Nitrogen and Microelements Co-Drive the Decomposition of Typical Grass Litter in the Loess Plateau, China
Abstract
1. Introduction
2. Results
2.1. Variation in the Mass Remaining Proportion of Different Grass Litter
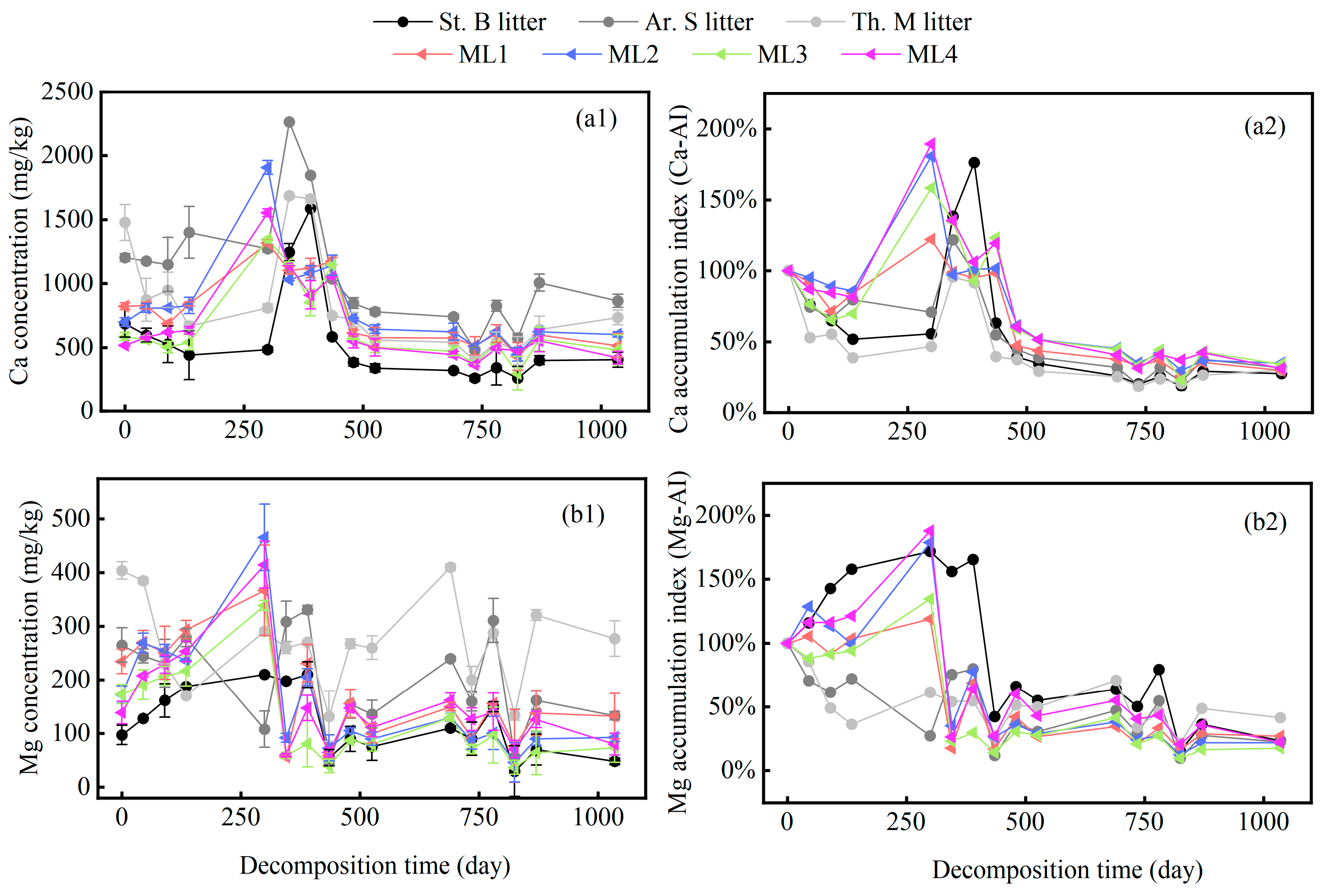
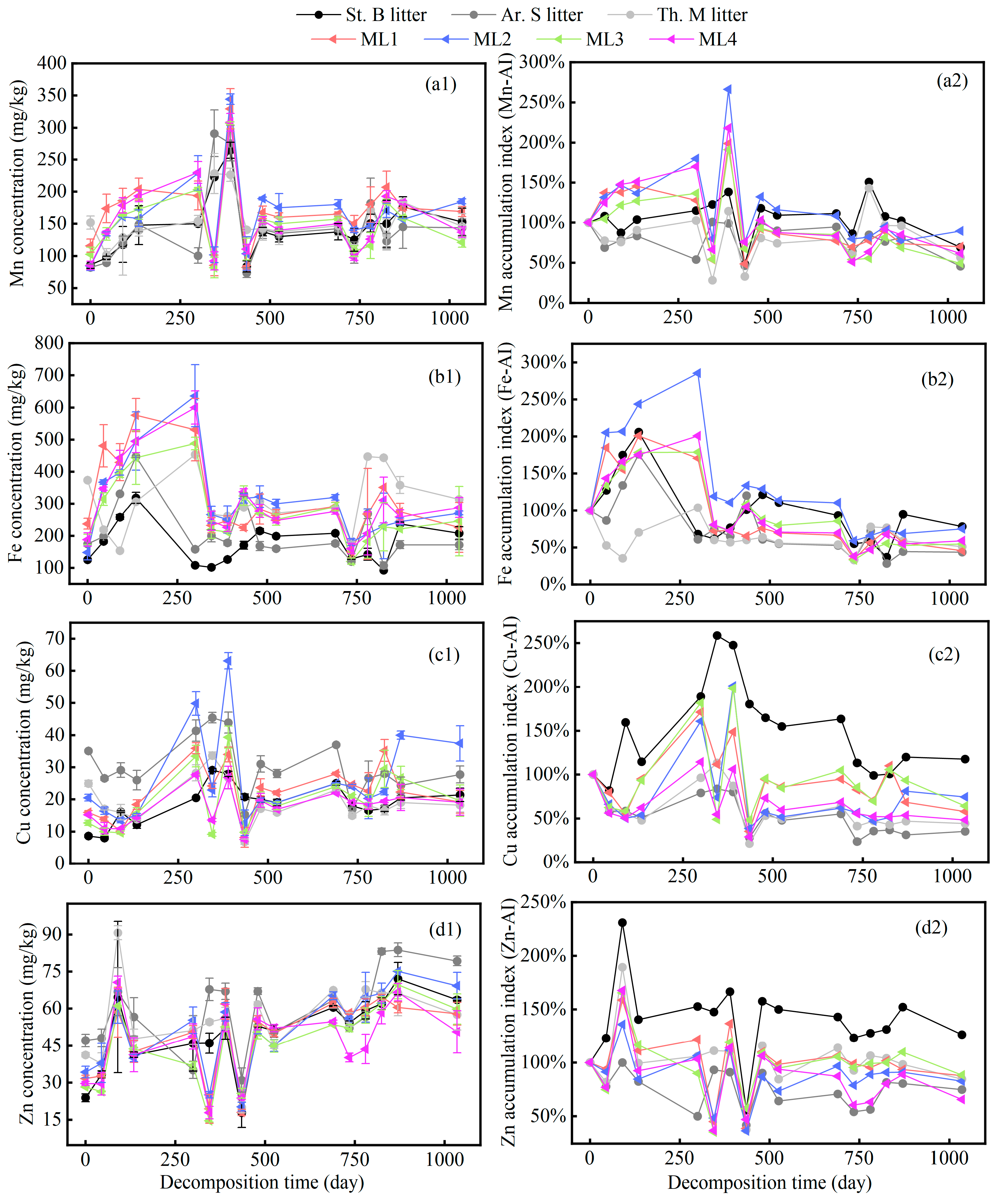
2.2. Variation in Microelements of Different Grass Litters
2.3. Non-Additive Effect of Litter Mixing on the Mass Remaining Rate and Element Accumulation
2.4. Relationship between the Mass Remaining Rate and Litter Chemical Elements
3. Discussion
3.1. Mixing of Different Grass Litters Promotes Decomposition
3.2. Release or Enrichment of Microelements during Grass Litter Decomposition
3.3. Correlation Analysis Reveals Important Factors Regulating Litter Decomposition
4. Materials and Methods
4.1. Study Sites
4.2. Litter Collection and Stimulated Field Decomposition Experiments
4.3. Determination
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krishna, M.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Aerts, R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Berg, B. Decomposing litter; limit values; humus accumulation, locally and regionally. Agric. Ecosyst. Environ. Appl. Soil Ecol. 2018, 123, 494–508. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Xie, F.; Wang, X.; Cheng, G.; Lu, X. Litter chemical structure is more important than species richness in affecting soil carbon and nitrogen dynamics including gas emissions from an alpine soil. Biol. Fertil. Soils 2015, 51, 791–800. [Google Scholar] [CrossRef]
- Setiawan, N.N.; Vanhellemont, M.; De Schrijver, A.; Schelfhout, S.; Baeten, L.; Verheyen, K. Mixing effects on litter decomposition rates in a young tree diversity experiment. Acta Oecologica 2016, 70, 79–86. [Google Scholar] [CrossRef]
- Gartner, T.B.; Cardon, Z.G. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Cuchietti, A.; Marcotti, E.; Gurvich, D.E.; Cingolani, A.M.; Pérez Harguindeguy, N. Leaf litter mixtures and neighbour effects: Low-nitrogen and high-lignin species increase decomposition rate of high-nitrogen and low-lignin neighbours. Agric. Ecosyst. Environ. Appl. Soil Ecol. 2014, 82, 44–51. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; West, J.B.; Hobbie, S.E.; Reich, P.B. Antagonistic effects of species on C respiration and net N mineralization in soils from mixed coniferous plantations. For. Ecol. Manag. 2009, 257, 1112–1118. [Google Scholar] [CrossRef]
