In Silico Analysis of Glutamate Receptors in Capsicum chinense: Structure, Evolution, and Molecular Interactions
Abstract
:1. Introduction
2. Results
2.1. Identification of the GLR Gene Family in the Capsicum Chinense Genome
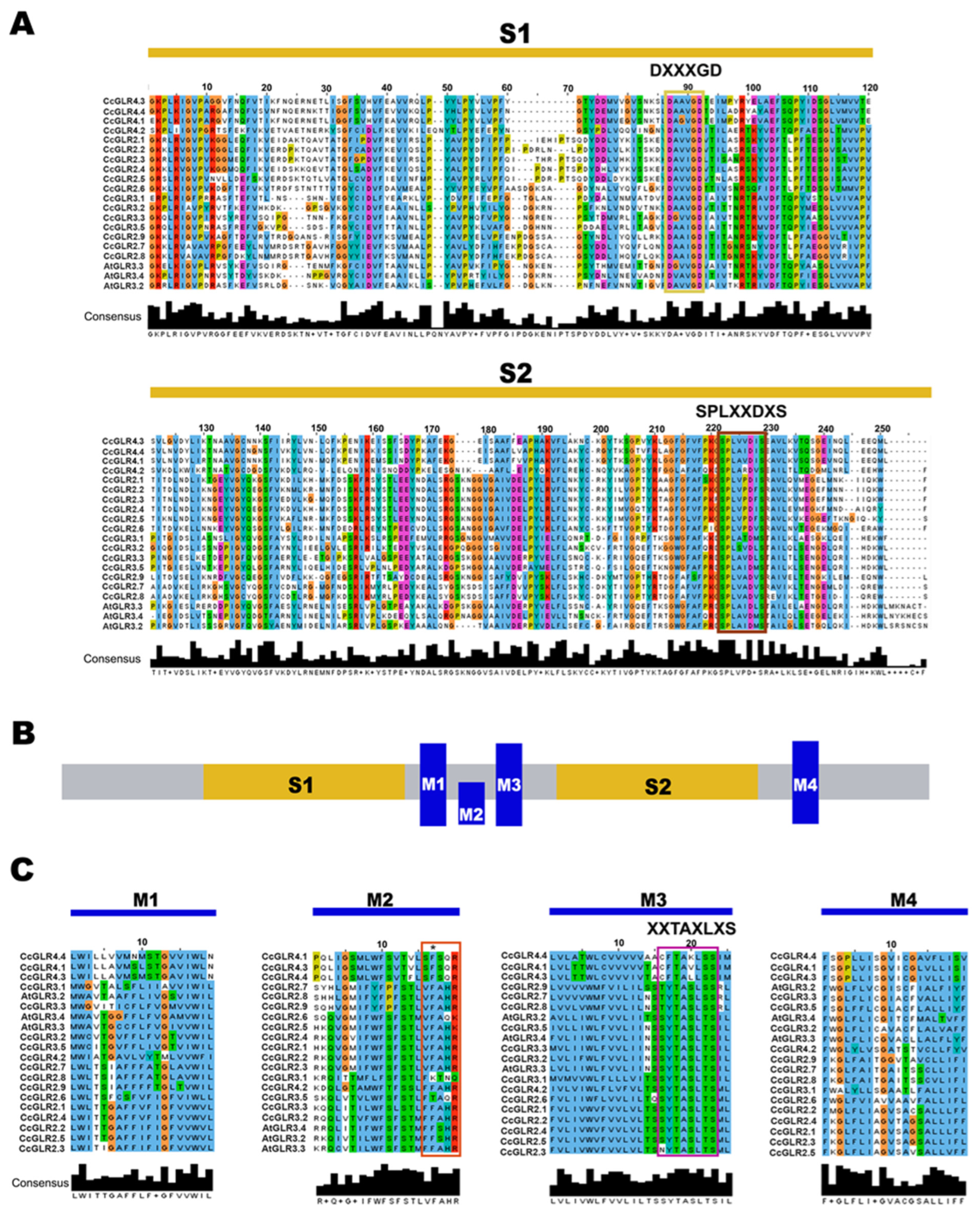
2.2. Structural Analysis and Main Characteristics of the CcGLR Family
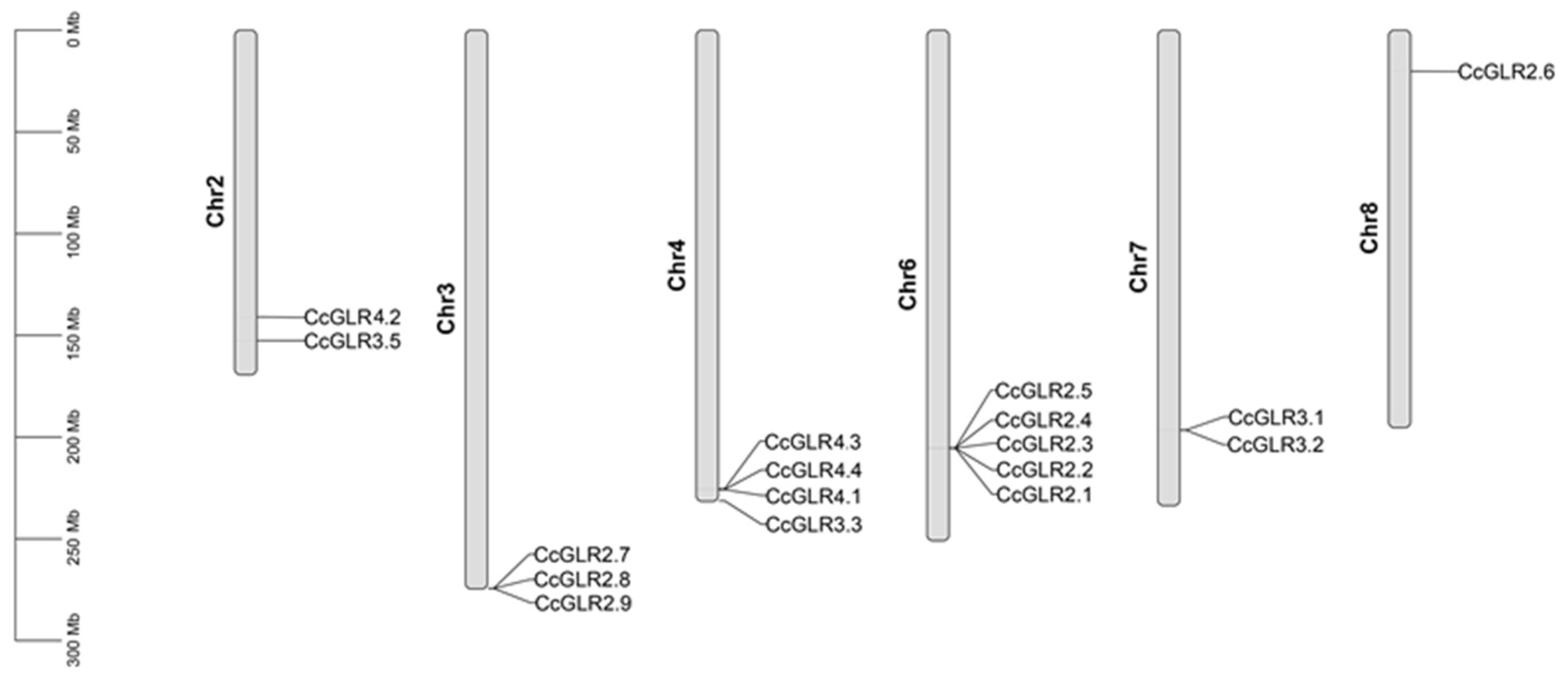
2.3. Structure of CcGLRs Genes
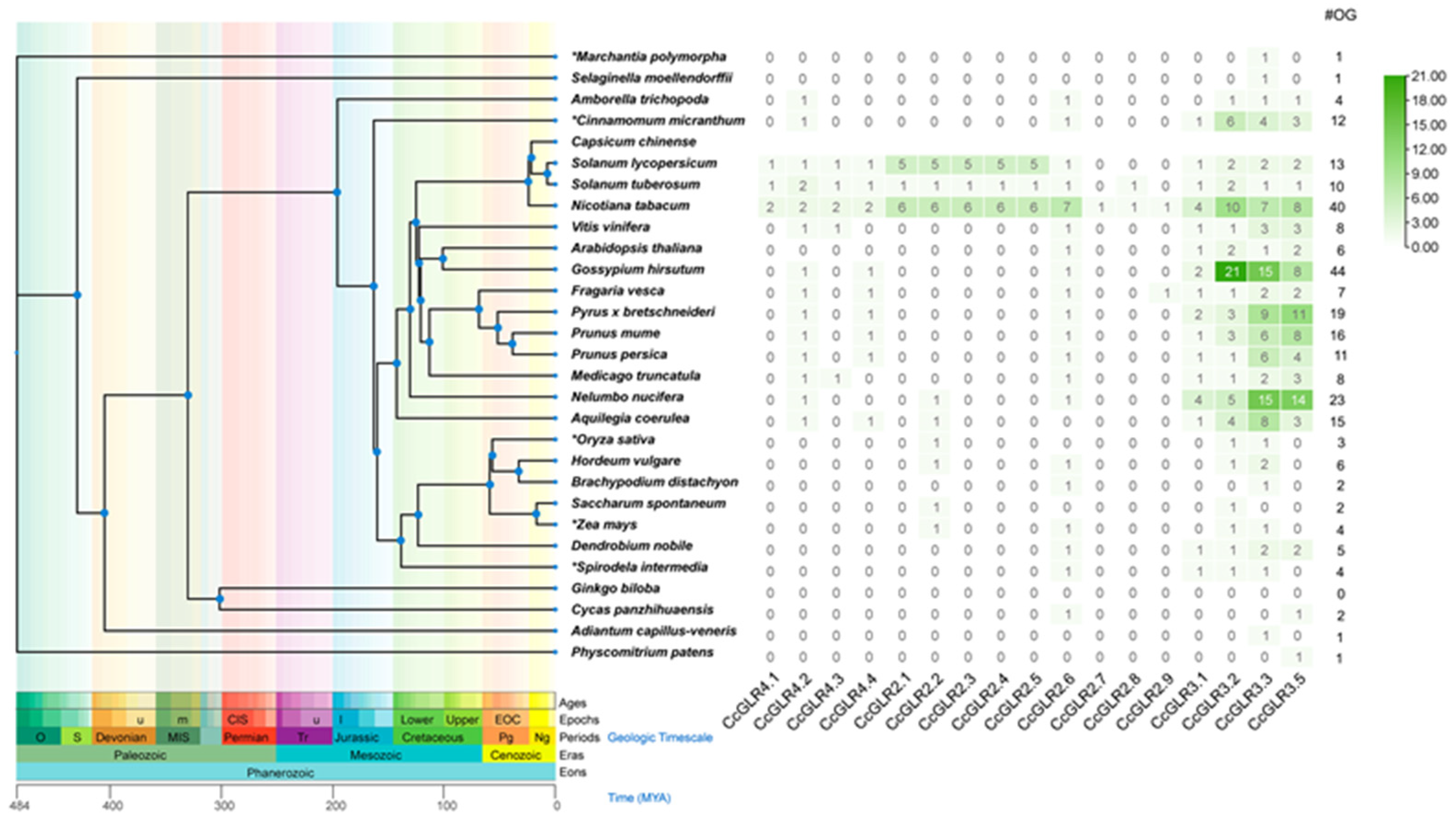
2.4. Orthologs of CcGLRs Present in Different Species
2.5. Phylogenetic Analysis of CcGLRs Proteins
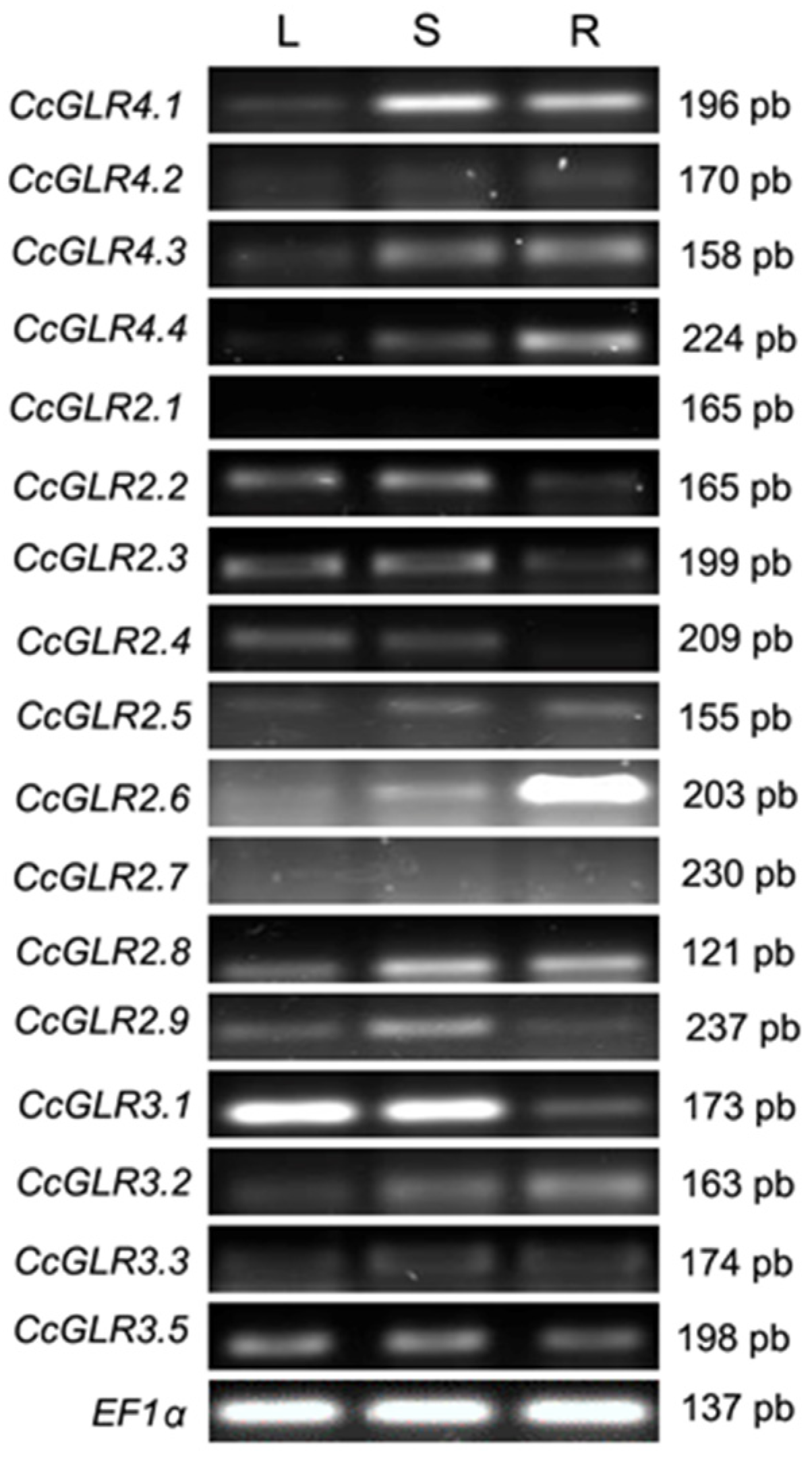
2.6. Transcription Pattern of CcGLRs
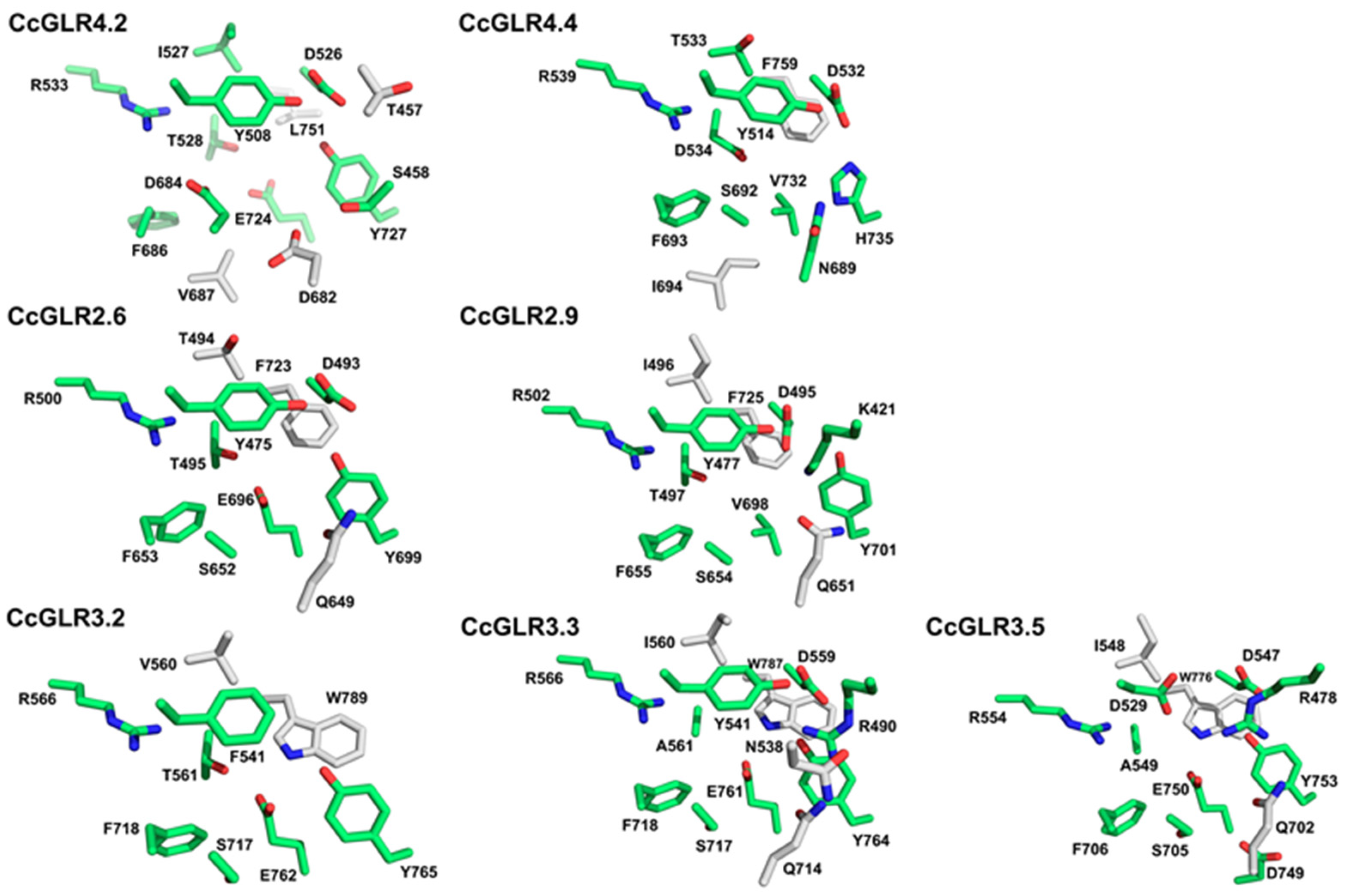
2.7. Molecular Docking Analysis
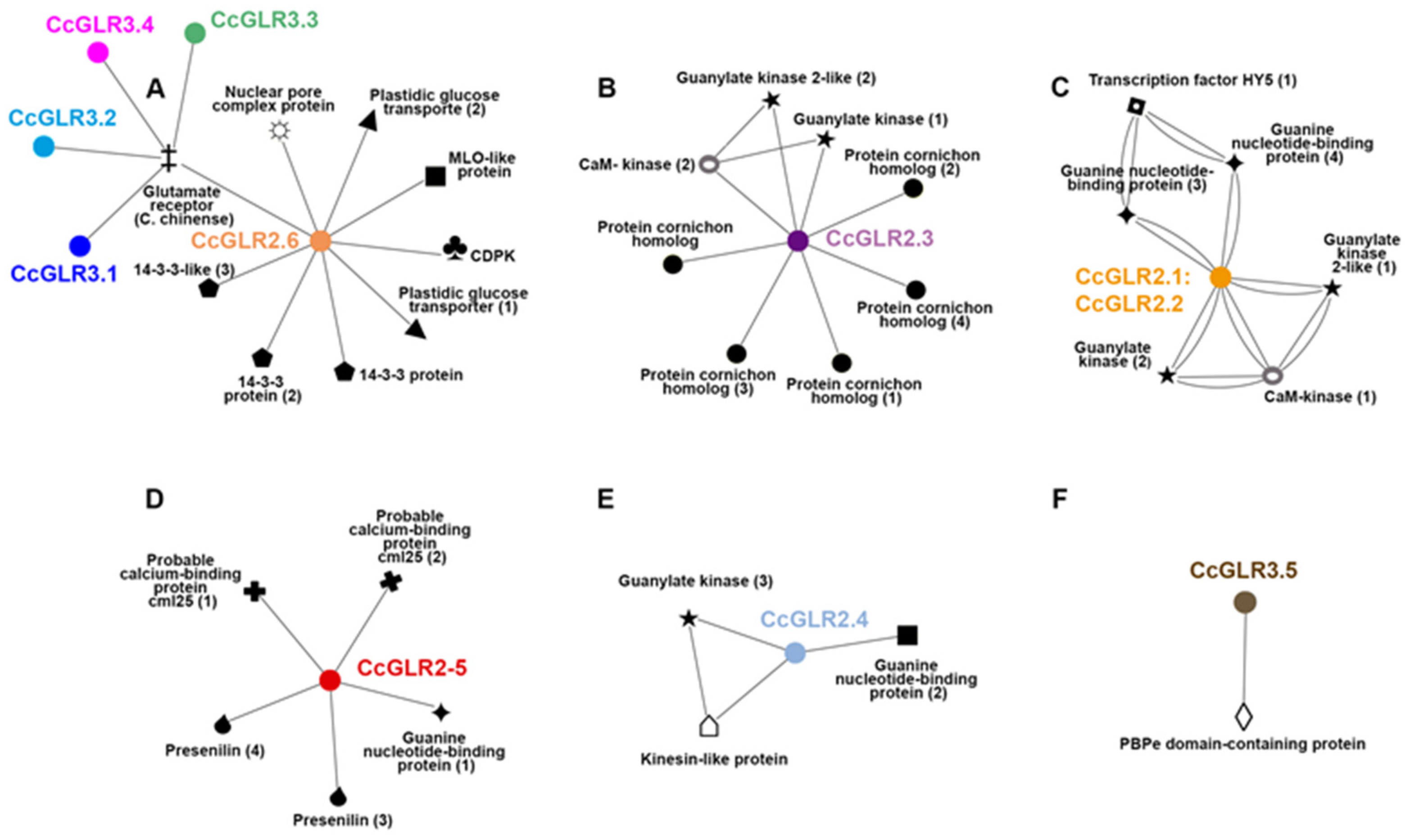
2.8. Protein–Protein Interaction
3. Discussion
3.1. Identification of CcGLR
3.2. Structure of CcGLRs Proteins
3.3. Phylogenetic Analysis and Transcription Patterns of CcGLRs
3.4. Molecular Docking
3.5. Protein–Protein Interaction
4. Materials and Methods
4.1. Identification and Annotation of GLR Family Members in Habanero Pepper
4.2. Structural Analysis and Main Characteristics of the CcGLR Family
4.3. Phylogenetic Analysis
4.4. Transcription Patterns of CcGLRs
4.5. CcGLRs Gene Structure, Chromosomal Localization, and Gene Duplication
4.6. Ortholog Analysis
4.7. Taxonomic Tree
4.8. Ka/Ks Ratio
4.9. Molecular Modeling and Docking
4.10. Protein Interaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kew, J.N.C.; Kemp, J.A. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology 2005, 179, 4–29. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.M.; Chiu, J.; Hsieh, M.H.; Meisel, L.; Oliveira, I.C.; Shin, M.; Coruzzi, G. Glutamate-receptor genes in plants. Nature 1998, 396, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.C.; Brenner, E.D.; Desalle, R.; Nitabach, M.N.; Holmes, T.C.; Coruzzi, G.M. Phylogenetic and expression analysis of the glutamate-receptor-like gene family in Arabidopsis thaliana. Mol. Biol. Evol. 2002, 19, 1066–1082. [Google Scholar] [CrossRef]
- Aouini, A.; Matsukura, C.; Ezura, H.; Asamizu, E. Characterisation of 13 glutamate receptor-like genes encoded in the tomato genome by structure, phylogeny and expression profiles. Gene 2012, 493, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kanwar, P.; Yadav, A.K.; Mishra, M.; Jha, S.K.; Baranwal, V.; Pandey, A.; Kapoor, S.; Tyagi, A.K.; Pandey, G.K. Genome-wide expressional and functional analysis of calcium transport elements during abiotic stress and development in rice. FEBS J. 2014, 281, 894–915. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, S.; Song, X.; Shen, Y.; Chen, H.; Yu, J.; Yi, K.; Liu, Y.; Karplus, V.J.; Wu, P.; et al. A rice glutamate receptor-like gene is critical for the division and survival of individual cells in the root apical meristem. Plant Cell 2006, 18, 340–349. [Google Scholar] [CrossRef]
- Philippe, F.; Verdu, I.; Morère-Le Paven, M.C.; Limami, A.M.; Planchet, E. Involvement of Medicago truncatula glutamate receptor-like channels in nitric oxide production under short-term water deficit stress. J. Plant Physiol. 2019, 236, 1–6. [Google Scholar] [CrossRef]
- Chen, J.; Jing, Y.; Zhang, X.; Li, L.; Wang, P.; Zhang, S.; Zhou, H.; Wu, J. Evolutionary and expression analysis provides evidence for the plant glutamate-like receptors family is involved in woody growth-related function. Sci. Rep. 2016, 6, 32013. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, T.; Su, Y.; Zang, S.; Zhao, Z.; Zhang, C.; Zou, W.; Chen, Y.; Cao, Y.; Chen, Y.; et al. Genome-Wide Identification, Characterization, and Expression Analysis of Glutamate Receptor-like Gene (GLR) Family in Sugarcane. Plants 2022, 11, 2440. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, X.; Xiao, S.; Ma, J.; Shi, W.; Qin, T.; Xi, H.; Nie, X.; You, C.; Xu, Z.; et al. A single-nucleotide mutation in a glutamate receptor-like gene confers resistance to Fusarium Wilt in Gossypium hirsutum. Adv. Sci. 2021, 8, 2002723. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Chiu, J.; Desalle, R.; Lam, H.M.; Meisel, L.; Coruzzi, G. Molecular evolution of glutamate receptors: A primitive signaling mechanism that existed before plants and animals diverged. Mol. Biol. Evol. 1999, 16, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Price, M.B.; Okumoto, S. Inter-subunit interactions between Glutamate-Like Receptors in Arabidopsis. Plant Signal. Behav. 