Effects of Different Mulching Practices on Soil Environment and Fruit Quality in Peach Orchards
Abstract
:1. Introduction
2. Results
2.1. Effects of Different Mulching Practices on Soil Physiochemical Properties and Mineral Contents
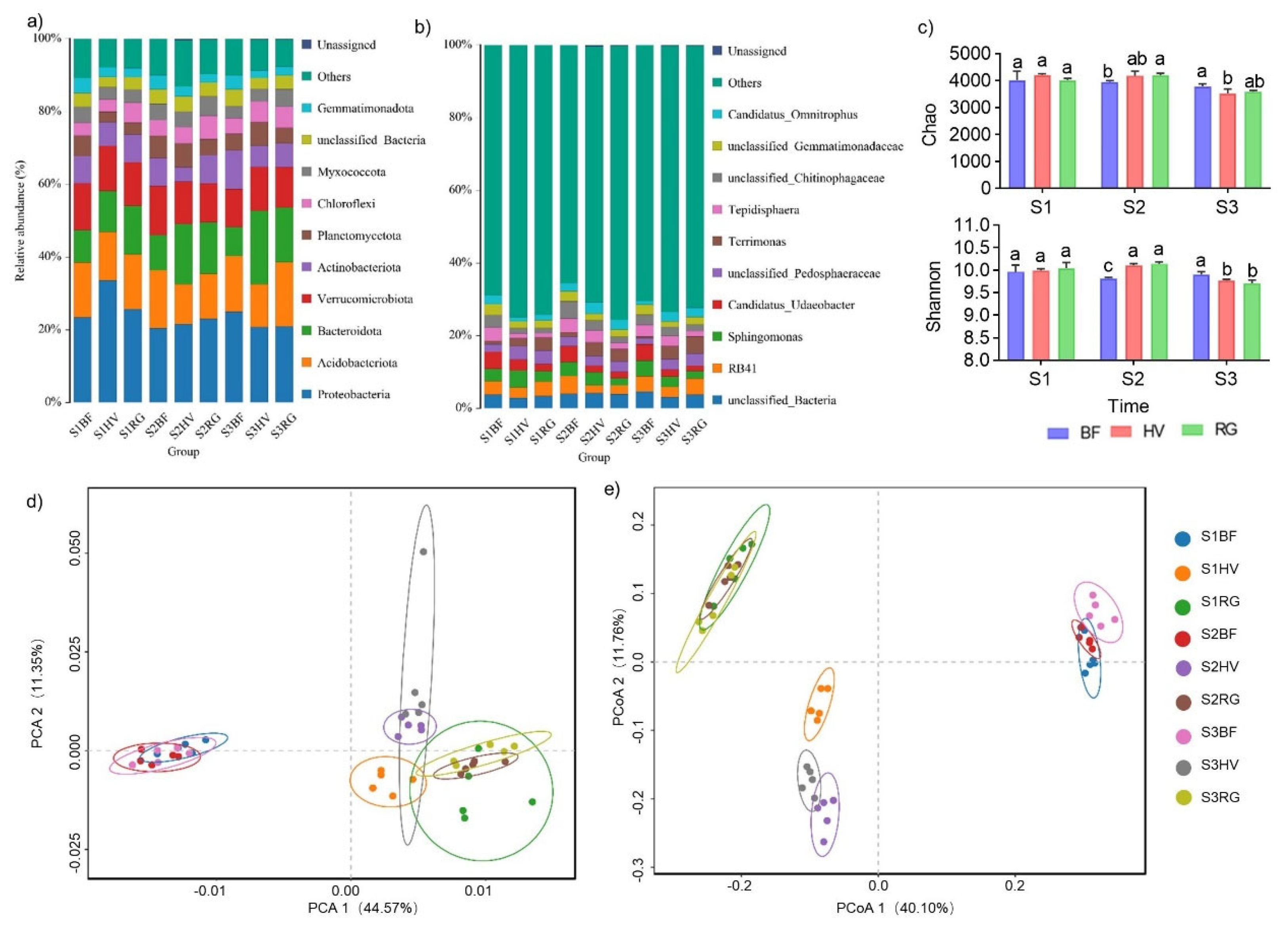
2.2. Effects of Different Mulching Practices on Soil Bacterial Communities
2.2.1. Effect of Mulching Practices on Soil Bacterial Abundance and Composition
2.2.2. Diversity Analysis of Bacterial Communities
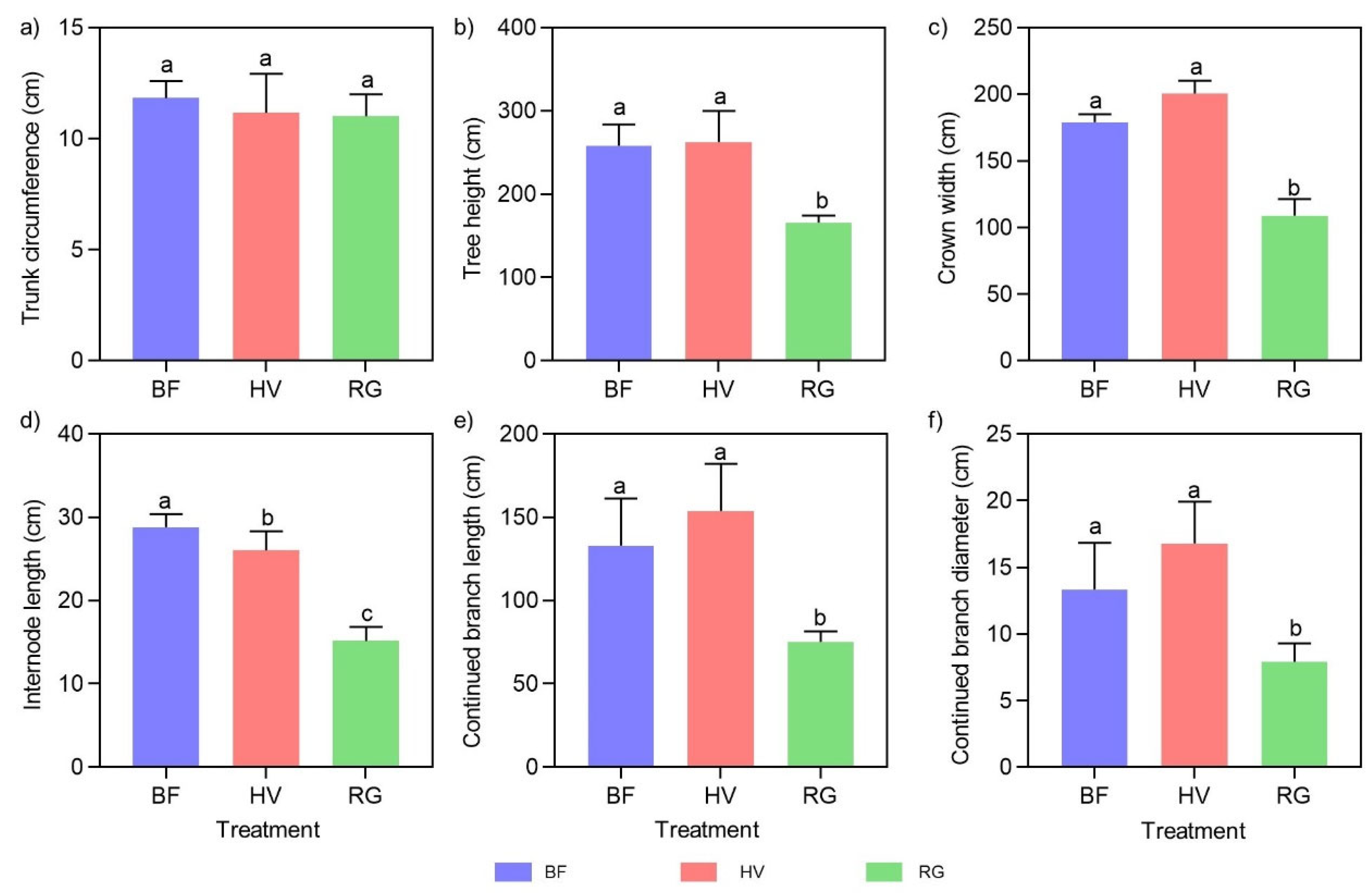
2.3. Effects of Mulching Practices on Tree Growth Indicators and Fruit Qualities
2.3.1. Effects of Mulching Practices on Tree Growth and Leaf Mineral Element Content
2.3.2. Effect of Mulching Practices on Peach Fruit Quality
2.4. Correlation Analysis between Soil Physicochemical Factors and Soil Microbial Communities
2.5. Correlation Analysis between Soil Physical and Chemical Properties and Fruit Quality
3. Discussion
3.1. Effects of Mulching Practices on Soil Characteristics, Microbial Community, and Nutrients
3.2. Effects of Mulching Practices on Tree Growth and Fruit Quality
4. Materials and Methods
4.1. Overview of the Test Sites
4.2. Test Materials
4.3. Test Methods
4.3.1. Soil Management
4.3.2. Soil Sampling and Collection
4.3.3. Determination of Soil Chemical Properties
4.3.4. Soil DNA Extraction, Amplification, and Sequencing
4.3.5. Tree Growth Index Measurement
4.3.6. Determination, Collection, and Sampling of Plant Tissues
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manganaris, G.A.; Minas, I.; Cirilli, M.; Torres, R.; Bassi, D.; Costa, G. Peach for the future: A specialty crop revisited. Sci. Hortic. 2022, 305, 111390. [Google Scholar] [CrossRef]
- Xiao, L.; Lai, S.; Chen, M.; Long, X.; Fu, X.; Yang, H. Effects of gras cultivation on soil arbuscular mycorrhizal fungi community in a tangerine orchard. Rhizosphere 2022, 24, 100583. [Google Scholar] [CrossRef]
- Singh, G.; Williard, K.W.J.; Schoonover, J.E. Cover crops and tillage influence on nitrogen dynamics in plant-soil-water pools. Soil Sci. Soc. Am. J. 2018, 82, 1572–1582. [Google Scholar] [CrossRef]
- Hobbs, P.R.; Sayre, K.; Gupta, R. The role of conservation agriculture in sustainable agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 543–555. [Google Scholar] [CrossRef]
- Daryanto, S.; Fu, B.; Wang, L.; Jacinthe, P.-A.; Zhao, W. Quantitative synthesis on the ecosystem services of cover crops. Earth-Sci. Rev. 2018, 185, 357–373. [Google Scholar] [CrossRef]
- Tejada, M.; Benítez, C. Effects of crushed maize straw residues on soil biological properties and soil restoration. Land Degrad. Dev. 2014, 25, 501–509. [Google Scholar] [CrossRef]
