Responses of Three Pedicularis Species to Geological and Climatic Changes in the Qinling Mountains and Adjacent Areas in East Asia
Abstract
1. Introduction
2. Results
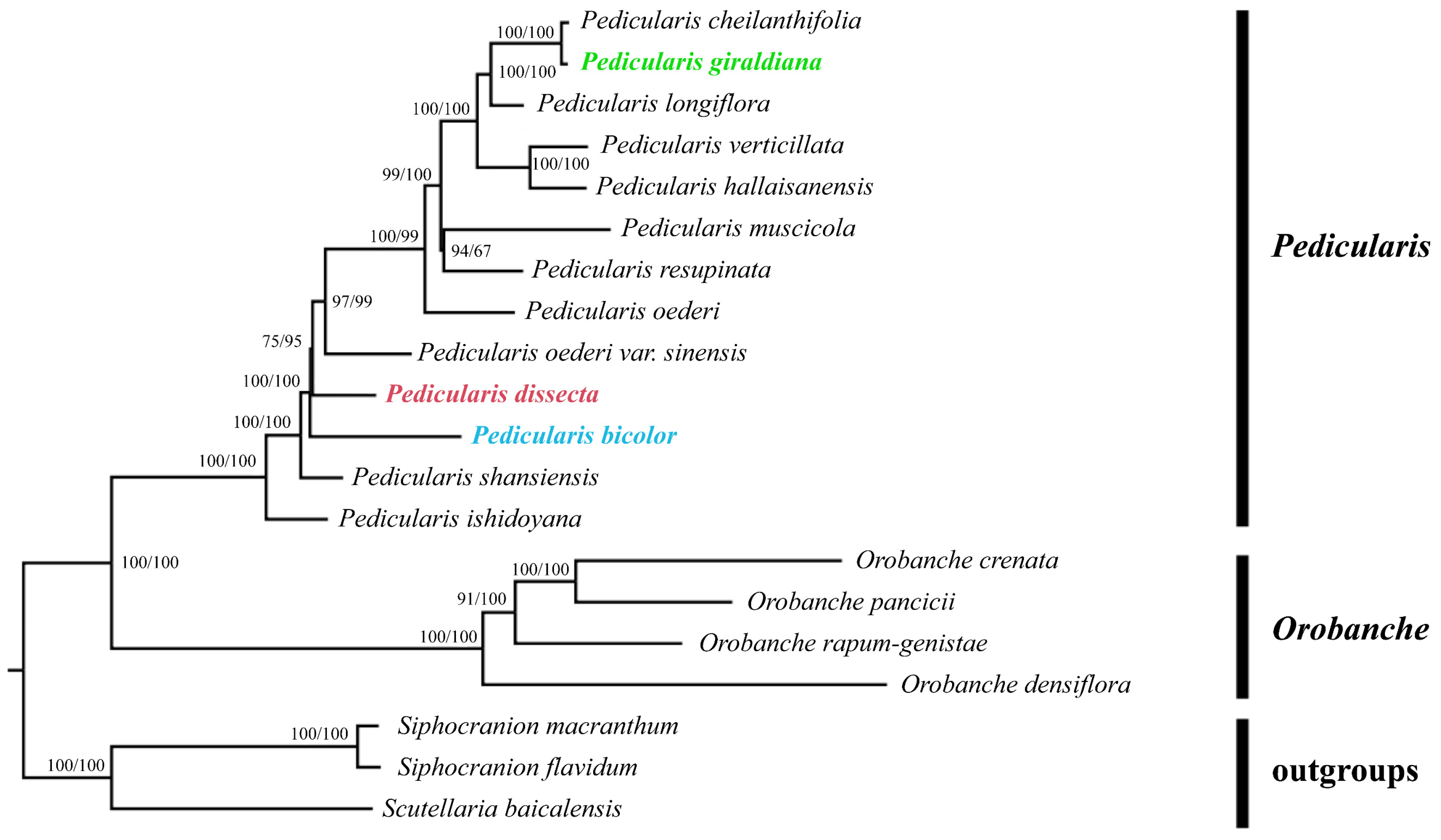
2.1. Phylogenetic Relationship
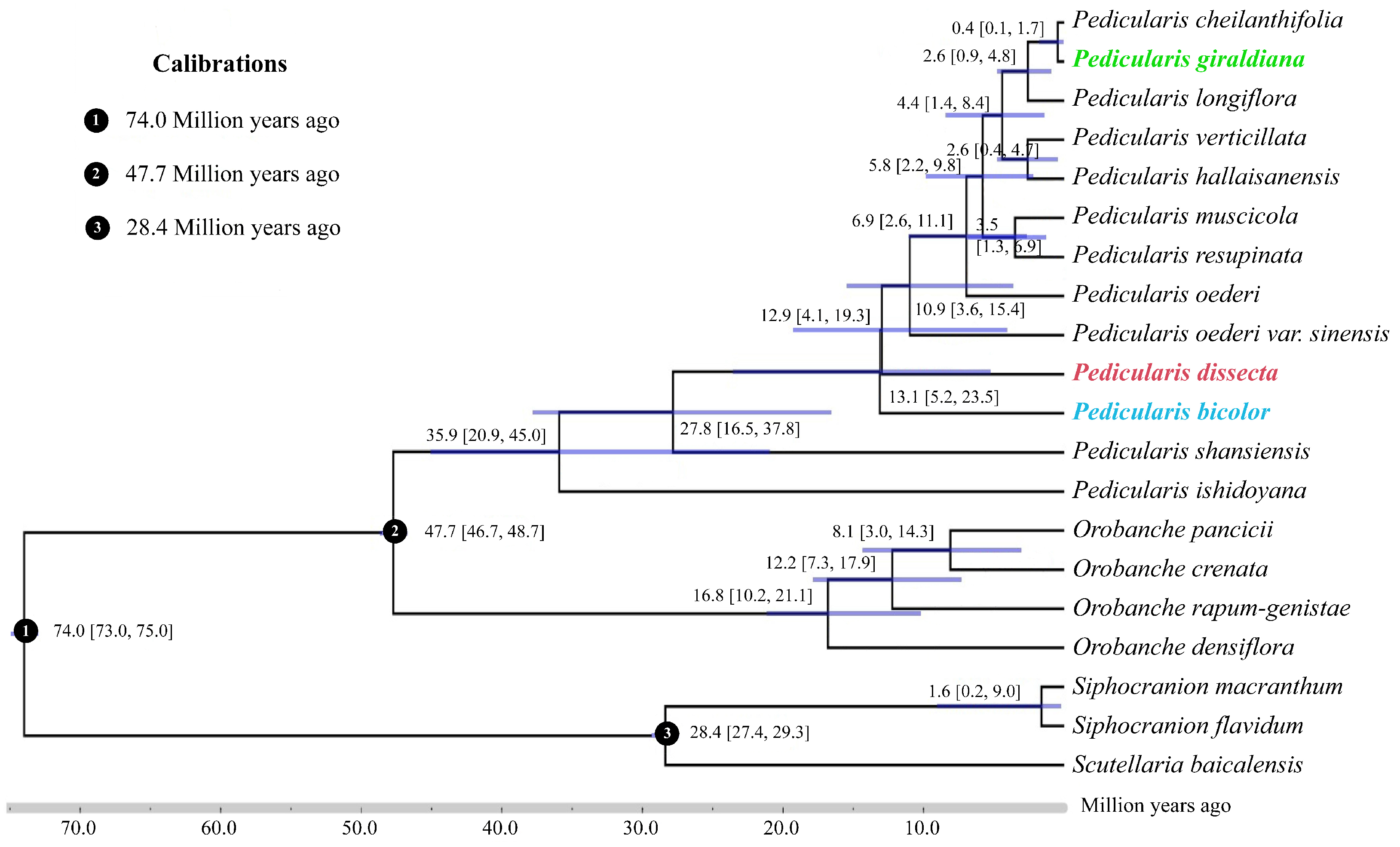
2.2. Divergence Time Estimation
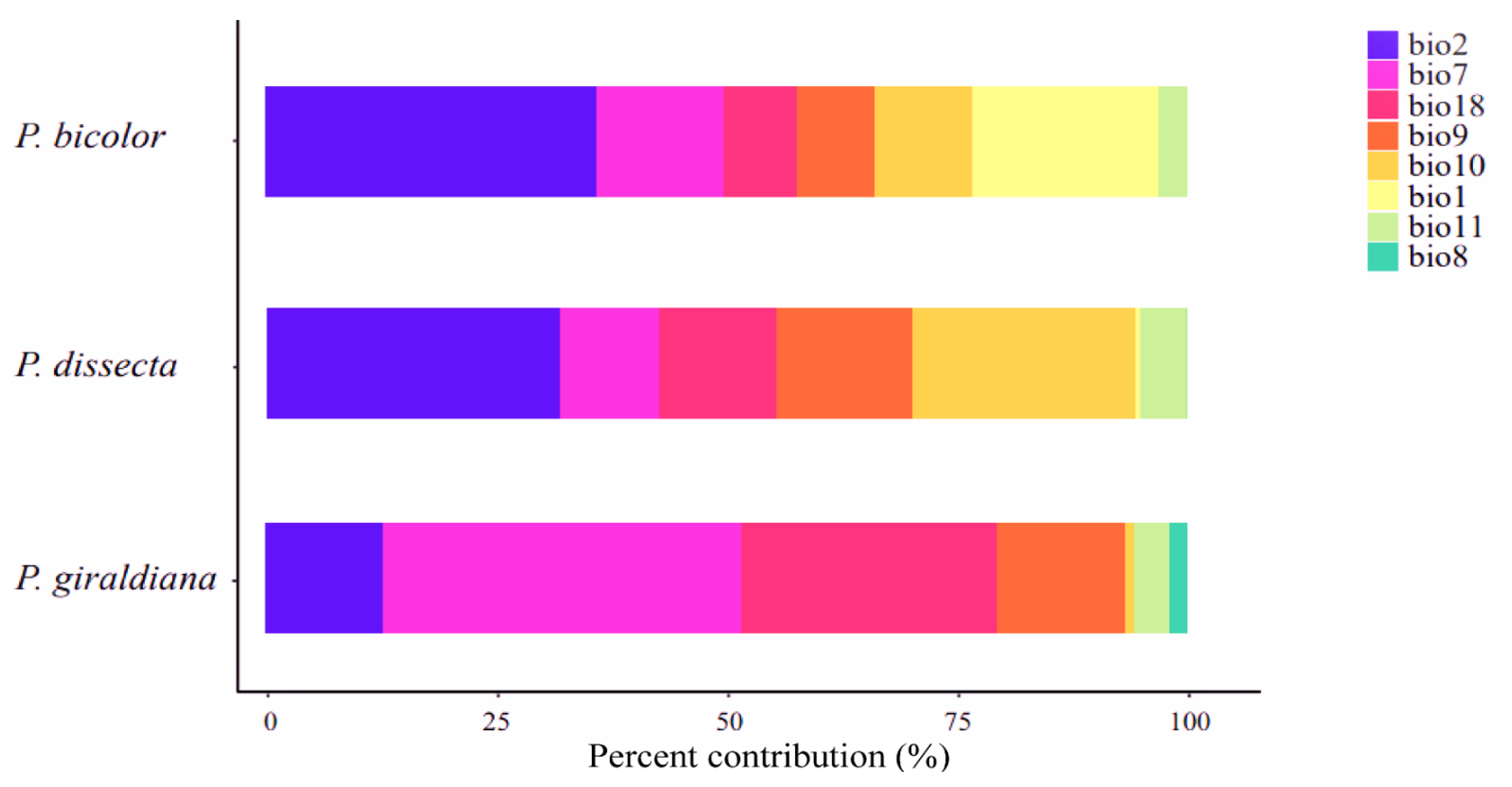
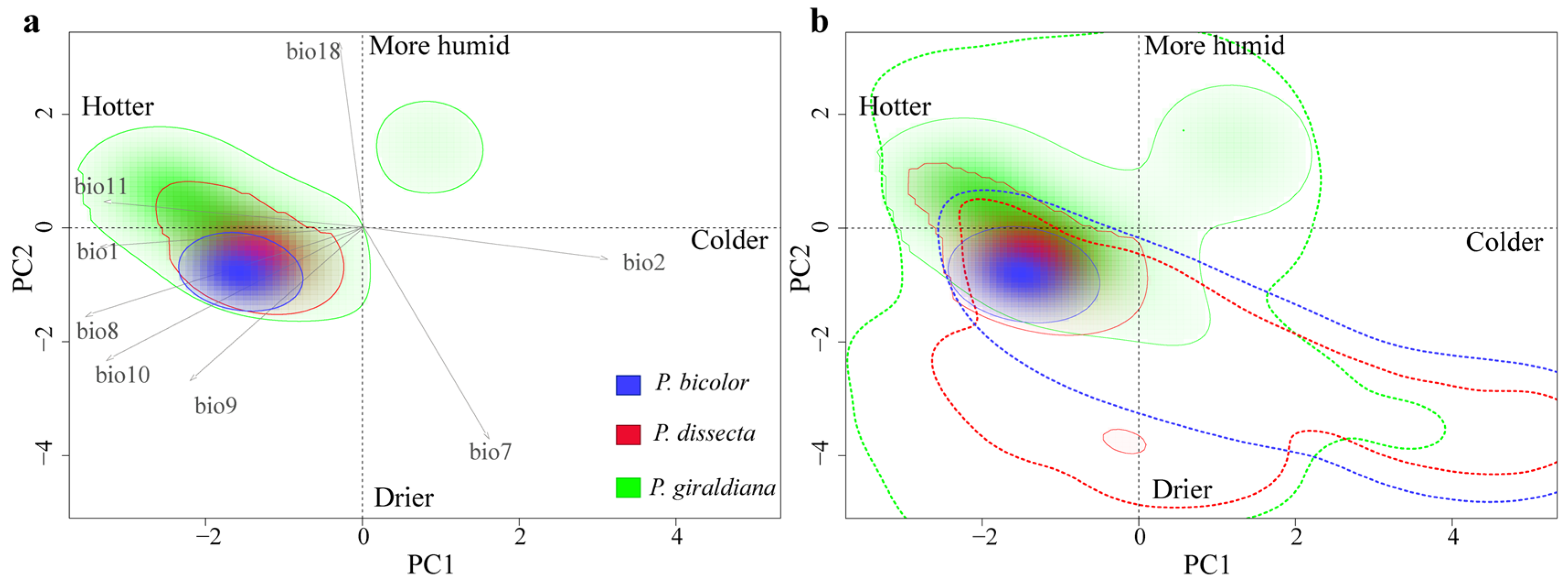
2.3. Environmental Variables Analysis
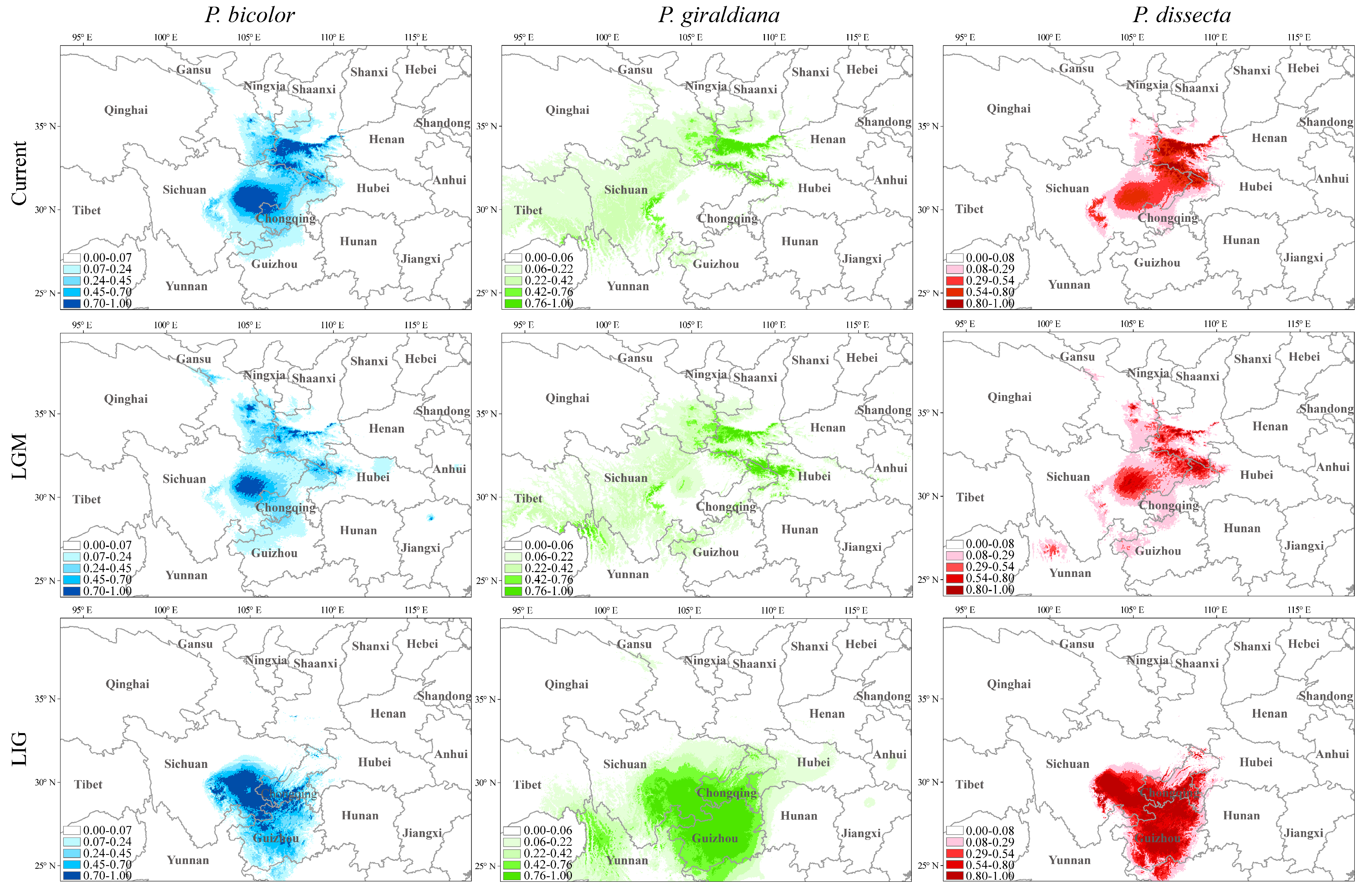
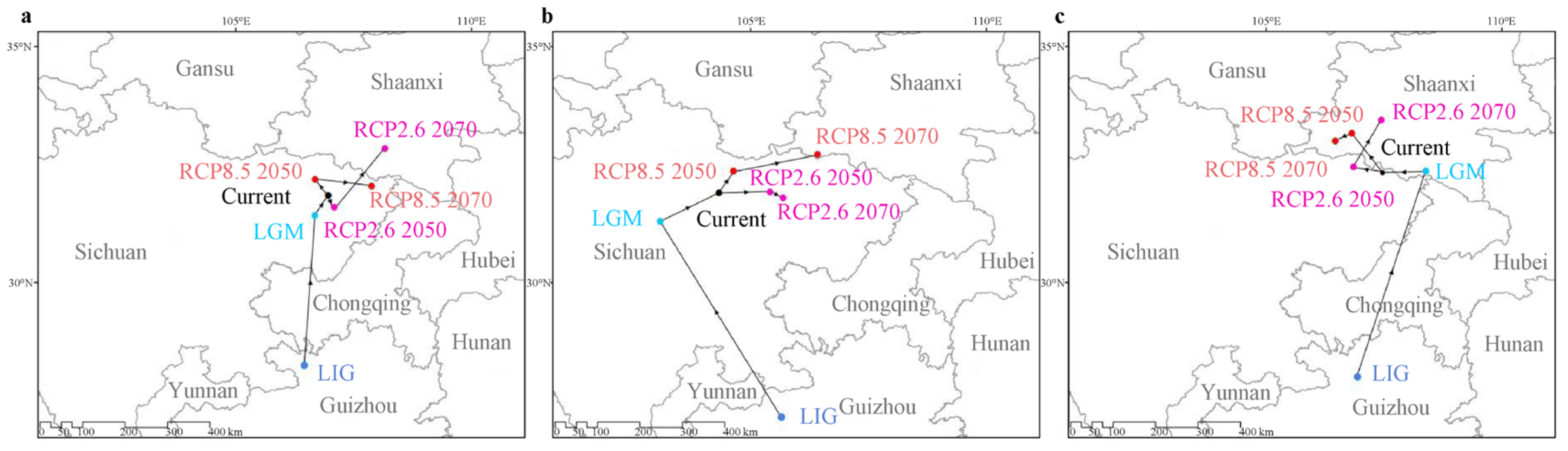
2.4. Dynamic Changes in Geographic Distribution from the Past to the Future
2.5. Migration Trends Based on the Centroid of Suitable Habitats
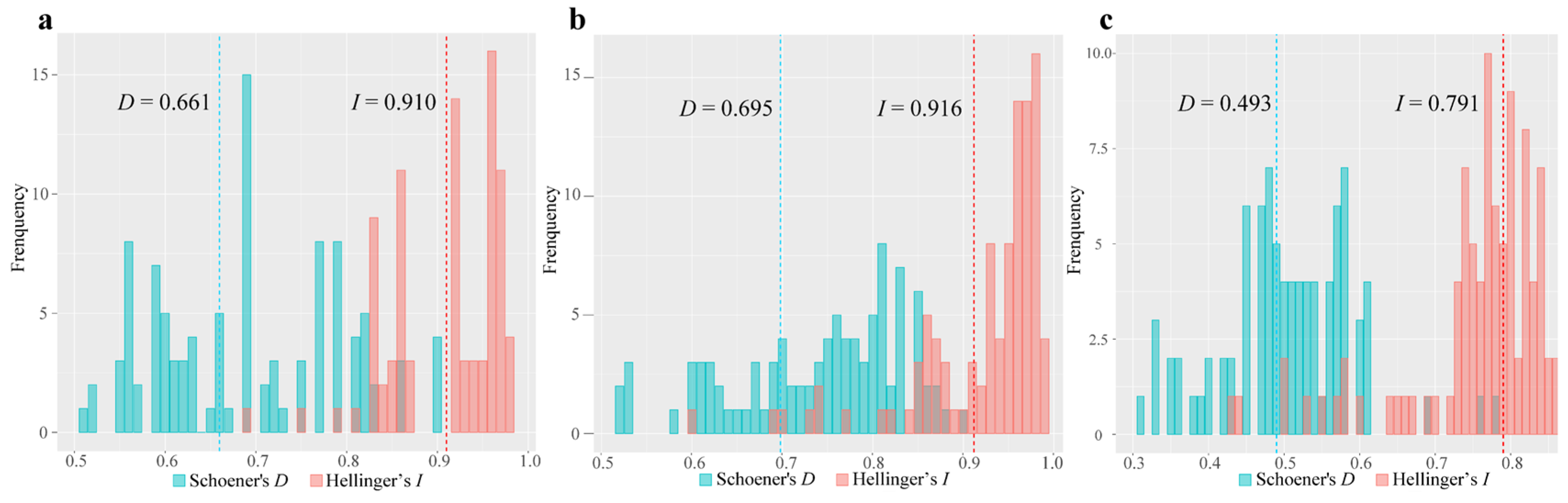
2.6. Niche Analysis
3. Discussion
3.1. Evolutionary Relationships
3.2. Species Divergence
3.3. Population Dynamics Changes
3.4. Climatic Niche Comparisons
4. Materials and Methods
4.1. Plant Materials, DNA Extraction, and Genome Assembly
4.2. Phylogenetic Analysis and Divergence Time Estimation
4.3. Distribution Data Collection
4.4. Ecological Niche Modeling
4.5. Niche Comparison Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hewitt, G.M. Genetic consequences of climatic oscillations in the Quaternary. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 183–195. [Google Scholar] [CrossRef]
- Davis, M.B.; Shaw, R.G. Range shifts and adaptive responses to Quaternary climate change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Qiu, Y.X.; Liu, Y.H.; Qi, X.S.; Kim, S.H.; Han, J.; Takeuchi, Y.; Worth, J.R.P.; Yamasaki, M.; Sakurai, S.; et al. Climate oscillation during the Quaternary associated with landscape heterogeneity promoted allopatric lineage divergence of a temperate tree Kalopanax septemlobus (Araliaceae) in East Asia. Mol. Ecol. 2012, 21, 3823–3838. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.H.; Li, S.; Qian, Z.Q.; Su, H.L.; Huang, Z.H.; Guo, Z.G.; Dai, P.F.; Liu, Z.L.; Zhao, G.F. Strong phylogeographic pattern of cpDNA variation reveals multiple glacial refugia for Saruma henryi Oliv. (Aristolochiaceae), an endangered herb endemic to China. Mol. Phylogenet. Evol. 2010, 57, 176–188. [Google Scholar] [CrossRef]
- Xu, X.X.; Cheng, F.Y.; Peng, L.P.; Sun, Y.Q.; Hu, X.G.; Li, S.Y.; Xian, H.L.; Jia, K.H.; Abbott, R.J.; Mao, J.F. Late Pleistocene speciation of three closely related tree peonies endemic to the Qinling–Daba Mountains, a major glacial refugium in Central China. Ecol. Evol. 2019, 9, 7528–7548. [Google Scholar] [CrossRef]