2013, 8, e27034. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.J.; Gilliham, M.; Berger, B.; Essah, P.A.; Cheffings, C.; Miller, A.J.; Davenport, R.J.; Liu, L.H.; Skynner, M.J.; Davies, J.M.; et al. Investigating glutamate receptor-like gene co-expression in Arabidopsis thaliana. Plant Cell Environ. 2008, 31, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Vincill, E.D.; Clarin, A.E.; Molenda, J.N.; Spalding, E.P. Interacting glutamate receptor-like proteins in phloem regulate lateral root initiation in Arabidopsis. Plant Cell 2013, 25, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Weiland, M.; Mancuso, S.; Baluska, F. Signalling via glutamate and GLRs in Arabidopsis thaliana. Funct. Plant Biol. 2016, 43, 1–25. [Google Scholar] [CrossRef]
- Chang, I.F.; Curran, A.; Woolsey, R.; Quilici, D.; Cushman, J.C.; Mittler, R.; Harmon, A.; Harper, J.F. Proteomic profiling of tandem affinity purified 14-3-3 protein complexes in Arabidopsis thaliana. Proteomics 2009, 9, 2967–2985. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, M.; Heinemann, S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994, 17, 31–108. [Google Scholar] [CrossRef]
- Standley, S.; Baudry, M. The role of glycosylation in ionotropic glutamate receptor ligand binding, function, and trafficking. Cell. Mol. Life Sci. 2000, 57, 508–1516. [Google Scholar] [CrossRef]
- Wudick, M.M.; Portes, M.T.; Michard, E.; Rosas-Santiago, P.; Lizzio, M.A.; Nunes, C.O.; Campos, C.; Santa Cruz Damineli, D.; Carvalho, J.C.; Lima, P.T.; et al. CORNICHON sorting and regulation of GLR channels underlie pollen tube Ca2+ homeostasis. Plant Sci. 2018, 360, 533–536. [Google Scholar]
- Vincill, E.D.; Bieck, A.M.; Spalding, E.P. Ca2+ conduction by an amino acid-gated ion channel related to glutamate receptors. Plant Physiol. 2012, 159, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Mehta, S.; Turano, F.J. The putative glutamate receptor 1.1 (AtGLR1.1) in Arabidopsis thaliana regulates abscisic acid biosynthesis and signaling to control development and water loss. Plant Cell Physiol. 2004, 45, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Turano, F.J. The putative glutamate receptor 1.1 (AtGLR1.1) functions as a regulator of carbon and nitrogen metabolism in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6872–6877. [Google Scholar] [CrossRef] [PubMed]
- Brenner, E.D.; Martinez-Barboza, N.; Clark, A.P.; Liang, Q.S.; Stevenson, D.W.; Coruzzi, G.M. Arabidopsis Mutants Resistant to S()-Methyl-,-Diaminopropionic Acid, a Cycad-Derived Glutamate Receptor Agonist 1. Plant Physiol. 2000, 212, 995–4204. [Google Scholar]
- Kim, S.A.; Myoung Kwak, J.; Jae, S.K.; Wang, M.H.; Gil Nam, H. Overexpression of the AtGluR2 gene encoding an Arabidopsis homolog of mammalian glutamate receptors impairs calcium utilization and sensitivity to ionic stress in transgenic plants. Plant Cell Physiol. 2001, 42, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Walch-Liu, P.; Liu, L.H.; Remans, T.; Tester, M.; Forde, B.G. Evidence that L-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 1045–1057. [Google Scholar] [CrossRef]
- Michard, E.; Lima, P.T.; Borges, F.; Silva, A.C.; Portes, M.T.; Carvalho, J.E.; Gilliham, M.; Liu, L.H.; Obermeyer, G.; Feijó, J.A. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-Serine. Science 2011, 332, 434–437. [Google Scholar] [CrossRef]
- Sivaguru, M.; Pike, S.; Gassmann, W.; Baskin, T.I. Aluminum Rapidly Depolymerizes Cortical Microtubules and Depolarizes the Plasma Membrane: Evidence that these Responses are Mediated by a Glutamate Receptor. Plant Cell Physiol. 2003, 44, 667–675. [Google Scholar] [CrossRef]
- Tapken, D.; Anschütz, U.; Liu, L.H.; Huelsken, T.; Seebohm, G.; Becker, D.; Hollmann, M. A plant homolog of animal glutamate receptors is an ion channel gated by multiple hydrophobic amino acids. Sci. Signal. 2013, 6, ra47. [Google Scholar] [CrossRef]
