Exchange or Eliminate: The Secrets of Algal-Bacterial Relationships
Abstract
:1. Introduction
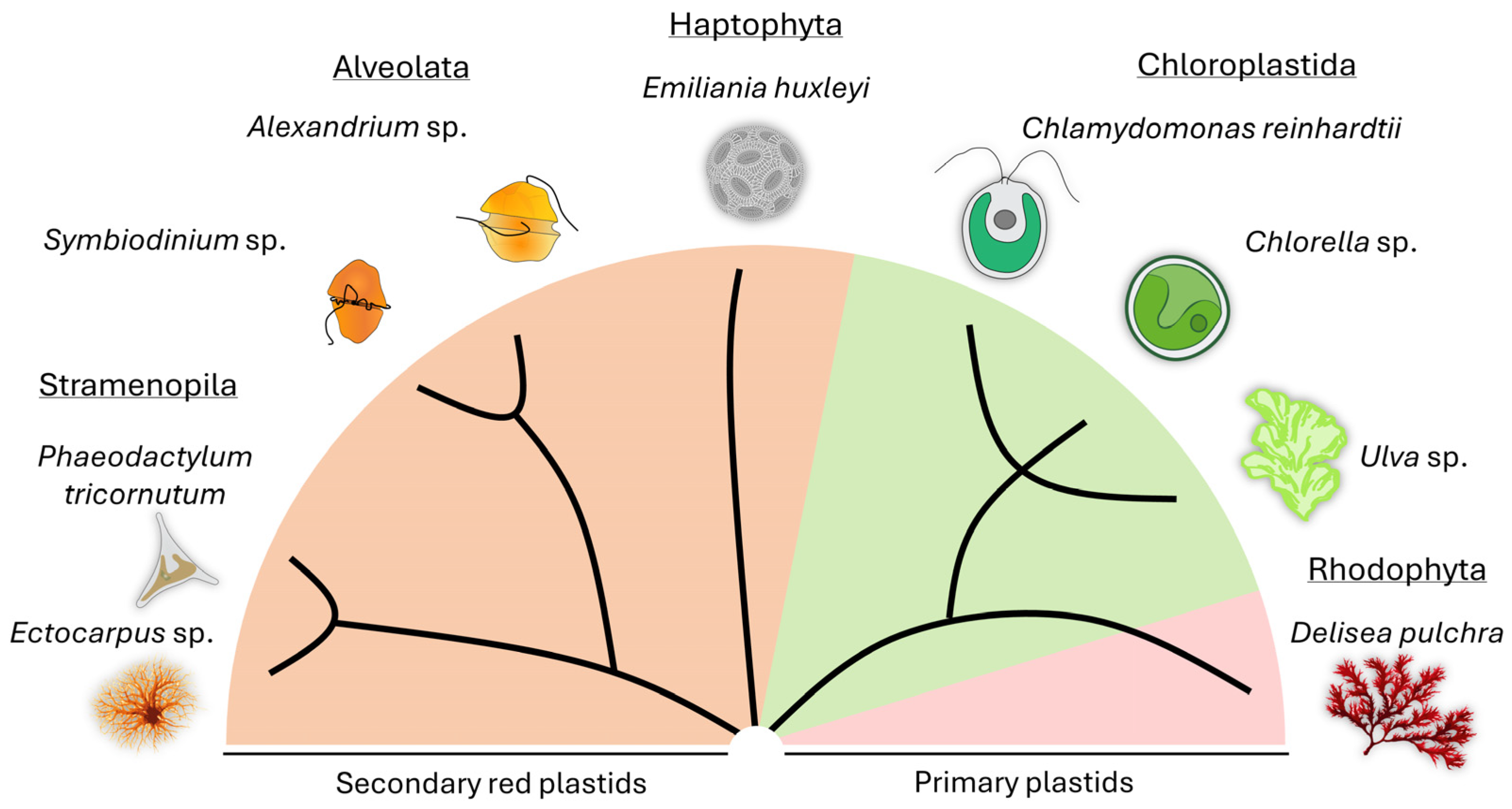
1.1. Definition, Phylogeny, Distribution, and Relevance of Algae
1.2. The Phycosphere and Algal-Microbial Interactions
2. Terrestrial and Freshwater Microalgae
2.1. The Green Microalga Chlamydomonas reinhardtii Emerges as a Soil Model
2.2. The Freshwater Green Alga Chlorella sp. and Its Potential for Biotechnology via Bacterial Interactions
3. Marine Microalgae
3.1. Phaeodactylum tricornutum: A Phytoplankton Diatom Model for Microbial Interactions
3.2. The Coccolithophore Emiliania huxleyi: An Algal Chameleon
3.3. Dinoflagellate Symbiotic Symbiodiniaceae and Toxic Alexandrium sp.
4. Marine Macroalgae
4.1. The Rhodophyceae Delisea pulchra and Its Bacterial Enemies
4.2. The Chlorophyceae Ulva sp. Needs Its Bacteria to Get in Shape
4.3. The Phaeophyceae Ectocarpus sp. Needs Bacteria for Its Shape, Sex, Fitness, and Environmental Adaptation
5. Conclusions and Futures Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHL | N-acyl-homoserine lactones |
| DMSP | Dimethylsulfoniopropionate |
| DOM | Dissolved organic matter |
| IAA | Indole-3-acetic acid |
| T6SS | Type 6 secretion system |
| TDA | Tropodithietic acid |
| TRP | Transient receptor potential |
| VOC | Volatile organic compounds |
References
- Parker, M.S.; Mock, T.; Armbrust, E.V. Genomic insights into marine microalgae. Annu. Rev. Genet. 2008, 42, 619–645. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.M.; De Clerck, O. Embracing algal models. Semin. Cell Dev. Biol. 2023, 134, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Gentil, J.; Hempel, F.; Moog, D.; Zauner, S.; Maier, U.G. Review: Origin of complex algae by secondary endosymbiosis: A journey through time. Protoplasma 2017, 254, 1835–1843. [Google Scholar] [CrossRef]
- Strassert, J.F.H.; Irisarri, I.; Williams, T.A.; Burki, F. A molecular timescale for eukaryote evolution with implications for the origin of red algal-derived plastids. Nat. Commun. 2021, 12, 1879. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.M.; Cock, J.M. Brown algal model organisms. Annu. Rev. Genet. 2020, 54, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Courties, C.; Vaquer, A.; Troussellier, M.; Lautier, J.; Chrétiennot-Dinet, M.J.; Neveux, J.; Machado, C.; Claustre, H. Smallest eukaryotic organism. Nature 1994, 370, 255. [Google Scholar] [CrossRef]
- Van Den Hoek, C.; Mann, D.G.; Jahns, H.M. Algae: An Introduction to Phycology; Cambridge University Press: Cambridge, MA, USA; New York, NY, USA, 1995; ISBN 978-0-521-30419-1. [Google Scholar]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 3rd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2022; ISBN 978-1-03-203512-3. [Google Scholar]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Falciatore, A.; Jaubert, M.; Bouly, J.-P.; Bailleul, B.; Mock, T. Diatom molecular research comes of age: Model species for studying phytoplankton biology and diversity. Plant Cell 2020, 32, 547–572. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Grube, M. Lichens redefined as complex ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef]
- Calatrava, V.; Tejada-Jimenez, M.; Sanz-Luque, E.; Fernandez, E.; Galvan, A.; Llamas, A. Chlamydomonas reinhardtii, a reference organism to study algal-microbial interactions: Why can’t they be friends? Plants 2023, 12, 788. [Google Scholar] [CrossRef]
- Krug, L.; Erlacher, A.; Markut, K.; Berg, G.; Cernava, T. The microbiome of alpine snow algae shows a specific inter-kingdom connectivity and algae-bacteria interactions with supportive capacities. ISME J. 2020, 14, 2197–2210. [Google Scholar] [CrossRef] [PubMed]
- Treves, H.; Siemiatkowska, B.; Luzarowska, U.; Murik, O.; Fernandez-Pozo, N.; Moraes, T.A.; Erban, A.; Armbruster, U.; Brotman, Y.; Kopka, J.; et al. Multi-omics reveals mechanisms of total resistance to extreme illumination of a desert alga. Nat. Plants 2020, 6, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.H. Marine food webs. In The Structure of Marine Ecosystems; Harvard University Press: Cambridge, MA, USA, 1974; pp. 2–98. ISBN 978-0-674-59251-3. [Google Scholar]
- McCutcheon, J.; Lutz, S.; Williamson, C.; Cook, J.M.; Tedstone, A.J.; Vanderstraeten, A.; Wilson, S.; Stockdale, A.; Bonneville, S.; Anesio, A.M.; et al. Mineral phosphorus drives glacier algal blooms on the Greenland Ice Sheet. Nat. Commun. 2021, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- B-Béres, V.; Stenger-Kovács, C.; Buczkó, K.; Padisák, J.; Selmeczy, G.B.; Lengyel, E.; Tapolczai, K. Ecosystem services provided by freshwater and marine diatoms. Hydrobiologia 2023, 850, 2707–2733. [Google Scholar] [CrossRef]
- Chung, I.K.; Oak, J.H.; Lee, J.A.; Shin, J.A.; Kim, J.G.; Park, K.-S. Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean Project Overview. ICES J. Mar. Sci. 2013, 70, 1038–1044. [Google Scholar] [CrossRef]
- Van Oostende, N.; Moerdijk-Poortvliet, T.C.W.; Boschker, H.T.S.; Vyverman, W.; Sabbe, K. Release of dissolved carbohydrates by Emiliania huxleyi and formation of transparent exopolymer particles depend on algal life cycle and bacterial activity. Environ. Microbiol. 2013, 15, 1514–1531. [Google Scholar] [CrossRef]
- Hartnett, H.E. Dissolved Organic Matter (DOM). In Encyclopedia of Engineering Geology; Bobrowsky, P., Marker, B., Eds.; Encyclopedia of Earth Sciences Series; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–3. ISBN 978-3-319-12127-7. [Google Scholar]
- González, J.M.; Simó, R.; Massana, R.; Covert, J.S.; Casamayor, E.O.; Pedrós-Alió, C.; Moran, M.A. Bacterial community structure associated with a dimethylsulfoniopropionate-producing North Atlantic algal bloom. Appl. Environ. Microbiol. 2000, 66, 4237–4246. [Google Scholar] [CrossRef]
- Durham, B.P.; Sharma, S.; Luo, H.; Smith, C.B.; Amin, S.A.; Bender, S.J.; Dearth, S.P.; Van Mooy, B.A.S.; Campagna, S.R.; Kujawinski, E.B.; et al. Cryptic carbon and sulfur cycling between surface ocean plankton. Proc. Natl. Acad. Sci. USA 2015, 112, 453–457. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Egan, S.; Harder, T.; Burke, C.; Steinberg, P.; Kjelleberg, S.; Thomas, T. The seaweed holobiont: Understanding seaweed-bacteria interactions. FEMS Microbiol. Rev. 2013, 37, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Araújo, R.M.; Assis, J.; Aguillar, R.; Airoldi, L.; Bárbara, I.; Bartsch, I.; Bekkby, T.; Christie, H.; Davoult, D.; Derrien-Courtel, S.; et al. Status, trends and drivers of kelp forests in Europe: An expert assessment. Biodivers. Conserv. 2016, 25, 1319–1348. [Google Scholar] [CrossRef]
- Diehl, N.; Li, H.; Scheschonk, L.; Burgunter-Delamare, B.; Niedzwiedz, S.; Forbord, S.; Sæther, M.; Bischof, K.; Monteiro, C. The sugar kelp Saccharina latissima I: Recent advances in a changing climate. Ann. Bot. 2024, 133, 183–212. [Google Scholar] [CrossRef] [PubMed]
- González-Olalla, J.M.; Medina-Sánchez, J.M.; Lozano, I.L.; Villar-Argaiz, M.; Carrillo, P. Climate-driven shifts in algal-bacterial interaction of high-mountain lakes in two years spanning a decade. Sci. Rep. 2018, 8, 10278. [Google Scholar] [CrossRef] [PubMed]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2008, 65, 535–543. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; FAO Fisheries Technical Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; ISBN 978-92-5-104958-7. [Google Scholar]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. 2014, 21, 6. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef]