- Liu, J.; Moller, M.; Provan, J.; Cao, L.M.; Poudel, R.C.; Li, D.Z. Geological and ecological factors drive cryptic speciation of yews in a biodiversity hotspot. New Phytol. 2013, 199, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Q.; Matsui, T.; Ohashi, H.; Dong, Y.F.; Momohara, A.; Herrando-Moraira, S.; Qian, S.; Yang, Y.; Ohsawa, M.; Luu, H.T.; et al. Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun. 2018, 9, 4488. [Google Scholar] [CrossRef]
- Xu, J.; Deng, M.; Jiang, X.L.; Westwood, M.; Song, Y.G.; Turkington, R. Phylogeography of Quercus glauca (Fagaceae), a dominant tree of East Asian subtropical evergreen forests, based on three chloroplast DNA interspace sequences. Tree Genet. Genomes 2014, 11, 805. [Google Scholar] [CrossRef]
- Rana, H.K.; Rana, S.K.; Luo, D.; Sun, H. Existence of biogeographic barriers for the long-term Neogene–Quaternary divergence and differentiation of Koenigia forrestii in the Himalaya–Hengduan Mountains. Bot. J. Linn. Soc. 2023, 201, 230–253. [Google Scholar] [CrossRef]
- Guan, B.C.; Chen, W.; Gong, X.; Wu, T.; Cai, Q.Y.; Liu, Y.Z.; Ge, G. Landscape connectivity of Cercidiphyllum japonicum, an endangered species and its implications for conservation. Ecol. Inform. 2016, 33, 51–56. [Google Scholar] [CrossRef]
- Bai, W.N.; Wang, W.T.; Zhang, D.Y. Phylogeographic breaks within Asian butternuts indicate the existence of a phytogeographic divide in East Asia. New Phytol. 2016, 209, 1757–1772. [Google Scholar] [CrossRef]
- Dong, Y.P.; Shi, X.H.; Sun, S.S.; Sun, J.P.; Hui, B.; He, D.F.; Chong, F.B.; Yang, Z. Co-evolution of the Cenozoic tectonics, geomorphology, environment and ecosystem in the Qinling Mountains and adjacent areas, central China. Geosyst. Geoenviron. 2022, 1, 100032. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, C.; Ratschbacher, L.; Enkelmann, E.; Jonckheere, R.; Wauschkuhn, B.; Dong, Y. Sichuan Basin and beyond: Eastward foreland growth of the Tibetan Plateau from an integration of Late Cretaceous-Cenozoic fission track and (U-Th)/He ages of the eastern Tibetan Plateau, Qinling, and Daba Shan. J. Geophys. Res. Solid Earth 2017, 122, 4712–4740. [Google Scholar] [CrossRef]
- Teng, Z.H.; Wang, X.H. Studies of the tectonic uplift at the Cenozoic era and the regionally environmental effects in the Qinling Orogenic Belt. Geol. Shaanxi 1996, 14, 33–42. [Google Scholar]