- Manzoor, H.; Kelloniemi, J.; Chiltz, A.; Wendehenne, D.; Pugin, A.; Poinssot, B.; Garcia-Brugger, A. Involvement of the glutamate receptor AtGLR3.3 in plant defense signaling and resistance to Hyaloperonospora arabidopsidis. Plant J. 2013, 76, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.D.; Brooks, T.L.D.; Assadi, A.H.; Spalding, E.P. Detection of a gravitropism phenotype in glutamate receptor-like 3.3 mutants of Arabidopsis thaliana using machine vision and computation. Genetics 2010, 186, 585–593. [Google Scholar] [CrossRef]
- Alfieri, A.; Doccula, F.G.; Pederzoli, R.; Grenzi, M.; Bonza, M.C.; Luoni, L.; Candeo, A.; Romano Armada, N.; Barbiroli, A.; Valentini, G.; et al. The structural bases for agonist diversity in an Arabidopsis thaliana glutamate receptor-like channel. Proc. Natl. Acad. Sci. USA 2020, 117, 752–760. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Ma, C.; Zhao, Y.; Wang, Y.; Hasi, A.; Qi, Z. Glutamate receptor-like channel3.3 is involved in mediating glutathione-triggered cytosolic calcium transients, transcriptional changes, and innate immunity responses in Arabidopsis. Plant Physiol. 2013, 162, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Stephens, N.R.; Spalding, E.P. Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol. 2006, 142, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Huggins, D.; Grant, G.H.; Knight, M.R.; Campbell, M.M. A role for glycine in the gating of plant NMDA-like receptors. Plant J. 2003, 35, 800–810. [Google Scholar] [CrossRef]
- Gangwar, S.P.; Green, M.N.; Michard, E.; Simon, A.A.; Feijó, J.A.; Sobolevsky, A.I. Structure of the Arabidopsis glutamate receptor-like channel GLR3.2 ligand-binding domain. Structure 2021, 29, 161–169. [Google Scholar] [CrossRef]
- Green, M.N.; Gangwar, S.P.; Michard, E.; Simon, A.A.; Portes, M.T.; Barbosa-Caro, J.; Wudick, M.M.; Lizzio, M.A.; Klykov, O.; Yelshanskaya, M.V.; et al. Structure of the Arabidopsis thaliana glutamate receptor-like channel GLR3.4. Mol. Cell 2021, 81, 3216–3226. [Google Scholar] [CrossRef]
- Samuels, J. Biodiversity of food species of the solanaceae family: A preliminary taxonomic inventory of subfamily Solanoideae. Resources 2015, 4, 277–322. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, B.; Wei, J.; Cai, W.; Huang, Z.; Chen, C.; Cao, B.; Chen, G.; Lei, J. Construction of a high density genetic map of an interspecific cross of Capsicum chinense and Capsicum annuum and QTL analysis of floral traits. Sci. Rep. 2019, 9, 1054. [Google Scholar] [CrossRef]
- Haile, M.; Ro, N.; Ko, H.C.; Oh, H.; Lee, G.A. A Comprehensive Genome-Wide Association Study of Carotenoid and Capsaicinoid Contents in Capsicum chinense Germplasm. Int. J. Mol. Sci. 2023, 24, 13885. [Google Scholar] [CrossRef]
- Lozada, D.N.; Bosland, P.W.; Barchenger, D.W.; Haghshenas-Jaryani, M.; Sanogo, S.; Walker, S. Chile Pepper (Capsicum) Breeding and Improvement in the “Multi-Omics” Era. Front. Plant Sci. 2022, 13, 879182. [Google Scholar] [CrossRef]
- Cruz, J.G.; Silveira, T.; Richter, V.; Wagner, J.G.; Neitzke, R.S.; Barbieri, R.L.; Vizzotto, M. Genetic variability of bioactive compounds in Capsicum chinense. Food Sci. Technol. 2022, 42, e123721. [Google Scholar] [CrossRef]
- Sarpras, M.; Chhapekar, S.S.; Ahmad, I.; Abraham, S.K.; Ramchiary, N. Analysis of bioactive components in Ghost chili (Capsicum chinense) for antioxidant, genotoxic, and apoptotic effects in mice. Drug Chem. Toxicol. 2020, 43, 182–191. [Google Scholar]
- Sharma, J.; Sharma, P.; Sharma, B.; Chaudhary, P. In-Vitro Estimation of Antioxidant Activity in Green Chilli (Capsicum annuum) and Yellow Lantern Chilli (Capsicum chinense). Int. J. Res. Rev. 2017, 4, 54. [Google Scholar]
- Sherova, G.; Pavlov, A.; Georgiev, V. Food Science and Applied Biotechnology Polyphenols profiles and antioxidant activities of extracts from Capsicum chinense in vitro plants and callus cultures. Food Sci. Appl. Biotechnol. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- Salehi, B.; Hernández-Álvarez, A.J.; del Mar Contreras, M.; Martorell, M.; Ramírez-Alarcón, K.; Melgar-Lalanne, G.; Matthews, K.R.; Sharifi-Rad, M.; Setzer, W.N.; Nadeem, M.; et al. Potential Phytopharmacy and Food Applications of Capsicum spp.: A Comprehensive Review. Prod. Commun. 2018, 13, 1543–1556. [Google Scholar]
- Toniolo Pozzobon, M.; Schifino-Wittmann, M.T.; De Bem Bianchetti, L. Chromosome numbers in wild and semidomesticated Brazilian capsicum L. (Solanaceae) species: Do x = 12 and x = 13 represent two evolutionary lines? Bot. J. Linn. Soc. 2006, 151, 259–269. [Google Scholar] [CrossRef]
