry 2017, 133, 89–100. [Google Scholar] [CrossRef]
- Williams, A.; de Vries, F.T. Plant root exudation under drought: Implications for ecosystem functioning. New Phytol. 2020, 225, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Pramanik, K.; Pal, P.; Mitra, S.; Ghosh, S.K.; Mondal, T.; Soren, T.; Maiti, T.K. Chapter 3—Multifaceted roles of root exudates in light of plant-microbe interaction. In Unravelling Plant-Microbe Synergy; Chandra, D., Bhatt, P., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 49–76. [Google Scholar]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef]
- Inderjit; Duke, S.O. Ecophysiological aspects of allelopathy. Planta 2003, 217, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.V.; Wren, I.F.; Herman, D.J.; Firestone, M.K. Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol. Lett. 2005, 8, 976–985. [Google Scholar] [CrossRef]
- Nuccio, E.E.; Hodge, A.; Pett-Ridge, J.; Herman, D.J.; Weber, P.K.; Firestone, M.K. An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ. Microbiol. 2013, 15, 1870–1881. [Google Scholar] [CrossRef] [PubMed]
- Duffner, A.; Hoffland, E.; Temminghoff, E.J.M. Bioavailability of zinc and phosphorus in calcareous soils as affected by citrate exudation. Plant Soil 2012, 361, 165–175. [Google Scholar] [CrossRef]
- Morris, K.A.; Stark, J.M.; Bugbee, B.; Norton, J.M. The invasive annual cheatgrass releases more nitrogen than crested wheatgrass through root exudation and senescence. Oecologia 2016, 181, 971–983. [Google Scholar] [CrossRef]
- Lo Presti, E.; Badagliacca, G.; Romeo, M.; Monti, M. Does Legume Root Exudation Facilitate Itself P Uptake in Intercropped Wheat? J. Soil Sci. Plant Nutr. 2021, 21, 3269–3283. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, J.; Tang, L.; Zheng, Y. Effect of nitrogen application rate on root soy isoflavone exudate of wheat/faba bean intercropping system. Chin. J. Eco-Agric. 2018, 26, 799–806. [Google Scholar]
- Latif, S.; Chiapusio, G.; Weston, L.A. Chapter Two—Allelopathy and the Role of Allelochemicals in Plant Defence. In Advances in Botanical Research; Becard, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 82, pp. 19–54. [Google Scholar]
- Macías, F.A.; Mejías, F.J.R.; Molinillo, J.M.G. Recent advances in allelopathy for weed control: From knowledge to applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of Phenolic Compounds in Plant-Defensive Mechanisms. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A.N., Eds.; Springer Singapore: Singapore, 2020; Volume 1, pp. 517–532. [Google Scholar]
- Dahlstrom, K.M.; McRose, D.L.; Newman, D.K. Keystone metabolites of crop rhizosphere microbiomes. Curr. Biol. 2020, 30, R1131–R1137. [Google Scholar] [CrossRef] [PubMed]
- Li, H.D.; Kang, Z.L.; Hua, J.; Feng, Y.L.; Luo, S.H. Root exudate sesquiterpenoids from the invasive weed Ambrosia trifida regulate rhizospheric Proteobacteria. Sci. Total Environ. 2022, 834, 155263. [Google Scholar] [CrossRef]
- Saraf, M.; Pandya, U.; Thakkar, A. Role of allelochemicals in plant growth promoting rhizobacteria for biocontrol of phytopathogens. Microbiol. Res. 2014, 169, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, L.D.R.; Chiappero, J.; Santoro, M.V.; Giordano, W.; Banchio, E. Inducing phenolic production and volatile organic compounds emission by inoculating Mentha piperita with plant growth-promoting rhizobacteria. Sci. Hortic. 2017, 220, 193–198. [Google Scholar] [CrossRef]
- Neal, A.L.; Ahmad, S.; Gordon-Weeks, R.; Ton, J. Benzoxazinoids in Root Exudates of Maize Attract Pseudomonas putida to the Rhizosphere. PLoS ONE 2012, 7, e35498. [Google Scholar] [CrossRef]
