Antimicrobial Activity on Streptococcus mutans and Enterococcus faecalis of Cyperus articulatus Ethanolic Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Committee Approval
2.2. Plant Material
2.3. Ethanolic Extract from C. articulatus Rhizomes
2.4. Ethanolic Extract from the Solid Waste of C. articulatus
2.5. GC-MS Analysis of Extracts
2.6. Antimicrobial Evaluation
2.6.1. Microorganisms and Culture Conditions
2.6.2. Determination of the Minimum Inhibitory Concentration (MIC)
2.7. Toxicity Assessment: Hens Egg Test-Chorion Allantoic Membrane (HET-CAM)
2.8. Statistical Analysis
3. Results
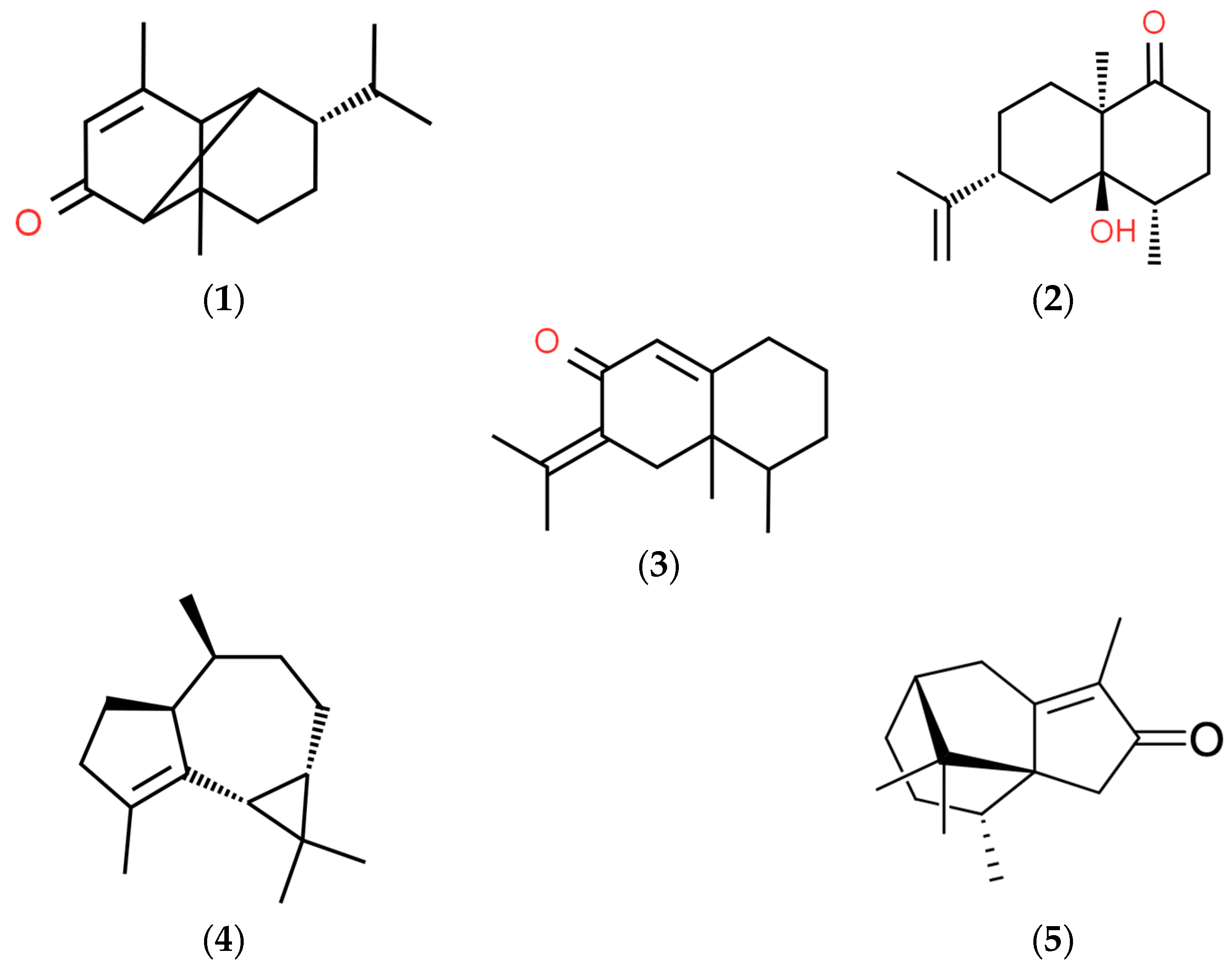
3.1. Analysis of the Chemical Composition of Volatile Compounds in the Extracts
3.2. Antimicrobial Evaluation
3.3. Toxicity Assessment: HET CAM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watt, R.G.; Daly, B.; Allison, P.; Macpherson, L.M.D.; Venturelli, R.; Listl, S.; Weyant, R.J.; Mathur, M.R.; Guarnizo-Herreño, C.C.; Celeste, R.K. Ending the Neglect of Global Oral Health: Time for Radical Action. Lancet 2019, 394, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Untreated Caries: A Systematic Review and Metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef]
- Brasil, S.B. Pesquisa Nacional de Saúde Bucal: Resultados Principais/Ministério Da Saúde. Secretaria de Atenção à Saúde. Secretaria de Vigilância Em Saúde. Brasília: Ministério da Saúde 2012, 116. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/pesquisa_nacional_saude_bucal.pdf (accessed on 10 September 2023).