- Hammermeister, A.M. Organic weed management in perennial fruits. Sci. Hortic. 2016, 208, 28–42. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Luo, Y.; Awasthi, M.K.; Yang, J.; Duan, Y.; Li, H.; Zhao, Z. Mulching practices alter the bacterial-fungal community and network in favor of soil quality in a semiarid orchard system. Sci. Total Environ. 2020, 725, 138527. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Liu, C.; Ding, Y.; Liu, L.; Tian, Y.; Wu, X.; Li, H.; Awasthi, M.K.; Zhao, Z. Mulching practices alter soil microbial functional diversity and benefit to soil quality in orchards on the loess plateau. J. Environ. Manag. 2020, 271, 110985. [Google Scholar] [CrossRef]
- Atucha, A.; Merwin, I.A.; Brown, M.G.; Gardiazabal, F.; Mena, F.; Adriazola, C.; Lehmann, J. Soil erosion, runoff and nutrient losses in an avocado (Persea Americana Mill) hillside orchard under different groundcover management systems. Plant Soil 2013, 368, 393–406. [Google Scholar] [CrossRef]
- Taguas, E.V.; Vanderlinden, K.; Pedrera-Parrilla, A.; Giráldez, J.V.; Gómez, J.A. Spatial and temporal variability of spontaneous grass cover and its influence on sediment losses in an extensive olive orchard catchment. CATENA 2017, 157, 58–66. [Google Scholar] [CrossRef]
- Hoyt, G.D.; Hargrove, W.L. Legume cover crops for improving crop and soil management in the southern united states. HortScience 1986, 21, 397–402. [Google Scholar] [CrossRef]
- Li, T.; Gao, J.; Bai, L.; Wang, Y.; Huang, J.; Kumar, M.; Zeng, X. Influence of green manure and rice straw management on soil organic carbon, enzyme activities, and rice yield in red paddy soil. Soil Tillage Res. 2019, 195, 104428. [Google Scholar] [CrossRef]
- Milgroom, J.; Soriano, M.A.; Garrido, J.M.; Gómez, J.A.; Fereres, E. The influence of a shift from conventional to organic olive farming on soil management and erosion risk in southern Spain. Renew. Agric. Food Syst. 2007, 22, 1–10. [Google Scholar] [CrossRef]
- Sharma, P.; Laor, Y.; Raviv, M.; Medina, S.; Saadi, I.; Krasnovsky, A.; Vager, M.; Levy, G.J.; Bar-Tal, A.; Borisover, M. Green manure as part of organic management cycle: Effects on changes in organic matter characteristics across the soil profile. Geoderma 2017, 305, 197–207. [Google Scholar] [CrossRef]
- Wardle, D.A.; Yeates, G.W.; Bonner, K.I.; Nicholson, K.S.; Watson, R.N. Impacts of ground vegetation management strategies in a kiwifruit orchard on the composition and functioning of the soil biota. Soil Biol. Biochem. 2001, 33, 893–905. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, Y.; Liu, Y.; Zhang, S.; Yue, X.; Yao, B.; Xue, J.; Lv, W.; Zhang, L.; Xu, X.; et al. Factors shaping soil organic carbon stocks in grass covered orchards across China: A meta-analysis. Sci. Total Environ. 2022, 807, 150632. [Google Scholar] [CrossRef]