- Das, S.; Teja, K.C.; Duary, B.; Agrawal, P.K.; Bhattacharya, S.S. Impact of nutrient management, soil type and location on the accumulation of capsaicin in Capsicum chinense (Jacq.): One of the hottest chili in the world. Sci. Hortic. 2016, 213, 354–366. [Google Scholar] [CrossRef]
- Medina-Lara, F.; Souza-Perera, R.; Martínez-Estévez, M.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M.; MacHado, I.E. Red and brown soils increase the development and content of nutrients in habanero pepper subjected to irrigation water with high electrical conductivity. HortScience 2019, 54, 2039–2049. [Google Scholar] [CrossRef]
- Medina-Lara, F.; Echevarría-Machado, I.; Pacheco-Arjona, R.; Ruiz-Lau, N.; Guzmá n-Antonio, A.; Martinez-Estevez, M. Influence of Nitrogen and Potassium Fertilization on Fruiting and Capsaicin Content in Habanero Pepper (Capsicum chinense Jacq.). HortScience 2008, 43, 1549–1554. [Google Scholar] [CrossRef]
- Zamudio-Moreno, E.; Echevarría-Machado, I.; Medina-Lara, M.D.F.; Calva-Calva, G.; Miranda-Ham, M.D.L.; Martínez-Estévez, M. Role of peroxidases in capsaicinoids degradation in habanero pepper (Capsicum chinense Jacq.) plants grown under water deficit conditions. Aust. J. Crop Sci. 2014, 8, 448–454. [Google Scholar]
- Burgos-Valencia, E.; García-Laynes, F.; Echevarría-Machado, I.; Medina-Lara, F.; Monforte-González, M.; Narváez-Zapata, J.; Martínez-Estévez, M. Differential Expression of Genes Related to Fruit Development and Capsaicinoids Synthesis in Habanero Pepper Plants Grown in Contrasting Soil Types. Phyton 2024, 93, 151–183. [Google Scholar] [CrossRef]
- Domínguez-May, Á.V.; Carrillo-Pech, M.; Barredo-Pool, F.A.; Martínez-Estévez, M.; Us-Camas, R.Y.; Moreno-Valenzuela, O.A.; Echevarría-Machado, I. A Novel Effect for Glycine on Root System Growth of Habanero Pepper. J. Am. Soc. Hortic. Sci. 2013, 138, 433–442. [Google Scholar] [CrossRef]
- Serralta-Interian, A.A.; Miranda-Ham, M.d.L.; Echevarría-Machado, I. Stimulation of root growth and enhanced nitrogenous metabolite content in habanero pepper (Capsicum chinense Jacq.) treated with a d-amino acid mixture. Theor. Exp. Plant Physiol. 2020, 32, 31–47. [Google Scholar] [CrossRef]
- Li, H.; Jiang, X.; Lv, X.; Ahammed, G.J.; Guo, Z.; Qi, Z.; Yu, J.; Zhou, Y. Tomato GLR3.3 and GLR3.5 mediate cold acclimation-induced chilling tolerance by regulating apoplastic H2O2 production and redox homeostasis. Plant Cell Environ. 2019, 42, 3326–3339. [Google Scholar] [CrossRef] [PubMed]
- Aouini, A.; Hernould, M.; Ariizumi, T.; Matsukura, C.; Ezura, H.; Asamizu, E. Overexpression of the tomato glutamate receptor-like genes SlGLR1.1 and SlGLR3.5 hinders Ca2+ utilization and promotes hypersensitivity to Na+ and K+ stresses. Plant Biotechnol. 2012, 29, 229–235. [Google Scholar] [CrossRef]
- Price, M.B.; Jelesko, J.; Okumoto, S. Glutamate receptor homologs in plants: Functions and evolutionary origins. Front. Plant Sci. 2012, 3, 235. [Google Scholar] [CrossRef]
- Peplow, P.V.; Martinez, B.; Dambinova, S.A. Stroke Biomarkers; Springer: New York, NY, USA, 2020. [Google Scholar]
- Panchenko, V.A.; Glasser, C.R.; Mayer, M.L. Structural Similarities between Glutamate Receptor Channels and K Channels Examined by Scanning Mutagenesis 7. J. Gen. Physiol. 2001, 117, 345–360. [Google Scholar] [CrossRef]
- Egebjerg, J.; Heinemann, S.F. Ca2+ permeability of unedited and edited versions of the kainate selective glutamate receptor GluR6. Proc. Natl. Acad. Sci. USA 1993, 90, 755–759. [Google Scholar] [CrossRef]
- Doyle, D.A.; Cabral, J.M.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The Structure of the Potassium Channel: Molecular Basis of K Conduction and Selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef]