- Indergaard, M.; Ostgaard, K. Polysaccharides for food and pharmaceutical uses. In Seaweed Resources in Europe: Uses and Potential; Guiry, M.D., Blunden, G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1991; pp. 169–183. ISBN 0-471-92947-6. [Google Scholar]
- Onen Cinar, S.; Chong, Z.K.; Kucuker, M.A.; Wieczorek, N.; Cengiz, U.; Kuchta, K. Bioplastic production from microalgae: A review. Int. J. Environ. Res. Public. Health 2020, 17, 3842. [Google Scholar] [CrossRef]
- Schmidtchen, L.; Roleda, M.Y.; Majschak, J.-P.; Mayser, M. Processing technologies for solid and flexible packaging materials from macroalgae. Algal Res. 2022, 61, 102300. [Google Scholar] [CrossRef]
- Karuppusamy, S.; Rajauria, G.; Fitzpatrick, S.; Lyons, H.; McMahon, H.; Curtin, J.; Tiwari, B.K.; O’Donnell, C. Biological properties and health-promoting functions of laminarin: A comprehensive review of preclinical and clinical studies. Mar. Drugs 2022, 20, 772. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae in medicine and human health. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 195–210. ISBN 978-0-12-811405-6. [Google Scholar]
- Blunden, G. Agricultural uses of seaweeds and seaweed extracts. In Seaweed Resources in Europe; Guiry, M.D., Blunden, G., Eds.; John Wiley & Sons: Chichester, UK, 1991; pp. 65–81. [Google Scholar]
- Castro, J.D.S.; Calijuri, M.L.; Ferreira, J.; Assemany, P.P.; Ribeiro, V.J. Microalgae based biofertilizer: A life cycle approach. Sci. Total Environ. 2020, 724, 138138. [Google Scholar] [CrossRef] [PubMed]
- Sæther, M.; Diehl, N.; Monteiro, C.; Huiru, L.; Niedzwiedz, S.; Burgunter-Delamare, B.; Scheschonk, L.; Bischof, K.; Forbord, S. The sugar kelp Saccharina latissima II: Recent advances in farming and applications. J. Appl. Phycol. 2024. [Google Scholar] [CrossRef]
- Khetkorn, W.; Rastogi, R.P.; Incharoensakdi, A.; Lindblad, P.; Madamwar, D.; Pandey, A.; Larroche, C. Microalgal hydrogen production—A review. Bioresour. Technol. 2017, 243, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- Branco-Vieira, M.; San Martin, S.; Agurto, C.; Freitas, M.A.V.; Martins, A.A.; Mata, T.M.; Caetano, N.S. Biotechnological potential of Phaeodactylum tricornutum for biorefinery processes. Fuel 2020, 268, 117357. [Google Scholar] [CrossRef]
- Kouhgardi, E.; Zendehboudi, S.; Mohammadzadeh, O.; Lohi, A.; Chatzis, I. Current status and future prospects of biofuel production from brown algae in North America: Progress and challenges. Renew. Sustain. Energy Rev. 2023, 172, 113012. [Google Scholar] [CrossRef]
- Broch, O.; Ellingsen, I.; Forbord, S.; Wang, X.; Volent, Z.; Alver, M.; Handå, A.; Andresen, K.; Slagstad, D.; Reitan, K.; et al. Modelling the cultivation and bioremediation potential of the kelp Saccharina latissima in close proximity to an exposed salmon farm in Norway. Aquac. Environ. Interact. 2013, 4, 187–206. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Bell, W.; Mitchell, R. Chemotactic and growth responses of marine bacteria to algal extracellular products. Biol. Bull. 1972, 143, 265–277. [Google Scholar] [CrossRef]
- Durán, P.; Flores-Uribe, J.; Wippel, K.; Zhang, P.; Guan, R.; Melkonian, B.; Melkonian, M.; Garrido-Oter, R. Shared features and reciprocal complementation of the Chlamydomonas and Arabidopsis microbiota. Nat. Commun. 2022, 13, 406. [Google Scholar] [CrossRef] [PubMed]
- Joint, I.; Tait, K.; Wheeler, G. Cross-kingdom signalling: Exploitation of bacterial quorum sensing molecules by the green seaweed Ulva. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Goecke, F.; Labes, A.; Wiese, J.; Imhoff, J. Chemical interactions between marine macroalgae and bacteria. Mar. Ecol. Prog. Ser. 2010, 409, 267–299. [Google Scholar] [CrossRef]
- Alsufyani, T.; Weiss, A.; Wichard, T. Time course exo-metabolomic profiling in the green marine macroalga Ulva (Chlorophyta) for identification of growth phase-dependent biomarkers. Mar. Drugs 2017, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Adouane, E.; Mercier, C.; Mamelle, J.; Willocquet, E.; Intertaglia, L.; Burgunter-Delamare, B.; Leblanc, C.; Rousvoal, S.; Lami, R.; Prado, S. Importance of quorum sensing crosstalk in the brown alga Saccharina latissima epimicrobiome. iScience 2024, 27, 109176. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.R.; Amin, S.A.; Raina, J.-B.; Stocker, R. Zooming in on the phycosphere: The ecological interface for phytoplankton-bacteria relationships. Nat. Microbiol. 2017, 2, 17065. [Google Scholar] [CrossRef] [PubMed]
- Wadhams, G.H.; Armitage, J.P. Making sense of it all: Bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 2004, 5, 1024–1037. [Google Scholar] [CrossRef]
- Stocker, R.; Seymour, J.R. Ecology and physics of bacterial chemotaxis in the ocean. Microbiol. Mol. Biol. Rev. 2012, 76, 792–812. [Google Scholar] [CrossRef]
- Amsler, C.D. Algal Chemical Ecology; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-74180-0. [Google Scholar]
- Govorunova, E.G.; Sineshchekov, O.A. Chemotaxis in the green flagellate alga Chlamydomonas. Biochemistry 2005, 70, 717–725. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Theis, K.R. Host biology in light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biol. 2015, 13, e1002226. [Google Scholar] [CrossRef]
- Rosenberg, E.; Zilber-Rosenberg, I. The hologenome concept of evolution after 10 years. Microbiome 2018, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Dittami, S.M.; Arboleda, E.; Auguet, J.-C.; Bigalke, A.; Briand, E.; Cárdenas, P.; Cardini, U.; Decelle, J.; Engelen, A.H.; Eveillard, D.; et al. A community perspective on the concept of marine holobionts: Current status, challenges, and future directions. PeerJ 2021, 9, e10911. [Google Scholar] [CrossRef] [PubMed]
- Brodie, J.; Ball, S.G.; Bouget, F.; Chan, C.X.; De Clerck, O.; Cock, J.M.; Gachon, C.; Grossman, A.R.; Mock, T.; Raven, J.A.; et al. Biotic interactions as drivers of algal origin and evolution. New Phytol. 2017, 216, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Hom, E.F.Y.; Aiyar, P.; Schaeme, D.; Mittag, M.; Sasso, S. A chemical perspective on microalgal-microbial interactions. Trends Plant Sci. 2015, 20, 689–693. [Google Scholar] [CrossRef]
- Cirri, E.; Pohnert, G. Algae-bacteria interactions that balance the planktonic microbiome. New Phytol. 2019, 223, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Tirichine, L.; Piganeau, G. Editorial: Algal symbiotic relationships in freshwater and marine environments. Front. Plant Sci. 2023, 14, 1155759. [Google Scholar] [CrossRef] [PubMed]
- Chomicki, G.; Kiers, E.T.; Renner, S.S. The evolution of mutualistic dependence. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 409–432. [Google Scholar] [CrossRef]
- Mathis, K.A.; Bronstein, J.L. Our current understanding of commensalism. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 167–189. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Su, J.; Tian, Y.; Ning, X.; Hong, H.; Zheng, T. Lysis of a red-tide causing alga, Alexandrium tamarense, caused by bacteria from its phycosphere. Biol. Control 2010, 52, 123–130. [Google Scholar] [CrossRef]
- Paul, C.; Pohnert, G. Interactions of the algicidal bacterium Kordia algicida with diatoms: Regulated protease excretion for specific algal lysis. PLoS ONE 2011, 6, e21032. [Google Scholar] [CrossRef]