- Cahill, J.F.; McNickle, G.G.; Haag, J.J.; Lamb, E.G.; Nyanumba, S.M.; Clair, C.C.S. Plants Integrate Information About Nutrients and Neighbors. Science 2010, 328, 1657. [Google Scholar] [CrossRef]
- Xu, K.; Li, F.; Gou, S.; Bao, W. Root functional traits and trade-offs in one-year-old plants of 25 species from the arid valley of Minjiang River. Acta Ecol. Sin. 2012, 32, 215–225. [Google Scholar]
- Cortina, J.; Green, J.J.; Baddeley, J.A.; Watson, C.A. Root morphology and water transport of Pistacia lentiscus seedlings under contrasting water supply: A test of the pipe stem theory. Environ. Exp. Bot. 2008, 62, 343–350. [Google Scholar] [CrossRef]
- Zhou, X.G.; Zhang, J.Y.; Rahman, M.K.U.; Gao, D.M.; Wei, Z.; Wu, F.Z.; Dini-Andreote, F. Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol. Plant 2023, 16, 849–864. [Google Scholar] [CrossRef]
- Gong, S.; Wang, X.; Zhang, T.; Li, Q.; Zhou, J. Release of inorganic phosphorus from red soils induced by low molecular weight organic acids. Acta Pedol. Sin. 2010, 47, 692–697. [Google Scholar]
- Stevens, G.G.; Pérez-Fernández, M.A.; Morcillo, R.J.L.; Kleinert, A.; Hills, P.; Brand, D.J.; Steenkamp, E.T.; Valentine, A.J. Roots and Nodules Response Differently to P Starvation in the Mediterranean-Type Legume Virgilia divaricata. Front. Plant Sci. 2019, 10, 73. [Google Scholar] [CrossRef]
- Omonode, R.A.; Vyn, T.J. Spatial dependence and relationships of electrical conductivity to soil organic matter, phosphorus, and potassium. Soil Sci. 2006, 171, 223–238. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, Y.L.; Wang, H.; Zhang, T.; Yu, L.Y.; Sun, H.; Zhang, Y.Q. Diversity of Bacteria and the Characteristics of Actinobacteria Community Structure in Badain Jaran Desert and Tengger Desert of China. Front. Microbiol. 2018, 9, 1068. [Google Scholar] [CrossRef]
- Gao, J.L.; Luo, Y.; Wei, Y.L.; Huang, Y.L.; Zhang, H.; He, W.L.; Sheng, H.M.; An, L.Z. Effect of aridity and dune type on rhizosphere soil bacterial communities of Caragana microphylla in desert regions of northern China. PLoS ONE 2019, 14, e0224195. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Ma, R.; Ma, Y.J.; Niu, D.N.; Liu, T.; Tian, Y.S.; Dong, Z.H.; Chai, Q.D. Soil Bacterial Community Structure and Physicochemical Influencing Factors of Artificial Haloxylon ammodendron Forest in the Sand Blocking and Fixing Belt of Minqin, China. Forests 2023, 14, 2244. [Google Scholar] [CrossRef]
- Mitra, D.; Mondal, R.; Khoshru, B.; Senapati, A.; Radha, T.K.; Mahakur, B.; Uniyal, N.; Myo, E.M.; Boutaj, H.; Guerra Sierra, B.E.; et al. Actinobacteria-enhanced plant growth, nutrient acquisition, and crop protection: Advances in soil, plant, and microbial multifactorial interactions. Pedosphere 2022, 32, 149–170. [Google Scholar] [CrossRef]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Ouhdouch, Y.; Hafidi, M.; Kouisni, L. Multifunctional role of Actinobacteria in agricultural production sustainability: A review. Microbiol. Res. 2022, 261, 127059. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Kobayashi, Y.; Wasaki, J.; Koyama, H. Organic acid excretion from roots: A plant mechanism for enhancing phosphorus acquisition, enhancing aluminum tolerance, and recruiting beneficial rhizobacteria. Soil Sci. Plant Nutr. 2018, 64, 697–704. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, N.; Huang, Q.W.; Raza, W.; Li, R.; Vivanco, J.M.; Shen, Q.R. Organic acids from root exudates of banana help root colonization of PGPR strain Bacillus amyloliquefaciens NJN-6. Sci. Rep. 2015, 5, srep13438. [Google Scholar] [CrossRef]
- Mafu, S.; Ding, Y.Z.; Murphy, K.M.; Yaacoobi, O.; Addison, J.B.; Wang, Q.; Shen, Z.X.; Briggs, S.P.; Bohlmann, J.; Castro-Falcon, G.; et al. Discovery, Biosynthesis and Stress-Related Accumulation of Dolabradiene-Derived Defenses in Maize. Plant Physiol. 2018, 176, 2677–2690. [Google Scholar] [CrossRef]