- Roy, B.C.; Mukherjee, A. Computational analysis of the glutamate receptor gene family of Arabidopsis thaliana. J. Biomol. Struct. Dyn. 2017, 35, 2454–2474. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Yu, Z.; Du, G.; Zhang, Y.; Taylor, J.L.; Shen, C.; Xu, J.; Liu, X.; Wang, Y.; Wu, Y. Heterologous Expression and Functional Analysis of Rice GLUTAMATE RECEPTOR-LIKE Family Indicates its Role in Glutamate Triggered Calcium Flux in Rice Roots. Rice 2016, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Teardo, E.; Carraretto, L.; De Bortoli, S.; Costa, A.; Behera, S.; Wagner, R.; Lo Schiavo, F.; Formentin, E.; Szabo, I. Alternative splicing-Mediated targeting of the arabidopsis GLUTAMATE RECEPTOR 3.5 to mitochondria affects organelle morphology. Plant Physiol. 2015, 167, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Gao, M.; Su, Z. Exploring the Roles of Proline in Three-Dimensional Domain Swapping from Structure Analysis and Molecular Dynamics Simulations. Protein J. 2018, 37, 13–20. [Google Scholar] [CrossRef]
- Twomey, E.C.; Sobolevsky, A.I. Structural Mechanisms of Gating in Ionotropic Glutamate Receptors. Biochemistry 2018, 57, 267–276. [Google Scholar] [CrossRef]
- Ortiz-Ramírez, C.; Michard, E.; Simon, A.A.; Damineli, D.S.; Hernández-Coronado, M.; Becker, J.D.; Feijó, J.A. GLUTAMATE RECEPTOR-LIKE channels are essential for chemotaxis and reproduction in mosses. Nature 2017, 549, 91–95. [Google Scholar] [CrossRef]
- Klein, R.M.; Howe, J.R. Effects of the lurcher mutation on GluR1 desensitation and activation kinetics. J. Neurosci. 2004, 24, 4941–4951. [Google Scholar] [CrossRef]
- Wudick, M.M.; Michard, E.; Oliveira Nunes, C.; Feijó, J.A. Comparing plant and animal glutamate receptors: Common traits but different fates? J. Exp. Bot. 2018, 69, 4151–4163. [Google Scholar] [CrossRef]
- Zeng, H.; Zhao, B.; Wu, H.; Zhu, Y.; Chen, H. Comprehensive in silico characterization and expression profiling of nine gene families associated with calcium transport in soybean. Agronomy 2020, 10, 1539. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Y.; Wu, X.; Zhang, Y.; Fu, Y.; Duan, Q.; Ma, W.; Huang, J. Genome-Wide Identification and Expression Analysis of BraGLRs Reveal Their Potential Roles in Abiotic Stress Tolerance and Sexual Reproduction. Cells 2022, 11, 3729. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Luo, L.; Wei, J.; Chen, Q.; Yang, Y.; Hu, X.; Kong, X. The glutamate receptors AtGLR1.2 and AtGLR1.3 increase cold tolerance by regulating jasmonate signaling in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2018, 506, 895–900. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Sun, T.; Tian, Q.; Zhang, W.H. Glutamate Receptor Homolog3.4 is Involved in Regulation of Seed Germination under Salt Stress in Arabidopsis. Plant Cell Physiol. 2018, 59, 978–988. [Google Scholar] [CrossRef]
- Fabrice, M.R.; Jing, Y.; Jiang, X.; Xiong, C.; Liu, X.; Chen, J.; Jiao, H.; Zhou, H.; Zhao, Z.; Zhang, S.; et al. PbGLR3.3 Regulates Pollen Tube Growth in the Mediation of Ca2+ Influx in Pyrus bretschneideri. J. Plant Biol. 2018, 61, 217–226. [Google Scholar] [CrossRef]
- Kang, S.; Kim, H.B.; Lee, H.; Choi, J.Y.; Heu, S.; Oh, C.J.; Kwon, S.I.; An, C.S. Overexpression in Arabidopsis of a Plasma Membrane-targeting Glutamate Receptor from Small Radish Increases Glutamate-mediated Ca2+ Influx and Delays Fungal Infection. Mol. Cells 2006, 21, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Teardo, E.; Formentin, E.; Segalla, A.; Giacometti, G.M.; Marin, O.; Zanetti, M.; Schiavo, F.L.; Zoratti, M.; Szabò, I. Dual localization of plant glutamate receptor AtGLR3.4 to plastids and plasmamembrane. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Kurenda, A.; Stolz, S.; Chételat, A.; Farmer, E.E. Identification of cell populations necessary for leaf-toleaf electrical signaling in a wounded plant. Proc. Natl. Acad. Sci. USA 2018, 115, 10178–10183. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Li, Y.J.; Kalyanaraman, C.; Qiu, L.L.; Chen, C.; Xiao, Q.; Liu, W.X.; Zhang, W.; Yang, J.J.; Chen, G.; et al. GluA1 signal peptide determines the spatial assembly of heteromeric AMPA receptors. Proc. Natl. Acad. Sci. USA 2016, 113, E5645–E5654. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Stolz, S.; Kumari, A.; Farmer, E.E. The carboxy-terminal tail of GLR3.3 is essential for wound-response electrical signaling. New Phytol. 2022, 236, 2189–2201. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.S.; Roche, K.W. Regulation of NMDA receptors by phosphorylation. Neuropharmacology 2007, 53, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Zhang, J. Genomic evidence for adaptation by gene duplication. Genome Res. 2014, 24, 1356–1362. [Google Scholar] [CrossRef]