- Petersen, P.E. The World Oral Health Report 2003: Continuous Improvement of Oral Health in the 21st Century—The Approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2003, 31, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, P.B. Variação Do Índice CPOD Do Brasil No Período de 1980 a 2010. Rev. Bras Odontol. 2016, 72, 10. [Google Scholar] [CrossRef][Green Version]
- Pitts, N.; Zero, D.; March, P.; Ekstrand, K.; Weintraub, J.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental Caries. Nat. Rev. Dis. Primers 2017, 3, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lobo, C.I.V.; Rinaldi, T.B.; Christiano, C.M.S.; De Sales Leite, L.; Barbugli, P.A.; Klein, M.I. Dual-Species Biofilms of Streptococcus Mutans and Candida Albicans Exhibit More Biomass and Are Mutually Beneficial Compared with Single-Species Biofilms. J. Oral. Microbiol. 2019, 11, 1581520. [Google Scholar] [CrossRef]
- Wade, W.G. The Oral Microbiome in Health and Disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral Diseases: A Global Public Health Challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental Diseases—Are These Examples of Ecological Catastrophes? Int. J. Dent. Hyg. 2006, 4 (Suppl. S1), 3–10. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. A Fitoterapia No SUS Eo Programa de Pesquisas de Plantas Medicinais Da Central de Medicamentos; Série B. Textos Básicos de Saúde; Departamento de Assistência Farmacêutica, Ministério da Saúde: Brasília, Brazil, 2006; p. 148. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/fitoterapia_no_sus.pdf (accessed on 10 September 2023).