- Chen, G.; Liu, S.; Xiang, Y.; Tang, X.; Liu, H.; Yao, B.; Luo, X. Impact of living mulch on soil C:N:P stoichiometry in orchards across China: A meta-analysis examining climatic, edaphic, and biotic dependency. Pedosphere 2020, 30, 181–189. [Google Scholar] [CrossRef]
- Haruna, S.I.; Anderson, S.H.; Udawatta, R.P.; Gantzer, C.J.; Phillips, N.C.; Cui, S.; Gao, Y. Improving soil physical properties through the use of cover crops: A review. Agrosystems Geosci. Environ. 2020, 3, e20105. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, K.; Zhang, J.; Li, D.; Zhang, Y.; Xiang, H. Grass cultivation alters soil organic carbon fractions in a subtropical orchard of southern China. Soil Tillage Res. 2018, 181, 110–116. [Google Scholar] [CrossRef]
- Monteiro, A.; Lopes, C.M. Influence of cover crop on water use and performance of vineyard in mediterranean Portugal. Agric. Ecosyst. Environ. 2007, 121, 336–342. [Google Scholar] [CrossRef]
- Hoagland, L.; Carpenter-Boggs, L.; Granatstein, D.; Mazzola, M.; Smith, J.; Peryea, F.; Reganold, J.P. Orchard floor management effects on nitrogen fertility and soil biological activity in a newly established organic apple orchard. Biol. Fertil. Soils 2008, 45, 11–18. [Google Scholar] [CrossRef]
- Paušič, A.; Tojnko, S.; Lešnik, M. Permanent, undisturbed, in-row living mulch: A realistic option to replace glyphosate-dominated chemical weed control in intensive pear orchards. Agric. Ecosyst. Environ. 2021, 318, 107502. [Google Scholar] [CrossRef]
- Neilsen, G.; Forge, T.; Angers, D.; Neilsen, D.; Hogue, E. Suitable orchard floor management strategies in organic apple orchards that augment soil organic matter and maintain tree performance. Plant Soil 2014, 378, 325–335. [Google Scholar] [CrossRef]
- Vicente-Vicente, J.L.; Gómez-Muñoz, B.; Hinojosa-Centeno, M.B.; Smith, P.; Garcia-Ruiz, R. Carbon saturation and assessment of soil organic carbon fractions in mediterranean rainfed olive orchards under plant cover management. Agric. Ecosyst. Environ. 2017, 245, 135–146. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, X.; Sun, Y.; Zhang, J.; Wu, W.; Liao, Y. Mulching practices altered soil bacterial community structure and improved orchard productivity and apple quality after five growing seasons. Sci. Hortic. 2014, 172, 248–257. [Google Scholar] [CrossRef]
- Muscas, E.; Cocco, A.; Mercenaro, L.; Cabras, M.; Lentini, A.; Porqueddu, C.; Nieddu, G. Effects of vineyard floor cover crops on grapevine vigor, yield, and fruit quality, and the development of the vine mealybug under a mediterranean climate. Agric. Ecosyst. Environ. 2017, 237, 203–212. [Google Scholar] [CrossRef]