- Aiyar, P.; Schaeme, D.; García-Altares, M.; Carrasco Flores, D.; Dathe, H.; Hertweck, C.; Sasso, S.; Mittag, M. Antagonistic bacteria disrupt calcium homeostasis and immobilize algal cells. Nat. Commun. 2017, 8, 1756. [Google Scholar] [CrossRef] [PubMed]
- Krespach, M.K.C.; García-Altares, M.; Flak, M.; Schoeler, H.; Scherlach, K.; Netzker, T.; Schmalzl, A.; Mattern, D.J.; Schroeckh, V.; Komor, A.; et al. Lichen-like association of Chlamydomonas reinhardtii and Aspergillus nidulans protects algal cells from bacteria. ISME J. 2020, 14, 2794–2805. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, D.C.; McKeown, D.A. Viruses of seaweeds. In Studies in Viral Ecology; Hurst, C.J., Ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 121–138. ISBN 978-1-119-60836-3. [Google Scholar]
- Lauritano, C.; Galasso, C. Microbial interactions between marine microalgae and fungi: From chemical ecology to biotechnological possible applications. Mar. Drugs 2023, 21, 310. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, G.; Kuhlisch, C.; Ziv, C.; Ben-Dor, S.; Malitsky, S.; Schatz, D.; Vardi, A. Lipid biomarkers for algal resistance to viral infection in the ocean. Proc. Natl. Acad. Sci. USA 2023, 120, e2217121120. [Google Scholar] [CrossRef] [PubMed]
- Walde, M.; Camplong, C.; De Vargas, C.; Baudoux, A.-C.; Simon, N. Viral infection impacts the 3D subcellular structure of the abundant marine diatom Guinardia delicatula. Front. Mar. Sci. 2023, 9, 1034235. [Google Scholar] [CrossRef]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef]
- Zuccaro, A.; Mitchell, J. Fungal communities of seaweeds. In The Fungal Community: Its Organization and Role in the Ecosystem; Dighton, J., White, J.F., Eds.; CRC Press: New York, NY, USA, 2005; pp. 533–580. ISBN 978-0-429-11640-7. [Google Scholar]
- Vallet, M.; Strittmatter, M.; Murúa, P.; Lacoste, S.; Dupont, J.; Hubas, C.; Genta-Jouve, G.; Gachon, C.M.M.; Kim, G.H.; Prado, S. Chemically-mediated interactions between macroalgae, their fungal endophytes, and protistan pathogens. Front. Microbiol. 2018, 9, 3161. [Google Scholar] [CrossRef]
- Tourneroche, A.; Lami, R.; Hubas, C.; Blanchet, E.; Vallet, M.; Escoubeyrou, K.; Paris, A.; Prado, S. Bacterial-fungal interactions in the kelp endomicrobiota drive autoinducer-2 quorum sensing. Front. Microbiol. 2019, 10, 1693. [Google Scholar] [CrossRef]
- Tourneroche, A.; Lami, R.; Burgaud, G.; Domart-Coulon, I.; Li, W.; Gachon, C.; Gèze, M.; Boeuf, D.; Prado, S. The bacterial and fungal microbiota of Saccharina latissima (Laminariales, Phaeophyceae). Front. Mar. Sci. 2020, 7, 587566. [Google Scholar] [CrossRef]
- Pauli, J.N.; Mendoza, J.E.; Steffan, S.A.; Carey, C.C.; Weimer, P.J.; Peery, M.Z. A syndrome of mutualism reinforces the lifestyle of a sloth. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133006. [Google Scholar] [CrossRef]
- Carrasco Flores, D.; Fricke, M.; Wesp, V.; Desirò, D.; Kniewasser, A.; Hölzer, M.; Marz, M.; Mittag, M. A marine Chlamydomonas sp. emerging as an algal model. J. Phycol. 2021, 57, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Mock, T. Algal model species for advancing biological sciences. J. Phycol. 2023, 59, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Burki, F.; Roger, A.J.; Brown, M.W.; Simpson, A.G.B. The new tree of Eukaryotes. Trends Ecol. Evol. 2020, 35, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Findinier, J.; Grossman, A.R. Chlamydomonas: Fast tracking from genomics. J. Phycol. 2023, 59, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Maréchal-Drouard, L.; et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef]
- Li, X.; Patena, W.; Fauser, F.; Jinkerson, R.E.; Saroussi, S.; Meyer, M.T.; Ivanova, N.; Robertson, J.M.; Yue, R.; Zhang, R.; et al. A genome-wide algal mutant library and functional screen identifies genes required for eukaryotic photosynthesis. Nat. Genet. 2019, 51, 627–635. [Google Scholar] [CrossRef]
- Fauser, F.; Vilarrasa-Blasi, J.; Onishi, M.; Ramundo, S.; Patena, W.; Millican, M.; Osaki, J.; Philp, C.; Nemeth, M.; Salomé, P.A.; et al. Systematic characterization of gene function in the photosynthetic alga Chlamydomonas reinhardtii. Nat. Genet. 2022, 54, 705–714. [Google Scholar] [CrossRef]
- Craig, R.J.; Gallaher, S.D.; Shu, S.; Salomé, P.A.; Jenkins, J.W.; Blaby-Haas, C.E.; Purvine, S.O.; O’Donnell, S.; Barry, K.; Grimwood, J.; et al. The Chlamydomonas Genome Project, version 6: Reference assemblies for mating-type plus and minus strains reveal extensive structural mutation in the laboratory. Plant Cell 2023, 35, 644–672. [Google Scholar] [CrossRef]
- Harris, E.H. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use; Academic Press Inc.: San Diego, CA, USA, 1989; ISBN 978-1-4832-8860-4. [Google Scholar]
- Sasso, S.; Stibor, H.; Mittag, M.; Grossman, A.R. From molecular manipulation of domesticated Chlamydomonas reinhardtii to survival in nature. eLife 2018, 7, e39233. [Google Scholar] [CrossRef]
- Cooper, M.B.; Smith, A.G. Exploring mutualistic interactions between microalgae and bacteria in the omics age. Curr. Opin. Plant Biol. 2015, 26, 147–153. [Google Scholar] [CrossRef]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, K.E.; Collins, S.; Kazamia, E.; Purton, S.; Wheeler, G.L.; Smith, A.G. Fundamental shift in vitamin B12 eco-physiology of a model alga demonstrated by experimental evolution. ISME J. 2015, 9, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Bunbury, F.; Deery, E.; Sayer, A.P.; Bhardwaj, V.; Harrison, E.L.; Warren, M.J.; Smith, A.G. Exploring the onset of B12-based mutualisms using a recently evolved Chlamydomonas auxotroph and B12-producing bacteria. Environ. Microbiol. 2022, 24, 3134–3147. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Bishop, S.; Stessman, D.; Wright, D.; Spalding, M.H.; Halverson, L.J. Chlamydomonas reinhardtii thermal tolerance enhancement mediated by a mutualistic interaction with vitamin B12-producing bacteria. ISME J. 2013, 7, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Teplitski, M.; Chen, H.; Rajamani, S.; Gao, M.; Merighi, M.; Sayre, R.T.; Robinson, J.B.; Rolfe, B.G.; Bauer, W.D. Chlamydomonas reinhardtii secretes compounds that mimic bacterial signals and interfere with quorum sensing regulation in bacteria. Plant Physiol. 2004, 134, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Calatrava, V.; Hom, E.F.Y.; Llamas, Á.; Fernández, E.; Galván, A. OK, thanks! A new mutualism between Chlamydomonas and methylobacteria facilitates growth on amino acids and peptides. FEMS Microbiol. Lett. 2018, 365, fny021. [Google Scholar] [CrossRef] [PubMed]
- Calatrava, V.; Hom, E.F.Y.; Guan, Q.; Llamas, A.; Fernández, E.; Galván, A. Genetic evidence for algal auxin production in Chlamydomonas and its role in algal-bacterial mutualism. iScience 2024, 27, 108762. [Google Scholar] [CrossRef] [PubMed]
- Fakhimi, N.; Torres, M.J.; Fernández, E.; Galván, A.; Dubini, A.; González-Ballester, D. Chlamydomonas reinhardtii and Microbacterium forte sp. nov., a mutualistic association that favors sustainable hydrogen production. Sci. Total Environ. 2024, 913, 169559. [Google Scholar] [CrossRef]
- Hotter, V.; Zopf, D.; Kim, H.J.; Silge, A.; Schmitt, M.; Aiyar, P.; Fleck, J.; Matthäus, C.; Hniopek, J.; Yan, Q.; et al. A polyyne toxin produced by an antagonistic bacterium blinds and lyses a Chlamydomonad alga. Proc. Natl. Acad. Sci. USA 2021, 118, e2107695118. [Google Scholar] [CrossRef]