- Yuan, J.H.; Cheng, F.Y.; Zhou, S.L. The phylogeographic structure and conservation genetics of the endangered tree peony, Paeonia rockii (Paeoniaceae), inferred from chloroplast gene sequences. Conserv. Genet. 2011, 12, 1539–1549. [Google Scholar] [CrossRef]
- Ree, R.H. Phylogeny and the evolution of floral diversity in Pedicularis (Orobanchaceae). Int. J. Plant Sci. 2005, 166, 595–613. [Google Scholar] [CrossRef]
- Hong, D.Y. The distribution of Scrophulariaceae in the Holarctic with special reference to the floristic relationships between Eastern Asia and Eastern North America. Ann. Mo. Bot. Gard. 1983, 70, 701. [Google Scholar]
- Wang, G.; Baskin, C.C.; Baskin, J.M.; Yang, X.; Liu, G.; Ye, X.; Zhang, X.; Huang, Z. Effects of climate warming and prolonged snow cover on phenology of the early life history stages of four alpine herbs on the southeastern Tibetan Plateau. Am. J. Bot. 2018, 105, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Tkach, N.; Ree, R.H.; Kuss, P.; Roser, M.; Hoffmann, M.H. High mountain origin, phylogenetics, evolution, and niche conservatism of arctic lineages in the hemiparasitic genus Pedicularis (Orobanchaceae). Mol. Phylogenet. Evol. 2014, 76, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Li, W.T.; Liu, Y.N.; Yang, F.S.; Wang, X.Q. Range-wide multilocus phylogenetic analyses of Pedicularis sect. Cyathophora (Orobanchaceae): Implications for species delimitation and speciation. Taxon 2015, 64, 959–974. [Google Scholar] [CrossRef]
- Yu, W.B.; Huang, P.H.; Li, D.Z.; Wang, H. Incongruence between Nuclear and Chloroplast DNA Phylogenies in Pedicularis Section Cyathophora (Orobanchaceae). PLoS ONE 2013, 8, e74828. [Google Scholar] [CrossRef]
- Wang, H.J.; Li, W.T.; Liu, Y.N.; Yang, F.S.; Wang, X.Q. Resolving interspecific relationships within evolutionarily young lineages using RNA-seq data: An example from Pedicularis section Cyathophora (Orobanchaceae). Mol. Phylogenet. Evol. 2017, 107, 345–355. [Google Scholar] [CrossRef]
- Yang, H.; Holmgren, N.H.; Mill, R.R. Pedicularis L. In Flora of China; Raven, P.H., Ed.; Science Press: Beijing, China, 1998; Volume 18, pp. 97–209. [Google Scholar]
- Qin, H.; Yang, Y.; Dong, S.; He, Q.; Jia, Y.; Zhao, L.; Yu, S.; Liu, H.; Liu, B.; Yan, Y. Threatened species list of China’s higher plants. Biodivers. Sci. 2017, 25, 744. [Google Scholar] [CrossRef]