- Butenschoen, O.; Krashevska, V.; Maraun, M.; Marian, F.; Sandmann, D.; Scheu, S. Litter mixture effects on decomposition in tropical montane rainforests vary strongly with time and turn negative at later stages of decay. Soil Biol. Biochem. 2014, 77, 121–128. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Zhang, N.; Ma, K. The research of mixed litter effects on litter decomposition in terrestrial ecosystems. Acta Ecol. Sin. 2016, 36, 4977–4987. [Google Scholar]
- Ward, S.E.; Ostle, N.J.; McNamara, N.P.; Bardgett, R.D. Litter evenness influences short-term peatland decomposition processes. Oecologia 2010, 164, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Kajino, H.; Kawai, K.; Nakai, W.; Ohnuki, M.; Okada, N. Diverse recalcitrant substrates slow down decomposition of leaf litter from trees in a serpentine ecosystem. Plant Soil 2019, 442, 247–255. [Google Scholar] [CrossRef]
- Liu, P.; Sun, O.J.; Huang, J.; Li, L.; Han, X. Nonadditive effects of litter mixtures on decomposition and correlation with initial litter N and P concentrations in grassland plant species of northern China. Biol. Fertil. Soils 2007, 44, 211–216. [Google Scholar] [CrossRef]
- Zhao, G.F.; Cai, Y.B.; Luo, Y.Y.; Li, M.H.; Yu, M.J. Nutrient dynamics in litter decomposition in an evergreen broad-leaved forest in East China. Acta Ecol. Sin. 2006, 26, 3286–3295. [Google Scholar]
- Wardle, D.A.; Nilsson, M.C.; Zackrisson, O.; Gallet, C. Determinants of litter mixing effects in a Swedish boreal forest. Soil Biol. Biochem. 2003, 35, 827–835. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Wang, J.; You, Y.; Yang, Y.; Shi, Z.; Huang, X.; Zheng, L.; Li, Z.; Ming, A.; et al. Mixed-species plantation with Pinus massoniana and Castanopsis hystrix accelerates C loss in recalcitrant coniferous litter but slows C loss in labile broadleaf litter in southern China. For. Ecol. Manag. 2018, 422, 207–213. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Chen, Y.; Xu, Z.; Zhang, J.; Liu, Y.; Joly, F.X. Litter diversity accelerates labile carbon but slows recalcitrant carbon decomposition. Soil Biol. Biochem. 2022, 168, 108632. [Google Scholar] [CrossRef]