- Rose, M.M.; Scheer, D.; Hou, Y.; Hotter, V.S.; Komor, A.J.; Aiyar, P.; Scherlach, K.; Vergara, F.; Yan, Q.; Loper, J.E.; et al. The bacterium Pseudomonas protegens antagonizes the microalga Chlamydomonas reinhardtii using a blend of toxins. Environ. Microbiol. 2021, 23, 5525–5540. [Google Scholar] [CrossRef]
- Bando, Y.; Hou, Y.; Seyfarth, L.; Probst, J.; Götze, S.; Bogacz, M.; Hellmich, U.A.; Stallforth, P.; Mittag, M.; Arndt, H. Total synthesis and structure correction of the cyclic lipodepsipeptide Orfamide A. Chem. Eur. J. 2022, 28, e202104417. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Bando, Y.; Carrasco Flores, D.; Hotter, V.; Das, R.; Schiweck, B.; Melzer, T.; Arndt, H.; Mittag, M. A cyclic lipopeptide produced by an antagonistic bacterium relies on its tail and transient receptor potential-type Ca2+ channels to immobilize a green alga. New Phytol. 2023, 237, 1620–1635. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.; Loper, J.E. Genomics of secondary metabolite production by Pseudomonas spp. Nat. Prod. Rep. 2009, 26, 1408. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Kobayashi, H.; Furukawa, J.; Namikoshi, M.; Okuda, S.; Sato, Z.; Matsuda, I.; Noda, T. Studies on macrocyclic lactone antibiotics. VII Structure of a phytotoxin “rhizoxin” produced by Rhizopus chinensis. J. Antibiot. 1984, 37, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yi, L.; Ren, S.; Yin, Q.; Xiang, W.; Zhang, X.; Xie, B. Algicidal interaction between Paenibacillus polymyxa MEZ6 and microalgae. J. Appl. Microbiol. 2022, 133, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Krespach, M.K.C.; Stroe, M.C.; Flak, M.; Komor, A.J.; Nietzsche, S.; Sasso, S.; Hertweck, C.; Brakhage, A.A. Bacterial marginolactones trigger formation of algal gloeocapsoids, protective aggregates on the verge of multicellularity. Proc. Natl. Acad. Sci. USA 2021, 118, e2100892118. [Google Scholar] [CrossRef] [PubMed]
- Cecchin, M.; Marcolungo, L.; Rossato, M.; Girolomoni, L.; Cosentino, E.; Cuine, S.; Li-Beisson, Y.; Delledonne, M.; Ballottari, M. Chlorella vulgaris genome assembly and annotation reveals the molecular basis for metabolic acclimation to high light conditions. Plant J. 2019, 100, 1289–1305. [Google Scholar] [CrossRef]
- Hovde, B.T.; Hanschen, E.R.; Steadman Tyler, C.R.; Lo, C.-C.; Kunde, Y.; Davenport, K.; Daligault, H.; Msanne, J.; Canny, S.; Eyun, S.; et al. Genomic characterization reveals significant divergence within Chlorella sorokiniana (Chlorellales, Trebouxiophyceae). Algal Res. 2018, 35, 449–461. [Google Scholar] [CrossRef]
- Wu, T.; Li, L.; Jiang, X.; Yang, Y.; Song, Y.; Chen, L.; Xu, X.; Shen, Y.; Gu, Y. Sequencing and comparative analysis of three Chlorella genomes provide insights into strain-specific adaptation to wastewater. Sci. Rep. 2019, 9, 9514. [Google Scholar] [CrossRef]
- Blanc, G.; Duncan, G.; Agarkova, I.; Borodovsky, M.; Gurnon, J.; Kuo, A.; Lindquist, E.; Lucas, S.; Pangilinan, J.; Polle, J.; et al. The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 2010, 22, 2943–2955. [Google Scholar] [CrossRef]
- Chow, K.-C.; Tung, W.L. Electrotransformation of Chlorella vulgaris. Plant Cell Rep. 1999, 18, 778–780. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhang, Y.; Chen, X.; Zhang, P.; Ma, S. Development of a new method for genetic transformation of the green alga Chlorella ellipsoidea. Mol. Biotechnol. 2013, 54, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Goud, V.V.; Yamamoto, Y.; Sahoo, L. Efficient Agrobacterium tumefaciens-mediated stable genetic transformation of green microalgae, Chlorella sorokiniana. 3 Biotech 2021, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-R.; Ng, I.-S. Development of CRISPR/Cas9 system in Chlorella vulgaris FSP-E to enhance lipid accumulation. Enzyme Microb. Technol. 2020, 133, 109458. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, C.D.; Colwell, R.R.; Prescott, J.M. Numerical taxonomy of heterotrophic bacteria growing in association with continuous-culture Chlorella sorokiniana. Appl. Microbiol. 1969, 18, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Takihana, N.; Aoyagi, H.; Hanada, S.; Watanabe, Y.; Ohmura, N.; Saiki, H.; Tanaka, H. Symbiotic association in Chlorella culture. FEMS Microbiol. Ecol. 2005, 51, 187–196. [Google Scholar] [CrossRef]
- Park, Y.; Je, K.-W.; Lee, K.; Jung, S.-E.; Choi, T.-J. Growth promotion of Chlorella ellipsoidea by co-inoculation with Brevundimonas sp. isolated from the microalga. Hydrobiologia 2008, 598, 219–228. [Google Scholar] [CrossRef]
- Thi Vu, H.; Otsuka, S.; Ueda, H.; Senoo, K. Cocultivated bacteria can increase or decrease the culture lifetime of Chlorella vulgaris. J. Gen. Appl. Microbiol. 2010, 56, 413–418. [Google Scholar] [CrossRef]
- Amavizca, E.; Bashan, Y.; Ryu, C.-M.; Farag, M.A.; Bebout, B.M.; de-Bashan, L.E. Enhanced performance of the microalga Chlorella sorokiniana remotely induced by the plant growth-promoting bacteria Azospirillum brasilense and Bacillus pumilus. Sci. Rep. 2017, 7, 41310. [Google Scholar] [CrossRef]
- Pereg, L.; de-Bashan, L.E.; Bashan, Y. Assessment of affinity and specificity of Azospirillum for plants. Plant Soil 2016, 399, 389–414. [Google Scholar] [CrossRef]
- Gonzalez, L.E.; Bashan, Y. Increased growth of the microalga Chlorella vulgaris when coimmobilized and cocultured in alginate beads with the plant-growth-promoting bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2000, 66, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- De-Bashan, L.E.; Antoun, H.; Bashan, Y. Involvement of indole-3-acetic acid produced by the growth-promoting bacterium Azospirillum spp. in promoting growth of Chlorella vulgaris. J. Phycol. 2008, 44, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Van Puyvelde, S.; Cloots, L.; Engelen, K.; Das, F.; Marchal, K.; Vanderleyden, J.; Spaepen, S. Transcriptome analysis of the rhizosphere bacterium Azospirillum brasilense reveals an extensive auxin response. Microb. Ecol. 2011, 61, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Cassan, F.D.; Coniglio, A.; Amavizca, E.; Maroniche, G.; Cascales, E.; Bashan, Y.; de-Bashan, L.E. The Azospirillum brasilense type VI secretion system promotes cell aggregation, biocontrol protection against phytopathogens and attachment to the microalgae Chlorella sorokiniana. Environ. Microbiol. 2021, 23, 6257–6274. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wang, Z.; Liu, F.; Wu, Z.; Chen, H.; Tang, D.; Liu, J. Effect of complex iron on the phosphorus absorption by two freshwater algae. Environ. Technol. 2021, 42, 4125–4133. [Google Scholar] [CrossRef]
- Amin, S.A.; Green, D.H.; Hart, M.C.; Küpper, F.C.; Sunda, W.G.; Carrano, C.J. Photolysis of iron-siderophore chelates promotes bacterial-algal mutualism. Proc. Natl. Acad. Sci. USA 2009, 106, 17071–17076. [Google Scholar] [CrossRef] [PubMed]
- Rawat, D.; Sharma, U.; Poria, P.; Finlan, A.; Parker, B.; Sharma, R.S.; Mishra, V. Iron-dependent mutualism between Chlorella sorokiniana and Ralstonia pickettii forms the basis for a sustainable bioremediation system. ISME Commun. 2022, 2, 83. [Google Scholar] [CrossRef]
- Gromov, B.V.; Mamkaeva, K.A. Electron microscopic study of parasitism by Bdellovibrio chlorellavorus bacteria on cells of the green alga Chlorella vulgaris. Tsitologiia 1972, 14, 256–260. [Google Scholar]
- Soo, R.M.; Woodcroft, B.J.; Parks, D.H.; Tyson, G.W.; Hugenholtz, P. Back from the dead; the curious tale of the predatory cyanobacterium Vampirovibrio chlorellavorus. PeerJ 2015, 3, e968. [Google Scholar] [CrossRef]