- Fu, J.M.; Liu, H.M.; Hu, J.J.; Liang, Y.Q.; Liang, J.J.; Wu, T.N. Five complete chloroplast genome sequences from Diospyros: Genome organization and comparative analysis. PLoS ONE 2016, 11, e0159566. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Hong, D. Unexpected high divergence in nrDNA ITS and extensive parallelism in floral morphology of Pedicularis (Orobanchaceae). Plant Syst. Evol. 2003, 240, 91–105. [Google Scholar] [CrossRef]
- McNeal, J.R.; Bennett, J.R.; Wolfe, A.D.; Mathews, S. Phylogeny and origins of holoparasitism in Orobanchaceae. Am. J. Bot. 2013, 100, 971–983. [Google Scholar] [CrossRef]
- Scheunert, A.; Fleischmann, A.; Olano-Marín, C.; Bräuchler, C.; Heubl, G. Phylogeny of tribe Rhinantheae (Orobanchaceae) with a focus on biogeography, cytology and re-examination of generic concepts. Taxon 2012, 61, 1269–1285. [Google Scholar] [CrossRef]
- Bennett, J.; Mathews, S. Phylogeny of the parasitic plant family Orobanchaceae inferred from phytochrome A. Am. J. Bot. 2006, 93, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.B.; Wang, H.; Liu, M.L.; Grabovskaya-Borodina, A.E.; Li, D.Z. Phylogenetic approaches resolve taxonomical confusion in Pedicularis (Orobanchaceae): Reinstatement of Pedicularis delavayi and discovering a new species Pedicularis milliana. PLoS ONE 2018, 13, e0200372. [Google Scholar] [CrossRef]
- Yu, W.B.; Liu, M.L.; Wang, H.; Mill, R.R.; Ree, R.H.; Yang, J.B.; Li, D.Z. Towards a comprehensive phylogeny of the large temperate genus Pedicularis (Orobanchaceae), with an emphasis on species from the Himalaya-Hengduan Mountains. BMC Plant Biol. 2015, 15, 176. [Google Scholar] [CrossRef]
- Liu, R.; Wang, H.; Yang, J.B.; Corlett, R.T.; Randle, C.P.; Li, D.Z.; Yu, W.B. Cryptic species diversification of the Pedicularis siphonantha complex (Orobanchaceae) in the mountains of Southwest China since the Pliocene. Front Plant Sci. 2022, 13, 811206. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.B.; Han, E.K.; Choi, H.J.; Lee, J.H. Complete chloroplast genome sequence of Pedicularis verticillata and chloroplast genome reconstruction of P. cheilanthifolia (Orobanchaceae). Mitochondrial DNA B 2020, 5, 1578–1579. [Google Scholar] [CrossRef]