- Grossman, J.J.; Cavender-Bares, J.; Hobbie, S.E. Functional diversity of leaf litter mixtures slows decomposition of labile but not recalcitrant carbon over two years. Ecol. Monogr. 2020, 90, e01407. [Google Scholar] [CrossRef]
- Einhellig, F.A.; Rasmussen, J.A.; Hejl, A.M.; Souza, I.F. Effects of root exudate sorgoleone on photosynthesis. J. Chem. Ecol. 1993, 19, 369–375. [Google Scholar] [CrossRef]
- Magiatis, P.; Melliou, E.; Skaltsounis, A.L.; Chinou, I.B.; Mitaku, S. Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. chia. Planta Med. 1999, 65, 749–752. [Google Scholar] [CrossRef]
- Maisto, G.; De Marco, A.; Meola, A.; Sessa, L.; Virzo De Santo, A. Nutrient dynamics in litter mixtures of four Mediterranean maquis species decomposing in situ. Soil Biol. Biochem. 2011, 43, 520–530. [Google Scholar] [CrossRef]
- Prescott, C.; Zabek, L.; Staley, C.; Kabzems, R. Decomposition of broadleaf and needle litter in forests of British Columbia: Influences of litter type, forest type, and litter mixtures. Can. J. For. Res. 2000, 30, 1742–1750. [Google Scholar] [CrossRef]
- Garcia-Palacios, P.; McKie, B.G.; Handa, I.T.; Frainer, A.; Haettenschwiler, S. The importance of litter traits and decomposers for litter decomposition: A comparison of aquatic and terrestrial ecosystems within and across biomes. Funct. Ecol. 2016, 30, 819–829. [Google Scholar] [CrossRef]
- Zhang, D.; Hui, D.; Luo, Y.; Zhou, G. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef]
- Berg, B. Decomposition patterns for foliar litter—A theory for influencing factors. Soil Biol. Biochem. 2014, 78, 222–232. [Google Scholar] [CrossRef]
- Waring, B.G. A Meta-analysis of Climatic and Chemical Controls on Leaf Litter Decay Rates in Tropical Forests. Ecosystems 2012, 15, 999–1009. [Google Scholar] [CrossRef]
- Berg, B.; Steffen, K.T.; McClaugherty, C. Litter decomposition rate is dependent on litter Mn concentrations. Biogeochemistry 2007, 82, 29–39. [Google Scholar] [CrossRef]
- Dai, G.; Zhu, S.; Cai, Y.; Zhu, E.; Jia, Y.; Ji, C.; Tang, Z.; Fang, J.; Feng, X. Plant-derived lipids play a crucial role in forest soil carbon accumulation. Soil Biol. Biochem. 2022, 168, 108645. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Laskowski, R.; Berg, B. Dynamics of some mineral nutrients and heavy metals in decomposing forest litter. Scand. J. For. Res. 1993, 8, 446–456. [Google Scholar] [CrossRef]