- Xi, Z.; Tao, Y.; Zhang, L.; Li, H. Impact of cover crops in vineyard on the aroma compounds of Vitis vinifera L. Cv Cabernet Sauvignon wine. Food Chem. 2011, 127, 516–522. [Google Scholar] [CrossRef]
- Bowen, P.; Freyman, S. Ground covers affect raspberry yield, photosynthesis, and nitrogen nutrition of primocanes. HortScience 1995, 30, 238–241. [Google Scholar] [CrossRef]
- Merwin, I.A.; Stiles, W.C. Orchard groundcover management impacts on apple tree growth and yield, and nutrient availability and uptake. J. Am. Soc. Hortic. Sci. 1994, 119, 209–215. [Google Scholar] [CrossRef]
- Chavarría, D.N.; Verdenelli, R.A.; Serri, D.L.; Restovich, S.B.; Andriulo, A.E.; Meriles, J.M.; Vargas-Gil, S. Effect of cover crops on microbial community structure and related enzyme activities and macronutrient availability. Eur. J. Soil Biol. 2016, 76, 74–82. [Google Scholar] [CrossRef]
- Mia, M.J.; Monaci, E.; Murri, G.; Massetani, F.; Facchi, J.; Neri, D. Soil nitrogen and weed biodiversity: An assessment under two orchard floor management practices in a nitrogen vulnerable zone in Italy. Horticulturae 2020, 6, 96. [Google Scholar] [CrossRef]
- Garbeva, P.; van Veen, J.A.; van Elsas, J.D. Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Vukicevich, E.; Lowery, T.; Bowen, P.; Úrbez-Torres, J.R.; Hart, M. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A Review. Agron. Sustain. Dev. 2016, 36, 48. [Google Scholar] [CrossRef]
- Trouvelot, S.; Bonneau, L.; Redecker, D.; van Tuinen, D.; Adrian, M.; Wipf, D. Arbuscular mycorrhiza symbiosis in viticulture: A review. Agron. Sustain. Dev. 2015, 35, 1449–1467. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, R.; Fu, L.; Tao, S.; Bao, J. Effects of orchard grass on soil fertility and nutritional status of fruit trees in Korla fragrant pear orchard. Horticulturae 2023, 9, 903. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Yue, S.; Tian, J.; Chen, H.; Jiang, H.; Siddique, K.H.M.; Zhan, A.; Fang, Q.; Yu, Q. Soil microbial community and network changes after long-term use of plastic mulch and nitrogen fertilization on semiarid farmland. Geoderma 2021, 396, 115086. [Google Scholar] [CrossRef]
- Qiu, Y.; Lv, W.; Wang, X.; Xie, Z.; Wang, Y. Long-Term Effects of Gravel Mulching and Straw Mulching on Soil Physicochemical Properties and Bacterial and Fungal Community Composition in the Loess Plateau of China. Eur. J. Soil Biol. 2020, 98, 103188. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Q.; Li, X.; Li, X.; Yu, Z.; Li, X.; Yang, T.; Su, Z.; Zhang, H.; Zhang, C. Effects of long-term no-tillage with different straw mulching frequencies on soil microbial community and the abundances of two soil-borne pathogens. Appl. Soil Ecol. 2020, 148, 103488. [Google Scholar] [CrossRef]