- Wang, Q.; He, Z.; Zhang, R.; Dang, X.; Zhao, X. Characterization of the complete chloroplast genome of Pedicularis shansiensis (Orobanchaceae), an endemic species of China. Mitochondrial DNA B 2022, 7, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, J.B.; Wang, H.; Song, Y.; Yu, W.B. Plastid NDH pseudogenization and gene loss in a recently derived lineage from the largest hemiparasitic plant genus Pedicularis (Orobanchaceae). Plant Cell Physiol. 2021, 62, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.S.; Wang, X.Q. Extensive length variation in the cpDNA trnT-trnF region of hemiparasitic Pedicularis and its phylogenetic implications. Plant Syst. Evol. 2007, 264, 251–264. [Google Scholar] [CrossRef]
- Magallon, S.; Castillo, S. Angiosperm diversification through time. Am. J. Bot. 2009, 96, 349–365. [Google Scholar] [CrossRef]
- Bell, C.D.; Soltis, D.E.; Soltis, P.S. The age and diversification of the angiosperms re-revisited. Am. J. Bot. 2010, 97, 1296–1303. [Google Scholar] [CrossRef]
- Wolfe, A.D.; Randle, C.P.; Liu, L.; Steiner, K.E. Phylogeny and biogeography of Orobanchaceae. Folia Geobot. 2005, 40, 115–134. [Google Scholar] [CrossRef]
- Drew, B.T.; Sytsma, K.J. Phylogenetics, biogeography, and staminal evolution in the tribe Mentheae (Lamiaceae). Am. J. Bot. 2012, 99, 933–953. [Google Scholar] [CrossRef]
- Song, Z.C.; Wang, W.M.; Huang, F. Fossil pollen records of extant angiosperms in China. Bot. Rev. 2004, 70, 425–458. [Google Scholar]
- Dong, Y.P.; Santosh, M. Tectonic architecture and multiple orogeny of the Qinling Orogenic Belt, Central China. Gondwana Res. 2016, 29, 1–40. [Google Scholar] [CrossRef]
- Dong, Y.P.; Zhang, G.W.; Neubauer, F.; Liu, X.; Genser, J.; Hauzenberger, C. Tectonic evolution of the Qinling orogen, China: Review and synthesis. J. Asian Earth Sci. 2011, 41, 213–237. [Google Scholar] [CrossRef]
- Ying, T.S. An analysis of the flora of Qinling Mountain range: Its nature, characteristics and origins. Acta Phytotaxon. Sin. 1994, 32, 389–410. [Google Scholar]
- Liu, M.L.; He, Y.L.; López-Pujol, J.; Jia, Y.; Li, Z.H. Complex population evolutionary history of four cold-tolerant Notopterygium herb species in the Qinghai-Tibetan Plateau and adjacent areas. Heredity 2019, 123, 242–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, H.J.; Zhang, W.; Chai, L. Global last glacial maximum climate inferred from reconstructing the Eryehai Valley, Mount Taibai, Qinling Mountains, eastern China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 590, 110858. [Google Scholar] [CrossRef]