- D’ávila, A.M.M.N.; de Cruz, J.H.A.; Guênes, G.M.T.; de Oliveira Filho, A.A.; dos Anjos, R.M. Interações Medicamentosas: Fitoterápicos Utilizados Na Odontologia e Fármacos de Uso Contínuo Dos Pacientes. Arq. Health Investig. 2021, 10, 468–473. [Google Scholar] [CrossRef]
- Pereira, L.D.P.; Da Silva, R.O.; Bringel, P.H.D.S.F.; Da Silva, K.E.S.; Assreuy, A.M.S.; Pereira, M.G. Polysaccharide Fractions of Caesalpinia Ferrea Pods: Potential Anti-Inflammatory Usage. J. Ethnopharmacol. 2012, 139, 642–648. [Google Scholar] [CrossRef]
- De Oliveira Conde, N.C.; Pereira, M.D.S.V.; Bandeira, M.F.C.L.; Venâncio, G.N.; De Oliveira, G.P.; Sampaio, F.C. In Vitro Antimicrobial Activity of Plants of the Amazon on Oral Biofilm Micro-Organisms. Rev. Odonto Cienc. 2015, 30, 179–183. [Google Scholar] [CrossRef]
- Gomes, M.S.; Pereira de Mendonça, A.K.; Cordeiro, T.O.; Barbosa, M.M. Uso De Plantas Medicinais Na Odontologia: Uma Revisão Integrativa. Rev. Ciências Saúde Nova Esperança 2020, 18, 118–126. [Google Scholar] [CrossRef]
- Mallavadhani, U.V.; Chandrashekhar, M.; Nayak, V.L.; Ramakrishna, S. Synthesis and Anticancer Activity of Novel Fused Pyrimidine Hybrids of Myrrhanone C, a Bicyclic Triterpene of Commiphora Mukul Gum Resin. Mol. Divers 2015, 19, 745–757. [Google Scholar] [CrossRef]
- Chandrashekhar, M.; Nayak, V.L.; Ramakrishna, S.; Mallavadhani, U.V. Novel Triazole Hybrids of Myrrhanone C, a Natural Polypodane Triterpene: Synthesis, Cytotoxic Activity and Cell Based Studies. Eur. J. Med. Chem. 2016, 114, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.E.S. As Espécies Cyperaceae Juss. Conhecidas Como Priprioca. In POTIGUARA, RC V.; ZOGHBI, MGB Priprioca: Um recurso aromático do Pará; MPEG, UEPA: Belém, Brazil, 2008; pp. 13–24. [Google Scholar]
- Zoghbi, M.G.B.; Guilhon, G.M.S.; Andrade, E.H.A.; Vilhena, K.S.S. Química Das Espécies de Cyperus Conhecidas Como Priprioca. In POTIGUARA, RC V.; ZOGHBI, MGB Priprioca: Um Recurso Aromático do Pará; MPEG, UEPA: Belém, Brazil, 2008; pp. 53–76. [Google Scholar]
- Oladosu, I.A.; Usman, L.A.; Olawore, N.O.; Atata, R.F. Antibacterial Activity of Rhizomes Essential Oils of Two Types of Cyperus articulatus Growing in Nigeria. Adv. Biol. Res. 2011, 5, 179–183. [Google Scholar]
- Bum, E.N.; Schmutz, M.; Meyer, C.; Rakotonirina, A.; Bopelet, M.; Portet, C.; Jeker, A.; Rakotonirina, S.V.; Olpe, H.R.; Herrling, P. Anticonvulsant Properties of the Methanolic Extract of Cyperus articulatus (Cyperaceae). J. Ethnopharmacol. 2001, 76, 145–150. [Google Scholar] [CrossRef]
- Metuge, J.A.; Babiaka, S.B.; Mbah, J.A.; Ntie-Kang, F.; Ayimele, G.A.; Cho-Ngwa, F. Anti-Onchocerca Metabolites from Cyperus articulatus: Isolation, in Vitro Activity and in Silico ‘Drug-Likeness’. Nat. Prod. Bioprospect. 2014, 4, 243–249. [Google Scholar] [CrossRef]