- Walela, C.; Daniel, H.; Wilson, B.; Lockwood, P.; Cowie, A.; Harden, S. The initial lignin: Nitrogen ratio of litter from above and below ground sources strongly and negatively influenced decay rates of slowly decomposing litter carbon pools. Soil Biol. Biochem. 2014, 77, 268–275. [Google Scholar] [CrossRef]
- Santonja, M.; Rancon, A.; Fromin, N.; Baldy, V.; Hättenschwiler, S.; Fernandez, C.; Montès, N.; Mirleau, P. Plant litter diversity increases microbial abundance, fungal diversity, and carbon and nitrogen cycling in a Mediterranean shrubland. Soil Biol. Biochem. 2017, 111, 124–134. [Google Scholar] [CrossRef]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S.; et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Raczka, N.C.; Piñeiro, J.; Tfaily, M.M.; Chu, R.K.; Lipton, M.S.; Pasa-Tolic, L.; Morrissey, E.; Brzostek, E. Interactions between microbial diversity and substrate chemistry determine the fate of carbon in soil. Sci. Rep. 2021, 11, 19320. [Google Scholar] [CrossRef]
- Xu, X.; Shibata, H.; Enoki, T. Decomposition patterns of leaf litter of seven common canopy species in a subtropical forest: Dynamics of mineral nutrients. J. For. Res. 2006, 17, 1–6. [Google Scholar] [CrossRef]
- Kalburtji, K.L.; Mosjidis, J.A.; Mamolos, A.P. Litter dynamics of low and high tannin sericea lespedeza plants under field conditions. Plant Soil 1999, 208, 271–281. [Google Scholar] [CrossRef]
- Laskowski, R.; Niklinska, M.; Maryanski, M. The dynamics of chemical elements in forest litter. Ecology 1995, 76, 1393–1406. [Google Scholar] [CrossRef]
- Chen, B.M.; Peng, S.L.; D’Antonio, C.M.; Li, D.J.; Ren, W.T. Non-additive effects on decomposition from mixing litter of the invasive Mikania micrantha HBK with native plants. PLoS ONE 2013, 8, e66289. [Google Scholar]
- Wu, D.; Li, T.; Wan, S. Time and litter species composition affect litter-mixing effects on decomposition rates. Plant Soil 2013, 371, 355–366. [Google Scholar] [CrossRef]
- De Santo, A.V.; Fierro, A.; Berg, B.; Rutigliano, F.A.; De Marco, A. Heavy metals and litter decomposition in coniferous forests. Dev. Soil Sci. 2002, 28, 63–78. [Google Scholar]