- Tu, A.; Xie, S.; Zheng, H.; Li, H.; Li, Y.; Mo, M. Long-term effects of living grass mulching on soil and water conservation and fruit yield of citrus orchard in south China. Agric. Water Manag. 2021, 252, 106897. [Google Scholar] [CrossRef]
- Zhu, L.; He, J.; Tian, Y.; Li, X.; Li, Y.; Wang, F.; Qin, K.; Wang, J. Intercropping wolfberry with gramineae plants improves productivity and soil quality. Sci. Hortic. 2022, 292, 110632. [Google Scholar] [CrossRef]
- Demoling, F.; Ola Nilsson, L.; Bååth, E. Bacterial and fungal response to nitrogen fertilization in three coniferous forest soils. Soil Biol. Biochem. 2008, 40, 370–379. [Google Scholar] [CrossRef]
- Tang, W.; Yang, H.; Wang, W.; Wang, C.; Pang, Y.; Chen, D.; Hu, X. Effects of living grass mulch on soil properties and assessment of soil quality in Chinese apple orchards: A meta-analysis. Agronomy 2022, 12, 1974. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Bailey, B.A. Role of Cover Crops in Improving soil and row crop productivity. Commun. Soil Sci. Plant Anal. 2005, 36, 2733–2757. [Google Scholar] [CrossRef]
- Guggenberger, G.; Zech, W. Composition and dynamics of dissolved carbohydrates and lignin-degradation products in two coniferous forests, N.E. Bavaria, Germany. Soil Biol. Biochem. 1994, 26, 19–27. [Google Scholar] [CrossRef]
- Kalbitz, K.; Solinger, S.; Park, J.-H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Sha, L.-Q.; Schaefer, D.A.; Zhang, Y.-P.; Song, Q.-H.; Tan, Z.-H.; Deng, Y.; Deng, X.-B.; Guan, H.-L. Direct effects of litter decomposition on soil dissolved organic carbon and nitrogen in a tropical rainforest. Soil Biol. Biochem. 2015, 81, 255–258. [Google Scholar] [CrossRef]
- Guo, Z.; Wan, S.; Hua, K.; Yin, Y.; Chu, H.; Wang, D.; Guo, X. Fertilization regime has a greater effect on soil microbial community structure than crop rotation and growth stage in an agroecosystem. Appl. Soil Ecol. 2020, 149, 103510. [Google Scholar] [CrossRef]
- Breidenbach, B.; Pump, J.; Dumont, M.G. Microbial Community structure in the rhizosphere of rice plants. Front. Microbiol. 2015, 6, 1537. [Google Scholar] [CrossRef]
- Zecchin, S.; Corsini, A.; Martin, M.; Cavalca, L. Influence of water management on the active root-associated microbiota involved in arsenic, iron, and sulfur cycles in rice paddies. Appl. Microbiol. Biotechnol. 2017, 101, 6725–6738. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.-X.; Zhang, N.; Hu, B.; Jin, T.; Xu, H.; Qin, Y.; Yan, P.; Zhang, X.; Guo, X.; et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 2019, 37, 676–684. [Google Scholar] [CrossRef]