- Kavaz, D.; Idris, M.; Onyebuchi, C. Physiochemical Characterization, Antioxidative, Anticancer Cells Proliferation and Food Pathogens Antibacterial Activity of Chitosan Nanoparticles Loaded with Cyperus articulatus Rhizome Essential Oils. Int. J. Biol. Macromol. 2019, 123, 837–845. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Mongelli, E.; Coussio, J.; Ciccia, G. Studies on the Cytotoxicity, Antimicrobial and DNA-Binding Activities of Plants Used by the Ese’ejas. J. Ethnopharmacol. 1996, 50, 91–96. [Google Scholar] [CrossRef]
- Rakotonirina, V.S.; Bum, E.N.; Rakotonirina, A.; Bopelet, M. Sedative Properties of the Decoction of the Rhizome of Cyperus articulatus. Fitoterapia 2001, 72, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Bersan, S.M.F.; Galvão, L.C.C.; Goes, V.F.F.; Sartoratto, A.; Figueira, G.M.; Rehder, V.L.G.; Alencar, S.M.; Duarte, R.M.T.; Rosalen, P.L.; Duarte, M.C.T. Action of Essential Oils from Brazilian Native and Exotic Medicinal Species on Oral Biofilms. BMC Complement. Altern. Med. 2014, 14, 451. [Google Scholar] [CrossRef] [PubMed]
- da Silva, É.B.S.; Barata, L.E.S.; Arevalo, M.R.; Vieira, L.Q.; Castro, W.; Ruiz, A.L.T.G.; Torre, A.D.; Castro, K.C.F.; Sartoratto, A.; Baratto, L.C.; et al. Chemical Composition and Antiproliferative Activity of the Ethanolic Extract of Cyperus articulatus L. (Cyperaceae). Plants 2021, 10, 2084. [Google Scholar] [CrossRef] [PubMed]
- Kasper, A.A.M.; de Sousa, S.F.; de San Martin, B.S.; Sartoratto, A.; Nunes, K.M.; de Sousa Júnior, J.J.V.; da Silva, S.K.R.; Barata, L.E.S. Aproveitamento Dos Resíduos de Priprioca (Cyperus articulatus L.) No Controle Alternativo de Fungos Fitopatogênicos. Rev. Ibero-Am. Ciências Ambient. 2020, 11, 80–88. [Google Scholar] [CrossRef]
- Assis, F.F.V.; Da Silva, N.C.; Moraes, W.P.; Barata, L.E.S.; Minervino, A.H.H. Chemical Composition and in Vitro Antiplasmodial Activity of the Ethanolic Extract of Cyperus articulatus Var. Nodosus Residue. Pathogens 2020, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- NIH ICCVAM. Test Method Evaluation Report: Current Validation Status of In Vitro Test Methods Proposed for Identifying Eye Injury Hazard Potential of Chemicals and Products; NIH Publication No 10-7553; NIH: Stapleton, NY, USA, 2010; p. 1324. [Google Scholar]
- Rukunga, G.M.; Muregi, F.W.; Omar, S.A.; Gathirwa, J.W.; Muthaura, C.N.; Peter, M.G.; Heydenreich, M.; Mungai, G.M. Anti-Plasmodial Activity of the Extracts and Two Sesquiterpenes from Cyperus articulatus. Fitoterapia 2008, 79, 188–190. [Google Scholar] [CrossRef] [PubMed]
- das Zoghbi, M.G.B.; Andrade, E.H.A.; Oliveira, J.; Carreira, L.M.M.; Guilhon, G.M.S.P. Yield and Chemical Composition of the Essential Oil of the Stems and Rhizomes of Cyperus articulatus L. Cultivated in the State of Pará, Brazil. J. Essent. Oil Res. 2006, 18, 10–12. [Google Scholar] [CrossRef]
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.G.; Delarmelina, C. Anti-Candida Activity of Brazilian Medicinal Plants. J. Ethnopharmacol. 2005, 97, 305–311. [Google Scholar] [CrossRef]