- Kovalenko, T.F.; Patrushev, L.I. Pseudogenes as Functionally Significant Elements of the Genome. Biochemistry 2018, 83, 1332–1349. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.R.; Chauvin, A.; Pascaud, F.; Kellenberger, S.; Farmer, E.E. Glutamate Receptor-Like genes mediate leaf-to-leaf wound signalling. Nature 2013, 500, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Grenzi, M.; Bonza, M.C.; Alfieri, A.; Costa, A. Structural insights into long-distance signal transduction pathways mediated by plant glutamate receptor-like channels. New Phytol. 2021, 229, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.A.; Navarro-Retamal, C.; Feijó, J.A. Annual Review of Plant Biology Merging Signaling with Structure: Functions and Mechanisms of Plant Glutamate Receptor Ion Channels. Annu. Rev. Plant. Biol. 2023, 74, 415–452. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Hu, H.C.; Okuma, E.; Lee, Y.; Lee, H.S.; Munemasa, S.; Cho, D.; Ju, C.; Pedoeim, L.; Rodriguez, B.; et al. L-Met Activates Arabidopsis GLR Ca2+ Channels Upstream of ROS Production and Regulates Stomatal Movement. Cell Rep. 2016, 17, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Lee, C.E.; Lin, Y.S.; Lee, M.H.; Chen, P.Y.; Chang, H.C.; Chang, I.F. The Glutamate Receptor-Like Protein GLR3.7 Interacts with 14-3-3ω and Participates in Salt Stress Response in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Coronado, M.; Araujo, P.C.D.; Ip, P.L.; Nunes, C.O.; Rahni, R.; Wudick, M.M.; Lizzio, M.A.; Feijó, J.A.; Birnbaum, K.D. Plant glutamate receptors mediate a bet-hedging strategy between regeneration and defense. Dev. Cell 2022, 57, 451–465.e6. [Google Scholar] [CrossRef]
- Brockie, P.J.; Jensen, M.; Mellem, J.E.; Jensen, E.; Yamasaki, T.; Wang, R.; Maxfield, D.; Thacker, C.; Hoerndli, F.; Dunn, P.J.; et al. Cornichons Control ER Export of AMPA Receptors to Regulate Synaptic Excitability. Neuron 2013, 80, 129–142. [Google Scholar] [CrossRef]
- Gardoni, F.; Schrama, L.H.; Kamal, A.; Gispen, W.H.; Cattabeni, F.; Di Luca, M. Hippocampal Synaptic Plasticity Involves Competition between Ca 2 /Calmodulin-Dependent Protein Kinase II and Postsynaptic Density 95 for Binding to the NR2A Subunit of the NMDA Receptor. J. Neurosci. 2001, 21, 1501–1509. [Google Scholar] [CrossRef]
- Iwamoto, T.; Yamada, Y.; Hori, K.; Watanabe, Y.; Sobue, K.; Inui, M. Differential modulation of NR1-NR2A and NR1-NR2B subtypes of NMDA receptor by PDZ domain-containing proteins. J. Neurochem. 2004, 89, 100–108. [Google Scholar] [CrossRef]
- Wilkinson, B.; Li, J.; Coba, M.P. Synaptic GAP and GEF complexes cluster proteins essential for GTP signaling. Sci. Rep. 2017, 7, 5272. [Google Scholar] [CrossRef]
- Fukata, Y.; Tzingounis, A.V.; Trinidad, J.C.; Fukata, M.; Burlingame, A.L.; Nicoll, R.A.; Bredt, D.S. Molecular constituents of neuronal AMPA receptors. J. Cell Biol. 2005, 169, 399–404. [Google Scholar] [CrossRef]
- Amparan, D.; Avram, D.; Thomas, C.G.; Lindahl, M.G.; Yang, J.; Bajaj, G.; Ishmael, J.E. Direct interaction of myosin regulatory light chain with the NMDA receptor. J. Neurochem. 2005, 92, 349–361. [Google Scholar] [CrossRef]
- Joiner, M.L.A.; Lisé, M.F.; Yuen, E.Y.; Kam, A.Y.; Zhang, M.; Hall, D.D.; Malik, Z.A.; Qian, H.; Chen, Y.; Ulrich, J.D.; et al. Assembly of a β2-adrenergic receptorGluR1 signalling complex for localized cAMP signalling. EMBO J. 2010, 29, 482–495. [Google Scholar] [CrossRef]
- Saura, C.A.; Choi, S.Y.; Beglopoulos, V.; Malkani, S.; Zhang, D.; Rao, B.S.; Chattarji, S.; Kelleher, R.J.; Kandel, E.R.; Duff, K.; et al. Loss of Presenilin Function Causes Impairments of Memory and Synaptic Plasticity Followed by Age-Dependent Neurodegeneration. Neuron 2004, 42, 23–36. [Google Scholar] [CrossRef]