- Zerbe, P. Small molecules with big impact: Terpenoid phytoalexins as key factors in maize stress tolerance. Plant Cell Environ. 2015, 38, 2193–2194. [Google Scholar] [CrossRef]
- Li, S.F.; Ding, J.Y.; Li, Y.T.; Hao, X.J.; Li, S.L. Antimicrobial Diterpenoids of Wedelia trilobata (L.) Hitchc. Molecules 2016, 21, 457. [Google Scholar] [CrossRef]
- Yao, Y.; He, F.; Hu, S.; Kong, L.; Liu, B.; Zeng, L.; Zhang, L.; Wu, Z. Effects of allelopathy of submerged macrophytes on the phytoplankton community collected from the west part of the West Lake wetland in Hangzhou, China. Acta Ecol. Sin. 2016, 36, 971–978. [Google Scholar]
- Bryant, J.P.; Chapin, F.S.; Klein, D.R.J.O. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 1983, 40, 357. [Google Scholar] [CrossRef]
- Matsuura, H.N.; Fett-Neto, A.G. Plant Alkaloids: Main Features, Toxicity, and Mechanisms of Action. In Plant Toxins; Gopalakrishnakone, P., Carlini, C.R., Ligabue-Braun, R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 1–15. [Google Scholar]
- Skoneczny, D.; Zhu, X.C.; Weston, P.A.; Gurr, G.M.; Callaway, R.M.; Weston, L.A. Production of pyrrolizidine alkaloids and shikonins in Echium plantagineum L. in response to various plant stressors. Pest Manag. Sci. 2019, 75, 2530–2541. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yao, Y.; Xu, H.; Xie, Z.; Guo, J.; Qi, Z.; Jiang, H. Soil metabolomics and bacterial functional traits revealed the responses of rhizosphere soil bacterial community to long-term continuous cropping of Tibetan barley. PeerJ 2022, 10, e13254. [Google Scholar] [CrossRef] [PubMed]
- Ervin, G.N.; Wetzel, R.G. Allelochemical autotoxicity in the emergent wetland macrophyte Juncus effusus (Juncaceae). Am. J. Bot. 2000, 87, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Huang, L.; Jiang, Y.; Zhu, Y.; Chen, B.; Zeng, Y.; Fu, G.; Fu, M. Bioactivity of extracts from rhizoma and rhizosphere soil of cultivated Atractylodes lancea DC. and identification of their allelopathic compounds. Acta Ecol. Sin. 2006, 26, 528–535. [Google Scholar]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000; pp. 27–38. [Google Scholar]
- Li, Q.; Song, X.Z.; Chang, S.X.; Peng, C.H.; Xiao, W.F.; Zhang, J.B.; Xiang, W.H.; Li, Y.; Wang, W.F. Nitrogen depositions increase soil respiration and decrease temperature sensitivity in a Moso bamboo forest. Agric. For. Meteorol. 2019, 268, 48–54. [Google Scholar] [CrossRef]
- Vranova, V.; Rejsek, K.; Skene, K.R.; Janous, D.; Formanek, P. Methods of collection of plant root exudates in relation to plant metabolism and purpose: A review. J. Plant Nutr. Soil Sci. 2013, 176, 175–199. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, N.; Luo, Q.; Shen, H. A new type of device and method for collecting plant root exudates. Acta Pedol. Sin. 2010, 47, 747–752. [Google Scholar]
- Jakoby, G.; Rog, I.; Megidish, S.; Klein, T. Enhanced root exudation of mature broadleaf and conifer trees in a Mediterranean forest during the dry season. Tree Physiol. 2020, 40, 1595–1605. [Google Scholar] [CrossRef]
- Tückmantel, T.; Leuschner, C.; Preusser, S.; Kandeler, E.; Angst, G.; Mueller, C.W.; Meier, I.C. Root exudation patterns in a beech forest: Dependence on soil depth, root morphology, and environment. Soil Biol. Biochem. 2017, 107, 188–197. [Google Scholar] [CrossRef]
- Williams, A.; Langridge, H.; Straathof, A.L.; Fox, G.; Muhammadali, H.; Hollywood, K.A.; Xu, Y.; Goodacre, R.; de Vries, F.T. Comparing root exudate collection techniques: An improved hybrid method. Soil Biol. Biochem. 2021, 161, 108391. [Google Scholar] [CrossRef]