- Silva, N.C.; Goncalves, S.F.; de Araújo, L.S.; Kasper, A.A.M.; da Fonseca, A.L.; Sartoratto, A.; Castro, K.C.F.; Moraes, T.M.P.; Baratto, L.C.; de Varotti, F.P. In Vitro and in Vivo Antimalarial Activity of the Volatile Oil of Cyperus articulatus (Cyperaceae). Acta Amazon 2019, 49, 334–342. [Google Scholar] [CrossRef]
- Freires, I.A.; Bueno-Silva, B.; Galvão, L.C.D.C.; Duarte, M.C.T.; Sartoratto, A.; Figueira, G.M.; De Alencar, S.M.; Rosalen, P.L. The Effect of Essential Oils and Bioactive Fractions on Streptococcus Mutans and Candida Albicans Biofilms: A Confocal Analysis. Evid.-Based Complement. Altern. Med. 2015, 2015, 871316. [Google Scholar] [CrossRef]
- Yang, Z.; He, S.; Wei, Y.; Li, X.; Shan, A.; Wang, J. Antimicrobial Peptides in Combination with Citronellal Efficiently Kills Multidrug Resistance Bacteria. Phytomedicine 2023, 120, 155070. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.L.S.; Bezerra, L.M.D.; Ribeiro, I.L.A.; Morais Júnior, R.C.D.; Castro, R.D. Susceptibility of Cariogenic Microorganisms to Phytoconstituents. Braz. J. Biol. 2018, 78, 691–696. [Google Scholar] [CrossRef] [PubMed]
| Effects | Points | ||
|---|---|---|---|
| 0.5 min (30 s) | 2 min (120 s) | 5 min (300 s) | |
| Lysis | 5 | 3 | 1 |
| Bleeding | 7 | 5 | 3 |
| Coagulation | 9 | 7 | 5 |
| Scoring Range | Irritation Category |
|---|---|
| 0 to 0.9 | not labeled |
| 1 to 4.9 | slight irritation |
| 5 to 8.9 | moderate irritation |
| 9 to 21 | severe irritation |
| Constituents | RT | % |
|---|---|---|
| Citronellal | 12.47 | 3.69 |
| Mustakone | 33.47 | 17.38 |
| Rotundone | 34.60 | 6.63 |
| Dehydrofukinone | 36.03 | 15.31 |
| Corymbolone | 40.82 | 14.27 |
| Palmitic acid | 42.05 | 11.04 |
| Oleic acid | 47.47 | 18.26 |
| Stearic acid | 48.28 | 4.28 |
| Oxygenated monoterpenes | 3.69 | |
| Oxygenated sesquiterpenes | 53.59 | |
| Fat acids | 33.58 | |
| Others unidentified | 6.41 | |
| Total (%) | 97.27 |
| Constituents | RT | % |
|---|---|---|
| (E)-Pinocarveol | 12.02 | 0.17 |
| Verbenol | 12.29 | 0.42 |
| (1R)-(-)-Myrtenal | 14.22 | 0.11 |
| Myrtenol | 14.44 | 0.27 |
| Verbenone | 14.77 | 0.40 |
| alpha-copaene | 21.64 | 0.13 |
| Cyperene | 22.57 | 0.12 |
| Cyperadiene | 22.86 | 0.71 |
| alpha.-Guaiene | 26.11 | 0.53 |
| trans-Calamenene | 27.62 | 0.17 |
| 9,10-dehydro-Isolongifolene | 28.06 | 0.20 |
| alpha-calacorene | 28.39 | 1.03 |
| Caryophyllene oxide | 29.90 | 2.34 |
| Ledene oxide-(II) | 30.18 | 1.29 |
| Calarene epoxide | 31.18 | 0.25 |
| β-Atlantol | 31.36 | 0.78 |
| Tumerone | 31.65 | 0.93 |
| Valencene | 32.17 | 0.33 |
| Pogostol | 32.68 | 2.30 |
| β-Selinene | 32.79 | 1.71 |
| 9-epi-(E)-Caryophyllene | 32.89 | 1.54 |
| Alloaromadendrene | 33.08 | 0.18 |
| Cedrol | 33.19 | 0.59 |
| Isogermacrene D | 33.29 | 1.09 |
| Mustakone | 33.50 | 11.36 |
| Isoaromadendrene epoxide | 33.75 | 2.94 |
| Cyperotundone | 34.04 | 6.84 |
| Eremophilene | 34.91 | 1.02 |
| (-)-Spathulenol | 35.05 | 0.96 |