- Mou, W.; Kao, Y.T.; Michard, E.; Simon, A.A.; Li, D.; Wudick, M.M.; Lizzio, M.A.; Feijó, J.A.; Chang, C. Ethylene-independent signaling by the ethylene precursor ACC in Arabidopsis ovular pollen tube attraction. Nat. Commun. 2020, 11, 4082. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Möller, S.; Croning, M.D.R.; Apweiler, R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 2001, 17, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, P.; Moretti, S.; Xenarios, I.; Orobitg, M.; Montanyola, A.; Chang, J.M.; Taly, J.F.; Notredame, C. T-Coffee: A web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 2011, 39 (Suppl. S2), W13–W17. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2-A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Sønderby, C.K.; Sønderby, S.K.; Nielsen, H.; Winther, O. DeepLoc: Prediction of protein subcellular localization using deep learning. Bioinformatics 2017, 33, 3387–3395. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35 (Suppl. S2), W585–W587. [Google Scholar] [CrossRef]
- Chou, K.C.; Shen, H.B. Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Ba, A.N.; Pogoutse, A.; Provart, N.; Moses, A.M. NLStradamus: A simple Hidden Markov Model for nuclear localization signal prediction. BMC Bioinform. 2009, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Käll, L.; Krogh, A.; Sonnhammer, E.L. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 2004, 338, 1027–1036. [Google Scholar] [CrossRef]
- Wang, D.; Zeng, S.; Xu, C.; Qiu, W.; Liang, Y.; Joshi, T.; Xu, D. MusiteDeep: A deep-learning framework for general and kinase-specific phosphorylation site prediction. Bioinformatics 2017, 33, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liang, Y.; Xu, D. Capsule network for protein post-translational modification site prediction. Bioinformatics 2019, 35, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, D.; Yuchi, J.; He, F.; Jiang, Y.; Cai, S.; Li, J.; Xu, D. MusiteDeep: A deep-learning based webserver for protein post-translational modification site prediction and visualization. Nucleic Acids Res. 2021, 48, W140–W146. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Velarde-Buendía, A.; Ku-González, Á.; Carillo-Pech, M.; Ortega-Camacho, D.; Echevarría-Machado, I.; Pottosin, I.; Martínez-Estévez, M. Mechanisms of salt tolerance in habanero pepper plants (Capsicum chinense Jacq.): Proline accumulation, ions dynamics and sodium root-shoot partition and compartmentation. Front. Plant Sci. 2014, 5, 605. [Google Scholar] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lu, F.; Luo, Y.; Bie, L.; Xu, L.; Wang, Y. OrthoVenn3: An integrated platform for exploring and visualizing orthologous data across genomes. Nucleic Acids Res. 2023, 51, W397–W403. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; Von Mering, C.; et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013, 41, D808–D815. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software Environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]


| Protein ID | Protein Name | # Exons | # Introns | CDS (bp) | Amino Acids (aa) | pI | Mw (kDa) | Subcellular Localization | Signal Peptide |
|---|---|---|---|---|---|---|---|---|---|
| PHU20802.1 | CcGLR4.1 | 8 | 7 | 2703 | 901 | 8.34 | 100.07 | MP | 1–25 |
| PHU26278.1 | CcGLR4.2 | 5 | 4 | 2784 | 928 | 6.72 | 104.44 | MP | 1–24 |
| PHU20786.1 | CcGLR4.3 | 5 | 4 | 2703 | 901 | 8.23 | 99.93 | MP | 1–21 |
| PHU20798.1 | CcGLR4.4 | 5 | 4 | 2703 | 901 | 8.69 | 100.18 | MP | 1–22 |
| PHU14992.1 | CcGLR2.1 | 5 | 4 | 2823 | 941 | 5.95 | 104.60 | MP | NOT |
| PHU14985.1 | CcGLR2.2 | 5 | 4 | 2898 | 966 | 5.88 | 107.48 | MP | NOT |
| PHU14982.1 | CcGLR2.3 | 5 | 4 | 2934 | 978 | 6.72 | 109.34 | MP | NOT |
| PHU14980.1 | CcGLR2.4 | 5 | 4 | 2874 | 958 | 6.48 | 106.73 | MP | NOT |
| PHU14979.1 | CcGLR2.5 | 6 | 5 | 3396 | 1132 | 6.2 | 125.73 | MP/Nu’ | NOT |
| PHU08778.1 | CcGLR2.6 | 6 | 5 | 2739 | 913 | 6.12 | 101.69 | MP | NOT |
| PHU24790.1 | CcGLR2.7 | 9 | 8 | 3336 | 1112 | 8.63 | 124.04 | MP | 1–15 |
| PHU24792.1 | CcGLR2.8 | 5 | 4 | 2913 | 971 | 8.67 | 108.06 | MP | 1–21 |
| PHU24793.1 | CcGLR2.9 | 5 | 4 | 2745 | 915 | 6.76 | 101.43 | MP | NOT |
| PHU12201.1 | CcGLR3.1 | 6 | 5 | 2721 | 907 | 6.94 | 101.78 | MP | 1–19 |
| PHU12202.1 | CcGLR3.2 | 6 | 5 | 2829 | 943 | 6.22 | 104.76 | MP | 1–27 |
| PHU21095.1 | CcGLR3.3 | 6 | 5 | 2835 | 945 | 8.26 | 104.86 | MP | 1–33 |
| PHU26821.1 | CcGLR3.5 | 6 | 5 | 2808 | 936 | 6.51 | 104.26 | MP | 1–18 |
| Genes | Genes | Ka | Ks | Ka_Ks | Type of Duplication |
|---|---|---|---|---|---|
| CcGLR4.4 | CcGLR4.3 | 0.092388823 | 0.215387561 | 0.428942242 | Tandem |
| CcGLR2.2 | CcGLR2.1 | 0.09404683 | 0.292663192 | 0.321348336 | Tandem |