| EC | pH | SOC | STN | STP | SAN | SNN | SAP | |
|---|---|---|---|---|---|---|---|---|
| EC | 1 | −0.509 | 0.695 * | 0.819 ** | 0.437 | −0.004 | 0.280 | 0.509 |
| pH | 1 | −0.731 ** | −0.714 ** | −0.616 * | 0.102 | −0.156 | −0.403 | |
| SOC | 1 | 0.850 ** | 0.800 ** | −0.278 | 0.144 | 0.598 * | ||
| STN | 1 | 0.639 * | 0.016 | 0.202 | 0.548 | |||
| STP | 1 | −0.552 | 0.403 | 0.460 | ||||
| SAN | 1 | −0.014 | 0.018 | |||||
| SNN | 1 | 0.320 | ||||||
| SAP | 1 |
| No. Root Exduates | D2 vs. N | D2 vs. P | N vs. P | % | ||||
|---|---|---|---|---|---|---|---|---|
| Up | Down | Up | Down | Up | Down | |||
| 1 | Alkaloids and derivatives | 5 | 0 | 7 | 6 | 2 | 4 | 0.92 |
| 2 | Benzenoids | 13 | 11 | 41 | 22 | 43 | 17 | 4.66 |
| 3 | Homogeneous nonmetal compounds | 0 | 0 | 0 | 4 | 0 | 0 | 0.12 |
| 4 | Hydrocarbon derivatives | 0 | 0 | 0 | 0 | 0 | 0 | 0.02 |
| 5 | Hydrocarbons | 2 | 0 | 6 | 0 | 5 | 0 | 0.20 |
| 6 | Lignans, neolignans, and related compounds | 0 | 1 | 0 | 1 | 1 | 1 | 0.35 |
| 7 | Lipids and lipid-like molecules | 77 | 12 | 168 | 58 | 112 | 29 | 13.92 |
| 8 | Nucleosides, nucleotides, and analogues | 7 | 3 | 24 | 24 | 20 | 3 | 2.32 |
| 9 | Organic acids and derivatives | 47 | 8 | 74 | 35 | 54 | 17 | 7.31 |
| 10 | Organic nitrogen compounds | 4 | 1 | 4 | 7 | 1 | 0 | 0.65 |
| 11 | Organic oxygen compounds | 15 | 3 | 44 | 22 | 41 | 12 | 4.91 |
| 12 | Organohalogen compounds | 0 | 0 | 1 | 0 | 1 | 0 | 0.02 |
| 13 | Organoheterocyclic compounds | 43 | 20 | 64 | 41 | 32 | 6 | 5.91 |
| 14 | Organosulfur compounds | 1 | 0 | 1 | 2 | 1 | 0 | 0.10 |
| 15 | Phenylpropanoids and polyketides | 13 | 1 | 63 | 18 | 66 | 12 | 5.66 |
| 16 | Other | 225 | 38 | 591 | 239 | 486 | 138 | 52.91 |
| Plant Secondary Metabolites | Up | Down | ||||
|---|---|---|---|---|---|---|
| D2 vs. N | D2 vs. P | N vs. P | D2 vs. N | D2 vs. P | N vs. P | |
| Alkaloids derived by amination reactions | 2 | 1 | 1 | |||
| Alkaloids derived from lysine | 1 | |||||
| Alkaloids derived from nicotinic acid | 1 | 1 | ||||
| Alkaloids derived from ornithine | ||||||
| Alkaloids derived from tryptophan and anthranilic acid | 2 | 6 | 2 | 1 | 2 | |
| Alkaloids derived from tyrosine | 1 | 2 | 1 | |||
| Betalains | 2 | |||||
| Cyanogenic glucosides | ||||||
| Glucosinolates | ||||||
| Carotenoids and apocarotenoids | 1 | |||||
| Diterpenoids (C20) | 3 | 1 | 1 | |||
| Monoterpenoids (C10) | 1 | 5 | 3 | 1 | ||
| Sesquiterpenoids (C15) | 2 | 2 | 2 | |||
| Steroids | 1 | 1 | ||||
| Triterpenoids (C30) | 1 | 4 | 1 | 1 | 1 | 1 |
| Coumarins | 3 | 1 | ||||
| Monolignols | 4 | 3 | ||||
| Fatty acids | 1 | 1 | ||||
| Flavonoids | 1 | 4 | 2 | |||
| Isoflavonoids | 2 | 2 | ||||
| Anthraquinones | 1 | 2 | ||||
| Pyrones | 1 | 1 | 1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Ji, S.; Wu, D.; Zhu, M.; Lv, G. Effects of Root–Root Interactions on the Physiological Characteristics of Haloxylon ammodendron Seedlings. Plants 2024, 13, 683. https://doi.org/10.3390/plants13050683
Yang H, Ji S, Wu D, Zhu M, Lv G. Effects of Root–Root Interactions on the Physiological Characteristics of Haloxylon ammodendron Seedlings. Plants. 2024; 13(5):683. https://doi.org/10.3390/plants13050683
Chicago/Turabian StyleYang, Huifang, Suwan Ji, Deyan Wu, Menghao Zhu, and Guanghui Lv. 2024. "Effects of Root–Root Interactions on the Physiological Characteristics of Haloxylon ammodendron Seedlings" Plants 13, no. 5: 683. https://doi.org/10.3390/plants13050683
APA StyleYang, H., Ji, S., Wu, D., Zhu, M., & Lv, G. (2024). Effects of Root–Root Interactions on the Physiological Characteristics of Haloxylon ammodendron Seedlings. Plants, 13(5), 683. https://doi.org/10.3390/plants13050683