- Hall, S.J.; Silver, W.L.; Timokhin, V.I.; Hammel, K.E. Iron addition to soil specifically stabilized lignin. Soil Biol. Biochem. 2016, 98, 95–98. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Y.; Gao, L.; Cui, X. A review of the factors influencing soil organic carbon stability. Chin. J. Eco-Agric. 2018, 26, 222–230. [Google Scholar]
- Wang, X.; Yang, X.; Yang, N.; Xin, X.; Qu, Y.; Zhao, N.; Gao, Y. Effects of litter diversity and composition on litter decomposition characteristics and soil microbial community. Acta Ecol. Sin. 2019, 39, 6264–6272. [Google Scholar]
- Eriksson, K.-E.; Blanchette, R.A.; Ander, P. Microbial and Enzymatic Degradation of Wood and Wood Components, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1990; 416p. [Google Scholar]
- Hattaka, A. Biodegradation of lignin. In Biopolymers. Biology, Chemistry, Biotechnology, Applications. Vol 1. Lignin, Humic Substances and Coal; Wiley-VCH: Weinheim, Germany, 2001; Volume 1. [Google Scholar]
- Berg, B.; Kjønaas, O.J.; Johansson, M.B.; Erhagen, B.; Åkerblom, S. Late stage pine litter decomposition: Relationship to litter N, Mn, and acid unhydrolyzable residue (AUR) concentrations and climatic factors. For. Ecol. Manag. 2015, 358, 41–47. [Google Scholar] [CrossRef]
- Guggenberger, G.J. Acidification effects on dissolved organic matter mobility in spruce forest ecosystems. Environ. Int. 1994, 20, 31–41. [Google Scholar] [CrossRef]
- Long, C.; Yang, F.; Chen, Q.; Zhang, Q.; Cheng, X. Different dynamics and controls of enzyme activities of leaf and root litter during decomposition. Funct. Ecol. 2024, 38, 586–599. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Zhang, W.; Zhang, Q.; Lu, D.; Zhang, Y.; Zheng, X.; Xu, S.; Wang, G. Litter decomposition and nutrient release from monospecific and mixed litters: Comparisons of litter quality, fauna and decomposition site effects. J. Ecol. 2022, 110, 1673–1686. [Google Scholar] [CrossRef]
- Voříšková, J.; Baldrian, P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 2013, 7, 477–486. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2022. [Google Scholar]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
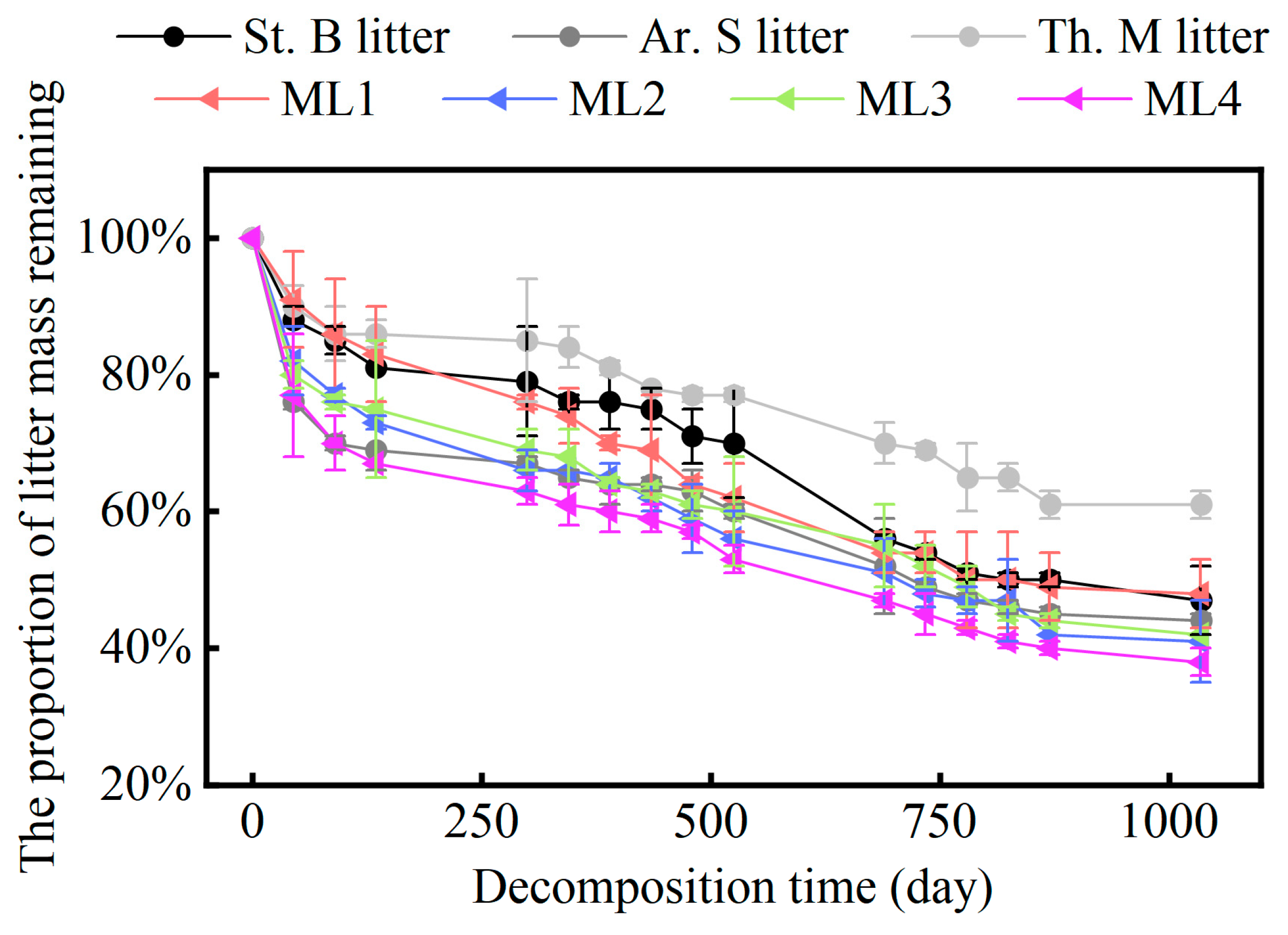