- Roper, M.M.; Gupta, V.V.S.R. Enhancing non-symbiotic N2 fixation in agriculture. Open Agric. J. 2016, 10, 7–27. [Google Scholar] [CrossRef]
- Xie, C.-H.; Yokota, A. Reclassification of [Flavobacterium] Ferrugineum as Terrimonas Ferruginea Gen. Nov., Comb. Nov., and Description of Terrimonas Lutea Sp. Nov., Isolated from Soil. Int. J. Syst. Evol. Microbiol. 2006, 56, 1117–1121. [Google Scholar] [CrossRef]
- Willms, I.M.; Rudolph, A.Y.; Göschel, I.; Bolz, S.H.; Schneider, D.; Penone, C.; Poehlein, A.; Schöning, I.; Nacke, H. Globally abundant “Candidatus Udaeobacter” benefits from release of antibiotics in soil and potentially performs trace gas scavenging. mSphere 2020, 5, e00186-20. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Kannan, V.M.; Saritha, V.N.; Loganathachetti, D.S.; Mohan, M.; Krishnan, K.P. Bacterial diversity and community structure along the glacier foreland of Midtre Lovénbreen, Svalbard, arctic. Ecol. Indic. 2021, 126, 107704. [Google Scholar] [CrossRef]
- Gao, Y.; Yuan, L.; Du, J.; Wang, H.; Yang, X.; Duan, L.; Zheng, L.; Bahar, M.M.; Zhao, Q.; Zhang, W.; et al. Bacterial community profile of the crude oil-contaminated saline soil in the yellow river delta natural reserve, China. Chemosphere 2022, 289, 133207. [Google Scholar] [CrossRef]
- Leys, N.M.; Bastiaens, L.; Verstraete, W.; Springael, D. Influence of the carbon/nitrogen/phosphorus ratio on polycyclic aromatic hydrocarbon degradation by mycobacterium and Sphingomonas in soil. Appl. Microbiol. Biotechnol. 2005, 66, 726–736. [Google Scholar] [CrossRef]
- Canali, S.; Di Bartolomeo, E.; Trinchera, A.; Nisini, L.; Tittarelli, F.; Intrigliolo, F.; Roccuzzo, G.; Calabretta, M.L. Effect of different management strategies on soil quality of citrus orchards in southern Italy. Soil Use Manag. 2009, 25, 34–42. [Google Scholar] [CrossRef]
- Marcelle, R. Mineral nutrition and fruit quality. Acta Hortic. 1995, 383, 219–226. [Google Scholar] [CrossRef]
- TerAvest, D.; Smith, J.L.; Carpenter-Boggs, L.; Hoagland, L.; Granatstein, D.; Reganold, J.P. Influence of orchard floor management and compost application timing on nitrogen partitioning in apple trees. HortScience 2010, 45, 637–642. [Google Scholar] [CrossRef]
- Tesic, D.; Keller, M.; Hutton, R.J. Influence of vineyard floor management practices on grapevine vegetative growth, yield, and fruit composition. Am. J. Enol. Vitic. 2007, 58, 1–11. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Qin, Y.; Yan, H.; Jiang, X.; Qin, Y. Nitrogen deficiency maintains the yield and improves the antioxidant activity of coreopsis tinctoria Nutt. Biosci. Biotechnol. Biochem. 2021, 85, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- Cordts, J.M.; Scorza, R.; Bell, R.L. Effects of carbohydrates and nitrogen on the development of anthocyanins of a red leaf peach (Prunus persica (L.) Batsch) in vitro. Plant Cell Tissue Organ Cult. 1987, 9, 103–110. [Google Scholar] [CrossRef]