- Miao, Y.F.; Herrmann, M.; Wu, F.L.; Yan, X.L.; Yang, S.L. What controlled Mid-Late Miocene long-term aridification in Central Asia—Global cooling or Tibetan Plateau uplift: A review. Earth Sci. Rev. 2012, 112, 155–172. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, W.J.; Zhu, Y.Z.; Cao, Y.T.; Yang, X.M.; Geng, Y.; Zhang, F.J.; Sun, R.Q.; Jia, R.W.; Yan, C.L.; et al. Impacts of Climate Changes on Geographic Distribution of Primula filchnerae, an Endangered Herb in China. Plants 2023, 12, 3561. [Google Scholar] [CrossRef]
- Zhao, H.R.; Yang, X.T.; Shi, S.Y.; Xu, Y.D.; Yu, X.P.; Ye, X.P. Climate-driven distribution changes for Bashania fargesii in the Qinling Mountains and its implication for panda conservation. Glob. Ecol. Conserv. 2023, 46, e02610. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, H.; Wang, W.; Yan, B. Prediction of the Distribution of Alpine Tree Species Under Climate Change Scenarios: Larix chinensis Taibai Mountain (China). Pol. J. Ecol. 2016, 64, 200–212. [Google Scholar]
- Alsos, I.G.; Eidesen, P.B.; Ehrich, D.; Skrede, I.; Westergaard, K.; Jacobsen, G.H.; Landvik, J.Y.; Taberlet, P.; Brochmann, C. Frequent long-distance plant colonization in the changing Arctic. Science 2007, 316, 1606–1609. [Google Scholar] [CrossRef]
- Chu, X.; Gugger, P.F.; Li, L.; Zhao, J.L.; Li, Q.J. Responses of an endemic species (Roscoea humeana) in the Hengduan Mountains to climate change. Divers. Distrib. 2021, 27, 2231–2244. [Google Scholar] [CrossRef]
- Zhan, P.; Wang, F.Y.; Xia, P.G.; Zhao, G.H.; Wei, M.T.; Wei, F.G.; Han, R.L. Assessment of suitable cultivation region for Panax notoginseng under different climatic conditions using MaxEnt model and high-performance liquid chromatography in China. Ind. Crops Prod. 2022, 176, 114416. [Google Scholar] [CrossRef]
- Zhao, G.H.; Cui, X.Y.; Sun, J.J.; Li, T.T.; Wang, Q.; Ye, X.Z.; Fan, B.G. Analysis of the distribution pattern of Chinese Ziziphus jujuba under climate change based on optimized biomod2 and MaxEnt models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- Antonelli, A.; Kissling, W.D.; Flantua, S.G.A.; Bermúdez, M.A.; Mulch, A.; Muellner-Riehl, A.N.; Kreft, H.; Linder, H.P.; Badgley, C.; Fjeldså, J.; et al. Geological and climatic influences on mountain biodiversity. Nat. Geosci. 2018, 11, 718–725. [Google Scholar] [CrossRef]