| Litter Groups | Regression Equation | The Decomposition Constant (k) | R2 | p |
|---|---|---|---|---|
| St. B | = 0.9506 × e−0.0209t | 0.0209 | 0.9512 | <0.0001 |
| Ar. S | = 0.8363 × e−0.0207t | 0.0207 | 0.8576 | <0.0001 |
| Th. M | = 0.9469 × e−0.0133t | 0.0133 | 0.9437 | <0.0001 |
| ML1 | = 0.9489 × e−0.0229t | 0.0229 | 0.9813 | <0.0001 |
| ML2 | = 0.8772 × e−0.0242t | 0.0242 | 0.9391 | <0.0001 |
| ML3 | = 0.8747 × e−0.0224t | 0.0224 | 0.9644 | <0.0001 |
| ML4 | = 0.8367 × e−0.0254t | 0.0254 | 0.8956 | <0.0001 |
| Time | Mass Remaining Proportion | Ca−AI | Mg−AI | Fe−AI | Mn−AI | Cu−AI | Zn−AI | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (d) | ML1 | ML2 | ML3 | ML4 | ML1 | ML2 | ML3 | ML4 | ML1 | ML2 | ML3 | ML4 | ML1 | ML2 | ML3 | ML4 | ML1 | ML2 | ML3 | ML4 | ML1 | ML2 | ML3 | ML4 | ML1 | ML2 | ML3 | ML4 |
| 0 | 2.79 | −0.25 | −9.58 | −12.34 | 39.57 | 26.26 | 9.14 | 23.79 | 2.89 | 34.65 | −18.76 | 7.29 | 97.13 | 88.28 | 23.23 | 31.81 | 68.26 | 42.88 | 16.98 | 39.78 | 9.44 | −6.91 | −18.40 | −26.94 | −9.82 | −11.08 | −33.59 | −31.62 |
| 45 | 0.35 | −2.30 | −10.52 | −17.64 | 17.93 | 34.72 | 4.45 | 34.80 | −9.01 | 6.75 | −23.45 | −2.68 | 38.87 | 32.20 | 14.43 | 18.71 | 42.45 | 31.85 | 15.13 | 39.73 | −51.31 | −55.87 | −56.50 | −62.21 | −25.24 | −21.15 | −24.96 | −24.00 |
| 90 | −0.72 | −3.92 | −8.51 | −19.23 | 82.92 | 33.25 | 43.22 | 67.47 | 0.28 | −16.69 | −26.21 | −4.85 | 38.52 | 26.59 | 3.72 | 2.04 | 29.86 | 2.92 | 2.29 | 21.59 | 12.61 | −37.24 | −4.69 | −36.61 | −8.95 | −26.11 | −10.39 | −28.91 |
| 135 | −7.14 | −10.28 | −14.78 | −22.11 | 136.22 | 188.44 | 196.31 | 254.28 | −2.59 | 67.41 | −6.70 | 30.23 | 102.69 | 340.00 | 132.39 | 161.13 | 10.93 | 59.03 | 9.03 | 35.56 | 16.31 | 15.25 | 9.55 | −31.00 | −7.68 | 0.31 | −36.04 | −26.63 |
| 300 | −7.63 | −7.09 | −13.11 | −21.57 | −17.02 | −25.61 | 5.42 | 6.14 | −83.93 | −70.56 | −82.29 | −79.73 | 18.36 | 76.69 | 29.27 | 31.23 | 9.77 | 11.69 | 4.01 | 17.15 | −42.18 | −59.00 | −77.96 | −75.41 | −65.59 | −60.70 | −74.34 | −73.32 |
| 345 | −11.21 | −7.77 | −17.61 | −22.40 | −31.16 | −28.35 | −39.99 | −31.50 | −40.55 | −38.85 | −78.39 | −53.52 | 7.22 | 55.07 | 0.69 | 1.15 | 8.25 | 18.25 | −7.29 | 5.77 | −15.39 | 16.57 | −4.56 | −48.90 | −3.49 | −15.77 | −21.89 | −25.25 |