- Landa, M.; Burns, A.S.; Durham, B.P.; Esson, K.; Nowinski, B.; Sharma, S.; Vorobev, A.; Nielsen, T.; Kiene, R.P.; Moran, M.A. Sulfur metabolites that facilitate oceanic phytoplankton–bacteria carbon flux. ISME J. 2019, 13, 2536–2550. [Google Scholar] [CrossRef]
- Moran, M.A.; Durham, B.P. Sulfur metabolites in the pelagic ocean. Nat. Rev. Microbiol. 2019, 17, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Vallet, M.; Pohnert, G. Temporal and spatial signaling mediating the balance of the plankton microbiome. Annu. Rev. Mar. Sci. 2022, 14, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.A.; Hmelo, L.R.; van Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Branscombe, L.; Harrison, E.L.; Choong, Z.Y.D.; Swink, C.; Keys, M.; Widdicombe, C.; Wilson, W.H.; Cunliffe, M.; Helliwell, K. Cryptic bacterial pathogens of diatoms peak during senescence of a winter diatom bloom. New Phytol. 2024, 241, 1292–1307. [Google Scholar] [CrossRef] [PubMed]
- Shibl, A.A.; Isaac, A.; Ochsenkühn, M.A.; Cárdenas, A.; Fei, C.; Behringer, G.; Arnoux, M.; Drou, N.; Santos, M.P.; Gunsalus, K.C.; et al. Diatom modulation of select bacteria through use of two unique secondary metabolites. Proc. Natl. Acad. Sci. USA 2020, 117, 27445–27455. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Falciatore, A. Phaeodactylum tricornutum . Trends Genet. 2019, 35, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.T.; Rogato, A.; Jaubert, M.; Karas, B.J.; Falciatore, A. Phaeodactylum tricornutum: An established model species for diatom molecular research and an emerging chassis for algal synthetic biology. J. Phycol. 2023, 59, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Allen, A.E.; Badger, J.H.; Grimwood, J.; Jabbari, K.; Kuo, A.; Maheswari, U.; Martens, C.; Maumus, F.; Otillar, R.P.; et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef]
- Apt, K.E.; Grossman, A.R.; Kroth-Pancic, P.G. Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Mol. Gen. Genet. 1996, 252, 572–579. [Google Scholar] [CrossRef]
- De Riso, V.; Raniello, R.; Maumus, F.; Rogato, A.; Bowler, C.; Falciatore, A. Gene silencing in the marine diatom Phaeodactylum tricornutum. Nucleic Acids Res. 2009, 37, e96. [Google Scholar] [CrossRef]
- Xie, W.-H.; Zhu, C.-C.; Zhang, N.-S.; Li, D.-W.; Yang, W.-D.; Liu, J.-S.; Sathishkumar, R.; Li, H.-Y. Construction of novel chloroplast expression vector and development of an efficient transformation system for the diatom Phaeodactylum tricornutum. Mar. Biotechnol. 2014, 16, 538–546. [Google Scholar] [CrossRef]
- De Martino, A.; Meichenin, A.; Shi, J.; Pan, K.; Bowler, C. Genetic and phenotypic characterization of Phaeodactylum tricornutum (Bacillariophyceae) accessions. J. Phycol. 2007, 43, 992–1009. [Google Scholar] [CrossRef]
- Chorazyczewski, A.M.; Huang, I.; Abdulla, H.; Mayali, X.; Zimba, P.V. The influence of bacteria on the growth, lipid production, and extracellular metabolite accumulation by Phaeodactylum tricornutum (Bacillariophyceae). J. Phycol. 2021, 57, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.W.; Berube, P.M.; Follett, C.L.; Waterbury, J.B.; Chisholm, S.W.; DeLong, E.F.; Repeta, D.J. Closely related phytoplankton species produce similar suites of dissolved organic matter. Front. Microbiol. 2014, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kimbrel, J.A.; Vaiana, C.A.; Wollard, J.R.; Mayali, X.; Buie, C.R. Bacterial response to spatial gradients of algal-derived nutrients in a porous microplate. ISME J. 2022, 16, 1036–1045. [Google Scholar] [CrossRef]
- Brisson, V.; Swink, C.; Kimbrel, J.; Mayali, X.; Samo, T.; Kosina, S.M.; Thelen, M.; Northen, T.R.; Stuart, R.K. Dynamic Phaeodactylum tricornutum exometabolites shape surrounding bacterial communities. New Phytol. 2023, 239, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Daly, G.; Decorosi, F.; Viti, C.; Adessi, A. Shaping the phycosphere: Analysis of the EPS in diatom-bacterial co-cultures. J. Phycol. 2023, 59, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Daly, G.; Perrin, E.; Viti, C.; Fondi, M.; Adessi, A. Scaling down the microbial loop: Data-driven modelling of growth interactions in a diatom-bacterium co-culture. Environ. Microbiol. Rep. 2021, 13, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Mayali, X.; Samo, T.J.; Kimbrel, J.A.; Morris, M.M.; Rolison, K.; Swink, C.; Ramon, C.; Kim, Y.-M.; Munoz-Munoz, N.; Nicora, C.; et al. Single-cell isotope tracing reveals functional guilds of bacteria associated with the diatom Phaeodactylum tricornutum. Nat. Commun. 2023, 14, 5642. [Google Scholar] [CrossRef] [PubMed]
- Zecher, K.; Hayes, K.R.; Philipp, B. Evidence of interdomain ammonium cross-feeding from methylamine- and glycine betaine-degrading Rhodobacteraceae to diatoms as a widespread interaction in the marine phycosphere. Front. Microbiol. 2020, 11, 533894. [Google Scholar] [CrossRef]
- Dow, L.; Stock, F.; Peltekis, A.; Szamosvári, D.; Prothiwa, M.; Lapointe, A.; Böttcher, T.; Bailleul, B.; Vyverman, W.; Kroth, P.G.; et al. The multifaceted inhibitory effects of an alkylquinolone on the diatom Phaeodactylum tricornutum. ChemBioChem 2020, 21, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, C.; Langer, G.; Wheeler, G.L. Coccolithophore calcification: Changing paradigms in changing oceans. Acta Biomater. 2021, 120, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Read, B.A.; Kegel, J.; Klute, M.J.; Kuo, A.; Lefebvre, S.C.; Maumus, F.; Mayer, C.; Miller, J.; Monier, A.; Salamov, A.; et al. Pan genome of the phytoplankton Emiliania underpins its global distribution. Nature 2013, 499, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wang, X.; Su, J.; Li, J.; Zeng, J.; Li, G.; Liu, J. Transformation of coccolithophorid Emiliania huxleyi harboring a marine virus (Coccolithoviruses) serine palmitoyltransferase (SPT) gene by electroporation. J. Oceanol. Limnol. 2021, 39, 693–704. [Google Scholar] [CrossRef]
- Frank, O.; Pradella, S.; Rohde, M.; Scheuner, C.; Klenk, H.-P.; Göker, M.; Petersen, J. Complete genome sequence of the Phaeobacter gallaeciensis type strain CIP 105210T (= DSM 26640T = BS107T). Stand. Genomic Sci. 2014, 9, 914–932. [Google Scholar] [CrossRef] [PubMed]
- Seyedsayamdost, M.R.; Case, R.J.; Kolter, R.; Clardy, J. The Jekyll-and-Hyde chemistry of Phaeobacter gallaeciensis. Nat. Chem. 2011, 3, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Seyedsayamdost, M.R.; Wang, R.; Kolter, R.; Clardy, J. Hybrid biosynthesis of roseobacticides from algal and bacterial precursor molecules. J. Am. Chem. Soc. 2014, 136, 15150–15153. [Google Scholar] [CrossRef]
- Segev, E.; Castañeda, I.S.; Sikes, E.L.; Vlamakis, H.; Kolter, R. Bacterial influence on alkenones in live microalgae. J. Phycol. 2016, 52, 125–130. [Google Scholar] [CrossRef]
- Wang, R.; Gallant, É.; Seyedsayamdost, M.R. Investigation of the genetics and biochemistry of roseobacticide production in the Roseobacter clade bacterium Phaeobacter inhibens. mBio 2016, 7, e02118-15. [Google Scholar] [CrossRef]
- Segev, E.; Wyche, T.P.; Kim, K.H.; Petersen, J.; Ellebrandt, C.; Vlamakis, H.; Barteneva, N.; Paulson, J.N.; Chai, L.; Clardy, J.; et al. Dynamic metabolic exchange governs a marine algal-bacterial interaction. eLife 2016, 5, e17473. [Google Scholar] [CrossRef]
- Beiralas, R.; Ozer, N.; Segev, E. Abundant Sulfitobacter marine bacteria protect Emiliania huxleyi algae from pathogenic bacteria. ISME Commun. 2023, 3, 100. [Google Scholar] [CrossRef]