| Myristic acid | 35.22 | 1.51 |
| Gurjunene | 36.06 | 9.32 |
| Premnaspirodiene | 36.31 | 0.44 |
| cis-Z-.alpha.-Bisabolene epoxide | 36.41 | 0.52 |
| Anhydro-.beta.-rotunol | 36.80 | 1.91 |
| 2-epi-(E)-β-Caryophyllene | 36.99 | 1.50 |
| Thujopsenal | 37.41 | 0.23 |
| trans-Z-.alpha.-Bisabolene epoxide | 38.03 | 0.65 |
| Cyperadione | 38.90 | 0.39 |
| Corymbolone | 40.84 | 3.94 |
| Palmitic acid | 42.05 | 4.75 |
| Linoleic acid | 47.27 | 2.43 |
| Oleic acid | 47.47 | 6.71 |
| Stearic acid | 48.28 | 1.24 |
| Oxygenated monoterpenes | 1.36 | |
| Sesquiterpene hydrocarbons | 18.99 | |
| Oxygenated sesquiterpenes | 39.23 | |
| Fat acids | 16.63 | |
| Others unidentified | 20.11 | |
| Total (%) | 96.32 |
| Bacterial Strains | Extr EtOH C. articulatus Waste | Extr EtOH C. articulatus Rizhomes | Chlorhexidine |
|---|---|---|---|
| MIC (mg/mL) | MIC (mg/mL) | MIC (mg/mL) | |
| S. mutans ATCC 25175 | >2.0 | 0.29 | 0.004 |
| E. faecalis ATCC 29212 | 1.17 | >2.0 | 0.002 |
| Treatment | II (±SEM) | Irritation Category |
|---|---|---|
| (NaOH) 0.1 mol/L (positive control) | 17.67 (±1.44) **** | Severe irritation |
| NaCl 0.9% (negative control) | 0 (±0.0) | Not labeled |
| Propylene glycol 1% | 0 (±0.0) | Not labeled |
| Extr EtOH C. articulatus rhizomes (4.68 mg/mL) | 0 (±0.0) | Not labeled |
| Extr EtOH C. articulatus waste (4.68 mg/mL) | 0 (±0.0) | Not labeled |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macambira, D.V.d.C.; Almeida Júnior, J.S.d.; Silveira, C.F.d.M.; Sarrazin, S.L.F.; Moraes, T.M.P.; da Silva, B.A.; Minervino, A.H.H.; Moraes, W.P.; Barata, L.E.S. Antimicrobial Activity on Streptococcus mutans and Enterococcus faecalis of Cyperus articulatus Ethanolic Extracts. Plants 2024, 13, 689. https://doi.org/10.3390/plants13050689
Macambira DVdC, Almeida Júnior JSd, Silveira CFdM, Sarrazin SLF, Moraes TMP, da Silva BA, Minervino AHH, Moraes WP, Barata LES. Antimicrobial Activity on Streptococcus mutans and Enterococcus faecalis of Cyperus articulatus Ethanolic Extracts. Plants. 2024; 13(5):689. https://doi.org/10.3390/plants13050689
Chicago/Turabian StyleMacambira, Daniela Vieira de Castro, José Sousa de Almeida Júnior, Claudia Fernandes de Magalhães Silveira, Sandra Layse Ferreira Sarrazin, Tânia Mara Pires Moraes, Bruno Alexandre da Silva, Antonio Humberto Hamad Minervino, Waldiney Pires Moraes, and Lauro Euclides Soares Barata. 2024. "Antimicrobial Activity on Streptococcus mutans and Enterococcus faecalis of Cyperus articulatus Ethanolic Extracts" Plants 13, no. 5: 689. https://doi.org/10.3390/plants13050689
APA StyleMacambira, D. V. d. C., Almeida Júnior, J. S. d., Silveira, C. F. d. M., Sarrazin, S. L. F., Moraes, T. M. P., da Silva, B. A., Minervino, A. H. H., Moraes, W. P., & Barata, L. E. S. (2024). Antimicrobial Activity on Streptococcus mutans and Enterococcus faecalis of Cyperus articulatus Ethanolic Extracts. Plants, 13(5), 689. https://doi.org/10.3390/plants13050689