- Zhao, J.L.; Gugger, P.F.; Xia, Y.M.; Li, Q.J. Ecological divergence of two closely related Roscoea species associated with late Quaternary climate change. J. Biogeogr. 2001, 43, 1990–2001. [Google Scholar] [CrossRef]
- Wang, B.; Mao, J.F.; Zhao, W.; Wang, X.R. Impact of geography and climate on the genetic differentiation of the subtropical pine Pinus yunnanensis. PLoS ONE 2013, 8, e67345. [Google Scholar] [CrossRef]
- Quintero, I.; González-Caro, S.; Zalamea, P.C.; Cadena, C.D. Asynchrony of seasons: Genetic differentiation associated with geographic variation in climatic seasonality and reproductive phenology. Am. Nat. 2014, 184, 352–363. [Google Scholar] [CrossRef]
- Chen, X.D.; Yang, J.; Guo, Y.F.; Zhao, Y.M.; Zhou, T.; Zhang, X.; Ju, M.M.; Li, Z.H.; Zhao, G.F. Spatial genetic structure and demographic history of the dominant forest oak Quercus fabri Hance in subtropical China. Front. Plant Sci. 2021, 11, 583284. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Dickson, E.E. Preservation of plant samples for DNA restriction endonuclease analysis. Taxon 1987, 36, 715–722. [Google Scholar] [CrossRef]
- Jin, J.J.; Yu, W.B.; Yang, J.B.; Song, Y.; dePamphilis, C.W.; Yi, T.S.; Li, D.Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Schultz, M.B.; Zobel, J.; Holt, K.E. Bandage: Interactive visualization of de novo genome assemblies. Bioinformatics 2015, 31, 3350–3352. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.J.; Moore, M.J.; Li, D.Z.; Yi, T.S. PGA: A software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods 2019, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Swofford, D.L. PAUP*. Phylogenetic Analysis Using Parsimony (and Other Methods), version 4; Sinauer Associates: Sunderland, MA, USA, 2003.
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Daehler, C.; Kueffer, C. Unifying niche shift studies: Insights from biological invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Wang, B.; Liu, D.L.; Waters, C.; Yu, Q. Incorporating machine learning with biophysical model can improve the evaluation of climate extremes impacts on wheat yield in south-eastern Australia. Agric. For. Meteorol. 2019, 275, 100–113. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.-F.; et al. The Representative Concentration Pathways: An Overview. Clim. Chang. 2011, 109, 5. [Google Scholar] [CrossRef]