| 390 | −9.65 | −10.75 | −16.89 | −22.46 | 86.64 | 70.72 | 114.44 | 107.78 | −50.76 | −7.72 | −62.58 | −28.03 | −21.02 | 21.75 | 19.48 | 15.63 | −32.57 | 16.60 | −6.64 | 5.01 | −67.62 | −65.44 | −65.61 | −79.38 | −18.11 | −25.85 | 11.37 | −8.28 |
| 435 | −13.90 | −12.65 | −15.96 | −22.06 | 22.67 | 46.97 | 51.51 | 54.50 | −28.93 | −32.04 | −50.62 | −3.33 | −20.43 | 36.84 | −18.06 | −21.97 | −3.27 | 18.76 | −9.77 | −0.13 | −17.65 | −50.99 | −30.39 | −46.60 | −20.48 | −31.68 | −25.71 | −27.56 |
| 480 | −14.92 | −14.65 | −16.88 | −25.78 | 35.77 | 41.13 | 54.08 | 55.16 | −49.55 | −33.85 | −48.70 | −19.88 | −19.24 | 32.78 | −17.88 | −27.25 | −3.91 | 13.20 | −9.82 | −9.48 | −19.96 | −51.47 | −34.04 | −53.62 | −18.18 | −34.12 | −28.88 | −29.76 |
| 525 | −13.28 | −6.69 | −7.04 | −20.75 | 45.95 | 56.69 | 72.16 | 56.66 | −48.50 | −32.16 | −36.73 | −16.03 | −11.78 | 47.03 | 2.87 | −16.54 | −3.41 | 20.25 | 1.04 | −1.21 | −20.26 | −46.03 | −24.44 | −50.77 | −18.15 | −12.34 | −22.28 | −35.58 |
| 690 | −11.46 | −6.82 | −10.68 | −22.84 | 70.47 | 74.74 | 65.41 | 57.62 | −51.46 | −40.75 | −54.60 | −12.20 | −24.86 | 29.39 | −33.49 | −23.40 | 6.84 | 12.25 | −23.97 | −28.26 | 2.38 | −22.73 | −10.38 | −41.60 | −9.65 | −14.39 | −17.51 | −47.84 |
| 735 | −12.85 | −4.12 | −10.30 | −21.53 | 49.01 | 47.62 | 77.18 | 63.43 | −48.39 | −59.64 | −61.62 | −38.91 | −15.44 | 2.81 | −24.25 | −24.90 | −5.66 | −14.52 | −35.25 | −25.76 | −6.62 | −33.73 | −18.94 | −39.46 | −19.05 | −6.73 | −18.72 | −48.39 |
| 780 | −12.36 | −3.23 | −15.77 | −24.64 | 31.63 | 46.87 | 19.18 | 92.60 | −11.13 | −3.10 | −43.77 | 21.49 | 34.15 | 121.10 | 17.58 | 43.53 | 20.82 | 19.70 | 2.19 | 15.20 | 47.22 | −28.92 | 22.44 | −39.85 | −14.41 | −16.64 | −19.40 | −35.53 |
| 825 | −11.47 | −13.34 | −16.19 | −25.39 | 26.80 | 11.84 | 50.24 | 48.27 | −31.42 | −32.90 | −58.40 | −8.99 | −27.84 | −4.76 | −38.73 | −36.31 | −17.94 | −16.31 | −27.85 | −11.24 | −21.32 | 1.25 | −8.12 | −47.38 | −26.99 | −23.90 | −20.75 | −35.80 |
| 870 | −9.97 | −10.94 | −17.79 | −24.05 | 4.08 | 18.60 | 20.47 | 10.39 | −14.45 | −4.05 | −37.14 | −20.68 | −30.73 | 19.90 | −23.71 | −17.39 | −3.28 | 10.53 | −36.53 | −20.29 | −31.74 | −7.21 | −35.35 | −51.37 | −18.34 | −19.68 | −23.71 | −43.35 |