- Barak-Gavish, N.; Dassa, B.; Kuhlisch, C.; Nussbaum, I.; Brandis, A.; Rosenberg, G.; Avraham, R.; Vardi, A. Bacterial lifestyle switch in response to algal metabolites. eLife 2023, 12, e84400. [Google Scholar] [CrossRef] [PubMed]
- Câmara dos Reis, M.; Romac, S.; Le Gall, F.; Marie, D.; Frada, M.J.; Koplovitz, G.; Cariou, T.; Henry, N.; De Vargas, C.; Jeanthon, C. Exploring the phycosphere of Emiliania huxleyi: From bloom dynamics to microbiome assembly experiments. Mol. Ecol. 2023, 32, 6507–6522. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Mittag, M.; Sczekan, S.; Morse, D.; Hastings, J.W. Molecular cloning and genomic organization of a gene for luciferin-binding protein from the dinoflagellate Gonyaulax polyedra. J. Biol. Chem. 1993, 268, 8842–8850. [Google Scholar] [CrossRef] [PubMed]
- Jaeckisch, N.; Yang, I.; Wohlrab, S.; Glöckner, G.; Kroymann, J.; Vogel, H.; Cembella, A.; John, U. Comparative genomic and transcriptomic characterization of the toxigenic marine dinoflagellate Alexandrium ostenfeldii. PLoS ONE 2011, 6, e28012. [Google Scholar] [CrossRef]
- Fogel, M.; Hastings, J.W. Bioluminescence: Mechanism and mode of control of scintillon activity. Proc. Natl. Acad. Sci. USA 1972, 69, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Hastings, J.W. Chemistries and colors of bioluminescent reactions: A review. Gene 1996, 173, 5–11. [Google Scholar] [CrossRef]
- Jacobovitz, M.R.; Hambleton, E.A.; Guse, A. Unlocking the complex cell biology of coral–dinoflagellate symbiosis: A model systems approach. Annu. Rev. Genet. 2023, 57, 411–434. [Google Scholar] [CrossRef]
- Dougan, K.E.; González-Pech, R.A.; Stephens, T.G.; Shah, S.; Chen, Y.; Ragan, M.A.; Bhattacharya, D.; Chan, C.X. Genome-powered classification of microbial eukaryotes: Focus on coral algal symbionts. Trends Microbiol. 2022, 30, 831–840. [Google Scholar] [CrossRef]
- Liu, H.; Stephens, T.G.; González-Pech, R.A.; Beltran, V.H.; Lapeyre, B.; Bongaerts, P.; Cooke, I.; Aranda, M.; Bourne, D.G.; Forêt, S.; et al. Symbiodinium genomes reveal adaptive evolution of functions related to coral-dinoflagellate symbiosis. Commun. Biol. 2018, 1, 95. [Google Scholar] [CrossRef]
- González-Pech, R.A.; Stephens, T.G.; Chen, Y.; Mohamed, A.R.; Cheng, Y.; Shah, S.; Dougan, K.E.; Fortuin, M.D.A.; Lagorce, R.; Burt, D.W.; et al. Comparison of 15 dinoflagellate genomes reveals extensive sequence and structural divergence in family Symbiodiniaceae and genus Symbiodinium. BMC Biol. 2021, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Wuerz, M.; Lawson, C.A.; Oakley, C.A.; Possell, M.; Wilkinson, S.P.; Grossman, A.R.; Weis, V.M.; Suggett, D.J.; Davy, S.K. Symbiont identity impacts the microbiome and volatilome of a model cnidarian-dinoflagellate symbiosis. Biology 2023, 12, 1014. [Google Scholar] [CrossRef] [PubMed]
- Maire, J.; Deore, P.; Jameson, V.J.; Sakkas, M.; Perez-Gonzalez, A.; Blackall, L.L.; Van Oppen, M.J.H. Assessing the contribution of bacteria to the heat tolerance of experimentally evolved coral photosymbionts. Environ. Microbiol. 2023, 25, 3298–3318. [Google Scholar] [CrossRef] [PubMed]
- Deschaseaux, E.; O’Brien, J.; Siboni, N.; Petrou, K.; Seymour, J.R. Shifts in dimethylated sulfur concentrations and microbiome composition in the red-tide causing dinoflagellate Alexandrium minutum during a simulated marine heatwave. Biogeosciences 2019, 16, 4377–4391. [Google Scholar] [CrossRef]
- Anderson, D.M. Bloom dynamics of toxic Alexandrium species in the northeastern U.S. Limnol. Oceanogr. 1997, 42, 1009–1022. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z. Identification and genomic analysis of Pseudosulfitobacter koreense sp. nov. isolated from toxin-producing dinoflagellate Alexandrium pacificum. Arch. Microbiol. 2023, 205, 245. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jiang, Z.; Zhou, X.; Zhang, R.; Xie, Z.; Zhang, S.; Wu, Y.; Ge, Y.; Zhang, X. Haliea alexandrii sp. nov., isolated from phycosphere microbiota of the toxin-producing dinoflagellate Alexandrium catenella. Int. J. Syst. Evol. Microbiol. 2020, 70, 1133–1138. [Google Scholar] [CrossRef]
- Amaro, A.M.; Fuentes, M.S.; Ogalde, S.R.; Venegas, J.A.; Suárez-Isla, B.A. Identification and characterization of potentially algal-lytic marine bacteria strongly associated with the toxic dinoflagellate Alexandrium catenella. J. Eukaryot. Microbiol. 2005, 52, 191–200. [Google Scholar] [CrossRef]
- Hattenrath-Lehmann, T.K.; Gobler, C.J. Identification of unique microbiomes associated with harmful algal blooms caused by Alexandrium fundyense and Dinophysis acuminata. Harmful Algae 2017, 68, 17–30. [Google Scholar] [CrossRef]
- Choi, C.J.; Jauzein, C.; Erdner, D.L. High-resolution phylogenetic analysis reveals long-term microbial dynamics and microdiversity in phytoplankton microbiome. J. Eukaryot. Microbiol. 2023, 70, e12966. [Google Scholar] [CrossRef]
- Hollants, J.; Leliaert, F.; De Clerck, O.; Willems, A. What we can learn from sushi: A review on seaweed-bacterial associations. FEMS Microbiol. Ecol. 2013, 83, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Burgunter-Delamare, B.; Rousvoal, S.; Legeay, E.; Tanguy, G.; Fredriksen, S.; Boyen, C.; Dittami, S.M. The Saccharina latissima microbiome: Effects of region, season, and physiology. Front. Microbiol. 2023, 13, 1050939. [Google Scholar] [CrossRef] [PubMed]
- Ricker, R.W. Taxonomy and Biogeography of Macquarie Island Seaweeds; British Museum (Natural History): London, UK, 1987; ISBN 978-0-565-00998-4. [Google Scholar]
- Bonin, D.R.; Hawkes, M.W. Systematics and life histories of New Zealand Bonnemaisoniaceae (Bonnemaisoniales, Rhodophyta): II. The genus Delisea. N. Z. J. Bot. 1988, 26, 619–632. [Google Scholar] [CrossRef]
- Millar, A. Marine red algae of the Coffs Harbour region, northern New South Wales. Aust. Syst. Bot. 1990, 3, 293. [Google Scholar] [CrossRef]
- Harder, T.; Campbell, A.H.; Egan, S.; Steinberg, P.D. Chemical mediation of ternary interactions between marine holobionts and their environment as exemplified by the red alga Delisea pulchra. J. Chem. Ecol. 2012, 38, 442–450. [Google Scholar] [CrossRef]
- Case, R.J.; Longford, S.R.; Campbell, A.H.; Low, A.; Tujula, N.; Steinberg, P.D.; Kjelleberg, S. Temperature induced bacterial virulence and bleaching disease in a chemically defended marine macroalga: Temperature, disease and algal chemical defense. Environ. Microbiol. 2011, 13, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Zozaya-Valdes, E.; Kjelleberg, S.; Thomas, T.; Egan, S. Multiple opportunistic pathogens can cause a bleaching disease in the red seaweed Delisea pulchra. Environ. Microbiol. 2016, 18, 3962–3975. [Google Scholar] [CrossRef]
- Campbell, A.H.; Vergés, A.; Steinberg, P.D. Demographic consequences of disease in a habitat-forming seaweed and impacts on interactions between natural enemies. Ecology 2014, 95, 142–152. [Google Scholar] [CrossRef]
- Campbell, A.H.; Harder, T.; Nielsen, S.; Kjelleberg, S.; Steinberg, P.D. Climate change and disease: Bleaching of a chemically defended seaweed. Glob. Chang. Biol. 2011, 17, 2958–2970. [Google Scholar] [CrossRef]
- Hudson, J.; Deshpande, N.; Leblanc, C.; Egan, S. Pathogen exposure leads to a transcriptional downregulation of core cellular functions that may dampen the immune response in a macroalga. Mol. Ecol. 2022, 31, 3468–3480. [Google Scholar] [CrossRef]
- Longford, S.; Tujula, N.; Crocetti, G.; Holmes, A.; Holmström, C.; Kjelleberg, S.; Steinberg, P.; Taylor, M. Comparisons of diversity of bacterial communities associated with three sessile marine eukaryotes. Aquat. Microb. Ecol. 2007, 48, 217–229. [Google Scholar] [CrossRef]