| CcGLR2.2 | CcGLR2.3 | 0.081302231 | 0.281706402 | 0.288606258 | Tandem |
| CcGLR2.3 | CcGLR2.7 | 0.486844643 | 2.313179137 | 0.210465603 | Segmental |
| CcGLR2.3 | CcGLR2.4 | 0.117837234 | 0.321347488 | 0.36669723 | Tandem |
| CcGLR2.4 | CcGLR2.5 | 0.136764142 | 0.328760158 | 0.415999746 | Tandem |
| CcGLR2.7 | CcGLR2.8 | 0.038893102 | 0.074861737 | 0.519532455 | Tandem |
| CcGLR2.8 | CcGLR2.9 | 0.275425921 | 1.216754722 | 0.226361087 | Tandem |
| CcGLR3.1 | CcGLR3.2 | 0.469517865 | 2.611359743 | 0.179798232 | Tandem |
| CcGLR3.3 | CcGLR3.5 | 0.315604474 | 1.822451838 | 0.173175756 | Segmental |
| String Protein | Amino Acids (AA) | Description | Orthologs in C. chinense | Amino Acids (AA) | Description |
|---|---|---|---|---|---|
| LOC104246719 GB1 Solyc01g109560.2.1 LOC104228583 | 377 | Guanine nucleotide-binding protein subunit beta-1 | PHU10599.1 | 377 | Guanine nucleotide-binding protein subunit beta |
| LOC104224515 XP_009606158.1 Solyc02g068340.2.1 | 1265 | Kinesin-like calmodulin-binding protein | PHU25623.1 | 1505 | Kinesin-like calmodulin-binding protein |
| LOC104214115 XP_009623672.1 Solyc11g005900.1.1 | 301 | Guanylate kinase 2 | PHU00386.1 | 301 | Guanylate kinase 3 |
| XP_009589304.1 | 139 | Protein cornichon homolog 4-like | PHU14641.1 | 139 | Protein cornichon -like protein 4 |
| XP_009607364.1 | 134 | Protein cornichon homolog 4-like | PHU17864.1 | 134 | Protein cornichon -like protein 4 |
| XP_009604924.1 | 135 | Protein cornichon homolog 4-like | PHU29157.1 | 134 | Protein cornichon -like protein 4 |
| XP_009616174.1 LOC104216467 | 402 | Guanylate kinase | PHU22297.1 | 424 | Guanylate kinase 1 |
| PGSC0003DMT400063334 | 431 | Presenilin | PHU23970.1 | 435 | Presenilin-like protein |
| PGSC0003DMT400075801 | 194 | Calcium-binding pollen allergen | PHU17438.1 | 191 | Putative calcium-binding protein CML15 |
| PGSC0003DMT400072494 | 426 | Presenilin | PHU05860.1 | 435 | Presenilin-like protein |
| A0A2G3D340 | 116 | 14-3-3-like protein B | PHU25375.1 | 116 | 14-3-3-like protein B |
| A0A2G3D887 | 310 | Plastidic glucose transporter 4 | PHU27196.1 | 310 | Plastidic glucose transporter 4 |
| A0A2G3D8D4 | 230 | Plastidic glucose transporter 4 | PHU27195.1 | 230 | Plastidic glucose transporter 4 |
| A0A2G3CNZ2 | 256 | 14-3-3 protein 10 | PHU20451.1 | 256 | 14-3-3 protein 10 |
| A0A2G3CE77 | 564 | Calcium-dependent protein kinase 1 | PHU17048.1 | 564 | Calcium-dependent protein kinase 1 |
| A0A2G3BWA2 A0A2G2XW54 | 604 | Glutamate receptor 3.2 | PHU10769.1 | 604 | Glutamate receptor 3.2 |
| A0A2G3BX92 | 446 | Nuclear pore complex protein NUP43 | PHU11111.1 | 446 | Nuclear pore complex protein NUP43 |
| A0A2G3B0C0 | 251 | 14-3-3 protein 10 | PHT99929.1 | 251 | 14-3-3 protein 10 |
| A0A2G3BE56 | 425 | MLO-like protein | PHU04736.1 | 425 | MLO-like protein 4 |
| LOC104219342 | 162 | Transcription factor HY5 isoform X1 | PHU22755.1 | 158 | Transcription factor HY5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-García, F.; García-Laynes, F.; Estrada-Tapia, G.; Monforte-González, M.; Martínez-Estevez, M.; Echevarría-Machado, I. In Silico Analysis of Glutamate Receptors in Capsicum chinense: Structure, Evolution, and Molecular Interactions. Plants 2024, 13, 812. https://doi.org/10.3390/plants13060812
León-García F, García-Laynes F, Estrada-Tapia G, Monforte-González M, Martínez-Estevez M, Echevarría-Machado I. In Silico Analysis of Glutamate Receptors in Capsicum chinense: Structure, Evolution, and Molecular Interactions. Plants. 2024; 13(6):812. https://doi.org/10.3390/plants13060812
Chicago/Turabian StyleLeón-García, Fabiola, Federico García-Laynes, Georgina Estrada-Tapia, Miriam Monforte-González, Manuel Martínez-Estevez, and Ileana Echevarría-Machado. 2024. "In Silico Analysis of Glutamate Receptors in Capsicum chinense: Structure, Evolution, and Molecular Interactions" Plants 13, no. 6: 812. https://doi.org/10.3390/plants13060812
APA StyleLeón-García, F., García-Laynes, F., Estrada-Tapia, G., Monforte-González, M., Martínez-Estevez, M., & Echevarría-Machado, I. (2024). In Silico Analysis of Glutamate Receptors in Capsicum chinense: Structure, Evolution, and Molecular Interactions. Plants, 13(6), 812. https://doi.org/10.3390/plants13060812