| 1035 | 2.79 | −0.25 | −9.58 | −12.34 | 39.57 | 26.26 | 9.14 | 23.79 | 2.89 | 34.65 | −18.76 | 7.29 | 97.13 | 88.28 | 23.23 | 31.81 | 68.26 | 42.88 | 16.98 | 39.78 | 9.44 | −6.91 | −18.40 | −26.94 | −9.82 | −11.08 | −33.59 | −31.62 |
| Correlations | TN | Lignin | Ca | Mg | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|
| Mass remaining rate | −0.778 * | 0.756 * | 0.674 | 0.790 * | 0.821 * | 0.873 * | 0.229 | 0.349 |
| Variables | TN | Lignin | Ca | Mg | Fe | Mn | Zn | Cu | Constant | p | R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MRsingle (0–525) | / | / | −0.011 (100%) | / | / | / | / | / | 0.910 | 0.002 | 0.299 |
| MRsingle (690–1035) | −0.018 (53.07%) | / | / | / | / | −0.001 (23.84%) | / | 0.007 (23.09%) | 0.951 | 0.027 | 0.807 |
| MRmixed (0–525) | / | / | −0.016 (18.37%) | 0.126 (43.39%) | / | −0.002 (38.24%) | / | / | 1.001 | 0.000 | 0.700 |
| MRmixed (0–525) | −0.010 (100%) | / | / | / | / | / | / | 0.637 | 0.016 | 0.238 |
| Treatments | Litter Composition (50 g) |
|---|---|
| St. B | St. B litter (100%) |
| Ar. S | Ar. S litter (100%) |
| Th. M | Th. M litter (100%) |
| ML1 | St. B litter (55%) + Th. M litter (45%) |
| ML2 | St. B litter (55%) + Ar. S litter (45%) |
| ML3 | St. B litter (75%) + Th. M litter (25%) |
| ML4 | St. B litter (75%) + Ar. S litter (25%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Y.; Chen, H.; Feng, W.; Wen, Y.; Xie, Y.; Cheng, M.; Li, H. Nitrogen and Microelements Co-Drive the Decomposition of Typical Grass Litter in the Loess Plateau, China. Plants 2024, 13, 753. https://doi.org/10.3390/plants13060753
Xiang Y, Chen H, Feng W, Wen Y, Xie Y, Cheng M, Li H. Nitrogen and Microelements Co-Drive the Decomposition of Typical Grass Litter in the Loess Plateau, China. Plants. 2024; 13(6):753. https://doi.org/10.3390/plants13060753
Chicago/Turabian StyleXiang, Yun, Haoning Chen, Weiqi Feng, Yongli Wen, Ying Xie, Man Cheng, and Hua Li. 2024. "Nitrogen and Microelements Co-Drive the Decomposition of Typical Grass Litter in the Loess Plateau, China" Plants 13, no. 6: 753. https://doi.org/10.3390/plants13060753
APA StyleXiang, Y., Chen, H., Feng, W., Wen, Y., Xie, Y., Cheng, M., & Li, H. (2024). Nitrogen and Microelements Co-Drive the Decomposition of Typical Grass Litter in the Loess Plateau, China. Plants, 13(6), 753. https://doi.org/10.3390/plants13060753