- Zozaya-Valdés, E.; Roth-Schulze, A.J.; Egan, S.; Thomas, T. Microbial community function in the bleaching disease of the marine macroalgae Delisea pulchra. Environ. Microbiol. 2017, 19, 3012–3024. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.; Steinberg, P.; Rusch, D.; Kjelleberg, S.; Thomas, T. Community structure and functional gene profile of bacteria on healthy and diseased thalli of the red seaweed Delisea pulchra. PLoS ONE 2012, 7, e50854. [Google Scholar] [CrossRef] [PubMed]
- De Nys, R.; Wright, A.D.; König, G.M.; Sticher, O. New halogenated furanones from the marine alga Delisea pulchra (cf. fimbriata). Tetrahedron 1993, 49, 11213–11220. [Google Scholar] [CrossRef]
- Steinberg, P.D.; De Nys, R.; Kjelleberg, S. Chemical cues for surface colonization. J. Chem. Ecol. 2002, 28, 1935–1951. [Google Scholar] [CrossRef]
- Dworjanyn, S.A.; De Nys, R.; Steinberg, P.D. Localisation and surface quantification of secondary metabolites in the red alga Delisea pulchra. Mar. Biol. 1999, 133, 727–736. [Google Scholar] [CrossRef]
- Wright, J.T.; Zuccarello, G.C.; Steinberg, P.D. Genetic structure of the subtidal red alga Delisea pulchra. Mar. Biol. 2000, 136, 439–448. [Google Scholar] [CrossRef]
- Wright, J.; De Nys, R.; Steinberg, P. Geographic variation in halogenated furanones from the red alga Delisea pulchra and associated herbivores and epiphytes. Mar. Ecol. Prog. Ser. 2000, 207, 227–241. [Google Scholar] [CrossRef]
- Zang, T.; Lee, B.W.K.; Cannon, L.M.; Ritter, K.A.; Dai, S.; Ren, D.; Wood, T.K.; Zhou, Z.S. A naturally occurring brominated furanone covalently modifies and inactivates LuxS. Bioorg. Med. Chem. Lett. 2009, 19, 6200–6204. [Google Scholar] [CrossRef]
- Manefield, M.; de Nys, R.; Naresh, K.; Roger, R.; Givskov, M.; Peter, S.; Kjelleberg, S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 1999, 145, 283–291. [Google Scholar] [CrossRef]
- Manefield, M.; Rasmussen, T.B.; Henzter, M.; Andersen, J.B.; Steinberg, P.; Kjelleberg, S.; Givskov, M. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 2002, 148, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Weinberger, F.; Saha, M.; Majzoub, M.E.; Egan, S. Cross-host protection of marine bacteria against macroalgal disease. Microb. Ecol. 2022, 84, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- De Clerck, O.; Kao, S.-M.; Bogaert, K.A.; Blomme, J.; Foflonker, F.; Kwantes, M.; Vancaester, E.; Vanderstraeten, L.; Aydogdu, E.; Boesger, J.; et al. Insights into the evolution of multicellularity from the sea lettuce genome. Curr. Biol. 2018, 28, 2921–2933.e5. [Google Scholar] [CrossRef] [PubMed]
- Alcolombri, U.; Ben-Dor, S.; Feldmesser, E.; Levin, Y.; Tawfik, D.S.; Vardi, A. Identification of the algal dimethyl sulfide–releasing enzyme: A missing link in the marine sulfur cycle. Science 2015, 348, 1466–1469. [Google Scholar] [CrossRef]
- Kessler, R.W.; Weiss, A.; Kuegler, S.; Hermes, C.; Wichard, T. Macroalgal-bacterial interactions: Role of dimethylsulfoniopropionate in microbial gardening by Ulva (Chlorophyta). Mol. Ecol. 2018, 27, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Kirst, G.O.; Wiencke, C. Dimethylsulphoniopropionate (DMSP) accumulation in green macroalgae from polar to temperate regions: Interactive effects of light versus salinity and light versus temperature. Polar Biol. 1992, 12, 603–607. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Puglisi, M.P. DMSP in marine macroalgae and macroinvertebrates: Distribution, function, and ecological impacts. Aquat. Sci. 2007, 69, 394–402. [Google Scholar] [CrossRef]
- Van Der Loos, L.M.; D’hondt, S.; Engelen, A.H.; Pavia, H.; Toth, G.B.; Willems, A.; Weinberger, F.; De Clerck, O.; Steinhagen, S. Salinity and host drive Ulva-associated bacterial communities across the Atlantic-Baltic Sea gradient. Mol. Ecol. 2022, 32, 6260–6277. [Google Scholar] [CrossRef]
- Burke, C.; Thomas, T.; Lewis, M.; Steinberg, P.; Kjelleberg, S. Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. ISME J. 2011, 5, 590–600. [Google Scholar] [CrossRef]
- Gemin, M.; Peña-Rodríguez, A.; Quiroz-Guzmán, E.; Magallón-Servín, P.; Barajas-Sandoval, D.; Elizondo-González, R. Growth-promoting bacteria for the green seaweed Ulva clathrata. Aquac. Res. 2019, 50, 3741–3748. [Google Scholar] [CrossRef]
- Singh, R.; Mantri, V.; Reddy, C.; Jha, B. Isolation of seaweed-associated bacteria and their morphogenesis-inducing capability in axenic cultures of the green alga Ulva fasciata. Aquat. Biol. 2011, 12, 13–21. [Google Scholar] [CrossRef]
- Ghaderiardakani, F.; Coates, J.C.; Wichard, T. Bacteria-induced morphogenesis of Ulva intestinalis and Ulva mutabilis (Chlorophyta): A contribution to the lottery theory. FEMS Microbiol. Ecol. 2017, 93, fix094. [Google Scholar] [CrossRef]
- Vesty, E.F.; Kessler, R.W.; Wichard, T.; Coates, J.C. Regulation of gametogenesis and zoosporogenesis in Ulva linza (Chlorophyta): Comparison with Ulva mutabilis and potential for laboratory culture. Front. Plant Sci. 2015, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Spoerner, M.; Wichard, T.; Bachhuber, T.; Stratmann, J.; Oertel, W. Growth and thallus morphogenesis of Ulva mutabilis (Chlorophyta) depends on a combination of two bacterial species excreting regulatory factors. J. Phycol. 2012, 48, 1433–1447. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Nishijima, M.; Nishimura, M.; Kuwano, K.; Saga, N. Bacteria that induce morphogenesis in Ulva pertusa (Chlorophyta) grown under axenic conditions. J. Phycol. 1996, 32, 479–482. [Google Scholar] [CrossRef]
- Tait, K.; Joint, I.; Daykin, M.; Milton, D.L.; Williams, P.; Camara, M. Disruption of quorum sensing in seawater abolishes attraction of zoospores of the green alga Ulva to bacterial biofilms. Environ. Microbiol. 2005, 7, 229–240. [Google Scholar] [CrossRef]
- Wichard, T. Exploring bacteria-induced growth and morphogenesis in the green macroalga order Ulvales (Chlorophyta). Front. Plant Sci. 2015, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Wichard, T. From model organism to application: Bacteria-induced growth and development of the green seaweed Ulva and the potential of microbe leveraging in algal aquaculture. Semin. Cell Dev. Biol. 2023, 134, 69–78. [Google Scholar] [CrossRef]
- Kessler, R.W.; Crecelius, A.C.; Schubert, U.S.; Wichard, T. In situ monitoring of molecular changes during cell differentiation processes in marine macroalgae through mass spectrometric imaging. Anal. Bioanal. Chem. 2017, 409, 4893–4903. [Google Scholar] [CrossRef]
- Vallet, M.; Kaftan, F.; Grabe, V.; Ghaderiardakani, F.; Fenizia, S.; Svatoš, A.; Pohnert, G.; Wichard, T. A new glance at the chemosphere of macroalgal-bacterial interactions: In situ profiling of metabolites in symbiosis by mass spectrometry. Beilstein J. Org. Chem. 2021, 17, 1313–1322. [Google Scholar] [CrossRef]
- Alsufyani, T.; Califano, G.; Deicke, M.; Grueneberg, J.; Weiss, A.; Engelen, A.H.; Kwantes, M.; Mohr, J.F.; Ulrich, J.F.; Wichard, T. Macroalgal-bacterial interactions: Identification and role of thallusin in morphogenesis of the seaweed Ulva (Chlorophyta). J. Exp. Bot. 2020, 71, 3340–3349. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Suzuki, M.; Kasai, H.; Shizuri, Y.; Harayama, S. Isolation and phylogenetic characterization of bacteria capable of inducing differentiation in the green alga Monostroma oxyspermum. Environ. Microbiol. 2003, 5, 25–35. [Google Scholar] [CrossRef]