- Van Proosdij, A.S.J.; Sosef, M.S.M.; Wieringa, J.J.; Raes, N. Minimum required number of specimen records to develop accurate species distribution models. Ecography 2016, 39, 542–552. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. Ecospat: An R package to support spatial analyses and modelling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Schoener, T.W. The Anolis lizards of Bimini: Resource partitioning in a complex fauna. Ecology 1968, 49, 704–726. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evol. Int. J. Org. Evol. 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.J.; Randin, C.; Zimmermann, N.E.; et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Petitpierre, B.; Kueer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic niche shifts are rare among terrestrial plant invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef]
| Niche Overlap | Niche Unfilling | Niche Stability | Niche Expansion | |
|---|---|---|---|---|
| P. bicolor—P. dissecta | 0.463 | 0.036 | 0.624 | 0.376 |
| P. dissecta—P. giraldiana | 0.204 | 0.126 | 0.427 | 0.573 |
| P. giraldiana—P. bicolor | 0.122 | 0.000 | 0.276 | 0.724 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Lu, Z.; Guo, M.; Kang, J.; Li, J.; He, X.; Wu, J.; Liu, R.; Dang, J.; Li, Z. Responses of Three Pedicularis Species to Geological and Climatic Changes in the Qinling Mountains and Adjacent Areas in East Asia. Plants 2024, 13, 765. https://doi.org/10.3390/plants13060765
Zhang Q, Lu Z, Guo M, Kang J, Li J, He X, Wu J, Liu R, Dang J, Li Z. Responses of Three Pedicularis Species to Geological and Climatic Changes in the Qinling Mountains and Adjacent Areas in East Asia. Plants. 2024; 13(6):765. https://doi.org/10.3390/plants13060765
Chicago/Turabian StyleZhang, Qijing, Zhaoping Lu, Mingchen Guo, Jia Kang, Jia Li, Xiaojing He, Jiayi Wu, Ruihang Liu, Jiaxin Dang, and Zhonghu Li. 2024. "Responses of Three Pedicularis Species to Geological and Climatic Changes in the Qinling Mountains and Adjacent Areas in East Asia" Plants 13, no. 6: 765. https://doi.org/10.3390/plants13060765
APA StyleZhang, Q., Lu, Z., Guo, M., Kang, J., Li, J., He, X., Wu, J., Liu, R., Dang, J., & Li, Z. (2024). Responses of Three Pedicularis Species to Geological and Climatic Changes in the Qinling Mountains and Adjacent Areas in East Asia. Plants, 13(6), 765. https://doi.org/10.3390/plants13060765