- Fallahi, E.; Mohan, S.K. Influence of nitrogen and rootstock on tree growth, precocity, fruit quality, leaf mineral nutrients, and fire blight in ‘Scarlet Gala’ apple. HortTechnology 2000, 10, 589–592. [Google Scholar] [CrossRef]
- Colaric, M.; Veberic, R.; Stampar, F.; Hudina, M. Evaluation of peach and nectarine fruit quality and correlations between sensory and chemical attributes. J. Sci. Food Agric. 2005, 85, 2611–2616. [Google Scholar] [CrossRef]
- Rubio Ames, Z.; Brecht, J.K.; Olmstead, M.A. Nitrogen fertilization rates in a subtropical peach orchard: Effects on tree vigor and fruit quality. J. Sci. Food Agric. 2020, 100, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.L. Kjeldahl method for total nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Ao, G.; Qin, W.; Wang, X.; Yu, M.; Feng, J.; Han, M.; Zhu, B. Linking the rhizosphere effects of 12 woody species on soil microbial activities with soil and root nitrogen status. Rhizosphere 2023, 28, 100809. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, P.; Wang, X.; Hakeem, A.; Niu, M.; Song, S.; Fang, J.; Shangguan, L. Comparative analysis of different bio-organic fertilizers on growth and rhizosphere environment of grapevine seedlings. Sci. Hortic. 2024, 324, 112587. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Li, S.; Zhang, T.; Zhang, W.; Zhai, P. Application of organic amendments to a coastal saline soil in north China: Effects on soil physical and chemical properties and tree growth. PLoS ONE 2014, 9, e89185. [Google Scholar] [CrossRef]
- Jin, J.; Fang, Y.; He, S.; Liu, Y.; Liu, C.; Li, F.; Khan, S.; Eltohamy, K.M.; Liu, B.; Liang, X. Improved phosphorus availability and reduced degree of phosphorus saturation by biochar-blended organic fertilizer addition to agricultural field soils. Chemosphere 2023, 317, 137809. [Google Scholar] [CrossRef] [PubMed]
- Corwin, D.L.; Scudiero, E. Field-scale apparent soil electrical conductivity. Soil Sci. Soc. Am. J. 2020, 84, 1405–1441. [Google Scholar] [CrossRef]
- Valette, T.; Leitwein, M.; Lascaux, J.-M.; Desmarais, E.; Berrebi, P.; Guinand, B. Redundancy analysis, genome-wide association studies and the pigmentation of brown trout (Salmo trutta L.). J. Fish Biol. 2023, 102, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Fang, X.; Wang, Z.; Shangguan, L.; Liu, T.; Chen, C.; Liu, Z.; Ge, M.; Zhang, C.; Zheng, T.; et al. Multi-omics analyses on the response mechanisms of ‘Shine Muscat’ grapevine to low degree of excess copper stress (Low-ECS). Environ. Pollut. 2021, 286, 117278. [Google Scholar] [CrossRef]