- Matsuo, Y.; Imagawa, H.; Nishizawa, M.; Shizuri, Y. Isolation of an algal morphogenesis inducer from a marine bacterium. Science 2005, 307, 1598. [Google Scholar] [CrossRef] [PubMed]
- Wichard, T. Identification of metallophores and organic ligands in the chemosphere of the marine macroalga Ulva (Chlorophyta) and at land-sea interfaces. Front. Mar. Sci. 2016, 3, 131. [Google Scholar] [CrossRef]
- Comba González, N.; Ramírez Hoyos, M.; López Kleine, L.; Montoya Castaño, D. Production of enzymes and siderophores by epiphytic bacteria isolated from the marine macroalga Ulva lactuca. Aquat. Biol. 2018, 27, 107–118. [Google Scholar] [CrossRef]
- Wienecke, P.; Ulrich, J.F.; Morales-Reyes, C.F.; Dhiman, S.; Wichard, T.; Arndt, H. Enantioselective total synthesis of the morphogen (−)-thallusin and mediated uptake of Fe(III) into the green seaweed Ulva. Chem. Eur. J. 2024, e202304007. [Google Scholar] [CrossRef]
- Charrier, B.; Coelho, S.M.; Le Bail, A.; Tonon, T.; Michel, G.; Potin, P.; Kloareg, B.; Boyen, C.; Peters, A.F.; Cock, J.M. Development and physiology of the brown alga Ectocarpus siliculosus: Two centuries of research. New Phytol. 2007, 177, 319–332. [Google Scholar] [CrossRef]
- Coelho, S.M.; Peters, A.F.; Müller, D.; Cock, J.M. Ectocarpus: An evo-devo model for the brown algae. EvoDevo 2020, 11, 19. [Google Scholar] [CrossRef]
- Cock, J.M.; Sterck, L.; Rouzé, P.; Scornet, D.; Allen, A.E.; Amoutzias, G.; Anthouard, V.; Artiguenave, F.; Aury, J.-M.; Badger, J.H.; et al. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 2010, 465, 617–621. [Google Scholar] [CrossRef]
- Prigent, S.; Collet, G.; Dittami, S.M.; Delage, L.; Ethis de Corny, F.; Dameron, O.; Eveillard, D.; Thiele, S.; Cambefort, J.; Boyen, C.; et al. The genome-scale metabolic network of Ectocarpus siliculosus (EctoGEM): A resource to study brown algal physiology and beyond. Plant J. 2014, 80, 367–381. [Google Scholar] [CrossRef]
- Badis, Y.; Scornet, D.; Harada, M.; Caillard, C.; Godfroy, O.; Raphalen, M.; Gachon, C.M.M.; Coelho, S.M.; Motomura, T.; Nagasato, C.; et al. Targeted CRISPR-Cas9-based gene knockouts in the model brown alga Ectocarpus. New Phytol. 2021, 231, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Prigent, S.; Frioux, C.; Dittami, S.M.; Thiele, S.; Larhlimi, A.; Collet, G.; Gutknecht, F.; Got, J.; Eveillard, D.; Bourdon, J.; et al. Meneco, a topology-based gap-filling tool applicable to degraded genome-wide metabolic networks. PLoS Comput. Biol. 2017, 13, e1005276. [Google Scholar] [CrossRef] [PubMed]
- Frioux, C.; Fremy, E.; Trottier, C.; Siegel, A. Scalable and exhaustive screening of metabolic functions carried out by microbial consortia. Bioinformatics 2018, 34, i934–i943. [Google Scholar] [CrossRef] [PubMed]
- Le Bail, A.; Billoud, B.; Kowalczyk, N.; Kowalczyk, M.; Gicquel, M.; Le Panse, S.; Stewart, S.; Scornet, D.; Cock, J.M.; Ljung, K.; et al. Auxin metabolism and function in the multicellular brown alga Ectocarpus siliculosus. Plant Physiol. 2010, 153, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Rabillé, H.; Billoud, B.; Tesson, B.; Le Panse, S.; Rolland, É.; Charrier, B. The brown algal mode of tip growth: Keeping stress under control. PLOS Biol. 2019, 17, e2005258. [Google Scholar] [CrossRef]
- KleinJan, H.; Jeanthon, C.; Boyen, C.; Dittami, S.M. Exploring the cultivable Ectocarpus microbiome. Front. Microbiol. 2017, 8, 2456. [Google Scholar] [CrossRef] [PubMed]
- Burgunter-Delamare, B.; KleinJan, H.; Frioux, C.; Fremy, E.; Wagner, M.; Corre, E.; Le Salver, A.; Leroux, C.; Leblanc, C.; Boyen, C.; et al. Metabolic complementarity between a brown alga and associated cultivable bacteria provide indications of beneficial interactions. Front. Mar. Sci. 2020, 7, 85. [Google Scholar] [CrossRef]
- Karimi, E.; Dittami, S.M. Maintaining beneficial alga-associated bacterial communities under heat stress: Insights from controlled co-culture experiments using antibiotic-resistant bacterial strains. FEMS Microbiol. Ecol. 2023, 99, fiad130. [Google Scholar] [CrossRef]
- Strittmatter, M.; Grenville-Briggs, L.J.; Breithut, L.; Van West, P.; Gachon, C.M.M.; Küpper, F.C. Infection of the brown alga Ectocarpus siliculosus by the oomycete Eurychasma dicksonii induces oxidative stress and halogen metabolism. Plant Cell Environ. 2016, 39, 259–271. [Google Scholar] [CrossRef]
- Tapia, J.E.; González, B.; Goulitquer, S.; Potin, P.; Correa, J.A. Microbiota influences morphology and reproduction of the brown alga Ectocarpus sp. Front. Microbiol. 2016, 7, 197. [Google Scholar] [CrossRef]
- Dittami, S.M.; Duboscq-Bidot, L.; Perennou, M.; Gobet, A.; Corre, E.; Boyen, C.; Tonon, T. Host-microbe interactions as a driver of acclimation to salinity gradients in brown algal cultures. ISME J. 2016, 10, 51–63. [Google Scholar] [CrossRef]
- Dittami, S.M.; Peters, A.F.; West, J.A.; Cariou, T.; KleinJan, H.; Burgunter-Delamare, B.; Prechoux, A.; Egan, S.; Boyen, C. Revisiting Australian Ectocarpus subulatus (Phaeophyceae) from the Hopkins River: Distribution, abiotic environment, and associated microbiota. J. Phycol. 2020, 56, 821579. [Google Scholar] [CrossRef]
- Karimi, E.; Geslain, E.; KleinJan, H.; Tanguy, G.; Legeay, E.; Corre, E.; Dittami, S.M. Genome sequences of 72 bacterial strains isolated from Ectocarpus subulatus: A resource for algal microbiology. Genome Biol. Evol. 2020, 12, 3647–3655. [Google Scholar] [CrossRef]
- Dittami, S.M.; Eveillard, D.; Tonon, T. A metabolic approach to study algal-bacterial interactions in changing environments. Mol. Ecol. 2014, 23, 1656–1660. [Google Scholar] [CrossRef]


| Toxin | Nature of Chemical Compound | Effect |
|---|---|---|
| Orfamide A [70,102,103] | Cyclic lipopeptide | Inhibits algal growth on plates and in liquid culture Changes cell morphology Inhibits algal motility (IC50 4.1 µM) Deflagellates algal cells |
| Protegencin [101] | Polyyne | Inhibits algal growth in liquid culture Destroys the algal eyespot Lyses the algal cells |
| Pyoluteorin [102] | Natural antibiotic (hybrid stemming from a nonribosomal peptide synthase and a polyketide synthase [105]) | Inhibits algal growth on plates and in liquid culture Changes cell morphology Inhibits algal motility (IC50 95 µM) Deflagellates algal cells |
| Pyrrolnitrin [102] | Phenylpyrrole compound | Inhibits algal growth on plates and in liquid culture Changes cell morphology Inhibits algal motility (IC50 20 µM) Deflagellates algal cells |
| Rhizoxin S2 [102] | 16-membered lactone ring connected to an oxazole ring by a long unsaturated chain [106] | Inhibits algal growth on plates and in liquid culture Changes cell morphology |
| 2,4-Diacetylphloroglucinol [102] | Phenol compound | Inhibits algal growth on plates and in liquid culture Changes cell morphology Inhibits algal motility (IC50 350 µM) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgunter-Delamare, B.; Shetty, P.; Vuong, T.; Mittag, M. Exchange or Eliminate: The Secrets of Algal-Bacterial Relationships. Plants 2024, 13, 829. https://doi.org/10.3390/plants13060829
Burgunter-Delamare B, Shetty P, Vuong T, Mittag M. Exchange or Eliminate: The Secrets of Algal-Bacterial Relationships. Plants. 2024; 13(6):829. https://doi.org/10.3390/plants13060829
Chicago/Turabian StyleBurgunter-Delamare, Bertille, Prateek Shetty, Trang Vuong, and Maria Mittag. 2024. "Exchange or Eliminate: The Secrets of Algal-Bacterial Relationships" Plants 13, no. 6: 829. https://doi.org/10.3390/plants13060829