- Yan, X. HPLC for Carbohydrate Analysis. In HPLC Principle, Practices and Procedures; Zou, Y., Ed.; Nova Science Publisher: Hauppauge, NY, USA, 2014; p. 22. [Google Scholar]
- Zhang, B.-B.; Xu, J.-L.; Zhou, M.; Yan, D.-H.; Ma, R.-J. Effect of light quality on leaf photosynthetic characteristics and fruit quality of peach (Prunus persica L. Batch). Photosynthetica 2018, 56, 1113–1122. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-Pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]



| Physiological Indexes | Period | BF | HV | RG |
|---|---|---|---|---|
| Total N content of leaves (g·kg−1) | S1 | 28.02 ± 0.37 c | 33.09 ± 1.22 a | 22.45 ± 0.90 b |
| S2 | 25.64 ± 0.81 b | 29.10 ± 0.71 a | 25.33 ± 0.68 b | |
| S3 | 30.88 ± 1.31 a | 30.40 ± 0.54 a | 31.20 ± 0.51 a | |
| Total P content of leaves (g·kg−1) | S1 | 2.83 ± 0.20 a | 2.71 ± 0.07 a | 2.03 ± 0.11 b |
| S2 | 2.26 ± 0.13 b | 3.25 ± 0.15 a | 3.20 ± 0.13 a | |
| S3 | 2.64 ± 0.06 b | 2.62 ± 0.20 b | 3.58 ± 0.25 a | |
| Total K content of leaves (g·kg−1) | S1 | 19.61 ± 0.13 b | 17.82 ± 0.75 c | 21.42 ± 0.72 a |
| S2 | 10.82 ± 0.06 b | 15.27 ± 0.63 a | 14.98 ± 0.80 a | |
| S3 | 16.26 ± 0.25 a | 17.27 ± 0.73 a | 15.89 ± 0.44 a |
| Treatment | Single Fruit Weight/g | Longitudinal Diameter/cm | Diameter/cm | Side Diameter/cm | L* | a* | b* | h* |
|---|---|---|---|---|---|---|---|---|
| BF | 132.77 ± 12.98 b | 63.36 ± 4.4 b | 61.2 ± 5.05 b | 61.74 ± 4.78 b | 49.4 ± 4.11 a | 29.2 ± 0.89 b | 26.69 ± 4.35 a | 41.53 ± 5.78 a |
| HV | 161.51 ± 19.26 a | 66.74 ± 4.85 a | 69.82 ± 5.87 a | 68.15 ± 4.4 a | 46.75 ± 4.98 a | 28.67 ± 0.65 b | 23.2 ± 5.22 b | 37.57 ± 6.3 b |
| RG | 109.85 ± 15.76 c | 58.08 ± 3.76 c | 55.4 ± 3.86 c | 58.36 ± 4.01 c | 43.12 ± 3.91 b | 31.16 ± 1.27 a | 19.68 ± 3.42 c | 31.77 ± 2.68 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Liu, S.; Zhang, P.; Hakeem, A.; Song, H.; Yu, M.; Wang, F. Effects of Different Mulching Practices on Soil Environment and Fruit Quality in Peach Orchards. Plants 2024, 13, 827. https://doi.org/10.3390/plants13060827
Guo L, Liu S, Zhang P, Hakeem A, Song H, Yu M, Wang F. Effects of Different Mulching Practices on Soil Environment and Fruit Quality in Peach Orchards. Plants. 2024; 13(6):827. https://doi.org/10.3390/plants13060827
Chicago/Turabian StyleGuo, Lei, Siyu Liu, Peizhi Zhang, Abdul Hakeem, Hongfeng Song, Mingliang Yu, and Falin Wang. 2024. "Effects of Different Mulching Practices on Soil Environment and Fruit Quality in Peach Orchards" Plants 13, no. 6: 827. https://doi.org/10.3390/plants13060827




